Abstract
Objective:
The Pediatric Heart Network sponsored the multicenter Collaborative Learning Study that implemented a clinical practice guideline to facilitate early extubation in infants after repair of isolated coarctation of the aorta and Tetralogy of Fallot. We sought to compare the anesthetic practice in the operating room and sedation-analgesia management in the ICU before and after the implementation of the guideline that resulted in early extubation.
Design:
Secondary analysis of data from a multicenter study from January 2013 to April 2015. Predefined variables of anesthetic, sedative and analgesia exposure were compared before and after guideline implementation. Propensity score weighted logistic regression analysis was used to determine the independent effect of intraoperative dexmedetomidine administration on early extubation.
Setting:
Five children’s hospitals.
Patients:
240 study subjects who underwent repair of coarctation of the aorta or Tetralogy of Fallot (119 pre and 121 post-guideline implementation).
Interventions:
None.
Measurements and Main Results:
Clinical practice guideline implementation was accompanied by a decrease in the median total intraoperative dose of opioids (49.7 vs 24.0 mcg/kg of Fentanyl Equivalents, p<0.001) and benzodiazepines (1.0 vs 0.4 mg/kg of Midazolam Equivalents, p<0.001), but no change in median volatile anesthetic agent exposure (1.3 vs 1.5 MAC-hrs. p=0.25). Intraoperative dexmedetomidine administration was associated with early extubation (Odds Ratio 2.5, 95% CI: 1.02, 5.99, p=0.04) when adjusted for other covariates. In the ICU, more patients received dexmedetomidine (43% vs 75%) but concomitant benzodiazepine exposure decreased in both the frequency (66% vs 57%, p<0.001) and cumulative median dose (0.5 vs 0.3 mg/kg of ME, p=0.003) post-guideline implementation.
Conclusions:
The implementation of an early extubation clinical practice guideline resulted in a reduction in the dose of opioids and benzodiazepines without a change in volatile anesthetic agent used in the operating room. Intraoperative dexmedetomidine administration was independently associated with early extubation. The total benzodiazepine exposure decreased in the early postoperative period.
Keywords: Early Extubation, Sedation, Dexmedetomidine, Infant cardiac surgery
INTRODUCTION
Early post-operative extubation, meaning extubation in the operating room or soon after arrival in the intensive care unit (ICU), is being increasingly utilized after congenital heart surgery. Careful patient selection and modification of perioperative analgesia and sedation practice can facilitate early extubation (EE) of many patient groups (1–4). Previously, a high-dose narcotic anesthetic was advocated to attenuate the physiological, hormonal response to stress after cardiac surgery (5). While providing excellent anesthesia and analgesia during surgery and in the early post-operative period, this approach has not been shown to improve outcome (6,7) and may prolong mechanical ventilation and ICU length of stay due to the resulting respiratory depression. Minimizing intra-operative exposure to opioids to facilitate EE may increase the volatile anesthetic agent (VAA) dose and may adversely impact the developing brain. Alternatively, use of dexmedetomidine (DEX), a novel sedative agent associated with less respiratory depression, has been shown to decrease opioid and benzodiazepine (BZD) use during and after pediatric cardiac surgery, resulting in shortened time to extubation (8,9).
The Collaborative Learning Study (CLS) was a Pediatric Heart Network sponsored study conducted from January 2013 to April 2015 that applied collaborative learning strategy to improve postoperative cardiac care (10,11). In that study, multi-disciplinary teams from the 5 CLS sites applied a shared learning model through site visits to develop a clinical practice guideline (CPG) aimed at EE within 6 hours of the repair of isolated coarctation (CoA) or tetralogy of Fallot (TOF) (Online Appendix 1, Supplemental Digital Content 1). One of the five sites already practiced EE, and aspects of that center’s peri-operative analgesia and sedation management were incorporated into the guideline. The implementation of CPG at the other four sites resulted in a substantial increase in the proportion of infants extubated early with no change in the rate of reintubation (10). The compliance rate for the implementation of the guideline about intraoperative anesthesia was 92.6 % (10). While the CPG provided recommendations regarding intra-operative and post-operative analgesia and sedation to maximize the chances of EE, the ultimate choice of drugs, dosages and time of administration were left to the discretion of the attending anesthesiologists and ICU teams.
This study aimed to explore the changes in intra-operative anesthetic and post-operative analgesia and sedation management after the introduction of the EE CPG. We hypothesized that implementation of the CPG would be associated with a reduction in the intraoperative exposure to opioids and BZDs and an increase in VAA exposure and the use of DEX. We also sought to study whether intraoperative DEX administration is associated with EE after adjusting for confounding variables independent of CPG implementation in this multicenter cohort.
MATERIALS AND METHODS
We conducted a retrospective analysis of 322 infants undergoing CoA or TOF repair, who were enrolled in the Collaborative Learning Study. Details of the patient cohorts and inclusion/exclusion criteria have been previously reported (10). The exposure to anesthetics and sedatives in the operating room and during the first 48 hours after surgery was analyzed using predefined variables before and after implementation of the CPG in this retrospective study design (Online Appendix 2, Supplemental Digital Content 2). Ancillary data relating to VAA exposure in Minimum Alveolar Concentration-hours (MAC-hrs.) based on expired gas concentrations, was calculated from anesthetic records and was managed using Research Electronic Data Capture (REDCap) electronic data tools hosted at the University of Utah. REDCap is a secure, web-based application designed to support data capture for research studies (12).
All analyses excluded the model site (n=82) where EE was already the standard of care, leaving 240 subjects (119 pre-CPG and 121 post-CPG). The primary outcome was the comparison of total intravenous opioid or BZD, and VAA exposure in the operating room and during the initial 48 hours in the ICU before and after CPG implementation. The postoperative pain and discomfort were assessed using Face, Leg, Activity, Cry, Consolability Score (FLACC score). Parameters were summarized by descriptive statistics including median (interquartile range) for continuous variables and frequencies and percentages for categorical variables. A Wilcoxon rank sum test was used to compare continuous variables between groups, and a chi-squared or Fisher’s exact test was used to compare categorical variables. The opioids were converted to equianalgesic dosages of fentanyl and reported as Fentanyl Equivalents (FE) while the BZDs were reported as Midazolam Equivalents (ME).
We used a propensity score weighted logistic regression analysis to study the effect of intraoperative DEX administration on EE. This approach reduces confounding by indication bias, in which patient characteristics potentially associated with treatment selection (e.g., comorbidities) are also associated with their outcomes. We restricted this analysis to intraoperative DEX exposure that occurred before observing the EE outcome in the ICU. Post-operative DEX exposure in the ICU was ignored as it potentially occurred concomitantly with or following EE. A logistic regression model was used to construct the propensity score model, predicting intraoperative DEX use from age, weight at the time of operation, Site ID (4 sites), cardiopulmonary bypass (CPB) time (> or < 120min), total intraoperative fentanyl dose (> or < 10mcg/kg), CPG implementation (pre or post) and type of operation (CoA or TOF). Missing values were handled as another level (“unknown”) for categorical predictors and were replaced by average values for continuous variables. Balance in baseline characteristics was checked using standardized differences, where a standardized difference < 0.2 was considered balanced (13). We considered both matched weights and average treatment effect in the treated (ATT) weights (14). Propensity score analysis requires a positivity assumption that all patients included in the analysis could potentially receive or not receive the DEX treatment. This assumption was checked by plotting the propensity score distributions as scaled density functions for the DEX and non-DEX treatment groups. Due to the limited overlap in the propensity score distributions, matched weights were selected for our outcome model. Matched weights emphasize patients who had a comparable likelihood of being treated based on their covariates (15,16). Our outcome model was a weighted logistic regression model predicting EE in the ICU from DEX use, adjusting for most of the variables that were used in the matching process as they could potentially be associated with both treatment status and outcomes (17). This model was implemented in R with the survey package to include the propensity-matched weights16. Statistical analyses were implemented using R v. 3.4.4 (18). Statistical significance was assessed at the 0.05 level, and all tests were two-tailed. The study was approved by the individual Institutional Review Board, and the requirement for written informed consent was waived given the retrospective nature of the study.
RESULTS
Demographics and operative characteristics of the study cohort, including the rates of EE from the primary study are shown in Table 1. CPG implementation was accompanied by a decrease in the median total intraoperative dose of opioids (49.7 vs 24.0 mcg/kg of FE, p<0.001) and BZD, (1.0 vs 0.4 mg/kg of ME, p<0.001), but no change in volatile anesthetic agent (VAA) exposure (1.3 vs 1.5 MAC-hrs. p=0.25) (Table 2). The total intraoperative opioid dose given after bypass also decreased (6.1 vs. 1.4 mcg/kg of FE, p<0.001). The introduction of the CPG was associated with an increase in the proportion of infants receiving intravenous acetaminophen (13 vs. 27%, p=0.01) and DEX (18 vs. 66 %, p< 0.001) in the operating room. Use of propofol also increased significantly from 5% to 17% (p=0.003).
Table 1.
Demographics, Operative and Clinical Characteristics of the Study Cohort
| Variablea,b | CoA | TOF | Total | ||||
|---|---|---|---|---|---|---|---|
| Pre-CPG (50) |
Post-CPG (43) |
Pre-CPG (69) |
Post-CPG (78) |
Pre- CPG (119) | Post-CPG (121) |
p-valuec | |
| Age (d) | 14 (7,33.8) |
24 (7.5,79) |
149 (115,194) |
170 (126.2,211) |
111 (21,161.5) |
132 (56,192) |
0.024 |
| Male | 32 (64%) | 26 (60%) | 44 (64%) | 38 (49%) | 76 (64%) | 64 (53%) | 0.08 |
| Race/Ethnicity | |||||||
| Black | 6 (12%) | 4 (10%) | 12 (18%) | 13 (18%) | 18 (16%) | 17 (15%) | 0.83 |
| White | 40 (83%) | 37 (90%) | 48 (74%) | 54 (74%) | 88 (78%) | 91 (80%) | 0.72 |
| Hispanic | 9 (19%) | 7 (18%) | 8 (13%) | 16 (21%) | 17 (15%) | 23 (20%) | 0.36 |
| Weight (kg) | 3.6 (3.2,4.5) |
3.7 (3 1,4.8) |
6.4 (5.5,7.3) |
6.4 (5.4,7.2) |
5.4 (3.7,6.9) |
5.6 (4.5,7) |
0.23 |
| Induction Inhalation |
28 (56%) | 26 (60%) | 57 (84%) | 62 (79%) | 85 (72%) | 88 (73%) | 0.90 |
| IV | 30 (60%) | 26 (60%) | 20 (29%) | 27 (35%) | 50 (42%) | 53 (44%) | 0.82 |
| IM | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0%) | 0 (0%) | - |
| Regional | 1 (2%) | 2 (5%) | 0 (0%) | 0 (0%) | 1 (1%) | 2 (2%) | >0.99 |
| Intercostal | 5 (10%) | 10 (23%) | 16 (24%) | 11 (14%) | 21 (18%) | 21 (17%) | 0.93 |
| Regional block | 6 (12%) | 6 (14%) | 16 (24%) | 12 (15%) | 22 (19%) | 18 (15%) | 0.44 |
| CPB | 0 (0%) | 4 (9%) | 69 (100%) | 78 (100%) | 69 (58%) | 82 (68%) | 0.12 |
| CPB time (min) | NA | NA | 103 (86,133) |
113 (94.2,157.5) |
103 (86,133) |
112 (88.5,154.5) |
0.22 |
| Early Extubation |
5 (10%) | 24 (56%) | 9 (13%) | 57 (73%) | 14 (12%) | 81 (67%) | <0.001 |
| Average FLACCd Score | 0.8 (0.0, 2.2) |
1.5 (0.6, 2.7) |
1.3 (0.7, 2.2) |
2.3 (12, 3.2) |
1.3 (0.4, 2.2) |
2.1 (10, 3.1) |
0.0002 |
Missing values: Black =13, White =13, Hispanic =12, Inhalation =1, IV =1, IM =1, Regional =1, Intercostal =1, Regional Block =1;
Continuous variables are reported as median (interquartile range). Categorical variables are reported as frequencies with percentages.
Comparing groups in total cohort
Face, Leg, Activity, Cry, Consolability Scale expressed as median scores with interquartile range.
Table 2.
Anesthesia, Sedation and Adjunct Medication Use in the OR
| Variablea,b | Pre-CPG (n=119) | Post-CPG (n=121) | p-value |
|---|---|---|---|
| VAA (Mac-hrs.) | 1.3 (1.06, 1.67) | 1.5 (1.12, 1.85) | 0.25 |
| Medication use (n) | |||
| Fentanyl | 117 (98%) | 118 (98%) | 1 |
| Ketamine | 13 (11%) | 7 (6%) | 0.15 |
| Midazolam | 68 (57%) | 60 (50%) | 0.24 |
| Propofol | 6 (5%) | 21 (17%) | 0.003 |
| Dexmedetomidine | 21 (18%) | 80 (66%) | <0.001 |
| Acetaminophen | 16 (13%) | 33(27%) | 0.008 |
| Remifentanil | 1 (1%) | 6 (5%) | 0.12 |
| Opioid post-bypass | 48(40%) | 42(35%) | 0.37 |
| Cumulative OR Dosages | |||
| Opioid (FE), mcg/kgc | 49.7 (19, 183.6) | 24 (11,43.4) | <0.001 |
| BZD (ME), mg/kgd | 1.0 (0.3,1.5) | 0.4 (0.2, 0.6) | <0.001 |
| Ketamine, mg/kg | 2.9 (2.4,3.6) | 2.0 (1.6,4.7) | 0.018 |
| DEX, mcg/kg | 2.5 (1,3.8) | 2.5 (1,5.1) | 0.58 |
| Opioid (FE), mcg/kg post-bypass | 6.1 (2.7, 14.3) | 1.4 (1.0,2.6) | <0.001 |
Missing values: Opioid (FE), mcg/kg = 28, DEX, mcg/kg = 16.
Continuous variables are reported as median (interquartile range). Categorical variables are reported as frequencies with percentages.
Fentanyl Equivalents; Midazolam Equivalents. VAA-Volatile Anesthetic Agent
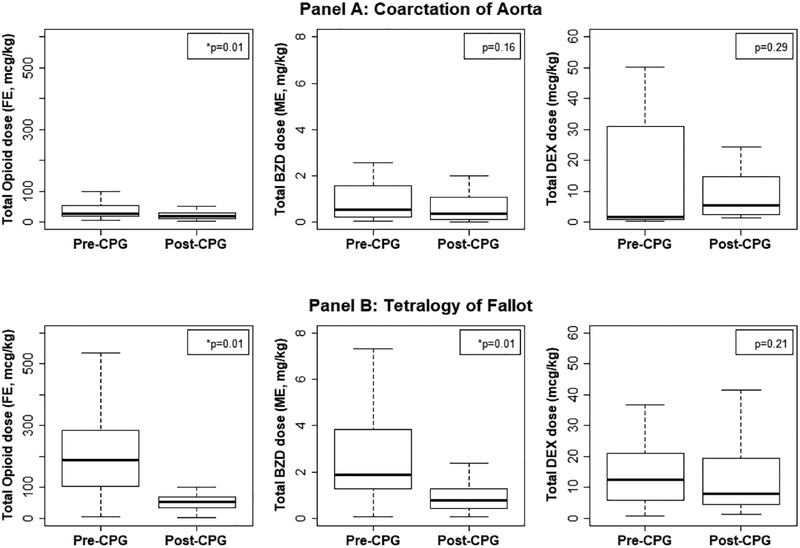
In the ICU, the use of DEX after the CPG implementation increased significantly with 75% of subjects receiving it as compared to 43% in the pre-CPG period (p<0.001). Even though the number of subjects exposed to DEX increased, the average cumulative 48 hr. dose among those who had received it was significantly less in the post-CPG period compared to the pre-CPG period (5.8 vs. 12.5 mcg/kg, p=0.04) (Table 3). Concomitant BZD use decreased with only 57% of post-CPG infants receiving the medication as compared to 66% among pre-CPG infants; and among those who received it, the dose was significantly less (0.3 vs. 0.5 mg/kg ME, p=0.027). The combined operating room and ICU usage of Opioid, BZD and DEX before and after implementation of CPG was compared in the cohort, separated by the lesion and shows a significant decrease in the opioid usage (Fig. 1). The average FLACC score in the post-CPG cohort increased from 1.3 (0.4, 2.2) to 2.1 (1.0, 3.1).
Table 3.
Sedation, Analgesia and Adjunct Medication Use for the first 48hrs of ICU stay
| Variablea,b | Pre-CPG (n=119) | Post-CPG (n=121) | p-value |
|---|---|---|---|
| Medication use (n) | |||
| Fentanyl | 36 (30%) | 24 (20%) | 0.06 |
| Morphine (IV/IM) | 116 (97%) | 117 (97%) | 1 |
| Midazolam (IV/IM) | 79 (66%) | 69 (57%) | <0.001 |
| Ketorolac | 6 (5%) | 14 (12%) | 0.07 |
| Dexmedetomidine | 51 (43%) | 91 (75%) | <0.001 |
| Acetaminophen (IVor PO) | 99 (83%) | 100 (83%) | 0.91 |
| Cumulative ICU Dosages | |||
| Opioid (FE)c,mcg/kg | 14 (5.5,52.6) | 9.1 (4.1,19.7) | 0.011 |
| BZD (ME)d,mg/kg | 0.5 (0.2,3.1) | 0.3 (0.1, 0.9) | 0.027 |
| Ketorolac, mg/kg | 0.9 (0.5,1.3) | 3.0 (1.6,4.3) | 0.006 |
| DEX, mcg/kg | 12.5(4.5,34) | 5.8 (2.9, 15.5) | 0.04 |
Missing values: Opioid (FE),mcg/kg = 8, BZD (ME),mg/kg = 12, Ketorolac, mg/kg = 4, DEX, mcg/kg = 22.
Continuous variables are reported as median (interquartile range). Categorical variables are reported as frequencies with percentages.
Fentanyl Equivalents;
Midazolam Equivalents.
Figure 1.
Boxplot comparison of Opioid, Benzodiazepine (BZD) and Dexmedetomidine (DEX) total drug dosages (OR and ICU combined) between Pre and Post Clinical Practice Guideline cohorts. Opioid dosage represented as Fentanyl Equivalents (FE) and Benzodiazepine dosage as Midazolam Equivalents (ME).
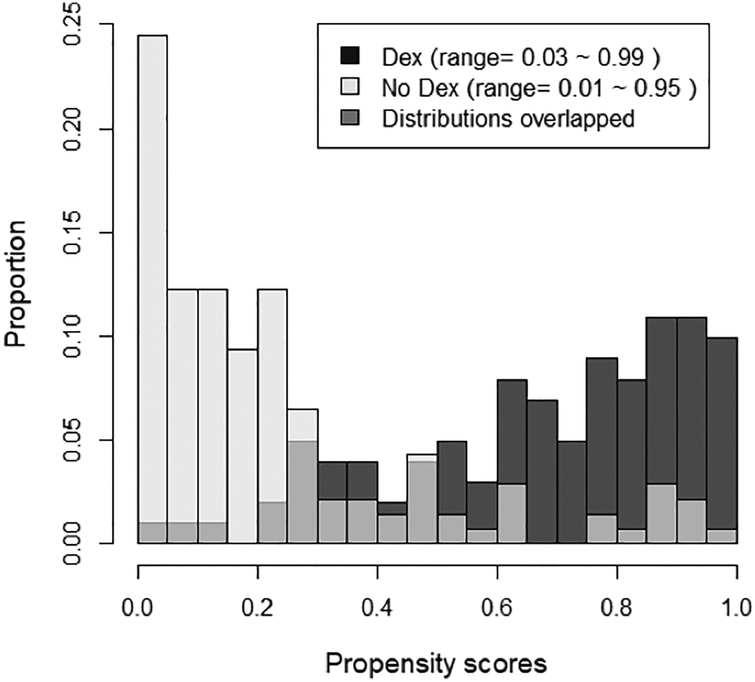
For our propensity model predicting DEX treatment, the variables age and weight at surgery had no missing values, but cardiopulmonary bypass time was not applicable in 89 cases (37%), and intraoperative fentanyl dose was missing in 6 (2.5%), and these missing values were classified as “unknown”. In an unadjusted comparison of patient characteristics, standardized differences ranged from 0.02–1.22. After adjustment with ATT weights the range was 0.06–0.46, and after adjusting for matched weights, all standardized differences were <0.2 with a range of <0.01–0.11, indicating balance in the patient characteristics (data not shown). The distributions of propensity scores for patients who received intraoperative DEX and those who did not, showed a lack of common support between the two distributions (Fig. 2). Hence we used matched weights for our outcome model.
Figure 2.
Overlay histograms of propensity scores showing the regions of common and uncommon support between the propensity score distributions in the Dex and no-Dex groups. The lightest color is “No Dex,” the darkest color is “Dex” and the intermediate color represents the overlap between the two distributions.
Univariable logistic regression analysis showed that age at surgery, weight at surgery, implementation of the CPG, total intraoperative fentanyl dose of < 10mcg/kg, and TOF repair were all significantly associated with EE (Online Appendix 3, Supplemental Digital Content 3). These predictors along with CPB time were selected for inclusion in the outcome model. CPB time was included even though it was not significant given the known effect of prolonged bypass time on surgical outcomes. Site ID was not included due to the limitation of the event rate.
A univariable propensity score weighted analysis showed that the odds of EE when using DEX in the operating room was 1.9 times (95% CI, 0.9–3.8, p = 0.09) higher than when not using DEX (data not shown).In the multivariable weighted analysis (Table 4), the signal of the association strengthened with the odds of EE when using DEX in the OR at 2.5 times (95% CI, 1.02–5.99, p = 0.04) the odds without using DEX adjusting for other covariates.
Table 4.
Multivariable propensity score weighted logistic regression of Early Extubation on selected predictors
| Predictor Variable | OR | 95% CL | p-value |
|---|---|---|---|
| DEX | 2.5 | (1.02, 5.99) | 0.04 |
| Age at surgery (months) | 1.1 | (0.84, 1.53) | 0.41 |
| Weight at surgery (kg) | 1.5 | (0.99, 2.4) | 0.05 |
| Post-Clinical Practice Guideline | 11.4 | (4.13, 31.42) | <0.001 |
| Cardiopulmonary bypass time >120 mina | 0.3 | (0.1, 1.16) | 0.08 |
| - Unknown | 1.1 | (0.09, 13.59) | 0.95 |
| Intraoperative Fentanyl Dose >10mcg/kga | 0.3 | (0.08, 1.09) | 0.06 |
| - Unknown | 0.2 | (0.05, 1.35) | 0.11 |
| Type of operation TOF | 0.5 | (0.03, 8.96) | 0.65 |
Missing values were imputed by mean value of non-missing or categorized as “unknown” level. DEX-Dexmedetomidine
When patients with DEX use in the OR were compared to those who did not receive DEX among the total cohort (101 vs. 139), there was no difference in bleeding requiring reoperation (0 vs 1%), cardiac dysfunction requiring low cardiac output (1vs 2%) or reintubation within 48 hrs. of primary extubation (4 vs 4%). The use of high flow nasal cannula was lower among the DEX group (19 vs. 30%). More subjects in the DEX group were associated with arrhythmias requiring therapy (14 vs. 6%). The most common arrhythmias occurred with junctional focus (19 of the 23 patients in the whole cohort). There was one patient with complete heart block, two patients with ventricular arrhythmia and one with atrial arrhythmia. The actual type of arrhythmias and the nature of the therapy were not captured in this retrospective dataset. The median time to discontinue all sedative infusions, time to oral feeds and ICU length of stay were significantly lower in the DEX group. (Online Appendix 4, Supplemental Digital Content 4). The overall complication rate was low to derive any meaningful statistical association with DEX use in this unadjusted comparison.
DISCUSSION
This study demonstrates that the implementation of a collaborative learning strategy derived CPG aimed at EE in infants after two index cardiac surgical procedures resulted in a significant change in anesthetic and early postoperative sedation-analgesia management. An anesthetic strategy consisting of reduction in the dose of opioids and BZDs and the use of adjuvant medications such as DEX and acetaminophen is associated with increased rate of EE without an increase in the rate of reintubation. This was accomplished without a significant change in VAA exposure. Intraoperative DEX administration was independently associated with EE. In the ICU, simultaneous exposure to BZDs decreased with increased DEX use.
Even though the concept of EE in children is not new, there is a lack of consensus among congenital cardiac care providers about the utility of such practice (19,20). Some question the safety and efficacy of the practice given the relative heterogeneity of pediatric cardiac surgical patients while others question cost savings by referring to methodologic flaws in the studies that linked EE to decreased resource utilization (20). Proponents of EE cite the advances in the anesthetic, surgical and cardiopulmonary bypass techniques along with the advent of newer and safe drugs as reasons to adopt such practice in patients who are hemodynamically stable and devoid of risk factors, regardless of age and complexity of surgery (21). In the PHN CLS study by Mahle et al. (10), the proportion of infants that achieved EE prior to the implementation of the CPG at active sites was low at 11.8% showing the relative lack of enthusiasm for EE even among the tertiary care centers represented in the PHN. The fact that the active sites increased the percentage of infants extubated within 6 hours of completion of their surgery with no adverse effects suggests the role of established practice patterns in determining readiness for extubation. The current analysis shows that changes in the anesthetic practice and sedative administration are feasible and provide positive outcomes in the care of children with CHD. The average FLACC score was higher in post CPG cohort but still in the range considered to be mild discomfort (10).
One of the major concerns of low dose narcotic anesthetic technique is whether it leads to an increase in VAA exposure. Several studies in animal models, including nonhuman primates, have shown adverse effects of anesthetic and sedative agents on developing brain (22–25). The clinical data in humans is mixed, with several large retrospective matched cohort studies concluding that single short anesthetic exposure in young healthy children does not contribute to adverse neurodevelopmental outcomes (26–29). The recent MASK study by Warner et al. (30) found no change in intelligence with multiple anesthetic exposures before age three years but suggested that problems with executive function, behavior and reading may occur. The current study analyzed the VAA exposure among the centers pre and post-CPG implementation and did not find a significant difference. These results suggest that it may be feasible to limit the narcotic dosage without a simultaneous increase in VAA exposure, mainly when adjunctive agents such as DEX and acetaminophen are utilized. However, further studies are needed to confirm these findings.
Dexmedetomidine, a selective alpha-2 adrenoceptor agonist, is increasing in use in perioperative period as it can provide sedation with minimal respiratory depression, decrease the use of deleriogenic agents, and provide favorable hemodynamics by attenuation of the postoperative neuroendocrine stress response (31,32). The use of DEX in children is off-label; however, it’s use is very common. A recent report from Information Medical Statistics Health (a vendor of US physician prescribing data) estimating the number of patients with inpatient and outpatient billing for Dexmedetomidine HCl Injection from U.S. Non-Federal Hospitals, from June 2014 through May 2015 showed 18.3% of the patients to be between 0–16 years of age with 22.1% of them being less than 1 year of age (33). Achuff et al. (8) showed that a DEX bolus in the OR at the time of the sternal closure is associated with reduced need for mechanical ventilation in the immediate postoperative period using a single center cohort. Our multicenter study supports their observation that intraoperative DEX use is associated with EE. In the current study, the frequency of DEX use increased after the guideline implementation but the average cumulative dose administered was significantly less, most likely related to early extubation and overall decreased the need for sedation.
The major side effects of DEX include hypotension, bradycardia and transient hypertension. No physiologic data were available in this dataset to evaluate these side effects, however, as described in the results section more subjects in the DEX group were associated with arrhythmias requiring therapy, mostly with junctional focus. The dataset did not separate arrhythmias into tachyarrhythmia or bradyarrhythmia, and hence it is hard to say whether the difference represents adverse effects of DEX use or it was used as a therapy to prevent or treat supraventricular or junctional tachyarrhythmias. Few studies have shown that DEX use is associated with decreased frequency of perioperative tachyarrhythmias (34, 35) while others showed increased odds of bradyarrhythmias (36).
In addition to the obvious benefits of decreased exposure to sedative agents, a more conservative anesthetic strategy may help in decreasing the incidence of delirium. In a recent multinational study, pediatric delirium was shown to be a common complication of critical illness, with a prevalence of 25% and identifiable risk factors of narcotic and benzodiazepine use, among several others. Younger age and mechanical ventilation were also strongly associated with delirium (37). The same authors have also demonstrated an independent association of delirium with mortality in a single center study (38). Although the current study did not assess for delirium in the postoperative period, this anesthetic strategy addresses some of the important modifiable risk factors of delirium by decreasing duration of mechanical ventilation, limiting opioid and BZD exposure and use of DEX.
The current study has several limitations inherent to the retrospective study design. The idea of low dose narcotic technique with adjuvant medication use to achieve EE is not unique. The authors felt the need to document the actual anesthetic and sedation practice change in the operating room and in the ICU that made EE feasible in this multicenter cohort, given the potential of extrapolation to all centers regardless of their previous extubation practices. Evaluation of VAA use in the setting of limited narcotic exposure is of added value, but the study was never powered to study the difference in the VAA exposure, so it is hard to draw definite conclusions. The preference to use DEX among practitioners to increase EE during the period of CPG implementation adds another limitation. However, CPG implementation was analyzed as part of the multivariable logistic regression analysis to account for preferential utilization of the DEX, and the association of intraoperative DEX use with EE remained significant after this adjustment. The applicability and extrapolation of this EE anesthetic strategy to other congenital heart surgeries will be interesting to study in the future. Currently, the experience at the model site indicates that the results apply to neonates and infants undergoing other open or closed cardiac procedures. TOF and isolated CoA were chosen for the CLS as they were more straight forward and commonly occurring procedures with least variation. As such, our conclusions in this study are limited to these two operations.
CONCLUSIONS
The current study showed that an anesthetic strategy of decreased intraoperative use of opioids and BZDs with simultaneous use of adjunctive medications is feasible and safe to accomplish the goal of EE. Intraoperative DEX administration is independently associated with EE and should be considered a part of the anesthetic strategy.
Supplementary Material
Online Appendix 1: Clinical Practice Guideline developed and implemented by the Pediatric Heart Network Collaborative Learning Study. The guideline provides decision algorithm for early extubation of pediatric cardiac post-op patients with two lesions : Isolated Coarctation of Aorta and Tetralogy of Fallot.
Online Appendix 2: Current ancillary study design comparing the anesthesia and sedative exposure before and after the implementation of the Clinical Practice Guideline.
Online Appendix 3: Univariable logistic regression of early extubation on candidate predictors: Age at surgery, weight at surgery, clinical practice guideline, cardiopulmonary bypass time, intraoperative fentanyl dose and type of operation were all significantly associated with early extubation. These predictors were selected for inclusion in the outcome model.
Online Appendix 4: Comparison of the total study cohort based on Dexmedetomidine use in the operating room (unadjusted)
Acknowledgments
We want to thank the primary investigators of the Pediatric Heart Network Collaborative Learning Study, specifically Dr. William T. Mahle, M.D., Department of Pediatrics, Emory University, Atlanta GA for his support to this ancillary study. The primary author also wishes to acknowledge the support of PHN Utah leadership Drs. LuAnn Minich M.D and Richard V. Williams M.D. in conducting this study.
Funding Disclosure:
Research reported in this publication was conducted as an ancillary study of the Pediatric Heart Network (PHN) supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number HL068270, HL068290, HL 109673, HL109737, HL109741, HL109741, HL109743, HL109777, HL109778, HL109781, HL109816, HL109818. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This investigation was also supported by the University of Utah Population Health Research (PHR) Foundation, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067–05 (formerly 8UL1TR000105 and UL1RR025764)
Footnotes
Copyright form disclosure:
Drs. Amula, Vener, Presson, and Nicolson received support for article research from the National Institutes of Health (NIH). Dr. Amula’s institution received funding from the NIH. Dr. Vener’s institution received funding from NIH (Pediatric Heart Network). Dr. Riegger’s institution received funding from the Pediatric Heart Network, and she received support for article research from Pediatric Heart Network. Dr. Presson disclosed that this investigation was supported by the University of Utah Population Health Research (PHR) Foundation, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067–05 (formerly 8UL1TR000105 and UL1RR025764). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Conflicts of Interest: None
Contributor Information
Venu Amula, Department of Pediatrics, University of Utah, Salt Lake City, UT
David F. Vener, Department of Pediatric Anesthesia, Pediatric Cardiac Anesthesia, Texas Children’s Hospital, Baylor College of Medicine, Houston, TX
Charles G. Pribble, Department of Pediatrics, University of Utah, Salt Lake City, UT
Lori Riegger, Department of Anesthesia, University of Michigan, Ann Arbor, MI
Elizabeth C. Wilson, Department of Anesthesia, Emory University, Children’s, Healthcare of Atlanta, Atlanta, GA
Lara S. Shekerdemian, Department of Pediatrics, Texas Children’s Hospital, Baylor College of Medicine, Houston, TX
Zhining Ou, Division of Epidemiology, Department of Internal Medicine, University of Utah, Salt Lake City, UT
Angela P. Presson, Division of Epidemiology, Department of Internal, Medicine, University of Utah, Salt Lake City, UT
Madolin K. Witte, Department of Pediatrics, University of Utah, Salt Lake City, UT
Susan C. Nicolson, Department of Anesthesiology and Critical Care Medicine, Children’s Hospital of Philadelphia, Philadelphia, PA
REFERENCES
- 1.Neirotti RA, Jones D, Hackbarth R, et al. : Early extubation in congenital heart surgery. Heart Lung Circ 2002; 11:157–161 [DOI] [PubMed] [Google Scholar]
- 2.Winch PD, Nicholson L, Isaacs J, et al. : Predictors of Successful Early Extubation Following Congenital Cardiac Surgery in Neonates and Infants. Heart Lung Circ 2009; 18:271–276 [DOI] [PubMed] [Google Scholar]
- 3.Miller JW, Vu D, Chai PJ, et al. : Patient and procedural characteristics for successful and failed immediate tracheal extubation in the operating room following cardiac surgery in infancy. Paediatr Anaesth 2014; 24:830–839 [DOI] [PubMed] [Google Scholar]
- 4.Mittnacht AJ, Thanjan M, Srivastava S, et al. : Extubation in the operating room after congenital heart surgery in children. J Thorac Cardiovasc Surg 2008; 136:88–93 [DOI] [PubMed] [Google Scholar]
- 5.Anand KJ, Hickey PR: Halothane–Morphine Compared with High-Dose Sufentanil for Anesthesia and Postoperative Analgesia in Neonatal Cardiac Surgery. N Engl J Med 1992; 326:1–9 [DOI] [PubMed] [Google Scholar]
- 6.Naguib AN, Tobias JD, Hall MW, et al. : The role of different anesthetic techniques in altering the stress response during cardiac surgery in children: a prospective, double-blinded, and randomized study. Pediatr Crit Care Med 2013; 14:481–490: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruber EM, Laussen PC, Casta A, et al. : Stress Response in Infants Undergoing Cardiac Surgery: A Randomized Study of Fentanyl Bolus, Fentanyl Infusion, and Fentanyl-Midazolam Infusion. Anesth Analg 2001; 92:882–890 [DOI] [PubMed] [Google Scholar]
- 8.Achuff BJ, Nicolson SC, Elci OU, et al. : Intraoperative dexmedetomidine reduces postoperative mechanical ventilation in infants after open heart surgery. Pediatr Crit Care Med 2015; 16:440–447. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Ye H, Xia Y, et al. : Clinical efficacy of dexmedetomidine in the diminution of fentanyl dosage in pediatric cardiac surgery. Minerva Pediatr 2017; 69:181–187 [DOI] [PubMed] [Google Scholar]
- 10.Mahle WT, Nicolson SC, Hollenbeck-Pringle D, et al. : Utilizing a Collaborative Learning Model to Promote Early Extubation Following Infant Heart Surgery. Pediatr Crit Care Med 2016; 17:939–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf MJ, Lee EK, Nicolson SC, et al. : Rationale and methodology of a collaborative learning project in congenital cardiac care. Am Heart J 2016; 17:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform 2009; 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanza ST, Moore JE, Butera NM: Drawing Causal Inferences Using Propensity Scores: A Practical Guide for Community Psychologists. Am J Community Psychol 2013; 52:380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC: An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011; 46:399–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Greene T: A Weighting Analogue to Pair Matching in Propensity Score Analysis Int J Biostat 2013; 9:215–34 [DOI] [PubMed] [Google Scholar]
- 16.Lumley T: Analysis of Complex Survey Samples. Journal of Statistical Software 2004; 9:1–19 [Google Scholar]
- 17.Harder VS, Stuart EA, Anthony JC: Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 2010; 15:234–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- 19.Mittnacht AJ: Pro: Early Extubation Following Surgery for Congenital Heart Disease. J Cardiothorac Vasc Anesth 2011; 25:874–876 [DOI] [PubMed] [Google Scholar]
- 20.DiNardo JA : Con: Extubation in the Operating Room Following Pediatric Cardiac Surgery. J Cardiothorac Vasc Anesth 2011; 25:877–879. [DOI] [PubMed] [Google Scholar]
- 21.Garg R, Rao S, John C, et al. : Extubation in the operating room after cardiac surgery in children: a prospective observational study with multidisciplinary coordinated approach. J Cardiothorac Vasc Anesth 2014; 28:479–487 [DOI] [PubMed] [Google Scholar]
- 22.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. : Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 2003; 23:876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez V, Feinstein SD, Lunardi N et al. : General anesthesia causes long-term impairment of mitochondrial morphogenesis and synaptic transmission in developing rat brain. Anesthesiology 2011; 115:992–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology 2010; 112:834–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Disma N, Mondardini MC, Terrando N, et al. : A systematic review of methodology applied during preclinical anesthetic neurotoxicity studies: Important issues and lessons relevant to the design of future clinical research. Paediatr Anaesth 2016; 26:6–36 [DOI] [PubMed] [Google Scholar]
- 26.O’Leary JD, Janus M, Duku E, et al. : A population-based study evaluating the & association between surgery in early life and child development at Primary School Entry. Anesthesiology 2016; 125:272–279. [DOI] [PubMed] [Google Scholar]
- 27.Graham MR, Brownell M, Chateau DG, et al. : Neurodevelopmental assessment in kindergarten in children exposed to General Anesthesia before the age of 4 years. Anesthesiology 2016; 125:667–677. [DOI] [PubMed] [Google Scholar]
- 28.Glatz P, Sandin RH, Pederson NL, et al. : Association of anesthesia and surgery & during childhood with long-term academic Performance. JAMA Pediatr 2017; 171:1–10. [DOI] [PubMed] [Google Scholar]
- 29.Guerra GG, Robertson CM, Alton GY et al. Western Canadian Complex Pediatric Therapies Follow-up Group. Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy. Paediatr Anaesth. 2011; 21:932–941 [DOI] [PubMed] [Google Scholar]
- 30.Warner DO, Zuccarello MJ, Katusic SK, et al. : Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring General Anesthesia. Anesthesiology, 2018; 129:89–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chrysostomouc C, Sanchez De Toledo J, Avolio T, et al. : Dexmedetomidine use in a pediatric cardiac intensive care unit: can we use it in infants after cardiac surgery? Pediatr Crit Care Med 2009; 10:654–660 [DOI] [PubMed] [Google Scholar]
- 32.Schwartz LI, Twite M, Gulack B, et al. : The Perioperative Use of Dexmedetomidine in Pediatric Patients with Congenital Heart Disease. Anesth Analg 2016; 123:715–721. [DOI] [PubMed] [Google Scholar]
- 33.Source: IMS Health, Inpatient Healthcare Utilization System (IHCarUS). June 2014-May 2015. Data extracted November 2015.
- 34.Chrysostomou C, Beerman L, Shiderly D, et al. : Dexmedetomidine: A Novel Drug for the Treatment of Atrial and Junctional Tachyarrhythmias During the Perioperative Period for Congenital Cardiac Surgery: A Preliminary Study. Anesth Analg 2008;107: 1514–1522 [DOI] [PubMed] [Google Scholar]
- 35.Chrysostomou C, Sanchez-de-Toledo J, Wearden P, et al. :. Perioperative Use of Dexmedetomidine Is Associated With Decreased Incidence of Ventricular and Supraventricular Tachyarrhythmias After Congenital Cardiac Operations. Ann Thorac Surg 2011; 92: 964–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shuplock JM, Smith AH, Owen J, et al. : Association Between Perioperative Dexmedetomidine and Arrhythmias After Surgery for Congenital Heart Disease. Circ Arrhythm Electrophysiol 2015; 8:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traube C, Silver G, Reeder RW, et al. Delirium in Critically Ill Patients: An International Point Prevalence Study. Crit Care Med 2017; 45:584–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traube C, Silver G, Gerber LM, et al. Delirium and Mortality in Critically Ill Children. Crit Care Med 2017; 45:891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Appendix 1: Clinical Practice Guideline developed and implemented by the Pediatric Heart Network Collaborative Learning Study. The guideline provides decision algorithm for early extubation of pediatric cardiac post-op patients with two lesions : Isolated Coarctation of Aorta and Tetralogy of Fallot.
Online Appendix 2: Current ancillary study design comparing the anesthesia and sedative exposure before and after the implementation of the Clinical Practice Guideline.
Online Appendix 3: Univariable logistic regression of early extubation on candidate predictors: Age at surgery, weight at surgery, clinical practice guideline, cardiopulmonary bypass time, intraoperative fentanyl dose and type of operation were all significantly associated with early extubation. These predictors were selected for inclusion in the outcome model.
Online Appendix 4: Comparison of the total study cohort based on Dexmedetomidine use in the operating room (unadjusted)