Abstract
Attending preferentially to social information in the environment is important in developing socio-communicative skills and language. Research using eye-tracking to explore how individuals with autism spectrum disorder (ASD) deploy visual attention has increased exponentially in the last decade; however, studies have typically not included minimally-verbal participants. In this study we compared 37 minimally-verbal children and adolescents with ASD (MV-ASD) with 34 age-matched verbally-fluent individuals with ASD (V-ASD) in how they viewed a brief video in which a young woman, surrounded by interesting objects, engages the viewer, and later reacts with expected or unexpected gaze shifts towards the objects. While both groups spent comparable amounts of time looking at different parts of the scene and looked longer at the person compared to the objects, the MV-ASD group spent significantly less time looking at the person’s face during the episodes where gaze following – a precursor of joint attention— was critical for interpreting her behavior. Proportional looking-time toward key areas of interest (AOIs) in some episodes correlated with receptive language measures. These findings underscore the connections between social attention and the development of communicative abilities in ASD.
Keywords: minimally-verbal ASD, visual social attention, eye tracking, dynamic scene
Investigations of visual social attention in autism spectrum disorders (ASD) have surged in the last decade, especially after unobtrusive eye-tracking technology became widely available for research. Enthusiasm for this topic was incited, in part, by the intriguing findings reported by Klin and colleagues in 2002 (Klin, Jones, Schultz, Volkmar, & Cohen, 2002a, 2002b), who examined the visual fixation patterns of adolescents and adults watching highly emotionally charged scenes from the 1967 movie version of Edward Albee’s play Who’s afraid of Virginia Woolf? These authors found that, in contrast to the visual scanning patterns showed by neurotypical peers, who consistently focused on the protagonists’ faces, in particular the eye region, the participants with ASD looked significantly less at the eyes and more at the protagonists’ mouth, body or various objects in the scenes. Since this seminal study, research using eye-tracking to explore how individuals with ASD orient to and engage attention towards social and nonsocial stimuli has increased rapidly, but findings of atypicalities in visual attention deployment remain mixed (see Frazier et al., 2017; Guillon, Hadjikhani, Baduel, & Rogé, 2014, Papagiannopoulou et al., 2014, for recent reviews and meta-analyses of eye-tracking studies).
Eye-movements have been studied as measures of attention monitoring, interest, problem solving and language comprehension in older verbal individuals with ASD (e.g., Bavin et al., 2014; Klin, et al., 2002b; Sasson, Turner-Brown, Holtzclaw, Lam, & Bodfish, 2008; Venker, Eernisse, Saffran, & Weismer, 2013) and, more recently, in infants and toddlers (e.g., Chawarska, Macari, & Shic, 2013; Elsabbagh et al., 2012; Jones & Klin, 2013; Pierce et al., 2016). Much research has been conducted on the deployment of attention to faces as potential windows into the mechanisms underlying the social impairments found in ASD (Dawson, Webb, & McPartland, 2005; Sasson, 2006; Schultz, 2005; Weigelt, Koldewyn, & Kanwisher, 2012). Difficulty processing information from faces early in development has been linked to socio-cognitive limitations that hinder the acquisition of language, a process heavily dependent on social interactive processes, such as initiating and responding to episodes of joint attention, which involve gaze monitoring (Bedford et al., 2012; Chawarska, & Shic, 2009; Chawarska, Macari, & Shic, 2012; Mundy, Sigman & Kasari, 1990). The ability to follow a person’s gaze is an important prerequisite for joint attention (Butler, Caron, & Brooks, 2009; Carpenter, Nagell, & Tomasello, 1998; Shepherd, 2010), which plays a significant role in the development of communication abilities and language in both typical development (e.g., Baldwin, 1995; Moore & Dunham, 1995; Tomasello & Farrar, 1986) and in autism (Adamson, Bakeman, Deckner & Romski, 2009; Akechi et al., 2911; Baron-Cohen, Baldwin, & Crowson, 1997; Charman, 2003; Leekam Lopez & Moore, 2000; Loveland & Landry, 1986; Mundy, Sigman, & Kasari, 1994; Toth, Munson, Meltzoff, & Dawson, 2006). Therefore, it is not surprising that an extensive body of research has examined this foundational ability in young children with ASD or in infants at risk for ASD, compared to those developing typically. A majority of these studies concluded that sensitivity to eye-gaze is atypical in ASD, as shown by children’s difficulties with spontaneously following another person’s eye gaze to share attention (Bedford et al., 2012; Gillespie-Lynch et al., 2013). However, evidence for typical attentional cueing from eye-gaze direction has also emerged, especially when evaluated using experimental tasks (see Nation & Penny, 2008; Falck-Ytter & von Hofsten, 2011 for reviews). Leekam and colleagues (1998) found that differences between school age children with ASD in their ability to orient spontaneously to another person’s head turn depended on their verbal mental ages, reporting that mainly children with mental ages under 48 months had difficulties with spontaneous gaze following. Research with older verbal individuals with ASD, using more complex stimuli, such as brief videos of social scenes, commonly focused on allocation of social attention during free viewing of the images/videos. Social attention in this context refers to the process of directing attention to aspects of people in a scene (Chevallier et al. 2015). Studies using eye-tracking technology usually compared looking time to people/faces versus nonsocial information (objects, background), and yielded mixed results across studies and tasks: some researchers reported that individuals with ASD without intellectual disabilities showed a reduced likelihood to follow a protagonist’s gaze spontaneously when viewing a social scene (Fletcher-Watson, Leekam, Benson, Frank, & Findlay, 2009; Norbury et al., 2009; Riby & Hancock, 2008; Riby, Hancock, Jones, & Hanley, 2013); in contrast, others have reported typical patterns of looking behavior in response to gaze cueing in participants with ASD who have IQ within normal range (Freeth, Chapman, Ropar, & Mitchell, 2010). Examining visual attention to social scenes in teenagers, Norbury and colleagues (2009) found differences in viewing patterns related to participants’ language status (e.g., between those with and without language impairments), while Rice and colleagues (Rice, Moriuchi, Jones, & Klin, 2012) reported significant variation in children’s visual scanning of complex social scenes based on four distinct cognitive profiles among non-intellectually disabled children with ASD.
In sum, numerous studies have documented atypical patterns of social attention orienting in individuals with ASD across a range of experimental paradigms, and in real or simulated social interactions (Caruana, McArthur, Woolgar & Brock, 2017; Franchini et al., 2017; Shic, Bradshaw, Klin, Scassellati & Chawarska, 2011; Senju, Tojo, Dairoku, & Hasegawa, 2004), but few have focused on individual differences across the wide spectrum of abilities in ASD. So far, eye tracking studies have shown that findings depended on the tasks and type of stimuli used (isolated faces/objects, complex scenes, static images or dynamic stimuli, cf. Chevalier et al., 2015; Speer, Cook, McMahon, & Clark, 2007), on the context and task demands (experimental, passive viewing, interactive, cf. Noris, Nadel, Barker, Hadjikani & Billard, 2012; Freeth, Foulsham, & Kingstone, 2013), as well as on sample characteristics (intellectual functioning, age and verbal mental age, or communication abilities, cf. Leekam et al, 2000; Norbury et al. 2009; Rice et al. 2012). A relatively small sample size and the exclusion of individuals with ASD with more severe intellectual disabilities are common limitations of many of these studies, restricting the generalizability of the findings with respect to the broad autism spectrum. Even when studies included larger, heterogeneous samples of individuals with ASD and focused on patterns of variability in visual social engagement (Rice, et al., 2012), participants’ average IQ was not in the range of intellectual disability (i.e., standard score below 70).
Only recently have investigators started to focus on associations between eye-movement data and other phenotypic characteristics besides autism symptom severity, such as expressive and receptive language. Findings of these studies generally supported the hypothesis of a significant relationship between social attention and communication ability profiles in both young children and adolescents with ASD (e.g., Chawarska et al., 2012; Murias et al. 2018; Norbury, et al. 2009). The span of verbal abilities among individuals with ASD ranges from those who remain nonverbal into adulthood to those who become highly proficient in their expressive language (Kim, Paul, Tager-Flusberg, & Lord, 2014). Yet the sources of this heterogeneity and their possible links to processes of social attention deployment are not well understood.
As noted, the majority of previous research focused either on young, preverbal infants and toddlers (Chawarska et al., 2013; Dawson et al., 1998; Elsabbagh et al., 2013; Jones & Klin, 2013; Klin, Lin, Gorrindo, Ramsay & Jones, 2009; Swettenham, et al., 1998) or on older children, adolescents and adults who are able to speak (Fletcher-Watson et al., 2009; Klin et al., 2002b; Riby & Hancock, 2008; Riby et al., 2013). To date, it is unknown whether the approximately 30% of individuals with ASD who do not develop functional speech by school-age differ in their attention allocation to social and nonsocial information in the environment, or whether their language and communication limitations are related to particular difficulties in attending to and processing socially relevant cues. Because of the challenges in testing this population, they have generally not been included as study participants in earlier research (Tager-Flusberg & Kasari, 2013; Tager-Flusberg et al., 2017).
The current study was motivated by two main goals: One was to investigate whether distinctive patterns of visual social attention differentiated minimally-verbal (MV-ASD) from verbally-fluent (V-ASD) individuals with ASD, when viewing naturalistic dynamic scenes. Given that the ability to follow gaze is an important prerequisite for joint attention, we were interested in examining whether MV-ASD children and adolescents were sensitive to the attentional focus of a protagonist in a naturalistic scenario, as indicated by following the gaze and head turn of a person shown in a brief video. Another goal was to examine whether visual social attention was related to measures of language ability and to diagnostic measures of autism symptomatology. We presented participants with a brief video modeled after a task used by Chawarska and colleagues (2012), which was adapted to make it more appropriate for older children and adolescents. The video depicted a young woman making a snack at a table, surrounded by four interesting objects. In the video, the protagonist addresses the viewer in greeting, comments on her activity, and then reacts to the sudden movement of one of the objects, a mechanical toy spider, by shifting her gaze appropriately toward the moving object. In a later episode when the spider moves again, the woman shifts her gaze unexpectedly, toward an object placed opposite the spider (a static panda). Our primary aim was to explore whether the two groups differed in their allocation of visual attention to the protagonist and the objects in the video as a function of the events presented. More specifically, we hypothesized that the V-ASD participants would pay more attention to the protagonist’s face and gaze behavior, especially in the unexpected gaze-shift episode, when her behavior should surprise typical viewers. We predicted that in the latter episode the V-ASD participants would demonstrate the tendency to spontaneously follow the protagonist’s gaze toward the target of her attention (i.e., will follow her gaze/head direction of movement toward the panda), whereas this viewing pattern will be diminished or absent in the MV-ASD group. We also predicted that visual attention toward the protagonist— in particular, her face and direction of gaze, as well as the target of her attention— would be positively related to measures of language ability and negatively related to aspects of autism symptom severity.
METHODS
Participants
Participants were 71 individuals with ASD, divided into two groups based on language ability. Thirty-seven participants (8 girls) ranging in age between 8.6 and 20.2 years (M = 13.56 years, SD =3.4) were described by their parents as having little to no functional speech used in a range of social contexts. Criteria for assignment to the minimally-verbal group (MV-ASD) included lack of spontaneous functional speech or inconsistent simple phrase speech of no more than three units, as defined by the Autism Diagnostic Observation Schedule-Second Edition (ADOS-2; Lord et al., 2012) Module 1. This definition of MV-ASD has been used in previous literature (Bal et al. 2016). The other 34 participants (8 girls), aged between 8.9 and 20.9 years, (M = 14.97 years, SD = 3.4) were verbally-fluent (V-ASD) and used complex phrase speech consistently. Diagnoses of all participants were confirmed using the ADOS-2 and the Autism Diagnostic Interview-Revised (ADI-R; Le Couteur, Lord, & Rutter, 2003). The MV-ASD participants were administered Module 1 of either the ADOS-2 or the Adapted ADOS (A-ADOS; Lord, Rutter, DiLavore & Risi, 2012), depending on their age: the MV-ASD participants over 12 years were assessed with the Adapted ADOS, which uses play materials more appropriate and engaging for adolescents. The V-ASD participants were administered Modules 3 or 4 of the ADOS-2, as appropriate for their age and language level. Social-affective and restrictive and repetitive behavior symptom severity were calculated with the ADOS calibrated symptom severity scores, which are comparable across ADOS modules (Hus, Gotham, & Lord, 2014). Table 1 summarizes the demographic characteristics of the two groups.
Table 1.
Demographic Characteristics of Participants
| MV-ASD (N = 37) |
V-ASD (N = 34) |
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| Chronological Age (years) | 13.56 | 3.5 | 14.97 | 3.4 |
| Gender: | ||||
| Male/Female | 29/8 | 26/8 | ||
| Race (N): | ||||
| African-American | 2 | 2 | ||
| Asian | 4 | 2 | ||
| White | 27 | 22 | ||
| Hispanic | 0 | 3 | ||
| Native Hawaiian or other Pacific islander | 1 | 0 | ||
| More than one race | 3 | 5 | ||
The Peabody Picture Vocabulary Test (PPVT-4; Dunn & Dunn, 2007) was administered to assess receptive word knowledge. Nonverbal IQ was assessed using the Leiter-3 (Roid, Miller, Pomplun & Koch, 2013) for the MV-ASD participants, and the WASI-II (Wechsler, 2011) for the V-ASD participants. The Leiter-3 is a test commonly used with minimally- and low-verbal individuals with ASD (Kasari, Brady, Lord, & Tager Flusberg, 2013) because it does not require verbal instructions or verbal responding, facilitating a reliable assessment of nonverbal reasoning abilities relatively independent of language. The Perceptual Reasoning Index of the WASI-II was used to obtain an estimate of nonverbal IQ for the V-ASD group. In addition to the ADI-R, parents completed the Vineland Adaptive Behavior Scales-2 (VABS-2; Sparrow, Cicchetti, & Balla, 2005), administered in an interview format. Table 2 summarizes the descriptive characteristics of the groups.
Table 2.
Behavioral Characteristics of Participants
| MV-ASD (N = 37) |
V-ASD (N = 34) |
|||
|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | |
| Receptive vocabulary1 | 25.88 (10.4) | 20 – 64 | 101.53 (25.9) | 31 – 135 |
| Nonverbal reasoning2 | 62.14 (14.7) | 30 – 94 | 104.15 (23.2) | 64 – 152 |
| VABS-2 Adaptive Behavior Composite | 48.94 (8.9) | 30 – 69 | 76.52 (13.4) | 37 – 104 |
| VABS-2 Communication | 47.72 (9.9) | 28 – 70 | 78.97 (17.8) | 42 – 118 |
| Receptive Language3 | 26.88 (14.5) | 1 – 59 | 102.58 (71.6) | 16 – 216 |
| Expressive Language4 | 20.61 (10.8) | 3 – 42 | 109.24 (81.3) | 5 – 276 |
| ADI-R | ||||
| Total A5 | 26.83 (3.4) | 15 – 30 | 20.58 (5.0) | 10 – 29 |
| Total B (NV)6 | 12.47 (1.8) | 9 – 14 | ||
| Total B (V)7 | 15.15 (5.2) | 7 – 25 | ||
| ADOS severity scores: | ||||
| Overall CSS | 7.73 (1.5) | 6 – 10 | 7.58 (2.3) | 1 – 10 |
| SA-CSS | 7.24 (1.5) | 5 – 10 | 7.27 (2.3) | 1 – 10 |
| RRB-CSS | 8.49 (1.5) | 5 – 10 | 7.67 (2.0) | 1 – 10 |
Standard scores from the PPVT-4 assessment.
Standard scores from the Leiter-3 for the MV-ASD group and from the WASI-perceptual reasoning scale for the V-ASD group.
Age equivalent scores in months.
ADI total on Qualitative abnormalities in reciprocal social interaction
ADI total on Qualitative abnormalities in communication – Nonverbal subjects
ADI total on Qualitative abnormalities in communication – Verbal subjects
All participants had normal or corrected to normal vision and no significant sensory or neurological impairments, according to a brief medical history survey completed by parents. Only participants from predominantly English-speaking homes were included in the study. Informed consent and participant assent were obtained from caregivers and from V-ASD participants, as appropriate. All study procedures were approved by the Institutional Review Board of the university where the study was conducted.
Procedures
Eye-Tracking Task
Participants’ eye movements were recorded with a TOBII T60 XL eye-tracker run by Tobii Studio 2.0.3 software (Tobii Technology AB, Danderyd, Sweden). This system requires no headgear and has relatively high tolerance for head movements. We used a 5-point calibration and adapted the choice of calibration method (adult or infant) to each participant. The choice of calibration method was dictated by the need to maximize the likelihood of attracting a fixation with minimum verbal instructions. Five and even 2-point calibration methods are commonly used with individuals with severe intellectual disabilities (Wilkinson & Mitchell, 2014).
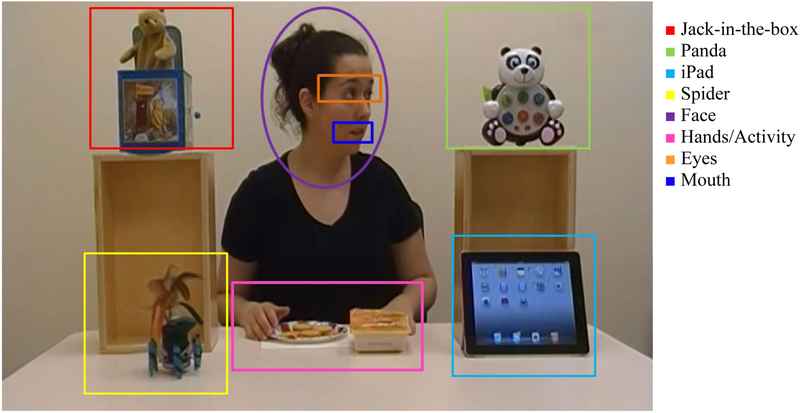
The eye-tracking task featured a video of a young woman making a snack. The movie display area was a rectangle subtending 35 × 23.4 degrees of visual angle. Four interesting objects were placed surrounding the woman, who was shown in the center of the scene seated at a table facing the camera. The objects (iPad, toy-Spider, toy-Panda, Jack-in-box toy) were about the same size, subtending 8.9 × 8.9 degrees of visual angle, and were positioned on the table and on top of two boxes placed on the left and the right side of the protagonist. Other AOIs included the face/head of the protagonist (8.7 × 10.4 degrees of visual angle) and the hands/activity area (11.8 × 7.4 degrees of visual angle). The sub-regions of the eyes and mouth subtended 5.2 × 2 and 4 × 1.6 degrees of visual angle, respectively (see Figure 2). The video was divided into 6 episodes based on the protagonist’s behavior (see Table 3). Three episodes were critical for assessing social visual attention: (A) episode 2: ‘Verbal greeting’ in which the protagonist lifts her head, looks toward the camera and addresses the viewer; (B) episode 3: ‘Expected gaze-shift’ showing the mechanical toy-spider starting to move on the table and the protagonist’s gaze following the spider’s movement; (C) episode 5: ‘Unexpected gaze-shift’ depicting the spider moving again, but the protagonist looking toward an unmoving object (a panda bear) placed diagonally opposite from the spider. It should be noted that in the gaze-shift episodes the young woman also turns her head, not just the eyes, toward the object of her attentional focus, so there is no ambiguity about her direction of gaze for the viewer watching her behavior. The total duration of the video is 75 seconds, and the duration of each episode is listed in Table 3.
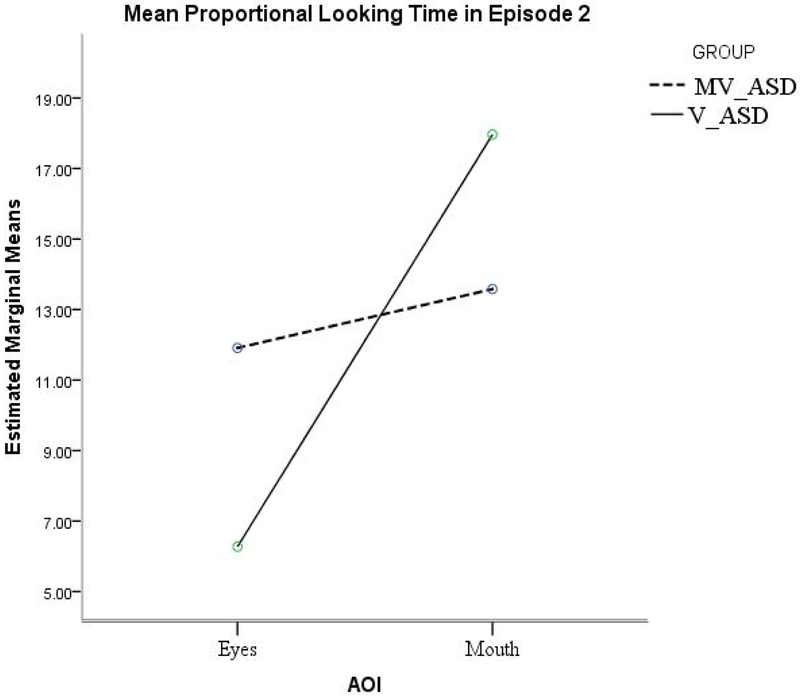
Figure 2.
Episode 2– Proportional looking-time (mean %) at the protagonist’s Eyes and Mouth, by group
Table 3.
Description of the movie episodes
| Duration (Seconds) | Event | Audio-track | |
|---|---|---|---|
| Episode 1 | 22 | Protagonist starts preparing a snack; she looks down at the box of crackers and plate she placed on the table | Silent episode |
| Episode 2 | 11 | Protagonist looks directly into the camera and speaks to the viewer, then resumes preparing the snack. |
Oh hi, how are you? It’s my snack time… |
| Episode 3 | 8 | Mechanical spider starts to move across the table; protagonist shifts gaze towards the spider and follows its movement until it stops. | Silent episode |
| Episode 4 | 15 | Protagonist turns her attention back to preparing the snack and speaks to the viewer | I’m making cheese and crackers… |
| Episode 5 | 12 | Spider starts moving across the table; Protagonist shifts gaze in opposite direction, looking at the toy panda bear. | Silent episode |
| Episode 6 | 7 | Protagonist resumes preparing the snack and speaks toward the camera. |
Now I’m ready to eat. I am hungry. |
Participants were seated approximately 60 cm from the monitor, with eye-level approximately even with the center of the scene. Up to five calibration attempts were conducted with each participant, at successive visits if needed, before the task was administered. After successful calibration, the participants’ compliance and interest in watching the movie varied significantly and the amount of valid data contributed by each participant across the video duration, according to the TOBII system, ranged from 1% to 93% in the MV-ASD group (M = 49.5%, SD = 29.1) and from 2% to 99% in the V-ASD group (M= 62.3%; SD = 35.1). We included in analyses participants with more than 15% valid data across the movie duration, with the additional constraint that they needed to provide data in at least 5 of the 6 episodes of the video. Nine MV-ASD participants who had no fixations in two or more episodes or provided less than 15% valid data across the video were excluded from further analyses. Five V-ASD participants were excluded based on these criteria, resulting in 28 MV-ASD participants and 29 V-ASD participants with gaze data included in analyses. Because our main interest was in capturing the characteristics of visual attention allocation to a complex scene by MV-ASD individuals who have ordinarily not been included in eye tracking studies, we could not afford to employ more stringent gaze data validity criteria without having to exclude a significant number of participants, potentially biasing the characterization of the attentional processes that may be distinctive to this ASD subpopulation. The MV-ASD and V-ASD groups were matched on chronological age, F (1, 56) = 0.25, p = .88 and on ADOS calibrated severity scores (Gotham, Pickles & Lord, 2009). The excluded participants within each group did not differ on age, receptive language, IQ or ADOS symptom severity (based on ADOS calibrated severity score; CSS) from those who were retained.
Analytic approach
First, we compared the groups in their overall attention across all episodes by calculating proportional looking time to the video (i.e., their gaze falling within the media frame) relative to the video total duration, to obtain an individual measure of general attention to the dynamic scene. Individual looking-time at the video was used in later analyses to calculate proportional looking-time within each AOI. More specifically, all analyses involving within-AOIs visual fixation data were conducted on proportional variables calculated as looking-time within a particular AOI divided by the participant’s total looking-time at the scene (i.e., within the media frame), considered both across the movie duration and within the duration of each episode. This approach was intended to mitigate the potential biasing effects of missing data in particular episodes when analyzing participants’ attention allocation to predefined AOIs relative to the key video events. Because of the differences in cognitive functioning between the two groups, we co-varied nonverbal IQ standard scores (NVIQ) in all analyses of proportional looking time data.
Next, we analyzed participants’ distribution of visual attention to the person and the four objects collapsed across all 6 episodes, to examine whether the salience – as indexed by proportional viewing time – of social (protagonist) and nonsocial (toys) elements of the dynamic scene, differed for the two groups.
The next set of analyses explored attention to specific AOIs that were tied to a priori predictions based on salient events in each of the key episodes. We tested whether AOIs and episodes differentially influenced viewing time in the MV-ASD and the V-ASD groups with a mixed model ANCOVA and followed main effects and interactions with post hoc comparisons reported by key episode. Because the primary purpose of the study was to determine whether and how MV-ASD individuals differ from V-ASD peers in their visual attention allocation to salient AOIs as a function of the events in the video, we prioritized reporting comparisons between participant groups, within key episodes, for particular AOIs relevant for interpreting the scene: face, in episodes 2 (protagonist addresses the viewer), 3 (protagonist shifts gaze toward the moving spider) and 5 (protagonist shifts gaze toward the stationary panda), spider in episodes 3 and 5 (in which it starts to move unexpectedly), and panda in episode 5 (because it is the target of the protagonist’s unexpected gaze shift).
We also compared the two groups in the proportion of individuals who made a responsive fixation toward the targets of the protagonist’s gaze after looking at her face in the two gaze-shifting episodes. This additional nonparametric approach was meant to test whether participants in the two groups showed a spontaneous gaze following tendency, regardless of the amount of viewing time spent within the relevant AOIs. Participants were categorized into those who did and those who did not make a fixation in the relevant AOIs in the key episodes, and chi square tests were used to compare the MV-ASD and V-ASD groups based on these categories of responders.
Finally, to determine whether social attention as indexed by looking-time data was related to language abilities and to autism symptom severity, we investigated correlations between proportional looking-time to the specific AOIs listed above and scores on measures of receptive and expressive language, and ratings of autism symptomatology.
RESULTS
Overall viewing of the video
A one-way ANCOVA conducted on looking-time at the scene relative to the total video duration, controlling for NVIQ, yielded a significant group effect, F(1, 56) = 4.83, p =.032, η2= .081 showing that the MV-ASD group spent on average less time (M = 56.5%) than did the VASD group (M = 72.2%) attending to the video overall. However, the groups did not differ in their initial attention to the video during the first episode, F (1, 56) = .538, p =.46. When controlling for individual looking time at the scene (i.e., within the media frame), the proportional viewing time spent within the 6 most relevant AOIs (i.e., the sum of looking time spent within the 6 non-overlapping AOIs –Face/Head, Hands/Activity area, Spider, Panda, iPad, Jack-in-the-box --- divided by the individual time spent looking at the entire screen) did not differ by group: F (1, 56) = .353, p =.56. Both groups looked at the relevant AOIs on average for over 85% of the time they attended to the screen (85.3% for the MV-ASD and 88% for the VASD, respectively). Table 4 presents the proportion of valid looking time by participant group for each of the three key episodes.
Table 4.
Proportional looking time per AOI and key episode, and percentage of participants who made a responsive fixation to selected AOIs in the episodes involving gaze-sifting.
| MV-ASD (N = 28) |
V-ASD (N = 29) |
|||||
|---|---|---|---|---|---|---|
| Episode | M | (SD) | M | (SD) | F / χ2 | p-value |
| 2. Verbal greeting | ||||||
| % valid time | 89.83 | 16.1 | 91.8 | 14.75 | .76 | ns |
| %Hands/activity | 32.33 | 25.02 | 40.51 | 20.74 | 3.98 | ns |
| %Face | 32.27 | 27.94 | 37.98 | 18.17 | 2.09 | ns |
| %Mouth | 13.91 | 21.75 | 17.38 | 15.93 | 1.59 | ns |
| %Eyes | 11.29 | 20.48 | 6.49 | 11.07 | 0.613 | ns |
| %Toy-Spider | 10.86 | 22.05 | 6.62 | 7.17 | 1.31 | ns |
| %iPad | 3.24 | 6.37 | 1.32 | 4.16 | 2.89 | ns |
| % Toy-Panda | 3.59 | 5.14 | 1.52 | 3.36 | 3.07 | .ns |
| % Jack in the box | 5.06 | 10.48 | 3.85 | 10.35 | 0.271 | ns |
| 3. Expected gaze-shift | ||||||
| % valid time | 85.16 | 28.98 | 88.02 | 22.37 | 1.64 | ns |
| %Hands/activity | 14.92 | 16.69 | 13.92 | 12.02 | 1.57 | ns |
| %Face | 10.48 | 15.37 | 21.17 | 15.51 | 10.16 | .002 |
| %Mouth | 4.53 | 11.87 | 7.08 | 9.32 | 3.77 | .ns |
| %Eyes | 1.75 | 3.76 | 5.01 | 10.93 | 2.37 | ns |
| %Toy-Spider | 36.88 | 30.34 | 47.03 | 28.5 | 3.61 | ns |
| %iPad | 6.01 | 19.32 | 0.79 | 1.55 | 2.45 | ns |
| % Toy-Panda | 5.12 | 11.82 | 1.29 | 3.22 | 1.73 | .ns |
| % Jack in the box | 4.56 | 9.07 | 3.89 | 8.43 | 0.123 | ns |
| % of N who made a responsive fixation to spider | 75% | 96.6% | 5.48 | .019 | ||
| 5. Unexpected gaze-shift | ||||||
| % valid time | 76.83 | 31.74 | 87.83 | 22.93 | 1.86 | ns |
| %Hands/activity | 35.68 | 26.76 | 33.47 | 18.59 | 0.630 | ns |
| %Face | 12.47 | 14.17 | 23.99 | 15.34 | 9.05 | .004 |
| %Mouth | 3.42 | 6.35 | 9.33 | 11.52 | 7.01 | .011 |
| %Eyes | 4.30 | 9.08 | 7.80 | 10.39 | 3.73 | .051 |
| %Toy-Spider | 16.22 | 16.68 | 21.67 | 14.95 | 3.28 | ns |
| %iPad | 4.46 | 12.08 | 0.753 | 1.35 | 1.16 | ns |
| % Toy-Panda | 2.79 | 8.05 | 5.56 | 7.07 | 6.9 | .011 |
| % Jack in the box | 5.21 | 14.97 | 1.32 | 2.37 | 0.78 | ns |
| % of N who made a responsive fixation to panda | 21.4% | 55.2% | 6.84 | .009 | ||
| % of N who made a responsive fixation to spider | 71.4% | 89.7% | 3.04 | ns | ||
As noted above, analyses of visual attention to particular AOIs were conducted on proportional looking-time data (i.e., variables of interest were standardized by individual looking-time at the scene across or within episodes, respectively). An inspection of these data revealed a positively skewed distribution; therefore, logarithmic transformations were applied to normalize the data distribution. For ease of interpretation, however, table 4 presents the untransformed percentages of looking-time within AOIs relative to individual time attending to the scene in the three key episodes.
Distribution of overall visual attention between the Person and Objects
We first compared the groups in their proportional attending to the Objects (i.e., the sum of looking at the iPad, Panda, Spider and Jack-in-the-box relative to individual looking at the scene) versus attending to the protagonist (i.e., looking at the face and the hands/activity area, relative to looking at the scene) during the entire duration of the movie. A mixed model ANCOVA with AOI (Person, Objects) as the within-subjects factor and group (MV-ASD vs. V-ASD) as the between-subjects factor on proportional looking-time measured across the movie duration yielded a significant main effect of AOI, F (1, 50) = 4.13, p = .04, ηp 2 = .076, but no main effect of group F (1, 50) = .55, p=.461 or interaction between group and AOI, F (1, 50) = 2.29, p=.14. Both groups looked proportionally longer at the Person (M = 54.87%, SD = 29.53 in the MV-ASD group and M = 62.52%, SD = 18.92 in the V-ASD group, respectively) than at the Objects (M= 29.53%, SD = 14.19 in the MV-ASD group and M= 25.5%, SD = 11.92 in the V-ASD group, respectively) across the six episodes.
Next, we examined whether the participants’ allocation of attention to the objects and to the protagonist depended on the content of the events viewed, as defined by the protagonist’s behavior toward the viewer in episode 2 (verbal greeting), and toward the moving and stationary objects in the scene (in episodes 3 and 5 in which the protagonist shifts her gaze to objects). We conducted analyses of proportional looking time in each AOI relative to individuals’ viewing time within each episode, to minimize potential biasing effects of missing data in particular episodes. All participants retained in analyses provided data in the three key episodes, 2, 3 and 5.
Distribution of attention within each AOI as a function of episode-content
An initial mixed model ANCOVA, with AOI (6) and episode (6) as within-subjects factors and group (2) as the between-subjects factor, covarying NVIQ, yielded a significant main effect of AOI, F (5, 250) = 6.25, p =.0001, ηp2 = .11, and a significant main effect of episode, F(5, 250) = 2.61, p =.02, ηp2 = .03, which were qualified by a significant 3-way interaction between AOI, episode and group, F (25, 1250) = 1.58, p = .035, ηp2 = .031. Following the significant 3-way interaction, we analyzed participant group differences in proportional looking-time to predicted AOIs within each key episode (2, 3 and 5). Table 4 presents the untransformed proportional looking-time data for every AOI by key episode and participant group.
Episode 2- Verbal greeting.
In this episode, we were primarily interested in whether the protagonist’s verbal greeting influenced how the MV-ASD vs V-ASD participants allocated attention to the face. Group differences for proportional attending to the face in this episode were not statistically significant, t (55) = −1.69, p = .096, with both groups spending about a third of their viewing time looking at the young woman’s face when she addressed the viewer (see Table 4).
An additional analysis was conducted for this episode involving only the eyes and mouth as AOIs: because the face AOI included both the mouth and the eye-region, we further investigated whether the group similarities in proportional viewing time of the face involved a similar or a different distribution of attention between the two face-features – eyes and mouth. A separate group (MV-ASD, V-ASD) X AOI (eyes, mouth) ANOVA for proportional looking time in episode 2 yielded a significant main effect of AOI, F (1, 55) = 5.24, p =.026, ηp2 = .088, but no significant group X AOI interaction, F(1, 55) = 1.72, p =.195, ηp2 = .03: both groups looked longer at the mouth than at the eyes in this episode (Figure 2 and Table 4).
Episode 3 – Expected gaze-shift
The primary comparisons of interest in this episode involved looking at the protagonist’s face as she turned her gaze toward a moving spider, and looking at the spider, which was the object of her attentional focus and was unexpectedly moving. Both groups looked significantly longer at the moving spider than at the protagonist’s face during episode 3, t (27) = −3.66, p = .001 in the MV-ASD group and t (28) = −2.6, p = .015 in the V-ASD group. However, the two groups differed significantly in their looking behavior at the face in this episode, as the MV-ASD participants spent on average proportionally less viewing time (10.5%) on the face AOI compared to the V-ASD group, who spent on average over 21% of their looking time on the protagonist’s face, t (55) = −3.18, p = .002. Proportional viewing time at the spider did not differ significantly between the MV-ASD and V-ASD groups in episode 3.
Episode 5- Unexpected gaze-shift
In episode 5, the primary comparisons of interest involved the protagonist’s face, the moving spider, and the panda toward which the young woman shifts her gaze unexpectedly. The groups differed significantly in their proportional viewing time for two AOIs: for the face, t (55) = −3.01, p = .004 and for the panda, t (55) = −2.63, p = .011, with the V-ASD participants looking proportionally longer at both these AOIs than the MV-ASD participants did (see Table 4).
Table 4 also presents the percentage of participants who made a responsive fixation to the panda after a fixation on the protagonist’s face, in each group. A significantly lower proportion of participants made at least a fixation on the panda among the MV-ASD individuals (21.4%) compared to 55.2 % of the V-ASD group, χ2 = 6.84, p = .009.
Relations between visual social attention and measures of cognition, language ability and autism symptomatology across and within episodes
First, we examined possible relations between proportional looking time in each relevant AOI, collapsed across episodes, and cognitive functioning (NVIQ), considering significance with Bonferroni correction at p = .008 (.05/6). Only the correlation between proportional looking time at the spider collapsed across episodes and NVIQ was significant, r (53) = .375, p =.002. Proportional looking time at the spider collapsed across episodes was also correlated with Vineland Adaptive Behavior composite score, r (50) = .376, p =.006, but no other gaze related variables were significantly correlated with any measures of cognition, communication, adaptive functioning or autism symptom severity when considered across episodes.
To address specific questions about possible relationships between gaze following ability, attending to another person’s attentional focus and language related skills, we conducted correlational analyses separately for the episodes involving the protagonist’s gaze shift (3 and 5), controlling for age and IQ. More specifically, we investigated whether looking-time at the AOIs that provided cues for interpreting the protagonist’s behavior in particular video segments (i.e., the young woman’s face and the spider in episodes 3 and 5; the panda in episode 5) correlated with language abilities.
In episode 3 (Expected gaze-shift), proportional looking-time at the protagonist’s face was positively correlated with PPVT-4 scores, after controlling for age and NVIQ, r (49) = .442, p = .001. Proportional looking time at the spider however, was not correlated with language measures in this episode, once NVIQ was partialled out. Proportional looking-time at the face was also positively correlated with PPVT-4 scores in episode 5 (Unexpected gaze-shift), r (48) = .348, p = .014. Interestingly, in episode 5, proportional looking-time at the panda – the object toward which the protagonist unexpectedly shifted her gaze when the spider started to move — was positively correlated with both PPVT-4 scores, r (47) = .309, p = .01, and with the Vineland Communication Domain score, r (47) =.364, p = .005, after controlling for age and NVIQ.
We further examined correlations among measures of autism symptom severity obtained from the ADOS and the ADI diagnostic assessments, and proportional looking-time spent on the protagonist’s mouth in episode 2, face/eyes and spider in episode 3, and face/eyes and panda in episode 5 (on both the ADOS and the ADI higher scores indicate more impairment). Only two looking time AOI-related-variables showed significant correlations with ASD symptomatology: in episode 3 proportional looking-time at the protagonist’s face was negatively related to scores on the ADI for qualitative abnormalities in reciprocal social interaction, r (41) = −.498, p = .001. In episode 5 looking-time at the protagonist’s eyes was negatively correlated with ADOS overall calibrated severity scores, r (51) = −.333, p = .007. No significant relationships were found between looking-time variables and ADOS calibrated severity scores for any other AOIs in any of the episodes.
Discussion
In this study, we compared MV-ASD children and adolescents with age-matched V-ASD participants in their viewing of naturalistic dynamic scenes, focusing on how they distributed attention to areas of the scene that involved social cues, such as a protagonist’s face and gaze-behavior. The majority of past research using eye-tracking methods to assess social attention in ASD has compared individuals with ASD with neurotypical controls. In the present study, we wanted to explore whether investigating similarities and differences between MV-ASD and V-ASD children and adolescents in their spontaneous viewing patterns of a naturalistic video clip could provide insights into the possible connections between social attention atypicalities and failure to acquire spoken language in individuals with autism. We hypothesized that proportional looking-time toward AOIs that provided social cues to interpreting the events in the video, especially in the episode when the protagonist’s behavior was unexpected, would be related positively to communication abilities and negatively to scores on ADOS and ADI items targeting joint attention and social reciprocity, an expectation that was largely supported by our findings.
Our results point to several commonalities and differences in how MV-ASD and V-ASD individuals deploy their attention to the components of a naturalistic scene involving a person and a set of interesting objects. Of note, although the MV-ASD participants tended to pay, on average, less attention to the entire video than their V-ASD peers, initial attention to the scene in the first episode was similar and relatively high in both groups, suggesting that they started similarly motivated to attend to the task. Relative to total movie duration, both V-ASD and MV-ASD participants spent proportionally more time looking at the protagonist compared to looking at the interesting objects placed around her in the scene. Consistent with findings reported by Chawarska and colleagues (2012) for toddlers, and Rice et al. (2012) for school-age children with ASD, our results do not suggest a generalized disinterest in looking at people in a social scene, even in the presence of an intriguing moving mechanical toy. Instead, our findings suggest that MV-ASD participants may be less motivated to attend to and interpret the protagonist’s behavior in a complex scene. Chawarska and colleagues (2012) found that toddlers with ASD showed diminished attention to an actor’s face compared to the comparison groups particularly in the condition where dyadic bids (child directed speech and eye contact) were present. In our study, we found group differences mainly in the segments that entailed interpreting the actor’s gaze shift toward and away from a surprising moving object (episodes 3 and 5): in these episodes, the MV-ASD participants spent proportionally less viewing time on the protagonist’s face than did the V-ASD group. Moreover, significantly fewer participants in the MV-ASD group compared to the V-ASD group followed the protagonist’s line of regard to look at the object of her attention when she shifted her gaze unexpectedly toward a static toy. It is notable however, that even in the V-ASD group there were participants who did not look at the panda in episode 5, despite spending viewing time on the protagonist’s face. Just over half of the V-ASD group followed the protagonist’s gaze shift to the panda. The tendency to follow spontaneously the direction of another person’s gaze toward the target of that person’s attention is of particular significance for establishing episodes of joint attention, particularly in an interactive context. In a social context, this tendency could reflect responsiveness to others’ bids for joint attention. In a free-viewing, passive paradigm, following spontaneously an actor’s direction of gaze may indicate the development of a foundational prerequisite for joint attention, although it does not constitute proof of joint attention abilities. Other studies conducted with verbal children and adolescents with ASD (Riby et al., 2013; Freeth et al., 2010) have reported subtle differences in gaze following between individuals with ASD and IQ matched typically developing children, or individuals with Williams syndrome. For instance, Riby et al. (2013), requested explicit responses from participants about the target of an actor’s gaze in a social scene, after a free-viewing phase. These authors showed that, when cued to follow an actor’s gaze in a naturalistic scene, participants with ASD looked more at the face and eyes but did not increase gaze to the correct targets of the actor’s attention, continuing to look much longer than their controls at implausible targets. In the spontaneous viewing phase, however, they spent less time on people’s faces and eyes than the control groups did. It appears from these results that atypicalities in spontaneous gaze following remain common among individuals with ASD across a wide range of verbal abilities. However, in our study, analyses relating visual social attention variables to language measures indicated that looking time at the most salient AOIs in the gaze shifting episodes (i.e., the face in episodes 3 and 5, and the panda in episode 5) was positively correlated with receptive language scores on a vocabulary test (PPVT-4), as well as with a parent report of communication abilities (Vineland Communication domain scores). Thus, participants who allocated more attention to the protagonist’s face and to the focus of her attention in the relevant episodes had better communication abilities according to these language assessments. Most significantly, proportional looking time at the protagonist’s face/eye-region in the unexpected gaze-shift episode was positively correlated with standardized measures of receptive vocabulary, suggesting a meaningful relationship between the ability to attend to visual social cues and language comprehension among children and adolescents with ASD. This relationship is particularly salient because the visual attention deployment measures in our sample were largely independent of nonverbal IQ or overall ASD symptom severity on the ADOS. The lack of sensitivity to the social cue of gaze shifting suggests that the MV-ASD participants may have difficulties understanding the referential nature of looking. In our paradigm, even school-age MV-ASD children and adolescents showed either a lack of understanding or a lack of interest in interpreting the protagonist’s gaze-shift, which was surprising in the context shown.
Reports in the literature relating the allocation of visual social attention to communication abilities, or to autism symptomatology vary widely. While some researchers found direct predictive relations between looking patterns and level of social competence or disability (e.g., Jones, Carr & Klin 2008; Klin, Jones, Schultz, Volkmar, & Cohen, 2002; Thorup et al., 2018), others have reported no correlations between gaze metrics and autism symptoms (see Guillon et al., 2013 for a review). In our study we found few and quite specific associations between gaze to the person-related AOIs and ASD symptomatology measured by the ADOS and the ADI: only for the unexpected gaze-shift episode (5), looking at the protagonist’s eyes was negatively correlated with the ADOS calibrated severity scores, while looking at the face in the expected gaze shift episode was negatively correlated with scores for qualitative abnormalities in reciprocal social interaction, on the ADI. Thus, the participants who showed more impairment in social interactive abilities on the two diagnostic assessments of autism were those who tended to look proportionally less at the protagonist’s eyes/face in the episodes when these AOIs provided cues for interpreting her behavior in the video. These correlations suggest a possible link between the ability to attend to the subtler social cue of gaze shifting and lower levels of ASD symptom severity.
To our knowledge, this is the first study to use a naturalistic video to directly compare the gaze allocation patterns of minimally-verbal and verbally-fluent age-matched children and adolescents with ASD. The group differences we found were mainly related to attention toward a protagonist’s face, eyes, or target of attention, when these AOIs provided or failed to provide relevant cues for interpreting the actor’s behavior in the scene. It is likely that the differences found between the MV-ASD and V-ASD groups in gaze time allocation to particular AOIs reflect decreased attention, among the MV-ASD participants, to behaviors that entail inferring the underlying intentions of the protagonist, suggesting either a lack of understanding, or a lack of interest in trying to interpret other people’s actions. The free-viewing paradigm used in our study, while less demanding than protocols that require an explicit response from participants, is not conducive to refuting such alternative explanations. Regardless of the underlying causes, these findings suggest that MV-ASD children may be less able to learn from interactive opportunities involving people’s shifts of attentional focus, which are critical for establishing joint attention; this limitation may further impair their ability to detect and interpret social cues, and may have downstream influences on their development of language and communication abilities.
Study limitations
In a first effort to characterize how MV-ASD children and adolescents deploy their visual attention to a dynamic scene showing a person involved in a routine activity, we started by documenting similarities and differences between individuals with ASD who remained non- or minimally verbal after age 8, and verbally fluent peers with ASD, in their viewing patterns. As designed and conducted, our study did not include a non-ASD control group and does not address larger theoretical questions about the nature of joint attention and gaze following atypicalities in autism, or their underlying mechanisms and neural underpinnings. We focused on viewing patterns in a passive paradigm to determine whether MV-ASD children and adolescents differ from V-ASD peers in their spontaneous allocation of attention to a protagonist’s gaze and target of looking (attentional focus), as gaze following ability is an important prerequisite of the ability to participate in joint attention episodes. We acknowledge that for probing joint attention abilities, interactive paradigms that involve social partners are more appropriate. Recent research has made tremendous progress using technology to record eye-movements during real life interactions or to employ virtual reality in simulating social interactive contexts, while ensuring tight experimental controls and even recording brain activity during such interactions (see Caruana, McArthur, Woolgar, & Brock, 2017 for a review of these studies). While watching another person in a video looking at various objects may not capture the essence of this social process, the ‘third person’ perspective involved in passive viewing paradigms is not without any merits. Indeed, these have been used extensively in research on the allocation of social attention, using eye-tracking technology. Our choice of a more ‘traditional’ free viewing paradigm was motivated by the need to facilitate comparisons between findings from research conducted with cognitively able participants with ASD using similar stimuli, and research with MV-ASD individuals who have usually been excluded from eye-tracking studies. The methodological limitations of our study are directly related to the difficulties of engaging MV-ASD participants in research tasks: for instance, we used only one video-clip as stimulus, without a comparable non-social viewing condition to match for non-social viewing differences; also, we did not fully control for the salience of the particular elements of the scene by a using another set of objects and a male protagonist. We also acknowledge as limitations the less stringent inclusion criteria for analyses of looking time data than those used in studies with cognitively able individuals, and the use of a 5-point calibration method instead of a 9-point calibration for eye-tracking. These constraining methodological decisions were dictated by the need to reduce testing time and attentional demands for the MV-ASD participants in particular. Caution in the interpretation of our results is also needed: even though we covaried nonverbal IQ in all our analyses, we cannot rule out the possibility that differences in proportional viewing time between the MV-ASD and the V-ASD groups may not be truly independent of non-social cognitive processes (e.g., oculomotor control, other aspects of attention or motivation to attend to the task), in the context of scene viewing. In sum, we acknowledge inherent methodological limitations driven by our goal to provide a realistic description of the social attention characteristics of this subpopulation of more severely impaired individuals with ASD, while minimizing task demands.
Conclusions
Our results suggest specific and subtle differences in viewing patterns between MV-ASD and V-ASD children and adolescents that were related to particular aspects of language and communication skills, primarily receptive vocabulary. These findings have important implications for the possibility of training social attention allocation to promote the development of other abilities, including those related to understanding and using language. Future research should explore whether interventions targeting basic social attention processes could improve outcomes in communication skills for MV- ASD children and adolescents. While research on individuals at the ‘neglected end of the spectrum’ (Tager-Flusberg, & Kasari, 2013) is slowly emerging, the wealth of phenotyping information provided by these efforts holds promise for better understanding the significant heterogeneity of ASD, as well as for developing ways to improve social functioning in affected individuals.
Figure 1.
Composition of the scene – Snapshot from episode 5
Acknowledgements:
This research was funded by an Autism Center of Excellence grant from NIH (PI: Tager-Flusberg): P01 DC 13027. We thank the participants and their families for their involvement in our research programs.
References
- Adamson LB, Bakeman R, Deckner DF, & Romski MA (2009). Joint engagement and the emergence of language in young children with autism and Down syndrome. Journal of Autism and Developmental Disorders, 39, 84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akechi H, Senju A, Kikuchi Y, Tojo Y, Osanai H, Hasegawa T (2011). Do children with ASD use referential gaze to learn the name of an object? An eye-tracking study. Research in Autism Spectrum Disorders, 5, 1230–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal VH, Katz T, Bishop SL, & Krasileva K (2016). Understanding definitions of minimally verbal across instruments: evidence for subgroups within minimally verbal children and adolescents with autism spectrum disorder. Journal of Child Psychology and Psychiatry, 57(12), 1424–1433. [DOI] [PubMed] [Google Scholar]
- Baldwin DA (1995). Understanding the link between joint attention and language In Moore C & Dunham P (Eds.), Joint attention: Its origins and role in development (pp. 131–158). Mahwah, NJ: Erlbau [Google Scholar]
- Baron-Cohen S, Baldwin DA, & Crowson M (1997). Do children with autism use the speaker’s direction of gaze strategy to crack the code of language? Child Development, 68(1), 48–57. [PubMed] [Google Scholar]
- Bavin EL, Kidd E, Prendergast L, Baker E, Dissanayake C, & Prior M (2014). Severity of autism is related to children’s language processing. Autism Research, 7(6), 687–694. [DOI] [PubMed] [Google Scholar]
- Bedford R, Elsabbagh M, Gliga T, Pickles A, Senju A, Charman T, et al. (2012). Precursors to Social and Communication Difficulties in Infants At-Risk for Autism: Gaze Following and Attentional Engagement. Journal of Autism and Developmental Disorders, 42:2208–2218. [DOI] [PubMed] [Google Scholar]
- Butler SC, Caron AJ, & Brooks R (2009). Infant understanding of the referential nature of looking. Journal of Cognition and Development, 1(4), 359–377. [Google Scholar]
- Carpenter M, Nagell K, & Tomasello M (1998). Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development, 63, 1–174. [PubMed] [Google Scholar]
- Caruana N, McArthur G, Woolgar A, & Brock J (2017). Simulating social interactions for the experimental investigation of joint attention. Neuroscience and Biobehavioral Reviews, 74, 115–125. [DOI] [PubMed] [Google Scholar]
- Caruana N, Stieglitz Ham H, Brock J, Woolgar A,Kloth N, Palermo R, McArthur G et al. (2017): Joint attention difficulties in autistic adults: an interactive eye-tracking study. Autism, 22 (4): 502–512. [DOI] [PubMed] [Google Scholar]
- Charman T (2003). Why is joint attention a pivotal skill in autism? Philosophical Transactions of the Royal Society, 358, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Klin A, & Volkmar F (2003). Automatic attention cueing through eye movement in 2-year old children with autism. Child Development, 74(4), 1108–1122 [DOI] [PubMed] [Google Scholar]
- Chawarska K, Macari S, & Shic F (2012). Context modulates attention to social scenes in toddlers with autism. Journal of Child Psychology and Psychiatry, 53(8), 903–913. doi: 10.1111/j.1469-7610.2012.02538.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Macari S, & Shic F (2013). Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological psychiatry, 74(3), 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, & Shic F (2009). Looking but not seeing: Atypical visual scanning and recognition of faces in 2 and 4-year- old children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 39(12), 1663–1672. doi: 10.1007/s10803-009-0803-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Parish-Morris J, McVey A, Rump KM, Sasson NJ, Herrington JD, & Schultz RT (2015). Measuring social attention and motivation in autism spectrum disorder using eye-tracking: Stimulus type matters. Autism research, 8(5), 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, & Brown E (1998). Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders, 28, 479–485. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, & McPartland J (2005). Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Development Neuropsychology, 27, 403–424. [DOI] [PubMed] [Google Scholar]
- Dunn LM, & Dunn LM (2007). Peabody Picture Vocabulary Test, Fourth Edition. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Elsabbagh M, Gliga T, Pickles A, Hudry K, Charman T, Johnson MH, & the BASIS Team. (2013). The development of face orienting mechanisms in infants at-risk for autism. Behavioural Brain Research, 251, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, & BASIS Team (2012). Infant Neural Sensitivity to Dynamic Eye Gaze Is Associated with Later Emerging Autism. Current Biology, 22, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck-Ytter T & von Hofsten C (2011). How special is social looking in ASD: a review. Progress in Brain Research, 189, 209–222. doi: 10.1016/B978-0-444-53884-0.00026-9. [DOI] [PubMed] [Google Scholar]
- Fletcher-Watson S, Leekam SR, Benson V, Frank MC, & Findlay JM (2009). Eye-movements reveal attention to social information in autism spectrum disorder. Neuropsychologia, 47(1), 248–257. [DOI] [PubMed] [Google Scholar]
- Franchini M, Glaser B, Wood de Wilde H, Gentaz E, Eliez S, & Schaer M (2017). Social orienting and joint attention in preschoolers with autism spectrum disorders. PloS one, 12(6), e0178859. doi: 10.1371/journal.pone.0178859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Strauss M, Klingemier EW, Zetzer EE, Hardan AY, Eng C, & Youngstrom EA (2017). A Meta-Analysis of Gaze Differences to Social and Nonsocial Information between Individuals with and without Autism. Journal of the American Academy of Child & Adolescent Psychiatry, 56(7), 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeth M, Chapman P, Ropar D, & Mitchell P (2010). Do gaze cues in complex scenes capture and direct attention of high functioning adolescents with ASD process social information in complex scenes. Combining evidence from eye movements and verbal descriptions. Journal of Autism and Developmental Disorders, 41, 364–371. [DOI] [PubMed] [Google Scholar]
- Freeth M, Foulsham T, Kingstone A (2013). What Affects Social Attention? Social Presence, Eye Contact and Autistic Traits. PLoS ONE 8(1): e53286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie-Lynch K, Elias R, Escudero P, Hutman T, & Johnson SP (2013). Atypical gaze following in autism: a comparison of three potential mechanisms. Journal of autism and developmental disorders, 43(12), 2779–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon Q, Hadjikhani N, Baduel S, & Rogé B (2014). Visual social attention in autism spectrum disorder: Insights from eye tracking studies. Neuroscience & Biobehavioral Reviews, 42, 279–297. [DOI] [PubMed] [Google Scholar]
- Jones W, & Klin A (2013). Attention to eyes is present but in decline in 2– 6-month-old infants later diagnosed with autism. Nature, 504(7480), 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasari C, Brady N, Lord C, & Tager Flusberg H (2013). Assessing the minimally verbal school aged child with autism spectrum disorder. Autism Research, 6(6), 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Paul R, Tager-Flusberg H & Lord C (2014). Language and communication in autism In Volkmar FR, Paul R, Rogers SJ, & Pelphrey KA (Eds). Handbook of autism and pervasive developmental disorders Volume 1: Diagnosis, development, and brain mechanisms. 4th Edition (pp. 230–262). New York: Wiley. [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, & Cohen D (2002a). Defining and quantifying the social phenotype in autism. The American Journal of Psychiatry, 159(6), 895. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, & Cohen D (2002b). Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry, 59(9), 809–816. [DOI] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, & Jones W (2009). Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature, 459 (7244), 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Lord C, & Rutter M (2003). Autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Leekam SR, Hunnisett E, & Moore C (1998). Targets and cues: Gaze following in children with autism. Journal of Child Psychology and Psychiatry, 39, 951–962. [PubMed] [Google Scholar]
- Leekam SR, Lopez B, Moore C (2000). Attention and joint attention in preschool children with autism. Developmental Psychology, 36: 261–273. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism Diagnostic Observation Schedule (ADOS), 2nd Edition Manual: Modules 1–4. Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, DiLavore P, & Risi S, (2012). Adapted Autism Diagnostic Observation Schedule (A-ADOS): Modules 1 & 2 (Unpublished).
- Loveland KA & Landry SH (1986) Joint attention and language in autism and developmental language delay. Journal of Autism and Developmental Disorders 16(3): 335–349. [DOI] [PubMed] [Google Scholar]
- Moore C & Dunham PJ (Eds. (1995). Joint attention: Its origins and role in development. Mahwah, NJ: Erlbaum. [Google Scholar]
- Mundy P, Sigman M, & Kasari C (1990). A longitudinal study of joint attention and language development in autistic children. Journal of Autism and Developmental Disorders, 20(1), 115–128. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, & Kasari C (1994). Joint attention, developmental level, and symptom presentation in autism. Development and Psychopathology, 6, 389–401. [Google Scholar]
- Murias M, Major S, Davlantis K, Franz L, Harris A, Rardin B, Sabatos DeVito M and Dawson G (2018), Validation of eye-tracking measures of social attention as a potential biomarker for autism clinical trials. Autism Research, 11, 166–174. [DOI] [PubMed] [Google Scholar]
- Nation K & Penny S (2008). Sensitivity to eye gaze in autism: Is it normal? Is it automatic? Is it social? Development and Psychopathology, 20, 79–97. [DOI] [PubMed] [Google Scholar]
- Norbury CF, Brock J, Cragg L, Einav S, Griffiths H & Nation K (2009). Eye-movement patterns are associated with communicative competence in autistic spectrum disorders. Journal of Child Psychology and Psychiatry. 50(7), 834–842. [DOI] [PubMed] [Google Scholar]
- Noris B, Nadel J, Barker M, Hadjikhani N, & Billard A (2012). Investigating gaze of children with ASD in naturalistic settings. PloS one, 7(9), e44144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagiannopoulou EA, Chitty KM, Hermens DF, Hickie IB, & Lagopoulos. J (2014). A systematic review and meta-analysis of eye-tracking studies in children with autism spectrum disorders. Social Neuroscience, 9(6), 610–632.doi:.1080/17470919.2014.934966. [DOI] [PubMed] [Google Scholar]
- Pierce K, Marinero S, Hazin R, McKenna B, Barnes CC, & Malige A (2016). Eye tracking reveals abnormal visual preference for geometric images as an early biomarker of an autism spectrum disorder subtype associated with increased symptom severity. Biological psychiatry, 79(8), 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riby DM, & Hancock PJB (2008). Viewing it differently: social scene perception in Williams syndrome and autism. Neuropsychologia, 46, 2855–2860. [DOI] [PubMed] [Google Scholar]
- Riby DM, Hancock PJB, Jones N, & Hanley M (2013). Spontaneous and cued gaze-following in autism and Williams syndrome. Journal of Neurodevelopmental Disorders, 5(13), doi: 10.1186/1866-1955-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice K, Moriuchi JM, Jones W, & Klin A (2012). Parsing Heterogeneity in Autism Spectrum Disorders: Visual Scanning of Dynamic Social Scenes in School-Aged Children. Journal of the American Academy of Child & Adolescent Psychiatry, 51(3), 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid GH, Miller LJ, Pomplun M, & Koch C (2013). Leiter International Performance Scale, (Leiter 3). Torrance CA: Western Psychological Services. [Google Scholar]
- Sasson NJ (2006). The Development of Face Processing in Autism. Journal of autism and developmental disorders, 36, 381–94. 10.1007/s10803-006-0076-3. [DOI] [PubMed] [Google Scholar]
- Sasson NJ, Turner-Brown LM, Holtzclaw TN, Lam KSL, & Bodfish JW (2008). Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research, 1, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT (2005). Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience, 23(2–3), 125–141. [DOI] [PubMed] [Google Scholar]
- Shic F, Bradshaw J, Klin A, Scassellati B, & Chawarska K (2011). Limited Activity Monitoring in Toddlers with Autism Spectrum Disorder. Brain Research, 1380: 246–254. doi: 10.1016/j.brainres.2010.11.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Dairoku H, & Hasegawa T (2004). Reflexive orienting in response to eye gaze and an arrow in children with and without autism. Journal of Child Psychology and Psychiatry, 45(3), 445–458. [DOI] [PubMed] [Google Scholar]
- Shepherd ST (2010). Following gaze: gaze-following behavior as a window into social cognition. Frontiers in Integrative Neuroscience, 4:5. doi: 10.3389/fnint.2010.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S, Cicchetti D, & Balla D (2005) Vineland adaptive behavior scales (2nd ed.). Circle Pines, MN: AGS. [Google Scholar]
- Speer LL, Cook AE, McMahon WM, & Clark E (2007). Face processing in children with autism. Autism 11: 265–277. [DOI] [PubMed] [Google Scholar]
- Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A, Rees L, & Wheelwright S (1998). The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. Journal of Child Psychology and Psychiatry and Allied Disciplines, 39(5), 747–753. [PubMed] [Google Scholar]
- Tager-Flusberg H, & Kasari C (2013). Minimally-verbal school-aged children with autism spectrum disorder: The neglected end of the spectrum. Autism Research 6: 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Plesa Skwerer D, Joseph RM, Brukilacchio B, Decker J, Eggleston B, Yoder A (2017). Conducting research with minimally-verbal participants with autism spectrum disorder. Autism, 21(7), 852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorup E, Nyström P, Gredebäck G, Bölte S, Falck-Ytter T, EASE Team (2018). Reduced Alternating Gaze During Social Interaction in Infancy is Associated with Elevated Symptoms of Autism in Toddlerhood. Journal of Abnormal Child Psychology, 46(7):1547–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M & Farrar MJ (1986). Joint attention and early language. Child Development, 57:1454–1463. [PubMed] [Google Scholar]
- Toth K, Munson J, Meltzoff AN, & Dawson G (2006). Early predictors of communication development in young children with autism spectrum disorder: Joint attention, imitation, and toy play. Journal of Autism and Developmental Disorders, 36, 993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venker CE, Eernisse ER, Saffran JR, & Weismer SE (2013). Individual differences in the real‐time comprehension of children with ASD. Autism Research, 6(5), 417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2011). Wechsler abbreviated scale of intelligence: Second edition. San Antonio, TX: Pearson. [Google Scholar]
- Weigelt S, Koldewyn K, & Kanwisher N (2012). Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neuroscience and Biobehavioral Reviews, 36(3) 1060–1084. [DOI] [PubMed] [Google Scholar]
- Wilkinson KM, & Mitchell T (2014). Eye Tracking Research to Answer Questions about Augmentative and Alternative Communication Assessment and Intervention. Augmentative and Alternative Communication, 30(2):106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]