Abstract
Objectives
To systematically summarize the risk relationship between different levels of alcohol consumption and incidence of liver cirrhosis.
Methods
Medline and Embase were searched up to March 6th, 2019 to identify case-control and cohort studies with sex-specific results and more than two categories of drinking in relation to incidence of liver cirrhosis. Study characteristics were extracted and random-effects meta-analyses and meta-regressions were conducted.
Results
A total of seven cohort studies and two case-control studies met the inclusion criteria, providing data from 2,629,272 participants with 5,505 cases of liver cirrhosis. There was no increased risk for occasional drinkers. Consumption of 1 drink per day in comparison to long-term abstainers showed an increased risk for liver cirrhosis in women, but not in men. The risk for women was consistently higher compared to men. Drinking ≥5 drinks per day was associated with a substantially increased risk in both women (RR = 12.44, 95% CI: 6.65 – 23.27 for 5–6 drinks, and RR = 24.58, 95% CI: 14.77 – 40.90 for ≥7 drinks) and men (RR = 3.80, 95% CI: 0.85 – 17.02, and RR = 6.93, 95% CI: 1.07 – 44.99, respectively). Heterogeneity across studies indicated the additional impact of other risk factors.
Conclusions
Alcohol is a major risk factor for liver cirrhosis with risk increasing exponentially. Women may be at higher risk compared to men even with little alcohol consumption. More high-quality research is necessary to elucidate the role of other risk factors, such as genetic vulnerability, body weight, metabolic risk factors, and drinking patterns over the life course. High alcohol consumption should be avoided, and people drinking at high levels should receive interventions to reduce their intake.
Keywords: Liver cirrhosis, Alcohol drinking, Cohort studies, Case-control studies, Systematic review, Meta-analysis
INTRODUCTION
Alcohol is a major risk factor for liver disease in general, and for liver cirrhosis in particular.(1–3) In fact, about half of the liver cirrhosis burden of morbidity and mortality would disappear in a world without alcohol.(4) Mortality from liver cirrhosis has been on the rise in the US(5) and Europe,(6) more so in women than in men. Alcohol consumption is partly responsible, but liver disease is increasingly recognized as a multifactorial disease process.(6)
The importance of alcohol in the etiology of liver disease has led to establishing different codes for categories of liver diseases, which are considered to be primarily caused by alcohol. Thus, the International Classification of Diseases (ICD-10(7)) recognizes several forms of alcoholic liver disease (ICD-10, K70), sometimes considered stages(8) that range from relatively mild and reversible alcoholic hepatic steatosis (fatty liver) (K70.0) and alcoholic hepatitis (K70.1), to alcoholic fibrosis and sclerosis of the liver (K70.2), and further to severe and irreversible stages such as alcoholic liver cirrhosis (K70.3) and alcoholic hepatic failure (K70.4). Alcohol consumption, in particular heavy use over time, has been found crucial in the etiology and progression of these diseases.(1, 9, 10) However, liver diseases are multifactorial, and alcohol use may play a role in the progression of all types of cirrhosis,(11) and even one drink per day may have an effect on the incidence of liver cirrhosis, (12). For scientific review of all liver cirrhosis, it is therefore crucial to include both alcoholic and non-alcoholic liver cirrhosis when examining the impact of alcohol use.
Most of the epidemiological literature to date has dealt with the level of drinking and incidence or mortality of liver cirrhosis.(13) It followed the epidemiological tradition of the early studies of Lelbach and others,(14, 15) who, based on studies in people with alcohol use disorders, postulated a clear association between volume of alcohol use and liver cirrhosis.(1) This association was corroborated in more rigorous studies.(1, 13, 16) It remains to be determined, however, if a threshold for alcohol-related damage to the liver exists, or whether any amount of alcohol increases the risk for liver cirrhosis, which has been discussed recently.(17–19) In fact, the last meta-analysis on the topic is now more than 10 years old and found some evidence for a protective association at low levels of alcohol intake in men.(13) Furthermore, several large-scale studies have been published since then.(20–22)
The present review provides an overview of the current knowledge on the dose-response relationship between alcohol consumption and risk of liver cirrhosis in comparison to abstainers, with particular consideration given to the effects of study design and sex, and other subgroups where data were available. As noted above, our review was not restricted to alcoholic liver cirrhosis.
Methods
Search strategy and selection criteria
Following the MOOSE guidelines,(23) we conducted a systematic electronic literature search using Medline and Embase from inception to March 6th, 2019 for keywords and MeSH terms relating to alcohol consumption, liver cirrhosis, and observational studies (Supplementary Table 1). Additionally, we searched reference lists of identified articles and published meta-analyses and reviews. Inclusion criteria were as follows:
cohort and case-control studies examining the sex-specific association between average alcohol consumption and liver cirrhosis,
analyses were adjusted for age at baseline,
data for at least two quantitatively defined categories of average alcohol consumption in relation to non-drinkers, or data for former drinkers in relation to long-term abstainers were reported,
more than 50 cases of liver cirrhosis occurred.
We did not apply language restrictions. At least two reviewers independently excluded articles based on title and abstract or full-text and abstracted the data. Any discrepancies were resolved in consultation with a third reviewer.
Data extraction
From all relevant articles we extracted authors’ names, year of publication, country, year(s) of baseline examination, follow-up period, setting of the study, study design, assessment of liver cirrhosis, age (range, mean or median) at baseline, sex, number of observed liver cirrhosis cases among participants by drinking group, number of total participants by drinking group, specific adjustment or stratification for potential confounders, and adjusted relative risks (RRs) and their confidence intervals (CIs) or standard errors. Risk estimates by sex were treated as independent samples. Where necessary, RRs within studies were re-calculated to contrast alcohol consumption categories against non-drinkers.(24)
Exposure and outcome assessment
Consolidating exposure measures across primary studies involved a two-step process. First, among drinkers, we converted reported alcohol intake categories in primary studies into an average of pure alcohol in grams per day (g/day) using the midpoints (mean or median) of reported drinking group categories. For open-ended categories, we added three quarters of the second highest category’s range to the lower limit of the open-ended category of alcohol intake if the mean was not reported. Standard drinks vary by country, with one standard drink containing approximately 8–14 g of pure alcohol.(25) We used reported conversion factors when standard drinks were the unit of measurement to convert all measures to grams per day. Then, for reporting of our analyses, we considered categories with a mean of up to 12 grams pure ethanol as one standard drink for a global representation. Qualitative descriptions, such as ‘social’ or ‘frequent’ drinkers with no clear total alcohol intake in g/day were excluded. When current non-drinkers were the reference group (i.e., including both long-term abstainers and former drinkers), we adjusted risk estimates for the effect of former drinking compared to long-term abstention, based on the pooled risk for former drinking from two studies included in this review to avoid the sick-quitter effect. Long-term abstainers were defined as people who stated that they never consumed alcohol,(20) people who stated that they never, or almost never, drank alcohol in the past,(26) and when people who had greatly decreased their consumption in the last 10 years were excluded from non-drinkers.(27) The logRR for former drinkers in comparison to long-term abstainers (RRformer drinkers = 2.52) was multiplied by the mean fraction of former drinkers among current non-drinkers (0.23) and added to the respective logRRs of current drinking groups from primary studies used in our analysis when current non-drinkers was the reference group.
Liver cirrhosis due to known aetiology such as alcohol, and unspecified liver cirrhosis was defined as in the primary studies, which included ICD codes for liver cirrhosis (ICD-7: 581; ICD-8: 571; ICD-10: K70, K73, K74) and unspecified liver cirrhosis (ICD-8: 571.9, 456.0, 785.3; ICD-10: I85.0, I85.9, K74.6, R18.9). Because we aimed to estimate the relative risk in comparison to abstainers, we excluded several studies (e.g., (28, 29)) which focused only on alcoholic liver cirrhosis (or included alcoholism in addition to liver cirrhosis in the outcome),(30) which, by definition, cannot occur in lifetime abstainers.
Quality assessment
Most quality scores are tailored for meta-analyses of randomized trials of interventions(31–33) and many criteria do not apply to epidemiological studies examined in this study. Additionally, quality score use in meta-analyses remains controversial.(34–36) As a result, study quality was enhanced by including quality components, such as study design, measurement of alcohol consumption and liver cirrhosis, adjustment for age in our inclusion and exclusion criteria, and further by investigating potential heterogeneity in several sensitivity analyses. We used the most adjusted RR reported and the most comprehensive data available for each analysis and gave priority to estimates where lifetime or long-term abstainers were used as the risk reference group.
In a formal risk of bias analysis, we used the Cochrane Risk of Bias Tool for Non-Randomized Studies (ROBINS-I(37)) to assess risk of bias in primary studies. We rated the evidence for the association between alcohol consumption and incidence of liver cirrhosis based on the Grades of Recommendation, Assessment, Development and Evaluation system (GRADE).(38)
Statistical analyses
In categorical analyses using standard drinks (12 grams pure alcohol) as the exposure measure, RRs were pooled with inverse-variance weighting using DerSimonian-Laird random-effect models to allow for between-study heterogeneity.(39) Small-study bias was examined using Egger’s regression-based test.(40) Variation in the effect size because of heterogeneity between studies was quantified using the I2 statistic.(41)
Using studies that reported data for four or more alcohol intake groups, we conducted two-stage restricted cubic spline regression analysis in multivariate meta-regression models, taking into account the variance-covariance matrix for risk estimates derived from one reference group(42, 43) to test for non-linear dose-response relationships in relation to long-term abstainers. All meta-analytical analyses were conducted on the natural log scale in Stata Statistical Software, Version 14.2.
Role of funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
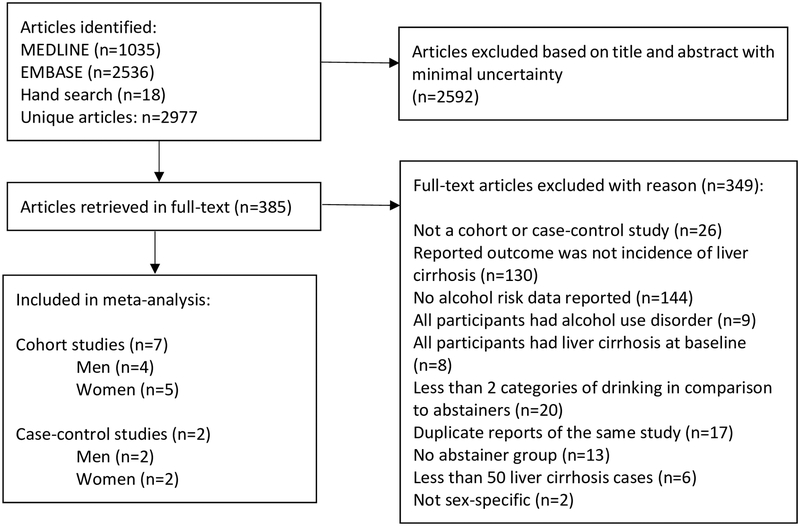
In total, out of 2,977 identified references, 385 articles were retrieved in full-text. Of these, seven cohort and two case-control studies fulfilled our inclusion criteria (Figure 1). Four studies were conducted in the US,(26, 27, 44, 45), two in Italy,(46, 47) and one each in China,(22) the UK,(21) and Denmark.(20) In total, data from 2,629,272 participants (579,592 men, 2,049,680 women) and 5,505 cases of liver cirrhosis (2,196 men, 3,309 women) were used in the analyses. All cohort studies included liver cirrhosis mortality as the outcome. The two case-control studies investigated first-time diagnosis of symptomatic liver cirrhosis in comparison to lifetime abstainers (Table 1). The study by Liu et al contributed 2,078 liver cirrhosis cases from the National Health Service Million Women Study linked to death and morbidity registries.(21) The proportion of non-drinkers varied widely, from 0.002% (lifetime abstainers) among men in the Danish study by Askgaard et al.(20) to 80% (current abstainers) in the study of women from the American Cancer Society I cohort by Garfinkel et al.(44) All cohort studies used a one-time measurement of alcohol consumption as the baseline alcohol intake, while the three case-control studies from Italy assessed lifetime alcohol consumption retrospectively. All but one cohort study were rated to be of moderate quality mainly because of the one-time measurement of alcohol consumption at baseline (cohort studies), and the observational study design (Supplementary Table 2). One cohort study(44) had potential serious bias because the results were adjusted only for age.
Fig. 1.
Flowchart of study selection
Table 1.
Characteristics of 7 cohort and 2 case-control studies investigating risk of liver cirrhosis by alcohol intake, 1988–2017.
| Reference | Baseline years, setting | Sex, age (years), country, cases (no.), participants (no.), follow-up time (years) | Exposure assessment | Outcome assessment | Adjustments |
|---|---|---|---|---|---|
| Askgaard et al, 2015(20) | 1993–1997, Danish men and women aged 50 to 64 participating in the Diet, Cancer, and Health study Exclusions: Previously diagnosed with cancer or alcoholic cirrhosis, missing or conflicting information on alcohol, smoking, education, and waist circumference. |
M, W 55.9 Denmark Women: 229 cases 29,221 participants Men: 393 cases 26,696 participants 14.9 |
Lifetime abstainers, former drinkers, current drinkers: (<14, 14–28, >28) d/week | Mortality from liver cirrhosis (obtained from the National Patient Register and the Danish Register of Causes of Death. ICD-8: 571.0 and ICD-10: K70.3, and codes for unspecified cirrhosis, ICD-8: 571.9, 456.0, 785.3 and ICD-10: I85.0, I85.9, K74.6, R18.9) | Age (underlying time axis), smoking, education, waist circumference. Subgroups: by drinking frequency. |
| Bofetta et al, 1990(45) | 1959, American Cancer Society volunteers (White men aged 40–59) from 25 states in the United States. |
M, 49.5 (40–59), USA 732 276,802 12 |
Current abstainers, occasional drinkers, irregular drinkers, current drinkers: (1, 2, 3, 4, 5, ≥6) d/day | Mortality from liver cirrhosis. ICD-7 code: 581 | Age (5-year groups), smoking (non-smoker, 1–20 cigarettes per day, 21+ cigarettes per day) |
| Corrao et al, 1993(46) | 1986–1990, Consecutive admissions from the emergency ward to the Division of Gastroenterology in Turin. Exclusion: Hepatocellular carcinoma, primary biliary cirrhosis. |
M, W 58.7 Italy Men: 207 cases, 207 controls Women: 113 cases, 113 controls N/A |
Lifetime abstainers, average lifetime intake: <50, 50–100, 100–150, 150–200, 200–250, >250) g/day | First-time diagnosis of liver cirrhosis based on signs of liver failure (ascites and/or encephalopathy and/or jaundice, or bleeding from ruptured oesophageal varices) | Matched on age (±5 years), sex. Subgroups: Stratified by duration of drinking (10, 20, ≥30 years); by age group (≤60 years, >60 years). |
| Corrao et al, 1997(47) | 1986–1990, Patients admitted to a) medical departments of 6 district hospitals (L’Aquila, 1989–1990), b) gastroenterology unit of a district hospital (Turin, 1993), c) medical departments of 16 hospitals nationwide (SIDECAR project, 1994–1996). Exclusion: Hepatic encephalopathy, hepatocellular carcinoma, primary biliary cirrhosis, Wilson’s disease, haemochromatosis, or acute causes of liver damage. Controls: Selected from the same hospitals for diseases unrelated to alcohol consumption (excluded were: orthopaedic and infectious diseases, patients from the psychiatry, gynaecology, obstetric departments, and gastroenterologic, metabolic, neoplastic, and cardiovascular diseases). |
M, W 57 Italy Men: 300 cases, 355 controls Women: 162 cases, 296 controls N/A |
Lifetime abstainers, average lifetime intake: <50, 50–100, >100) g/day | First-time diagnosis of liver cirrhosis based on signs of liver decompensation (ascites, jaundice, oedema, or bleeding from ruptured oesophageal varices), confirmed by liver biopsy in 319 cases. | Age (3 categories), area of residence, HCV status, HBsAg status Subgroups(48): HBsAg and/or anti-HCV positive participants were excluded. |
| Fuchs et al, 1995(27) | 1980, The Nurses’ Health Study (female registered nurses). Exclusion: ≥10 or more food items left blank, implausibly high or low scores for total food intake, history of cancer, angina, myocardial infarction, or stroke, women who reported no alcohol intake at baseline in 1980 but had greatly decreased their alcohol intake in the previous 10 years. |
W, 42.5 (30–55), USA 52 85,709 12 |
Long-term abstainers, current drinkers: (0.1–1.4, 1.5–4.9, 5–14.9, 15–29.2, ≥30) g/day | Mortality from liver cirrhosis. ICD-8 code: 571 | Age (5-year groups), smoking (never, <15, 15–24, >24 cigarettes per day), BMI, aspirin use, regular cholesterol level, diabetes, hypertension, myocardial infarction in a parent at 60 years of age, past or present oral-contraceptive use’ menopausal status, past or present postmenopausal hormone use, and energy-adjusted intake of dietary fiber and saturated fat |
| Garfinkel et al, 1988(44) | 1959–1960, American Cancer Society’s study (A prospective study of 581 321 women) Exclusion: Women with breast cancer |
W, 45, USA 589 581,321 12 |
Current abstainers, current drinkers: (occasional, 1, 2, 3, 4, 5, ≥6) d/day | Mortality from liver cirrhosis | Age (standardized mortality ratio stratified into 5-year age groups) |
| Klatsky et al, 2003(26) | 1978–1998, Kaiser Permanente Medical Care Program |
M, W, 40.6 USA Men: 146 cases 56,836 participants Women: 86 cases 72,008 participants 20 |
Lifetime abstainers, ex-drinkers, current drinkers: (<1 drink/month, >1 drink/month but <1 drink/day, 1–2, 3–5, ≥6) d/day | Mortality from liver cirrhosis ascertained by using an automated matching system to ascertain death in California | Age (undefined), race, BMI, education, marital status, smoking (never, ex, <1 pack, >=1 pack a day), coronary disease risk/symptoms |
| Liu et al, 2009(21) | 1996–2005, Women who were recruited through the United Kingdom National Health Service (NHS) Breast Screening Service (The Million Women Study). Exclusion: Hospital admission for or reported a history of either of the diagnoses of interest (liver cirrhosis or gallbladder disease) before recruitment, a diagnosis of cancer (except nonmelanomatous skin cancer (ICD-10 code C44)), self-reported hepatitis or had a record of viral hepatitis (ICD-10 codes B15–B19) at recruitment or during follow-up. |
W, 56, UK 2078 1,280,737 6.1 |
Current abstainers, current drinkers: (1–2, 3–6, 7–14, > 15) units/week | Mortality and incidence of liver cirrhosis with record linkage to the NHS central registries for deaths, cancers, and emigrations, and to the Hospital Episode Statistics for England, and Scottish Morbidity Records for hospital admission data: ICD-10 codes: K70, K73, K74 | Age (underlying time axis), region of recruitment, SES, BMI, smoking (never, past, current: 1–9, 10–19, >=20 cigarettes per day). Subgroups: by smoking status. Subgroups among drinkers by drinking frequency, drinking with or without meals, and type of beverage.(49) |
| Yang et al, 2012(22) | 1990–1991, Men randomly selected from China’s National Disease Surveillance Points (23 urban and 22 rural areas). Exclusion: A prior disease, deaths within the first 3 years of follow-up, missing values. |
M, 54.3, China 418 218,189 15 |
Current abstainers, current drinkers: (<140, 140–279, 280–419, 420–699, ≥700) g/week | Mortality due to liver cirrhosis, obtained from official death certificates using ICD-9 | Age (stratified by 5-year groups), area, smoking, education |
Abbreviations: BMI, body mass index; M, men; W, women; M, W, men and women stratified; M/W, men and women combined; SES, socioeconomic status; NHS, National Health System.
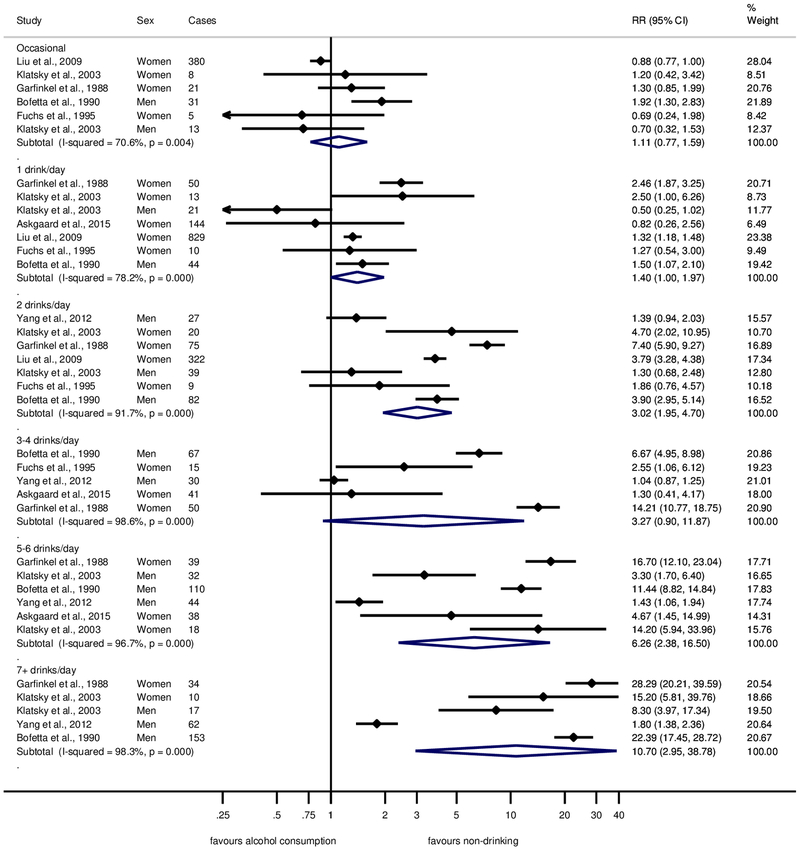
The pooled proportion of former drinkers among current abstainers(20, 26) was 23%, and the pooled RR for liver cirrhosis in comparison to long-term abstainers was 2.56 (95% CI: 0.93 – 6.79). Figure 2 displays the RRs for liver cirrhosis in cohort studies by alcohol intake in reference to long-term abstainers after current abstention at baseline was adjusted for the proportion and risk in former drinkers. Alcohol consumption beyond occasional drinking, which showed a similar risk compared to long-term abstainers, was associated with increasing risk for liver cirrhosis (Figure 2) with a pooled RR of 10.70 (95% CI: 2.95–38.78) for consumption of 7 drinks or more per day. However, all drinking categories showed substantial heterogeneity across studies (I2 between 70 and 98%, all P-values <0.001), resulting in large confidence intervals. We restricted analyses of small-study effects and influential studies to drinkers of 1 or 2 drinks per day for both sexes because of the small number of studies identified. We found no statistical evidence for small study bias for drinkers of 1 or 2 drinks per day (P = 0.94), the funnel plot showed similar results (Supplementary Figure 1). None of the studies had an overly large impact on the pooled estimates (Supplementary Figure 2).
Fig. 2. Forest plot of liver cirrhosis risk by alcohol consumption (in comparison to long-term abstainers) in cohort studies, 1988–2017.
Relative risk on the log scale. 1 standard drink = 12 grams pure ethanol per day. RR = relative risk.
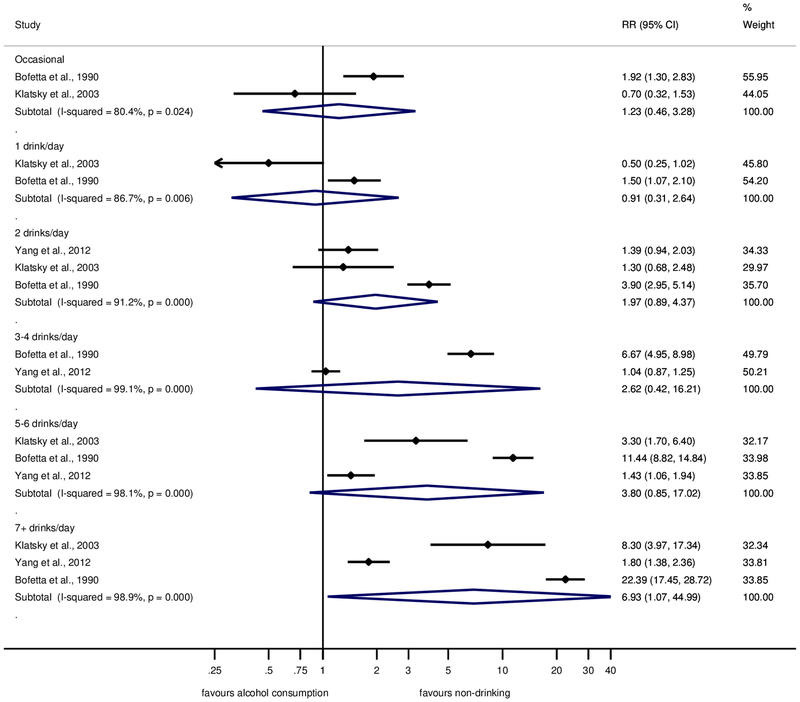
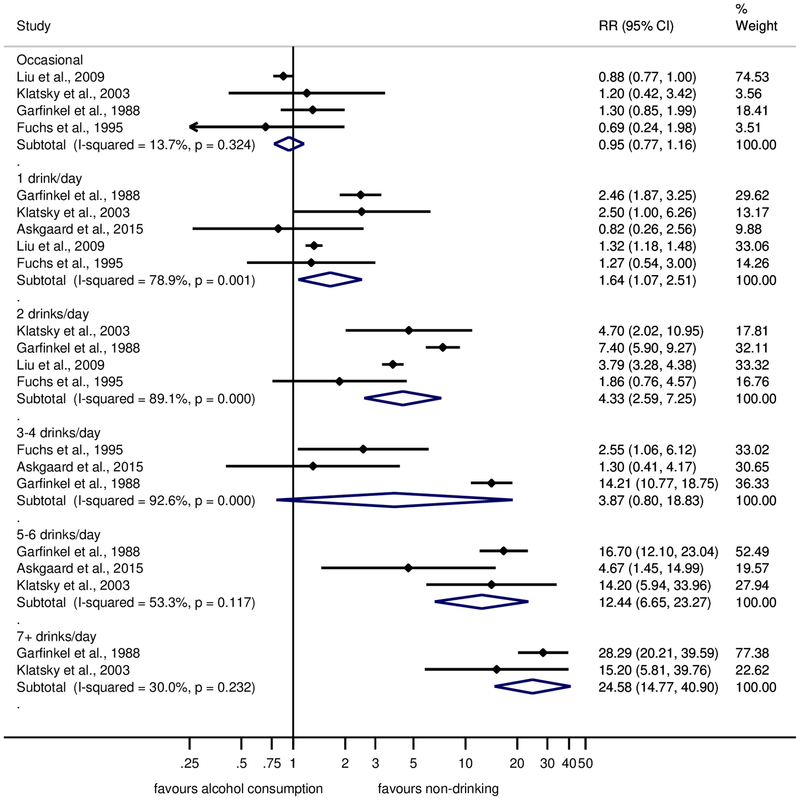
Results for men and women are shown separately in Figures 3 and 4, respectively. Across all consumption levels, RRs in women were higher, reaching RR = 24.58 (95% CI: 14.77–40.90) for ≥7 drinks. While consumption of 1–2 drinks was associated with a substantially elevated risk for liver cirrhosis in women, this was not the case in men. However, these results need to be interpreted with caution because of the small number of studies available. Four cohort studies(20, 21, 26, 27) in women were adjusted for age, BMI or waist circumference, and smoking. The relationship was similar to the main analysis with an elevated and linearly increasing risk for consumption of 1 drink and beyond (Supplementary Figure 3).
Fig. 3. Forest plot of liver cirrhosis risk by alcohol consumption (in comparison to long-term abstainers) in cohort studies in men, 1988–2017.
Relative risk on the log scale. 1 standard drink = 12 grams pure ethanol per day. RR = relative risk.
Fig. 4. Forest plot of liver cirrhosis risk by alcohol consumption (in comparison to long-term abstainers) in cohort studies in women, 1988–2017.
Relative risk on the log scale. 1 standard drink = 12 grams pure ethanol per day. RR = relative risk.
In both men and women, there was no evidence for a non-linear dose-response relationship on the log scale (P=0.24 and 0.27, respectively, Supplementary Figures 4 and 5). However, the number of studies available was low, resulting in little power to detect non-linearity.
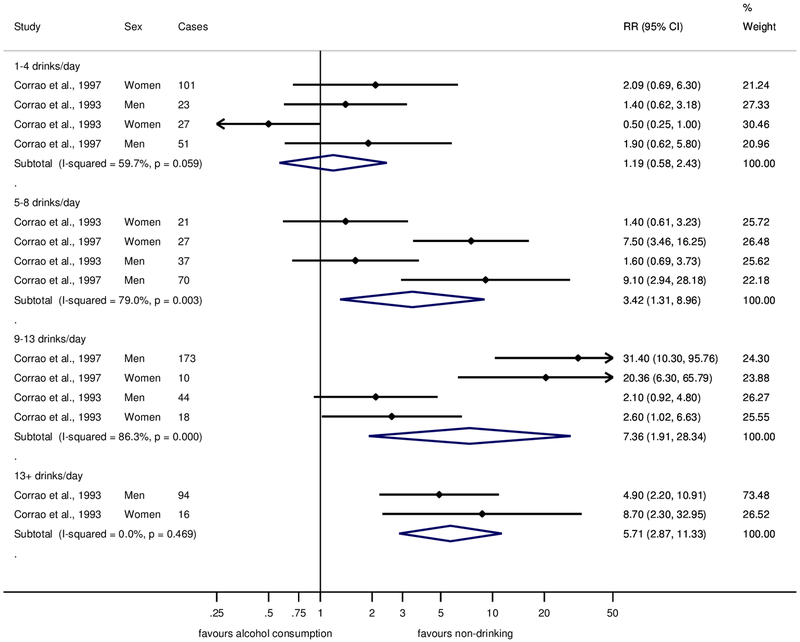
The two case-control studies with liver cirrhosis morbidity as the outcome yielded smaller risks associated with alcohol consumption, with 1–4 drinks showing no risk increase compared to lifetime abstainers (pooled RR=1.19, 95% CI: 0.58–2.43, Figure 5). Risks for consumption of 5–8 and 9–13 drinks were associated with large heterogeneity with one study(47) showing substantial risk increases for both men and women, while the other study(46) showed no or marginally statistically significant risk increases for either men or women (see also Supplementary Figures 6 and 7).
Fig. 5. Forest plot of liver cirrhosis risk by alcohol consumption (in comparison to lifetime abstainers) in case-control studies, 1988–2017.
Relative risk on the log scale. 1 standard drink = 12 grams pure ethanol per day. RR = relative risk.
Subgroup analyses
One cohort study(21) showed that while the risk in smokers was higher than in never-smokers, the risk of liver cirrhosis by alcohol intake increased in never-smokers similar to current smokers, indicating that smoking is a confounder but not an effect modifier. In another report(48) from the case-control studies by Corrao et al, it was shown that the relationship between alcohol and liver cirrhosis in all participants was similar to participants without serum HBsAg and/or positive anti-HCV status. One of the case-control studies(46) included in our main analysis also showed that the risk for liver cirrhosis was greatest in drinkers who drank heavily for 10 or 20 years, but not for 30 years, indicating potentially a survivor bias. Similarly, the same study(46) reported risk by age (≤60 years and > 60 years), showing that the risk increase was stronger in younger participants for both sexes. The cohort study by Askgaard et al.(20) reported results by frequency of drinking days adjusted for weekly alcohol intake. In men, there was an increased risk for daily drinking in comparison to drinking on 2–4 days per week (RR = 2.25, 95% CI: 1.68–3.00). In women, the RR was 1.28 (95% CI: 0.82–2.02). Results from the UK Million Women study(49) showed that among drinkers daily drinking in comparison to non-daily drinking (RR = 1.61, 95% CI: 1.40–1.85) and drinking with meals in comparison to drinking outside of meals (RR = 0.69, 95% CI: 0.62–0.77) were associated with incidence of liver cirrhosis. These associations were similar in strata of BMI, smoking status, and type of alcoholic beverage. Women who both drank daily and outside of meals had a 2.47 (95% CI: 1.96–3.11) increased risk for liver cirrhosis with adjustment for amount and type of beverage.(49)
Given the observational nature of the studies included in this report, we rate the evidence for a causal effect of alcohol consumption and risk for liver cirrhosis as moderate. However, the dose-response relationship in addition to established biological pathways confirmed in randomized controlled trials(50) give rise to high confidence in a causal dose-response relationship. There was no clear indication for a threshold effect, but we rate the quality of the evidence as low because of imprecision and the small number of studies reporting sex-specific RRs for low levels of drinking.
Discussion
We conducted a systematic review and various meta-analyses on alcohol consumption and risk of liver cirrhosis. Contrary to prior analyses,(13) we found overall no protective effects at any level of drinking when compared to long-term abstainers, and a steadily increasing dose-response relationship in women, and some evidence for a threshold effect in men. However, risks varied widely and the analysis of case-control studies showed no risk increase for consumption of 1–4 drinks per day. The high risk for heavy drinkers found in our meta-analysis of cohort studies is in line with prior research on risk for liver cirrhosis in people with alcohol use disorders.(11, 51, 52) The pooled RRs from case-control studies were much smaller; however, the more recent case-control study(47) corresponds with the risks found in people with alcohol use disorder. One of the cohort studies and one of the case-control studies reported very small RRs compared to the other studies. The reasons for this are unclear, although some outliers are to be expected in any statistical analysis.
Additionally, many studies were not well adjusted and of generally moderate methodological quality, mostly related to potential bias due to confounding and selection bias. While the increase in risk was stronger in women, confidence intervals were large and overlapped with those for men. Stronger effects in women are supported by studies in people with alcohol use disorder with or without liver cirrhosis(53, 54), and higher hepatotoxicity. While there is no doubt that heavy alcohol consumption is one of the main risk factors for liver cirrhosis, the large heterogeneity observed indicates that the multifactorial nature of development of liver cirrhosis has not been reflected in the epidemiological literature. Liver cirrhosis has a complex and not fully understood etiology, and the contributory role of other risk factors for liver cirrhosis, such as BMI, metabolic syndrome, diabetes, drinking frequency and outside of meals, and others, at any given level of alcohol intake over the life course, need more attention in both research and prevention efforts.
While several important confounders have been identified and should be adjusted for in epidemiological studies, it is likely that some of them are in fact effect modifiers that impact the risk associated with alcohol consumption. Most important here is genetic vulnerability. Twin studies have shown a three-fold higher disease concordance between monozygotic twins and dizygotic twins, but the genetic case-control studies have not yet led to conclusive results.(55) Genetic vulnerability is seen as the major reason why only a minority of very heavy drinkers develop liver problems. With respect to other potential effect modifiers, it seems that the drinking frequency modifies the risk for liver cirrhosis associated with a given total weekly alcohol intake with fewer drinking days being associated with lower risk supporting the notion of a ‘liver holiday’.(56–58) A report from the Singapore Chinese Health Study(59) showed that among daily drinkers, consumption of even one drink a day was associated with a RR = 2.72 (95% CI: 0.98–7.50) for liver cirrhosis in comparison to non-drinkers. In more recent years, patterns of drinking, especially binge drinking, were introduced as potentially important for the etiology and progression of liver cirrhosis.(60, 61) However, evidence is limited and inconclusive at this point.(62, 63) Future research should include standard measures on patterns of drinking, such as measures of irregular heavy drinking in addition to average volume of drinking and drinking frequency, to test hypotheses about such patterns, and to determine whether there is a positive effect of abstinence days.(64) The consumption of mostly wine, as opposed to beer or liquor, has been shown to modify the risk for alcoholic liver cirrhosis in some studies;(29, 58) however, as the UK Million Women study showed,(49) this may be explained by the consumption of alcohol with meals, which is more common in wine drinkers than consumers of other types of alcohol.
An investigation of Midspan cohorts(65) in Scotland indicated that BMI modifies the effect of alcohol consumption on liver disease, with obese participants being more susceptible to the harms from alcohol consumption than participants with lower BMI. An analysis of the Million Women Study(66) confirmed that BMI and alcohol consumption interact in development of liver cirrhosis, in particular at alcohol intake of more than 150 g/week and BMI above 30. Potential interaction with drinking patterns seem possible.(62) The effect of smoking in relation to alcohol consumption on liver cirrhosis is not clear. Several studies have reported an effect independent of alcohol consumption(21, 67), and no clear effect.(68, 69) Meta-analyses of the association of coffee consumption and risk for liver disease consistently show a decreased risk.(70, 71) Potential interaction with alcohol consumption should be explored. Liver cirrhosis severity may also play a role. Another analysis from the series of case-control studies from Italy showed the risk increase from alcohol consumption was characterized by a threshold effect at approximately 150 g/day, and a smaller risk at higher consumption for asymptomatic liver cirrhosis than for symptomatic liver cirrhosis. In other reports including the same participants,(69, 72) it was shown that HBV and HCV infection were risk factors independent from alcohol consumption. The role of nutrient intake is unclear. Several nutrients were investigated in reports from the Italian case-control studies. Possible interaction effects were observed for dietary intake of lipids,(73) vitamin A,(74) and iron.(74) However, larger sample sizes are required to detect an effect with sufficient power. More and higher quality epidemiological studies are needed to reach firm conclusions about confounding and interaction effects of these risk and protective factors in men and women.(51)
Other limitations of this review are based on the underlying literature. First, the number of original articles was limited. This is surprising given the fact that the majority of liver cirrhosis cases would not exist in a counterfactual scenario without alcohol. Second, the quality of the contributions was limited. Because of the small number of studies published, we were unable to investigate in detail the role of many study design characteristics, such as adjustment for potential confounders, follow-up length, race/ethnicity, and others that may play a role in the development of liver cirrhosis. Low response rates and inclusion criteria in primary studies, such as participants in screening programs, may limit the generalizability of our findings. Although self-reported alcohol consumption is generally reliable,(75) it may result in underestimation of the real consumption. No cohort study measured alcohol consumption more than once, thus opening the research to measurement and regression dilution bias, and underestimation of the real effect.(76) While the two case-control studies from Italy were able to assess lifetime drinking retrospectively, these types of studies are prone to recall bias, and categories of alcohol consumption were large, and adjustments for other risk factors for liver cirrhosis were minimal. Again, even with similar methodology in the same country, the two studies observed large differences in risk for liver cirrhosis for a given total alcohol intake. One possibility for the difference in risk observed between cohort and case-control studies is because of the difference in outcome assessment (mortality vs morbidity).
In comparison to our earlier meta-analysis,(13) the strengths of this meta-analysis lie in its clear definition of the outcome, and its methodological rigour. For example, we excluded studies with insufficient number of cases or adjustment,(77) and provide an examination of age, drinking patterns, and type of beverage where data were available. This strength came at a cost - some of the most well-known studies in the field, which were limited to subcategories of liver cirrhosis, had to be excluded.(28, 29), which was crucial to quantify the risk of liver cirrhosis in comparison to abstainers, which by definition cannot develop alcoholic liver cirrhosis.
What are the clinical conclusions of this study? The exponential dose-response curve on the relative risk level indicates that the highest levels of average volume of alcohol consumption confer exponentially higher risks and should be avoided.(78) For people at the high end of this trajectory, the risk for liver cirrhosis is very high,(16) and reductions of the highest levels are associated with the highest health gains.(79) This can be achieved on the individual level in two ways: first, the trajectory towards these levels should be interrupted early, and more than once. This should best be done at the general practitioner level with screening and brief interventions or treatment;(80) however, screening for unhealthy alcohol use is still not conducted routinely.(81, 82)` Second, (79)to prevent liver cirrhosis and subsequent complications including death in people with continued high consumption, it is most important to reduce high levels, even if the new drinking level are still high, and even if the patients still qualify for alcohol use disorders. Of course, the larger the reduction from a given level, the larger the reduction of relative risk, but it should be taken into consideration that any reduction of high volume drinking will be beneficial.(83) Finally, there are alcohol control policy measures. Measures like increase in price via taxation(84) or restrictions in availability have historically shown to impact on liver cirrhosis deaths.(85) Thus, the current high impact of alcohol consumption on liver cirrhosis is avoidable, and both individual interventions in the health care sector and alcohol control policies can contribute to reduce this impact.
Supplementary Material
Study Highlights.
What is the current knowledge
Alcohol is involved in all types of liver disease, and high alcohol consumption is associated with high disease risk.
Prior systematic evidence syntheses have included inconsistent definitions of alcohol exposure and liver cirrhosis.
What is new here
The risk for incidence of liver cirrhosis for former drinkers in comparison to long-term abstainers was three-fold.
With any alcohol consumption, the risk for liver cirrhosis increased exponentially among women; among men, the risk increased beyond consumption of 1 drink or more per day.
Drinking daily and outside of meals increases the risk for liver cirrhosis at any given level of overall alcohol intake. Several other risk factors for liver cirrhosis may modify the association of alcohol with liver cirrhosis, such as genetics, age, BMI, metabolic risk factors, and others.
Financial support
Research reported in this publication was supported by the National Institute On Alcohol Abuse And Alcoholism (NIAAA) of the National Institutes of Health under Award Number R21AA023521 to MR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsor of the study (NIAAA) had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The authors collected the data, and had full access to all of the data in the study. The authors also had final responsibility for the decision to submit the study results for publication.
Declaration of interests
MR and JR report grants from National Institutes of Health (NIH), National Institute on Alcohol Abuse and Alcoholism (NIAAA), during the conduct of the study. JR reports grants and personal fees from Lundbeck and D&A Pharma outside of this work. AV, OSMH, BRC, MGN, RL, MC report no conflicts of interest.
References
- 1.Becker U. Epidemiology and risk factors in alcohol liver disease In: Preedy VR, Watson RR, editors. Comprehensive handbook of alcohol related pathology. London, UK: Elsevier Academic Press; 2005. p. 467–480. [Google Scholar]
- 2.Lopez AD, Williams TN, Levin A, et al. Remembering the forgotten non-communicable diseases. BMC Med 2014;12:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749–1761. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Status Report on Alcohol and Health. Geneva: World Health Organization; 2018. [Google Scholar]
- 5.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pimpin L, Cortez-Pinto H, Negro F, et al. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol 2018;69:718–735. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision. In; 2016.
- 8.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Hepatology 2010;51:307–328. [DOI] [PubMed] [Google Scholar]
- 9.Menon KV, Gores GJ, Shah VH. Pathogenesis, diagnosis, and treatment of alcoholic liver disease. Mayo Clin Proc 2001;76:1021–1029. [DOI] [PubMed] [Google Scholar]
- 10.EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012;57:399–420. [DOI] [PubMed] [Google Scholar]
- 11.Schwarzinger M, Baillot S, Yazdanpanah Y, et al. Alcohol use disorders and the burden of chronic hepatitis C in France, 2008–2013: a nationwide retrospective cohort study. J Hepatol 2017;67:454–461. [DOI] [PubMed] [Google Scholar]
- 12.Roerecke M, Nanau R, Rehm J, et al. Ethnicity matters: a systematic review and meta-analysis of the non-linear relationship between alcohol consumption and prevalence and incidence of hepatic steatosis. EBioMedicine 2016;8:317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehm J, Taylor B, Mohapatra S, et al. Alcohol as a risk factor for liver cirrhosis - a systematic review and meta-analysis. Drug Alcohol Rev 2010;29:437–445. [DOI] [PubMed] [Google Scholar]
- 14.Lelbach WK. Liver damage in chronic alcoholism. Results of a clinical, clinical-chemical and bioptic-histological study in 526 alcoholic patients during a low-calorie diet in an open drinking sanatorium. Acta Hepatosplenol 1966;13:321–349. [PubMed] [Google Scholar]
- 15.Lelbach WK. Cirrhosis in the alcoholic and its relation to the volume of alcohol abuse. Ann N Y Acad Sci 1975;252:85–105. [DOI] [PubMed] [Google Scholar]
- 16.Askgaard G, Kjaer MS, Tolstrup JS. Opportunities to Prevent Alcoholic Liver Cirrhosis in High-risk Populations: A Systematic Review With Meta-analysis. Am J Gastroenterol 2018. [DOI] [PubMed] [Google Scholar]
- 17.Askgaard G, Tolstrup JS, Leon DA. A measure of alcohol consumption in late adolescence associated with liver disease after 39years of follow-up is insufficient to guide alcohol safe limits. J Hepatol 2018;69:251–252. [DOI] [PubMed] [Google Scholar]
- 18.Hagstrom H, Hemmingsson T, Discacciati A, et al. Reply to: “A measure of alcohol consumption in late adolescence associated with liver disease after 39years of follow-up is insufficient to guide alcohol safe limits”. J Hepatol 2018;69:252–253. [DOI] [PubMed] [Google Scholar]
- 19.Hagstrom H, Stal P, Hultcrantz R, et al. Overweight in late adolescence predicts development of severe liver disease later in life: A 39years follow-up study. J Hepatol 2016;65:363–8. [DOI] [PubMed] [Google Scholar]
- 20.Askgaard G, Gronbaek M, Kjaer MS, et al. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J Hepatol 2015;62:1061–7. [DOI] [PubMed] [Google Scholar]
- 21.Liu B, Balkwill A, Roddam A, et al. Separate and joint effects of alcohol and smoking on the risks of cirrhosis and gallbladder disease in middle-aged women. Am J Epidemiol 2009;169:153–60. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Zhou M, Sherliker P, et al. Alcohol drinking and overall and cause-specific mortality in China: nationally representative prospective study of 220,000 men with 15 years of follow-up. Int J Epidemiol 2012;41:1101–1113. [DOI] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 24.Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008;27:954–70. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. International guide for monitoring alcohol consumption and related harm. Geneva, Switzerland; 2000. [Google Scholar]
- 26.Klatsky AL, Friedman GD, Armstrong MA, et al. Wine, liquor, beer, and mortality. Am J Epidemiol 2003;158:585–95. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs CS, Stampfer MJ, Colditz GA, et al. Alcohol consumption and mortality among women. N Engl J Med 1995;332:1245–50. [DOI] [PubMed] [Google Scholar]
- 28.Becker U, Deis A, Sorensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 1996;23:1025–9. [DOI] [PubMed] [Google Scholar]
- 29.Becker U, Gronbaek M, Johansen D, et al. Lower risk for alcohol-induced cirrhosis in wine drinkers. Hepatology 2002;35:868–75. [DOI] [PubMed] [Google Scholar]
- 30.Thun MJ, Peto R, Lopez AD, et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med 1997;337:1705–14. [DOI] [PubMed] [Google Scholar]
- 31.Chalmers TC, Smith H Jr., Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials 1981;2:31–49. [DOI] [PubMed] [Google Scholar]
- 32.Detsky AS, Naylor CD, O’Rourke K, et al. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol 1992;45:255–65. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609–13. [DOI] [PubMed] [Google Scholar]
- 34.Herbison P, Hay-Smith J, Gillespie WJ. Adjustment of meta-analyses on the basis of quality scores should be abandoned. J Clin Epidemiol 2006;59:1249–56. [DOI] [PubMed] [Google Scholar]
- 35.Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics 2001;2:463–71. [DOI] [PubMed] [Google Scholar]
- 36.Shamliyan T, Kane RL, Dickinson S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. J Clin Epidemiol 2010;63:1061–70. [DOI] [PubMed] [Google Scholar]
- 37.Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- 39.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 40.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins J, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 42.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal 2006;6:40–57. [Google Scholar]
- 43.Orsini N, Li R, Wolk A, et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garfinkel L, Boffetta P, Stellman SD. Alcohol and breast cancer: a cohort study. Prev Med 1988;17:686–93. [DOI] [PubMed] [Google Scholar]
- 45.Boffetta P, Garfinkel L. Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology 1990;1:342–8. [DOI] [PubMed] [Google Scholar]
- 46.Corrao G, Arico S, Lepore R, et al. Amount and duration of alcohol intake as risk factors of symptomatic liver cirrhosis: a case-control study. J Clin Epidemiol 1993;46:601–7. [DOI] [PubMed] [Google Scholar]
- 47.Corrao G, Arico S, Zambon A, et al. Female sex and the risk of liver cirrhosis. Collaborative Groups for the Study of Liver Diseases in Italy. Scand J Gastroenterol 1997;32:1174–80. [DOI] [PubMed] [Google Scholar]
- 48.Corrao G, Arico S, Zambon A, et al. Is alcohol a risk factor for liver cirrhosis in HBsAg and anti-HCV negative subjects? Collaborative Groups for the Study of Liver Diseases in Italy. J Hepatol 1997;27:470–6. [DOI] [PubMed] [Google Scholar]
- 49.Simpson RF, Hermon C, Liu B, et al. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million Women Study. Lancet Public Health 2019;4:e41–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol 2015;12:231–42. [DOI] [PubMed] [Google Scholar]
- 51.Roerecke M, Rehm J. Cause-specific mortality risk in alcohol use disorder treatment patients: a systematic review and meta-analysis. Int J Epidemiol 2014;43:906–919. [DOI] [PubMed] [Google Scholar]
- 52.Schwarzinger M, Thiebaut SP, Baillot S, et al. Alcohol use disorders and associated chronic disease - a national retrospective cohort study from France. BMC Public Health 2017;18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen JK, Olafsson S, Bergmann OM, et al. Lifetime drinking history in patients with alcoholic liver disease and patients with alcohol use disorder without liver disease. Scand J Gastroenterol 2017;52:762–767. [DOI] [PubMed] [Google Scholar]
- 54.Stokkeland K, Hilm G, Spak F, et al. Different drinking patterns for women and men with alcohol dependence with and without alcoholic cirrhosis. Alcohol Alcohol 2008;43:39–45. [DOI] [PubMed] [Google Scholar]
- 55.Stickel F, Hampe J. Genetic determinants of alcoholic liver disease. Gut 2012;61:150–9. [DOI] [PubMed] [Google Scholar]
- 56.Marugame T, Yamamoto S, Yoshimi I, et al. Patterns of alcohol drinking and all-cause mortality: results from a large-scale population-based cohort study in Japan. Am J Epidemiol 2007;165:1039–46. [DOI] [PubMed] [Google Scholar]
- 57.Hatton J, Burton A, Nash H, et al. Drinking patterns, dependency and life-time drinking history in alcohol-related liver disease. Addiction 2009;104:587–92. [DOI] [PubMed] [Google Scholar]
- 58.Kamper-Jorgensen M, Gronbaek M, Tolstrup J, et al. Alcohol and cirrhosis: dose--response or threshold effect? J Hepatol 2004;41:25–30. [DOI] [PubMed] [Google Scholar]
- 59.Goh GB, Chow WC, Wang R, et al. Coffee, alcohol and other beverages in relation to cirrhosis mortality: the Singapore Chinese Health Study. Hepatology 2014;60:661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Åberg F, Helenius-Hietala J, Puukka P, et al. Binge drinking and the risk of liver events: A population-based cohort study. Liver Int 2017;37:1373–1381. [DOI] [PubMed] [Google Scholar]
- 61.Dawson DA, Li TK, Grant BF. A prospective study of risk drinking: At risk for what? Drug Alcohol Depend 2008;95:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ventura-Cots M, Watts AE, Bataller R. Binge drinking as a risk factor for advanced alcoholic liver disease. Liver Int 2017;37:1281–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathurin P, Deltenre P. Effect of binge drinking on the liver: an alarming public health issue? Gut 2009;58:613–7. [DOI] [PubMed] [Google Scholar]
- 64.Rehm J, Roerecke M. Patterns of drinking and liver cirrhosis - what do we know and where do we go? J Hepatol 2015;62:1061–1067. [DOI] [PubMed] [Google Scholar]
- 65.Hart CL, Morrison DS, Batty GD, et al. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ 2010;340:c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu B, Balkwill A, Reeves G, et al. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. BMJ 2010;340:c912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dam MK, Flensborg-Madsen T, Eliasen M, et al. Smoking and risk of liver cirrhosis: a population-based cohort study. Scand J Gastroenterol 2013;48:585–91. [DOI] [PubMed] [Google Scholar]
- 68.Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol 1992;136:1248–57. [DOI] [PubMed] [Google Scholar]
- 69.Corrao G, Lepore AR, Torchio P, et al. The effect of drinking coffee and smoking cigarettes on the risk of cirrhosis associated with alcohol consumption. A case-control study. Provincial Group for the Study of Chronic Liver Disease. Eur J Epidemiol 1994;10:657–64. [DOI] [PubMed] [Google Scholar]
- 70.Liu F, Wang X, Wu G, et al. Coffee Consumption Decreases Risks for Hepatic Fibrosis and Cirrhosis: A Meta-Analysis. PLoS One 2015;10:e0142457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kennedy OJ, Roderick P, Buchanan R, et al. Systematic review with meta-analysis: coffee consumption and the risk of cirrhosis. Aliment Pharmacol Ther 2016;43:562–74. [DOI] [PubMed] [Google Scholar]
- 72.Corrao G, Zambon A, Bagnardi V, et al. Coffee, caffeine, and the risk of liver cirrhosis. Ann Epidemiol 2001;11:458–65. [DOI] [PubMed] [Google Scholar]
- 73.Corrao G, Lepore AR, Torchio P, et al. Interaction between dietary pattern and alcohol intake on the risk of liver cirrhosis. The Provincial Group for the Study of Chronic Liver Disease. Rev Epidemiol Sante Publique 1995;43:7–17. [PubMed] [Google Scholar]
- 74.Corrao G, Torchio P, Zambon A, et al. Alcohol consumption and micronutrient intake as risk factors for liver cirrhosis: a case-control study. The Provincial Group for the study of Chronic Liver Disease. Ann Epidemiol 1998;8:154–9. [DOI] [PubMed] [Google Scholar]
- 75.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction 2003;98 Suppl 2:1–12. [DOI] [PubMed] [Google Scholar]
- 76.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ 2010;23:c2289. [DOI] [PubMed] [Google Scholar]
- 77.Rothman KJ GS, Lash TL. Modern Epidemiology. Philadelphia, US: Lippincott Williams & Wilkens; 2008. [Google Scholar]
- 78.Nutt DJ, Rehm J. Doing it by numbers: a simple approach to reducing the harms of alcohol. J Psychopharmacol 2014;28:3–7. [DOI] [PubMed] [Google Scholar]
- 79.Rehm J, Roerecke M. Reduction of drinking in problem drinkers and all-cause mortality. Alcohol Alcohol 2013;48:509–513. [DOI] [PubMed] [Google Scholar]
- 80.Rehm J, Anderson P, Manthey J, et al. Alcohol use disorders in primary health care – what do we know and where do we go? Alcohol Alcohol 2015;51:422–427. [DOI] [PubMed] [Google Scholar]
- 81.US Preventive Services Task Force. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA 2018;320:1899–1909. [DOI] [PubMed] [Google Scholar]
- 82.Heather N Can screening and brief intervention lead to population-level reductions in alcohol-related harm? Addict Sci Clin Pract 2012;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roerecke M, Gual A, Rehm J. Reduction of alcohol consumption and subsequent mortality in alcohol use disorders: systematic review and meta-analysis. J Clin Psychiatry 2013;74:e1181–e1189. [DOI] [PubMed] [Google Scholar]
- 84.Chaloupka FJ, Grossman M, Saffer H. The effects of price on alcohol consumption and alcohol-related problems. Alcohol Res Health 2002;26:22–34. [PMC free article] [PubMed] [Google Scholar]
- 85.Zatonski W, Sulkowska U, Manczuk M, et al. Liver cirrhosis mortality in Europe, with special attention to central and eastern Europe. Eur Addict Res 2010;16:193–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.