Abstract
Background & Aims:
Depression is a major health issue in the United States and is highly comorbid with gastrointestinal conditions. We collected data from the National Health and Nutrition Examination Survey (NHANES), a representative sample of the US population, to study the relationship between depression and bowel habits.
Methods:
Using data from the NHANES (2009–2010), we identified 495 depressed and 4709 non-depressed adults who filled out the Bowel Health Questionnaire. Depression was defined according to a validated questionnaire. We used multivariable analysis, controlling for clinical and demographic variables, to evaluate the relationship between mood and bowel habits.
Results:
In our weighed sample, 24.6% of depressed individuals and 12.6% of non-depressed individuals reported disordered bowel habits. Chronic diarrhea was significantly more prevalent in depressed individuals (15.53%; 95% CI, 11.34%–20.90%) than non-depressed individuals (6.05%; 95% CI, 5.24%–6.98%; P=.0001). Chronic constipation was also more common in depressed individuals (9.10%; 95% CI, 7.02%–11.69%) than non-depressed individuals (6.55%; 95% CI, 5.55%–7.70% CI; P=.003). Mean depression scores in patients with chronic diarrhea (4.9±5.8) and with chronic constipation (4.4±4.93) were significantly higher than mean depression scores for individuals with normal bowel habits (3.2±4.6) (P<.001). Moderate and severe depression were significantly associated with chronic diarrhea but not chronic constipation. Only mild depression was significantly associated with chronic constipation.
Conclusions:
In an analysis of the NHANES database, we found a higher proportion of depressed individuals to have chronic diarrhea and constipation than non-depressed individuals; chronic diarrhea was more strongly associated with depression. Our findings provide support for the relationship between mood and specific bowel habits, accounting for multiple co-variables in a large sample of the general US population.
Keywords: psychology, GI, irritable bowel syndrome, IBS, IBD
Introduction
Depression is a common comorbidity of many gastrointestinal disorders. For example, Irritable Bowel Syndrome (IBS) and Inflammatory Bowel Disease (IBD) are both associated with significantly higher rates and severity of depression in the general population1–3 and up to 50% of patients with IBS4,5 and 15–25% of patients with IBD6,7 meet criteria for depression in clinical settings. Similarly, patients with diagnosed depression report significantly more frequent gastrointestinal symptoms compared to individuals without depression8, with bowel-related symptoms highly associated with depression severity scores9.
Although the relationship between mood and gastrointestinal comorbidities has been well established, there is less data evaluating the relationship between mood and specific bowel habits (i.e. constipation or diarrhea). There are two large, population-based studies evaluating factors associated with either diarrhea or constipation. One, in the United States, evaluated diarrhea in the general population and found depression to be a significant predictor of chronic diarrhea10. The other, in Iran, evaluated factors associated with chronic constipation and found depression to be significantly associated11. In addition, there are two meta-analyses evaluating depression in IBS subtypes. One found that both IBS-C and IBS-D were associated with higher levels of depression compared to controls12, while the other only found this to be true for IBS-D13.
Previous studies have suggested that mood may impact intestinal transit, with depression hypothesized to increase intestinal transit (IT) and anxiety to decrease IT. For example, in a sample of 21 psychiatric outpatients and 21 controls, Gorard et al found that whole gut transit (WGT) and orocaecal transit times were shorter in anxiety patients compared to patients with depression and healthy controls and that depression scores were significantly correlated with WGT14. Another small study similarly found that IT was faster in patients with anxiety and slower in patients with depression compared to controls15. More recently, a study of 110 outpatients with functional gastrointestinal disorders reported that depression, but not anxiety, was significantly associated with delayed transit16. However, these studies are limited by small sample sizes and high potential for selection bias.
To-date, there are no large, population-based studies evaluating mood in both constipation and diarrhea assessing both stool consistency and frequency as well as adjusting for other possible confounding variables such as diet and exercise. Therefore, the current research study aims to investigate the relationship between depression and bowel habit, controlling for clinical and demographic factors, in a representative sample of the United States population using the National Health and Nutrition Examination Survey (NHANES).
Methods
Study Cohort
Data was extracted from the 2009–2010 National Health and Nutrition Examination Survey (NHANES) dataset. The NHANES offers a publically-available, nationally representative sample of non-institutionalized individuals in the United States. The NHANES survey program is conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control (Atlanta, GA, USA). Participants are selected using stratified multistage probability design with oversampling of certain ethnic and age groups in order to allow for sample-weighted inference to the U.S. population. All participants provide written informed consent prior to completing the NHANES and there are no patient identifiers in the publicly available NHANES database.
Participants in the NHANES 2009–2010 database were included in this study if they completed the bowel health questionnaire and were 20 years of age or older. Individuals with self-reported history of inflammatory bowel disease, celiac disease, and/or colon cancer were excluded from the analysis.
Bowel Health Questionnaire and Mood Questionnaires
Subjects were considered to have chronic diarrhea or Chronic Constipation based on their responses to The Bowel Health Questionnaire. This questionnaire was completed using a Computer-Assisted Personal Interview (CAPI) System in a Mobile Examination Center (MEC) Interview Room. Participants were shown a card with colored pictures and descriptions of the seven Bristol Stool Form Scale types (BSFS; Type 1-Type 7) and asked to ‘Please look at this card and tell me the number that corresponds with your usual or most common stool type.’ Consistent with previous research (10,17,18), chronic constipation was defined as a usual or most common stool type of BSFS Type 1 (separate hard lumps, like nuts) or Type 2 (sausage-like, but lumpy) and chronic diarrhea was defined as a usual or most common stool type of BSFS Type 6 (fluffy pieces with ragged edges, a mushy stool) or Type 7 (watery, no solid pieces). Remaining subjects were classified as having normal bowel habits.
Depression Screener from the 2009–2010 NHANES was used to identify individuals with depression. This screener consisted of the Patient Health Questionnaire 9 (PHQ-9), which is a 9-item validated, and publicly available depression questionnaire19. The PHQ-9 asks about symptoms of depression on a 4 point scale (“0”=not at all; “3”=nearly every day) over the past 2 weeks with scores ranging from 0–27. A clinical cutoff score of 10 or higher on the PHQ-9, which has a sensitivity and specificity of 88% in the detection of major depression19, was used to identify subjects with depression. The following, validated depression severity cutoff scores were also analyzed: a total score of 0–4 was identified as “no depression”, 5–9 was identified as “mild depression”, 10–14 as “moderate depression” and ≥15 as “moderately severe to severe”19
Co-variables
A number of co-variables were evaluated as factors hypothesized or previously shown to associate with chronic diarrhea and/or chronic constipation and depression and/or anxiety20. Calculations of adjusted risk ratios were performed following calculations of unadjusted risk ratios for all variables.
The co-variables included in this study included: gender, age (decade), race (white), higher education, living above poverty income threshold, SSRI use, laxative use, obese BMI, vigorous physical activity, heavy/moderate alcohol drinker, high caffeine intake, frequent milk intake, highest quartile fiber intake, highest quartile liquid intake, highest quartile carbohydrates intake, highest quartile sugar intake, highest quartile protein intake, highest quartile fat intake, and total number of reported medical comorbidities. Age was divided into groups by decade (20–29, 30–30, 40–49, 50–59, 60–69, and ≥70 years old). Race/ethnicity categories were Non-Hispanic White, Non-Hispanic Black, Hispanic, and other race/ethnicity (including multi-racial). Education levels were “less than high school”, “high school or GED”, or “greater than high school”. Poverty income ratio was grouped as less than two or greater than or equal to two. SSRI use was classified as “yes/no” based on participant report. Patients were considered laxative users if they reported having taken a laxative in the past 30 days. BMI classifications were normal (BMI less than 25.0), overweight (BMI from 25.0 to 29.9), and obese (BMI greater than or equal to 30). Vigorous physical activity was classified as ‘vigorous-intensity activity that causes large increases in breathing or heart rate like carrying or lifting heavy loads, digging or construction work for at least 10 minutes continuously.’ Alcohol intake was divided into six categories: never drink, former drinker, rare drinker, light drinker, moderate drinker, and heavy drinker. Milk intake was divided into four categories: never consume, rarely consume (less than once a week), sometimes consume (once a week or more, but less than once a day), and often consume (once a day or more). Gram values from all other dietary intake parameters were obtained from the first day of the 24-hour dietary recall period and divided into quartiles based on previous literature20. Finally, total number of medical comorbidities was calculated based on responses to the Medical Conditions Questionnaire. This questionnaire asks about asthma, anemia, psoriasis, arthritis, gout, congenital heart failure, coronary heart disease, angina, heart attack, stroke, emphysema, thyroid problems, chronic bronchitis, liver conditions, and cancer. We calculated a total sum of these 15 comorbidities for each respondent.
As mentioned above, Selective Serotonin Reuptake Inhibitors (SSRIs; n=41 with chronic diarrhea and n=33 with chronic constipation) were included in our analyses. Due to very low numbers taking antidepressants, we were not able to control for Tricyclic Antidepressants (TCAs; n=6 with chronic diarrhea and n=3 with chronic constipation) or Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs; n=8 with chronic diarrhea and 4 with chronic constipation). Similarly, there were very low numbers of respondents taking other medications that may impact bowel function (e.g. anticholinergics n=17) and, therefore, we did not control for these medications either.
Statistics
Differences between patients with and without depression within stool consistency categories (BSFS 1 & 2 for chronic constipation, BSFS 3, 4, & 5 for normal bowel habits, and BSFS 6 & 7 for chronic diarrhea) were first evaluated for statistical significance. Subsequently, log-binomial models provided mutually adjusted estimates of risk ratios (RRs) for chronic constipation and chronic diarrhea using our pre-defined co-variables. Both unadjusted and adjusted risk ratios were determined. The unadjusted regressions observed the association between the level of depression with chronic diarrhea or chronic constipation without accounting for co-variables, while adjusted regressions provided these associations balancing for all other factors. Adjusted RRs were evaluated for statistical significance against a value of 1.0. Wald Tests were used to test for differences between depression severity risk ratios. All CIs reported were 95% CIs. Chi-square analysis and ANOVA were used to detect statistically significant differences among groups. When ANOVA was employed, p-values from post-hoc analyses were reported based on the Bonferroni correction method. P values of ≤ 0.05 were considered to indicate statistically significant differences.
Estimates, association measures, and standard errors were all calculated with sampling weights to account for the complex nature of the NHANES database’s survey design. Statistical analyses were performed using STATA statistical software version 14.2 (College Station, TX, U.S.A.).
Results
A total of 5,160 subjects completed the Bowel Health Questionnaire and met our eligibility criteria (age > 20 and did not report a history of Inflammatory Bowel Disease or Celiac). Of these, there were 491 depressed and 4,669 non-depressed.
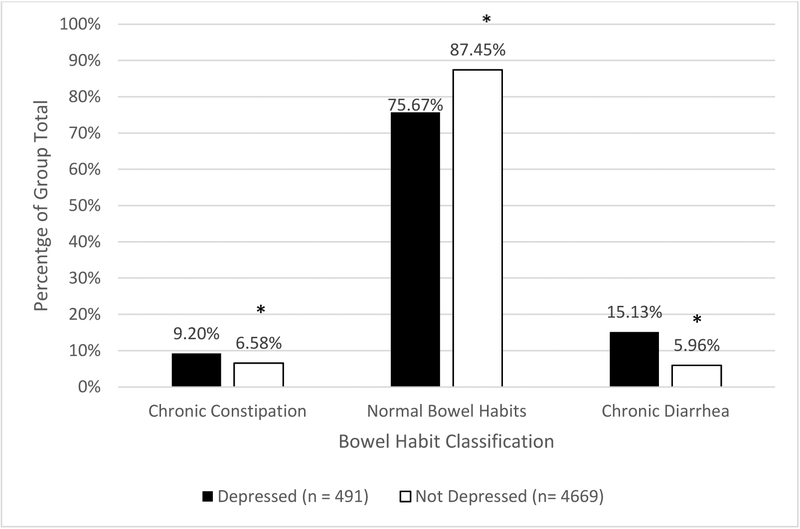
In our weighted sample, 24.33% (19.57%−29.81% CI) of depressed subjects and 12.54% (11.17%−14.07% CI) of non-depressed subjects reported disordered bowel habits (p<0.0001). We found that chronic diarrhea was significantly more prevalent in depressed individuals (15.13%, 10.83%−20.73% CI) than in non-depressed individuals (5.96%, 5.12%−6.93% CI; p = 0.0001). Chronic constipation was also more common in depressed individuals (9.20%, 7.11%−11.82% CI) than non-depressed (6.58%, 5.59%−7.74% CI; p = 0.003, Figure 1).
Figure 1.
Percentages of depressed and non-depressed individuals with chronic constipation, normal bowel habits, and chronic diarrhea.
There were no significant differences in percent reporting suicidal ideation (based on item 9 of the PHQ-9) between chronic constipation (5.6%) and chronic diarrhea (4.9%). Only chronic constipation was significantly more likely to report suicidal ideation when compared to normal bowels habits (2.7%, p=0.036).
Univariate analysis
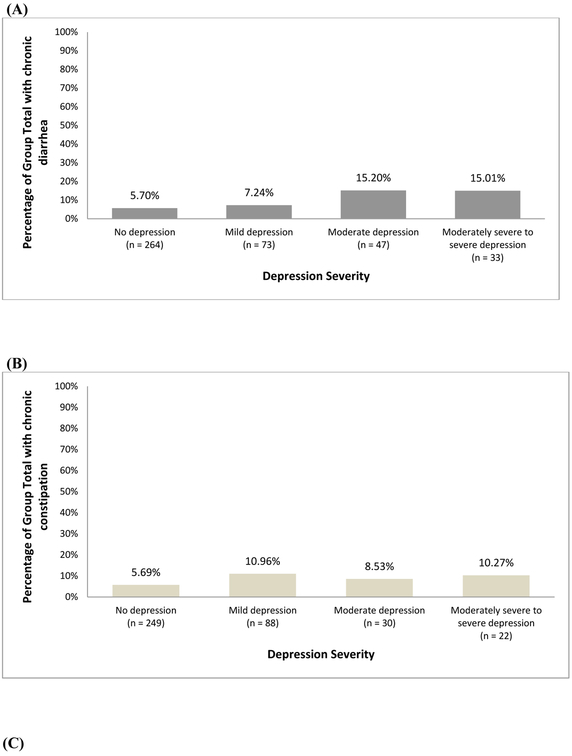
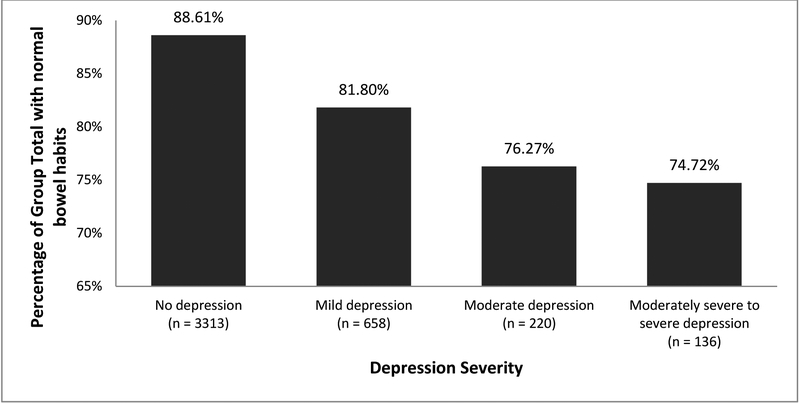
Figure 2 shows the percentage of chronic constipation, chronic diarrhea, and normal bowel habits in each depression severity subgroup. Of the individuals who reported no depression, 5.70% had chronic diarrhea and 5.69% had chronic constipation.
Figure 2.
Weighed percentage of population with (A) chronic diarrhea according to depression severity (B) chronic constipation according to depression severity and (C) normal bowel habits according to depression severity
Unadjusted risk ratios for each depression severity category are presented in Tables 1 and 2. Individuals without depression were half as likely to have chronic diarrhea or chronic constipation as individuals with any level of reported depression (mild, moderate, or moderately severe to severe).
Table 1.
Univariate and Multivariable analyses predicting chronic diarrhea
| Univariate analyses | Multivariable analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| None | 0.55 | .85 | 0.010 | Omitted | Omitted | Omitted | ||
| Mild | 1.29 | 2.05 | 0.258 | 1.21 | 1.88 | 0.347 | ||
| Moderate | 2.97 | 5.26 | 0.001 | 3.05 | 5.85 | 0.002* | ||
| Moderately severe to severe | 2.92 | 5.08 | 0.001 | 2.30 | 4.37 | 0.014* | ||
Multivariable analysis controlling for the following variables: gender, age (decade), race (white), higher education, living above poverty income, SSRI use, laxative use, obese BMI, vigorous physical activity, heavy/moderate alcohol drinker, high caffeine intake, frequent milk intake, highest quartile fiber intake, highest quartile liquid intake, highest quartile carbohydrates intake, highest quartile sugar intake, highest quartile protein intake, highest quartile fat intake, and total number of reported comorbidities.
All multivariable models are mutually adjusted and included appropriate sampling weight. Bold text and asterisk indicate a statistically significant difference with a p-value<0.05
CI, confidence interval; RR, risk ratio.
Table 2.
Univariate and Multivariable analyses predicting chronic constipation
| Univariate analyses | Multivariable analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| None | 0.52 | 0.73 | 0.001 | Omitted | Omitted | Omitted | ||
| Mild | 2.04 | 3.15 | 0.003 | 1.79 | 2.95 | 0.027* | ||
| Moderate | 1.55 | 2.56 | 0.086 | 0.95 | 2.04 | 0.890 | ||
| Moderately severe to severe | 1.90 | 3.27 | 0.024 | 1.15 | 2.22 | 0.661 | ||
Multivariable analysis controlling for the following variables: gender, age (decade), race (white), higher education, living above poverty income, SSRI use, laxative use, obese BMI, vigorous physical activity, heavy/moderate alcohol drinker, high caffeine intake, frequent milk intake, highest quartile fiber intake, highest quartile liquid intake, highest quartile carbohydrates intake, highest quartile sugar intake, highest quartile protein intake, highest quartile fat intake, and total number of reported comorbidities.
All multivariable models are mutually adjusted and included appropriate sampling weight. Bold text and asterisk indicate a statistically significant difference with a p-value<0.05
CI, confidence interval; RR, risk ratio.
Multivariable analysis
In multivariable analysis, controlling for co-variables detailed above, “moderate” and “moderately severe to severe” depression independently predicted chronic diarrhea. (Table 1) Only “mild” depression severity was a significant predictor of chronic constipation in adjusted analyses, while moderate and moderate-severe were not (Table 2).
Discussion
This is the first study to evaluate the relationship between depression and bowel habits (constipation, diarrhea, and normal) in a nationally representative adult sample in the United States. In this sample, 24.3% of patients with depression reported disordered bowel habits, compared to 12.5% of those without depression. Chronic diarrhea was present in nearly 16% of individuals with depression and chronic constipation was present in 9% of depressed patients.
In this study, depression severity was significantly associated with chronic diarrhea (controlling for SSRI use), with more severe depression predicting higher likelihood of diarrhea. Surprisingly, this was not true for chronic constipation (only mild depression was associated with constipation). This finding is in contrast to previous studies evaluating bowel transit times in anxiety and depression in which depression was associated with slower bowel transit14–16,21. However, those studies were limited by small sample sizes and high potential for selection bias. Our findings are consistent with previous studies that have found depression to be associated with both diarrhea and constipation12,13,20.
The association between depression and gastrointestinal symptoms is often attributed to two main factors: the burden of chronic illness and dysfunction in the brain-gut axis. Living with chronic illness, regardless of the diagnosis, is known to cause significant physical and emotional distress. In a large population-based analysis22 and a recent systemic review and meta-analysis23, it was reported that individuals who suffered from one or more chronic medical conditions were found to have an increased risk of major depression, with 45% greater odds of having a depressive disorder with each additional medical diagnosis23. Likewise, patients with depression are estimated to have two- to three-fold more medical symptoms compared to controls without depression24. The relationship between chronic illness and emotional distress has been described as multifaceted, with patients perceiving the relationship between mood and physical health as causal, cyclical, or unrelated25.
The relationship between mood and gastrointestinal disorders is unique from other chronic illnesses due to the significant interplay between the central nervous system and the gastrointestinal tract, also known as the brain-gut axis. For example, studies of neuronal stress pathways have found that the corticotropin-releasing factor (CRF) in the brain plays a significant role in mediating the relationship between emotional distress and changes in both upper and lower GI motor function26,27. In functional GI disorders, such as IBS, functional dyspepsia, and chronic constipation or diarrhea, dysfunction of the autonomic nervous system, which acts directly on CRF, may play a role in alteration in bowel habits and gastric emptying28. Similarly, depression is associated with hyperactivity of CRF neuronal pathways29 and CRF receptors have been suggested as a possible treatment target for both depression and GI disorders30,31. It is possible that consistent activation of the stress pathways mentioned above may lead to dysfunction in the brain-gut axis, making depressed patients more susceptible to symptoms such as chronic diarrhea or chronic constipation.
Given the significant overlap between altered bowel habits and mood, it is important for clinicians treating patients with chronic GI conditions to be prepared to screen for concerns regarding low mood and to refer to appropriate general mental health or health psychology services. Psychogastroenterology is a growing subspecialty field of health psychology with a range of evidence-based treatment options to address the complicated interplay between mood, stress, and gastrointestinal health32–34. These therapies are typically short-term and problem-focused interventions (such as Cognitive Behavioral Therapy or gut-focused hypnotherapy) that are distinct from traditional psychotherapy treatments for depression or anxiety.
Psychogastroenterology interventions have been shown to have direct effects on gastrointestinal symptoms independent of their effects on mood or quality of life35,36, presumably by promoting improvements in the brain-gut axis. However, per Rome guidelines, most studies evaluating psychogastroenterology interventions have used global symptom improvement scales as their primary outcomes and have not reported the effects of these interventions on specific bowel habits. One study evaluating Cognitive Behavioral Therapy (CBT) and despipramine, a tricyclic antidepressant, for IBS reported that CBT was equally effective in treating constipation and diarrhea predominant IBS, but did not report any statistical tests regarding bowel habits37. There have also been several studies evaluating gut-focused hypnotherapy, which have indicated improvements in both diarrhea and constipation after treatment36. There is a need for additional research regarding the specific impact of psychogastroenterology treatment on bowel function and mood in order to further understand the complex relationship between depression and bowel habits.
There were several limitations to this study. First, although there are many benefits to using a large, nationally representative database such as the NHANES, there are inherent limitations of these databases such as their cross-sectional nature and risk of recall bias in self-reported data. Second, this study only reported most common stool consistency and did not assess other gastrointestinal symptoms such as abdominal pain. Therefore, it was not possible to identify individuals who would meet criteria for IBS. Third, we approximated ‘chronicity’ based on the wording of the Bowel Health Questionnaire, which inquired about respondents’ “usual or most common” stool type. Thus, we cannot be sure that respondents met Rome criteria for chronic constipation or diarrhea. Lastly, there was no available data about rates or severity of anxiety in this patient population and, therefore, we are unable to compare our data about bowel habits in depression with anxiety, which is also significantly associated with gastrointestinal conditions.
In summary, this is the first study to use a nationally representative sample of the United States to evaluate the relationship between depression and disordered bowel habits, including both diarrhea and constipation. We found that both constipation and diarrhea were more common in depressed than non-depressed individuals. Moderate and severe depression were significant predictors of diarrhea, but not constipation. Only mild depression was a significant predictor of constipation.
Need to Know.
Background:
Many patients with gastrointestinal conditions have depression. We evaluate the relationship between depression and bowel habits using a representative sample of the United States population.
Findings:
In our weighed sample, 24.6% of depressed individuals and 12.6% of non-depressed individuals reported disordered bowel habits. Significantly higher proportions of depressed individuals had chronic diarrhea (15.5%) and constipation (9.1%) than non-depressed individuals (6.1% and 6.6%).
Implications for patient care:
Given the significant overlap between altered bowel habits and mood, it is important for clinicians treating patients with chronic gastrointestinal disorders to screen for mood disorders and to refer patients to appropriate mental health or psychology services.
Acknowledgments
Grant Support: This project was funded in part by NIH/NIDDK grant # T32DK007760 (SB)
Abbreviations:
- NHANES
National Health and Nutrition Examination Survey
- IBS
Irritable Bowel Syndrome
- IBD
Inflammatory Bowel Disease
- NCHS
National Center for Health Statistics
- CAPI
Computer-Assisted Personal Interview
- MEC
Mobile Examination Center
- BSFS
Bristol Stool Form Scale
- PHQ-9
Patient Health Questionnaire 9
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Mykletun A, Jacka F, Williams L, et al. Prevalence of mood and anxiety disorder in self reported irritable bowel syndrome (IBS). An epidemiological population based study of women. BMC Gastroenterol 2010;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflamm Bowel Dis 2006;12:697–707. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari S, Larson ME, Kumar N, et al. Association of Inflammatory Bowel Disease (IBD) with Depressive Symptoms in the United States Population and Independent Predictors of Depressive Symptoms in an IBD Population: A NHANES Study. Gut Liver 2017;11:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh P, Agnihotri A, Pathak MK, et al. Psychiatric, somatic and other functional gastrointestinal disorders in patients with irritable bowel syndrome at a tertiary care center. J Neurogastroenterol Motil 2012;18:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandvik PO, Wilhelmsen I, Ihlebaek C, et al. Comorbidity of irritable bowel syndrome in general practice: a striking feature with clinical implications. Aliment Pharmacol Ther 2004;20:1195–1203. [DOI] [PubMed] [Google Scholar]
- 6.Byrne G, Rosenfeld G, Leung Y, et al. Prevalence of Anxiety and Depression in Patients with Inflammatory Bowel Disease. Can J Gastroenterol Hepatol 2017;2017:6496727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuendorf R, Harding A, Stello N, et al. Depression and anxiety in patients with Inflammatory Bowel Disease: A systematic review. J Psychosom Res 2016;87:70–80. [DOI] [PubMed] [Google Scholar]
- 8.Hillilä MT, Hämäläinen J, Heikkinen ME, et al. Gastrointestinal complaints among subjects with depressive symptoms in the general population. Aliment Pharmacol Ther 2008;28:648–654. [DOI] [PubMed] [Google Scholar]
- 9.Dipnall JF, Pasco JA, Berk M, et al. Into the Bowels of Depression: Unravelling Medical Symptoms Associated with Depression by Applying Machine-Learning Techniques to a Community Based Population Sample. PloS One 2016;11:e0167055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh P, Mitsuhashi S, Ballou S, et al. Demographic and Dietary Associations of Chronic Diarrhea in a Representative Sample of Adults in the United States. Am J Gastroenterol March 2018. [DOI] [PubMed] [Google Scholar]
- 11.Moezi P, Salehi A, Molavi H, et al. Prevalence of Chronic Constipation and Its Associated Factors in Pars Cohort Study: A Study of 9000 Adults in Southern Iran. Middle East J Dig Dis 2018;10:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee C, Doo E, Choi JM, et al. The Increased Level of Depression and Anxiety in Irritable Bowel Syndrome Patients Compared with Healthy Controls: Systematic Review and Meta-analysis. J Neurogastroenterol Motil 2017;23:349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fond G, Loundou A, Hamdani N, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2014;264:651–660. [DOI] [PubMed] [Google Scholar]
- 14.Gorard DA, Gomborone JE, Libby GW, et al. Intestinal transit in anxiety and depression. Gut 1996;39:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhary HR. Study of intestinal transit time in patient with anxiety and depression. J Assoc Physicians India 1989;37:156–157. [PubMed] [Google Scholar]
- 16.Bennett EJ, Evans P, Scott AM, et al. Psychological and sex features of delayed gut transit in functional gastrointestinal disorders. Gut 2000;46:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sommers T, Mitsuhashi S, Singh P, et al. Prevalence of Chronic Constipation and Chronic Diarrhea in Diabetic Individuals in the United States. Am J Gastroenterol November 2018. [DOI] [PubMed] [Google Scholar]
- 18.Markland AD, Palsson O, Goode PS, et al. Association of low dietary intake of fiber and liquids with constipation: evidence from the National Health and Nutrition Examination Survey. Am J Gastroenterol 2013;108:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh P, Mitsuhashi S, Ballou S, et al. Demographic and Dietary Associations of Chronic Diarrhea in a Representative Sample of Adults in the United States. Am J Gastroenterol 2018;113:593–600. [DOI] [PubMed] [Google Scholar]
- 21.Haj Kheder S, Heller J, Bär JK, et al. Autonomic dysfunction of gastric motility in major depression. J Affect Disord 2018;226:196–202. [DOI] [PubMed] [Google Scholar]
- 22.Patten SB. Long-term medical conditions and major depression in a Canadian population study at waves 1 and 2. J Affect Disord 2001;63:35–41. [DOI] [PubMed] [Google Scholar]
- 23.Read JR, Sharpe L, Modini M, et al. Multimorbidity and depression: A systematic review and meta-analysis. J Affect Disord 2017;221:36–46. [DOI] [PubMed] [Google Scholar]
- 24.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci 2011;13:7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeJean D, Giacomini M, Vanstone M, et al. Patient experiences of depression and anxiety with chronic disease: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser 2013;13:1–33. [PMC free article] [PubMed] [Google Scholar]
- 26.Mönnikes H, Tebbe JJ, Hildebrandt M, et al. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis Basel Switz 2001;19:201–211. [DOI] [PubMed] [Google Scholar]
- 27.Taché Y, Martinez V, Million M, et al. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol 2001;280:G173–177. [DOI] [PubMed] [Google Scholar]
- 28.Mayer EA, Naliboff BD, Chang L, et al. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2001;280:G519–524. [DOI] [PubMed] [Google Scholar]
- 29.Arborelius L, Owens MJ, Plotsky PM, et al. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999;160:1–12. [DOI] [PubMed] [Google Scholar]
- 30.Kehne JH. The CRF1 receptor, a novel target for the treatment of depression, anxiety, and stress-related disorders. CNS Neurol Disord Drug Targets 2007;6:163–182. [DOI] [PubMed] [Google Scholar]
- 31.Tacheé Y, Kiank C, Stengel A. A Role for Corticotropin-releasing Factor in Functional Gastrointestinal Disorders. Curr Gastroenterol Rep 2009;11:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballou S, Keefer L. Psychological Interventions for Irritable Bowel Syndrome and Inflammatory Bowel Diseases. Clin Transl Gastroenterol 2017;8:e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCombie AM, Mulder RT, Gearry RB. Psychotherapy for inflammatory bowel disease: A review and update. J Crohns Colitis 2013;7:935–949. [DOI] [PubMed] [Google Scholar]
- 34.Ford AC, Quigley EMM, Lacy BE, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol 2014;109:1350–1365; quiz 1366. [DOI] [PubMed] [Google Scholar]
- 35.Lackner JM, Jaccard J, Krasner SS, et al. How does cognitive behavior therapy for irritable bowel syndrome work? A mediational analysis of a randomized clinical trial. Gastroenterology 2007;133:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palsson OS. Hypnosis Treatment of Gastrointestinal Disorders: A Comprehensive Review of the Empirical Evidence. Am J Clin Hypn 2015;58:134–158. [DOI] [PubMed] [Google Scholar]
- 37.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology 2003;125:19–31. [DOI] [PubMed] [Google Scholar]