Abstract
Background
Overprescribing of opioid medications for patients to be used at home after surgery is common. We sought to ascertain important patient and procedural characteristics that are associated with low vs. high rates of self-reported utilization of opioids at home, 1–4 weeks after discharge following gastrointestinal surgery.
Methods
We developed a survey consisting of questions from NIH PROMIS tools for pain intensity/interference and queries on postoperative analgesic use. Adult patients completed the survey weekly during the first month after discharge. Using regression procedures we determined the patient and procedure characteristics that predicted high post-discharge opioid use operationalized as 75mg oral morphine equivalents / 50mg oxycodone reported taken.
Results
The survey response rate was 86% (201/233). High opioid use was reported by 52.7% of patients (106/201). Median reported intake of opioid pain pills was 7 for week #1 and 0 for weeks #2–4. Combinations of acetaminophen and non-steroidal and anti-inflammatory drugs were used by 8.9% - 12.5% of patients after discharge. Following adjustment for significant variables of the univariate analysis, last 24-hour in-hospital opioid intake remained as a significant covariate for post-discharge opioid intake.
Conclusions
After gastrointestinal surgery, the equivalent of each oxycodone 5mg tablet taken in the last 24 hours before discharge increases the likelihood of taking the equivalent of >10 oxycodone 5mg tablets by 5%. Non-opioid analgesia was utilized in less than half of the cases. Maximizing nonopioid analgesic therapy and basing opioid prescriptions on 24-hour pre-discharge opioid intake may improve the quality of post-discharge pain management.
Keywords: Pain, Analgesics, Opioid, Digestive System Surgical Procedures, Self Report, Patient Discharge
INTRODUCTION
Compassionate and effective pain management after surgery represents a foundation of humane medical care. During the late 1990s pain was coined “the fifth vital sign”.[1] Since that time there has been a marked rise in the sales of prescription opioids paralleled by dramatically increased morbidity and mortality related to opioids that has more recently plateaued.[2–4] Given that more than two-thirds of opioids used for non-medical purposes were originally prescribed to another person,[5] prescribed, yet not needed opioids after surgery likely contribute to the adverse effects of opioids on public health.[6] Recent studies have shown overprescribing of opioid pain medications for patients to be used at home after a surgical procedure is common.[7–11] Yet, the majority of approaches to reducing the number of opioids prescribed have focused on limiting opioid therapy for the medical treatment for chronic pain.[12, 13] Following gastrointestinal surgery in particular, patients are prone to experience opioid-induced delayed recovery of bowel function.[14] Rates of new persistent opioid use in previously opioid-naïve patients are especially high (up to 10%) after selected gastrointestinal surgical procedures.[15] Analgesic adjuncts such as intravenous acetaminophen have been shown to be effective in reducing in-hospital opioid consumption after colorectal surgery, [16] yet if such non-opioid based approaches are continued by patients at home following discharge is unclear.
Here, we sought to test the hypothesis that there are important patient and perioperative characteristics that predict which patient and gastrointestinal procedure characteristics are associated with self-reported utilization of opioids and non-opioid pain medications in the first four weeks after discharge. Additionally, we assessed pain intensity and interference scores as well as opioid storage behaviors for four weeks after discharge following surgery.
MATERIALS AND METHODS
Institutional Review Board approval was obtained before patient enrollment into this study. This study was performed according to the STROBE reporting guideline for observational cohort studies.[17] A specially developed survey consisting of existing questions (PROMIS tools for pain intensity and pain interference) was developed.[18] Readability was assessed using the Flesch-Kincaid Grade level score,[19] and the instrument was tested for ease of administration and face validity in a pilot study.[20]
Patient enrollment
Adult inpatients who underwent gastrointestinal surgery were screened for eligibility using the electronic medical record. Exclusion criteria included patients who were non-English speaking, infectious at the time of enrollment, incarcerated, to be discharged to a long-term hospital or treatment program, or persons who were homeless and thus unable to complete study surveys. A list of 21 common gastrointestinal procedures was used to determine patient eligibility from the daily operating room schedule. Once screened and deemed eligible, the patients were compiled into a spreadsheet and randomized using a digital randomization procedure. This randomly sorted list determined the order in which patients were approached in the hospital. Patients were contacted in person with an introductory letter that described the content and purpose of the study. A U.S. $10 voucher for completion of each of the four weekly surveys (U.S. $40 per patient) was offered. Consent was obtained by completion of a contact information form at initial intake in the hospital.
Data collection
Demographic variables and information on perioperative care including the type of surgical procedure, duration of the procedure, American Society of Anesthesiology (ASA) physical status, and medications administered in the hospital were obtained from the electronic medical record. The type and amount of opioids prescribed to an individual patient at discharge was at the discretion of the individual surgeon. Details regarding type, dose, and amount of opioid prescribed were determined from the prescription stored in the electronic medical record (paper prescription pads are no longer in use at the study site). Depending on patient preference, the survey instrument was administered online, over the phone, or via hard copy by mail. Starting one week post-discharge, four weekly surveys were sent to participants. Data was collected using REDCap, a secure web application designed to support data capture for research studies.[21].
Outcomes
The primary outcome for this study was the amount of opioid medications reported taken by patients following discharge. Oral morphine equivalents (OME) were calculated based on drug and route of administration using commonly used conversion recommendations.[22] Opioid intake was operationalized as equal or less versus more than 75mg oral morphine equivalents (equivalent to 10 oxycodone 5mg tablets) reported taken. Secondary post-discharge outcomes included the amount of opioids prescribed and not taken, use of non-opioid medications, pain intensity and pain interference, as well as information on storage and disposal of leftover opioids.
Data analysis and sample size
For a sample size of 200 patients, we estimated 81% power to detect an odds ratio of 2.3 or higher as statistically significant, assuming about 50% of patients used 75mg or less OME.[23] Groups were characterized using descriptive statistical measures, including percentages, mean, median, and standard deviations. Potential predictor variables for low versus high opioid use were chosen based on the prior literature[24–26] and our own hypotheses. We first evaluated associations between high versus low self-reported opioid use and each independent variable with separate binomial regressions. A multiple binomial regression model was next attempted to jointly evaluate the significant variables but had questionable convergence so we used a modified Poisson regression approach[27] to estimate adjusted risk ratios for predicting high versus low opioid use after hospital discharge.
RESULTS
Study enrollment is summarized in Figure 1. Demographic, procedural characteristics are summarized in Table 1. Non-opioid analgesic medications were taken in the 24 hours prior to discharge at the following rates: acetaminophen (120/201=59.7%), NSAIDs (47/201=23.4%), and gabapentin or pregabalin (37/201=18.4%). Only pre-discharge use of pregablin/gabapentin was statistically different between patients using 10 oxycodone 5mg tablets or less versus more after discharge (Table 1). After discharge and paralleling the reduction in pain intensity and pain interference measures, median reported intake of opioid pain pills was 7 for week #1 and 0 for weeks #2–4. The most common reason for not taking opioid pain medications was adequate pain control without them. Combinations of acetaminophen and non-steroidal and anti-inflammatory drugs were used by 8.9% - 12.5% of patients. Only a minority, 16.3%−19.2% of patients reported storage of left-over opioids in a locked location during a given week. Disposal of left-over medications was rare, with 0.8%−3.7% of patients reporting disposal of left-over pills (Table 2).
Figure 1:
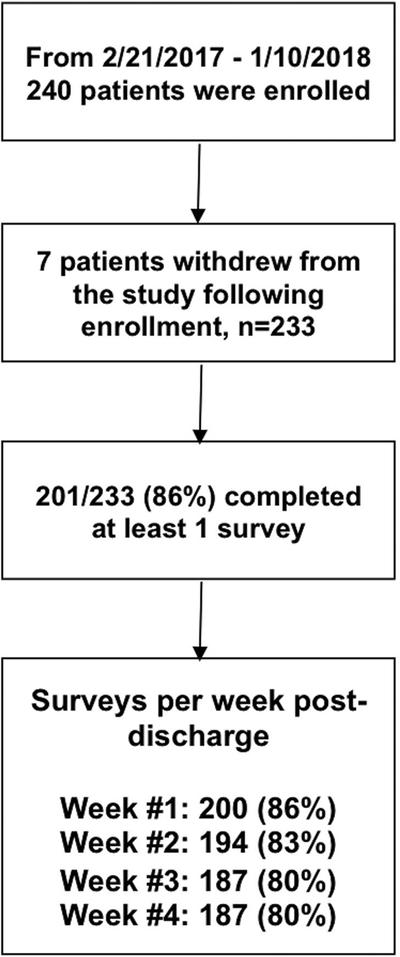
Study flow diagram.
Table 1: Patient, procedural, and perioperative characteristics.
Continuous variables are reported using mean, standard deviation in parentheses, and median in brackets. Categorical variables are reported with column percentages relative to the number of responses for the specific question. Comparisons between high and low OME groups were made with separate binomial regressions. Characteristics with multiple categorical variables were collapsed for statistical comparison as follows: Race (White vs. all other categories), Type of Insurance (Medicare vs. Medicaid vs. Commercial, vs. all other categories), ASA physical status (I and II vs. III and IV), Type of opioid medication prescribed (Oxycodone vs. all other opioids).
| Characteristic | Low OME n=95 | High OME n=106 | Chi-Square (degrees of freedom); p-value |
|---|---|---|---|
| Age | 53.9 (14.7) [56.0] |
46.4 (14.3) [45.5] |
11.78 (1) 0.0006 |
| Female | 57, 60.0% | 70, 66.0% | 0.78 (1) 0.3757 |
| Race | |||
| American Indian or Alaskan Native | 0, 0% | 1, 0.9% | 0.42 (1) 0.5172 |
| Asian | 0, 0% | 1, 0.9% | |
| Black or African American | 4, 4.2% | 5, 4.7% | |
| Native Hawaiian or Other Pacific Islander | 0, 0% | 2, 1.9% | |
| White | 82, 86.3% | 88, 83% | |
| More than one race | 4, 4.2% | 6, 5.7% | |
| Other | 3, 3.2% | 2, 1.9% | |
| Unknown | 2, 2.1% | 0, 0% | |
| Would rather not answer | 0, 0% | 1, 0.9% | |
| Ethnicity | |||
| Hispanic | 9, 8.5% | 0.53 (1) 0.4655 | |
| 11, 11.6% | |||
| Type of Insurance | |||
| Medicare | 23, 24.2% | 13, 12.3% | 7.09 (3) 0.690 |
| Medicaid | 15, 15.8% | 24, 22.6% | |
| Commercial Insurer (Cigna, Aetna, Anthem, etc.) | 54, 56.8% | 61, 57.6% | |
| Tri-Care or other government insurance | 3, 3.2% | 7, 6.6% | |
| Self pay | 0, 0.0% | 0, 0.0% | |
| Other | 0, 0.0% | 0, 0.0% | |
| Unknown’ | 0, 0.0% | 1, 0.9% | |
| Pre-operative opioids | 18, 19% | 36, 34% | 5.85 (1) 0.0156 |
| Pre-operative benzodiazepines | 12, 12.4% | 17, 16% | 0.47 (1) 0.4914 |
| ASA physical status | |||
| I | 1, 1.1% | 2, 1.9% | |
| II | 48, 50.5% | 50, 47.2% | |
| III | 45, 47.4% | 52, 49.1% | 0.13 (1) 0.7210 |
| IV | 1, 1.1% | 2, 1.9% | |
| ASA Emergency | 4, 4.2% | 6, 5.7% | 0.22 (1) 0.6355 |
| Surgical Procedure Duration (min) | 173.4 (100.8) [141.5] | 164.9 (98.0) [152] | 0.38 (1) 0.5352 |
| Primary Surgical procedure | |||
| Appendectomy or cholecystectomy | 10, 10.5% | 14, 13.2% | |
| Closure of enterostomy (large or small intestine) | 8, 8.4% | 9, 8.5% | 2.24 (6) 0.8960 |
| Colectomy, enterectomy or proctectomy | 26, 27.4% | 28, 26.4% | |
| Exploratory laparotomy | 7, 7.4% | 9, 8.5% | |
| Gastric restrictive procedure | 23, 24.2% | 30, 28.3% | |
| Pancreatectomy | 8, 8.4% | 5, 4.7% | |
| Other | 13, 13.7% | 11, 10.4% | |
| >1 surgical procedure | 25, 26.3% | 31, 29.3% | 0.21 (1) 0.6435 |
| 24-hour prior to discharge oral morphine equivalent (OME mg/7.5) | 3.2 (3.8) [2.0] | 9.8 (6.4) [9.2] | 74.93 (1) <0.0001 |
| 24-hour prior to discharge acetaminophen | 53, 55.8% | 67, 63.2% | 1.15 (1) 0.2844 |
| 24-hour prior to discharge NSAIDs | 21, 22.1% | 26, 24.5% | 0.16 (1) 0.6851 |
| 24-hour prior to discharge gabapentin or pregabalin | 12, 12.6% | 25, 23.6% | 4.09 (1) 0.0432 |
| Type of opioid medication prescribed | |||
| None | 9, 9.5% | 0, 0% | 3.99 (1) |
| Oxycodone | 65, 68.4% | 92, 86.8% | 0.0458 |
| Hydrocodone | 9, 9.5% | 2, 1.9% | |
| Hydromorphone | 4, 4.2% | 7, 6.6% | |
| Tramadol | 8, 8.4% | 2, 1.9% | |
| Other | 0, 0% | 3, 2.8% | |
| Amount of Opioids Prescribed at Discharge (OME mg/7.5) | 107.3 (168.7) [40] | 146.3 (181.5) [50] | 2.2 (1) 0.1382 |
| Disease Type | |||
| Acute | 18, 18.9% | 19, 17.9% | 4.24 (2) |
| Chronic and Non-Acute | 48, 50.5% | 67, 63.2% | 0.1200 |
| Cancer | 29, 30.5% | 20, 18.9% |
Table 2: Postoperative Patient outcomes.
Continuous variables are reported using mean, standard deviation in parentheses, and median in brackets. Categorical variables are reported with n and column percentages relative to the number of responses for the specific question. NIH PROMIS Pain Intensity and Pain Interference are reported as T-score and standard error.
| Outcome | Week 1 n=200 | Week 2 n=194 | Week 3 n=187 | Week 4 n=187 |
|---|---|---|---|---|
| Opioid # pain pills taken | 13.5 (15.9) [7] | 6.0 (12.0) [0] | 3.3 (9.0) [0] | 2.4 (7.5) [0] |
| Opioids taken (OME mg/7.5) | 14.6 (18.3) [7] | 6.9 (16.8) [0] | 3.7 (10.6) [0] | 2.7 (8.8) [0] |
| Opioid # pain pills left over | 28.1 (27.4) [24] | 23.7 (25.1) [17] | 23.6 (26.5) [15] | 22.2 (23.9) [15] |
| Opioids left over (OME mg/7.5) | 30.0 (37.2) [23] | 24.9 (31) [17] | 24.5 (33.7) [15] | 22.8 (26.8) [15] |
| Acetaminophen | 98, 49% | 83, 42.8% | 75, 39.9% | 64, 33.7% |
| NSAIDs | 56, 28% | 46, 23.7% | 38, 20.2% | 30, 15.8% |
| NSAIDs and Acetaminophen | 25, 12.5% | 20, 10.3% | 20, 10.6% | 17, 8.9% |
| Pain Intensity | 49.9 (6.3) | 45.5 (7.8) | 42.5 (8.3) | 39.8 (8.6) |
| Pain Interference | 62.3 (7.9) | 56.7 (9.1) | 53.0 (9.3) | 50.3 (10.1) |
| Reason for not taking any opioid pain pills: | ||||
| a. My pain was controlled without taking opioid pain pills | 44, 22% | 82, 42.3% | 108, 57.4% | 122, 64.2% |
| b. Side effects were too strong | 14, 7% | 14, 7.2% | 13, 6.9% | 12, 6.3% |
| c. I was concerned about becoming addicted | 4, 2% | 9, 4.6% | 9, 4.8% | 8, 4.2% |
| d. I was concerned because I was breast-feeding | 0, 0% | 0, 0% | 0, 0% | 0, 0% |
| e. I had no opioid pain pills left | 3, 1.5% | 9, 4.6% | 9, 4.8% | 13, 6.8% |
| f. Other | 6, 3% | 14, 7.2% | 8, 4.3% | 9, 4.7% |
| Storage location of left-over opioid pain pills | ||||
| a. cupboard or wardrobe | 38, 24.2% | 35, 26.1% | 38, 28.8% | 42, 33.6% |
| b. medicine cabinet/other box | 83, 52.9% | 78, 58.2% | 75, 56.8% | 66, 52.8% |
| c. Fridge | 5, 3.2% | 4, 3% | 2, 1.5% | 1, 0.8% |
| d. Other | 25, 15.9% | 11, 8.2% | 14, 10.6% | 11, 8.8% |
| e. Left-over pills were disposed of | 4, 2.5% 2, 1.3% | 5, 3.7% 1, 0.7% | 1, 0.8% 2, 1.5% | 4, 3.2% 1, 0.8% |
| f. Don’t have any leftover pain pills | ||||
| Locked Storage Location | 26, 17.2% | 23, 18.0% | 21, 16.3% | 23, 19.2% |
| Opioid Disposal Location | ||||
| a. Household garbage | 1, 0.50% | 0, 0.0% | 0, 0.0% | 1, 0.50% |
| b. Sink or toilet | 2, 1.0% | 2, 1.% | 1 0.5% | 3, 1.6% |
| c. Returned to pharmacy | 0, 0.0% | 0, 0.0% | 0, 0.0% | 0, 0.0% |
| d. Returned to other medication take-back program | 1, 0.50% | 1, 0.50% | 0, 0.0% | 0, 0.0% |
| e. Other | 0, 0.0% | 2, 1.0% | 0, 0.0% | 0, 0.0% |
The results of the Poisson regression approach to estimate adjusted risk ratios for predicting high versus low opioid use are depicted in Table 3. Following adjustment for age, pre-operative opioid use, last 24 hour prior to discharge opioid intake, and prior to discharge intake of gabapentin or pregabalin, only age and last 24-hour in-hospital opioid intake remained as significant predictors of high opioid use. In our model, every 10 years of additional age reduced the likelihood of being in the high use by approximately 8%. However, in a repeat-analysis including only patients that completed all four surveys, age was only a trend and no longer a significant co-variate (p=0.063).
Table 3: Poisson regression model.
Model to estimate adjusted risk ratios for predicting high opioid use operationalized as more than 75mg OME (oral morphine equivalents), equivalent to 10 oxycodone 5mg tablets. The 95% Likelihood Ratio confidence limits are reported. Significant variables are in bold. In a repeat-analysis including only patients that completed all four surveys, age was only a trend and no-longer a significant co-variate (p=0.0632).
| Characteristic | Relative Risk Estimate | 95% confidence limits | p-value | |
|---|---|---|---|---|
| 24h prior to discharge opioid intake (OME/7.5) | 1.0547 | 1.0358 | 1.0740 | <0.0001 |
| Age (in decades) | 0.9168 | 0.8405 | 0.9999 | 0.0477 |
| Pre-operative opioid prescription (Y/N) | 1.1267 | 0.8793 | 1.4438 | 0.3585 |
| 24h prior-to-discharge pregabalin or gabapentin taken | 1.308 | 0.433 | 3.956 | 0.1974 |
DISCUSSION
In this prospective cohort study of 201 gastrointestinal surgery patients, opioid intake 24-hours prior to discharge was significantly associated with opioid use after discharge. Each opioid equivalent of a 5mg oxycodone tablet administered during the last 24 hours prior to discharge increased the likelihood of taking an equivalent opioid total of more than ten 5mg oxycodone tablets after hospital discharge by 5%. Paralleling the reduction in pain intensity and pain interference, the median reported intake of opioid pain pills was 7 for week #1 and 0 for weeks #2–4. Combinations of acetaminophen and any non-steroidal anti-inflammatory drugs occurred in only 8.9% - 12.5% of patients at home, indicating a potential to leverage these drugs to minimize opioid use and associated side effects at home. Also, over-prescription of opioids was common with mean number of pills left over after four weeks being 22.2. Only in 16.3%−19.2% of cases were opioids stored in a locked location. Our results point to opportunities to improve post-discharge opioid safety by basing the amount prescribed on pre-discharge use.
Others have recently shown that the amount of opioids prescribed after discharge from surgery usually does not correlate with the documented opioid intake while an inpatient.[28] Our study confirms these findings, as we also did not detect a significant difference in the amount of opioids prescribed between the low and high user groups. By showing that prior to discharge inpatient use is, in fact, associated with total opioid use at home, our findings can help develop need-based prescribing approaches that can be implemented via quality improvement initiatives. The high amounts of left over opioids found in our study are further indicative of the need to tailor opioid prescriptions to individual patient requirements as previously suggested for patients after dental procedures.[29]
Maximizing non-opioid analgesics in conjunction with early goal-directed fluid therapy, immediate postoperative feeding, and early ambulation and mobilization are the hallmarks of successful inpatient early recovery after surgery standardization of care in gastrointestinal surgery patients.[30] Indeed, acetaminophen in addition to non-steroidal anti-inflammatory drugs such as ibuprofen has been shown to achieve highly effective analgesia in inpatient settings.[31] Our findings showed that only a small minority of patients used such a combination for pain therapy at home. Maximizing non-opioid therapy, similarly as is done using ERAS for inpatients, may be a successful approach to reducing reliance on opioid-based pain medications for analgesia at home.[32]
Limitations
Our study has several limitations. First, the validity of the primary outcome relies on accurate self-reporting by patients. To improve accuracy, we increased the reporting frequency to weekly compared to monthly in our pilot study.[20] Further, the validity of self-reported medication intake compared to pharmacy records has been previously found to be very strong (kappa=0.87). Second, our study was limited to gastrointestinal surgery patients undergoing inpatient surgery. While this enabled us substantial granularity (e.g., for the administered opioids before discharge), our findings may not be easily transferred to other types of surgery or the ambulatory setting. Third, we did not quantify the amounts of non-opioid medications taken. This was done deliberately, as considerable complexity would have been added to the survey by trying to ascertain specific types and doses of over-the-counter medications. Lastly, our survey response rate was 86%, and non-responder bias represents a possibly unaccounted confounder.
Conclusions
In summary, we found opioid intake after hospital discharge following gastrointestinal surgery is associated with 24-hour pre-discharge opioid intake. After gastrointestinal surgery, the equivalent of each oxycodone 5mg tablet taken in the last 24 hours before discharge increases the likelihood of taking the equivalent of >10 oxycodone 5mg tablets by 5%. These findings may be leveraged to design personalized, parsimonious opioid prescribing strategies in the future. Strategies to maximize safe non-opioid over-the-counter medications use for pain therapy should be extended from the inpatient to the outpatient setting.
ACKNOWLEDGEMENTS
None.
Funding:
This work was supported by the National Institutes of Health (NIH), Award Number K23DA040923 to Karsten Bartels and NIH Award Number UL1TR002535. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The NIH had no involvement in study design, collection, analysis, interpretation of data, writing of the report, or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Prior presentation:
Interim data from this work were presented at the 2018 Annual Meeting of The College on Problems of Drug Dependence, June 9–14, 2018, San Diego, CA, USA.
DISCLOSURES
Karsten Bartels, M.D, Ph.D. reports grants from National Institutes of Health during the conduct of the study.
Katharine Mahoney, B.A. has no conflict of interest.
Kristen M. Raymond, B.A. has no conflict of interest.
Shannon K. McWilliams, M.A. has no conflict of interest.
Ana Fernandez-Bustamante, M.D., Ph.D. has no conflict of interest.
Richard Schulick, M.D. has no conflict of interest.
Christian J. Hopfer, M.D. has no conflict of interest.
Susan K. Mikulich Gilbertson, Ph.D. has no conflict of interest.
REFERENCES
- 1.McCaffery M, Pasero CL (1997) Pain ratings: the fifth vital sign. Am J Nurs 97:15–16 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (2011) Vital signs: overdoses of prescription opioid pain relievers - United States, 1999–2008.. MMWR Morb Mortal Wkly Rep 60:1487–1492 [PubMed] [Google Scholar]
- 3.Volkow ND, Frieden TR, Hyde PS, Cha SS (2014) Medication-assisted therapies--tackling the opioid-overdose epidemic. N Engl J Med 370:2063–2066 [DOI] [PubMed] [Google Scholar]
- 4.Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL (2015) Trends in Opioid Analgesic Abuse and Mortality in the United States. N Engl J Med 372:241–248 [DOI] [PubMed] [Google Scholar]
- 5.Jones CM, Paulozzi LJ, Mack KA (2014) Sources of prescription opioid pain relievers by frequency of past-year nonmedical use United States, 2008–2011. JAMA Intern Med 174:802–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Compton WM, Volkow ND (2006) Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend 81:103–107 [DOI] [PubMed] [Google Scholar]
- 7.Rodgers J, Cunningham K, Fitzgerald K, Finnerty E (2012) Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am 37:645–650 [DOI] [PubMed] [Google Scholar]
- 8.Harris K, Curtis J, Larsen B, Calder S, Duffy K, Bowen G, Hadley M, Tristani-Firouzi P (2013) Opioid pain medication use after dermatologic surgery: a prospective observational study of 212 dermatologic surgery patients. JAMA Dermatol 149:317–321 [DOI] [PubMed] [Google Scholar]
- 9.Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL (2017) Prescription Opioid Analgesics Commonly Unused After Surgery: A Systematic Review. JAMA Surg 152:1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii MH, Hodges AC, Russell RL, Roensch K, Beynnon B, Ahern TP, Holoch P, Moore JS, Ames SE, MacLean CD (2018) Post-Discharge Opioid Prescribing and Use after Common Surgical Procedure. J Am Coll Surg 226:1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowell D, Haegerich TM, Chou R (2016) CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA 315:1624–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrico JA, Mahoney K, Raymond KM, Mims L, Smith PC, Sakai JT, Mikulich-Gilbertson SK, Hopfer CJ, Bartels K (2018) The Association of Patient Satisfaction-Based Incentives with Primary Care Physician Opioid Prescribing. J Am Board Fam Med 31:941–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beard TL, Leslie JB, Nemeth J (2011) The opioid component of delayed gastrointestinal recovery after bowel resection. J Gastrointest Surg 15:1259–1268 [DOI] [PubMed] [Google Scholar]
- 14.Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, Bohnert ASB, Kheterpal S, Nallamothu BK (2017) New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg 152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aryaie AH, Lalezari S, Sergent WK, Puckett Y, Juergens C, Ratermann C, Ogg C (2018) Decreased opioid consumption and enhance recovery with the addition of IV Acetaminophen in colorectal patients: a prospective, multi-institutional, randomized, double-blinded, placebo-controlled study (DOCIVA study). Surg Endosc 32:3432–3438 [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S (2014) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 12:1495–149925046131 [Google Scholar]
- 17.Jones RS, Stukenborg GJ (2017) Patient-Reported Outcomes Measurement Information System (PROMIS) Use in Surgical Care: A Scoping Study. J Am Coll Surg 224:245–254 e241 [DOI] [PubMed] [Google Scholar]
- 18.Kincaid JP, Braby R, Mears JE (1988) Electronic authoring and delivery of technical information. Journal of instructional development 11:8–13 [Google Scholar]
- 19.Bartels K, Mayes LM, Dingmann C, Bullard KJ, Hopfer CJ, Binswanger IA (2016) Opioid Use and Storage Patterns by Patients after Hospital Discharge following Surgery. PLoS One 11:e0147972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berdine HJ, Nesbit SA (2006) Equianalgesic dosing of opioids. J Pain Palliat Care Pharmacother 20:79–84 [PubMed] [Google Scholar]
- 22.Faul F, Erdfelder E, Buchner A, Lang AG (2009) Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41:1149–1160 [DOI] [PubMed] [Google Scholar]
- 23.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN (2014) Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM (2012) Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med 172:425–430 [DOI] [PubMed] [Google Scholar]
- 25.Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK (2018) Long-term opioid use after inpatient surgery - A retrospective cohort study. Drug Alcohol Depend 187:61–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou G (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159:702–706 [DOI] [PubMed] [Google Scholar]
- 27.Chen EY, Marcantonio A, Tornetta P 3rd (2018) Correlation Between 24-Hour Predischarge Opioid Use and Amount of Opioids Prescribed at Hospital Discharge. JAMA Surg 153:e174859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maughan BC, Hersh EV, Shofer FS, Wanner KJ, Archer E, Carrasco LR, Rhodes KV (2016) Unused opioid analgesics and drug disposal following outpatient dental surgery: A randomized controlled trial. Drug Alcohol Depend 168:328–334 [DOI] [PubMed] [Google Scholar]
- 29.Thiele RH, Rea KM, Turrentine FE, Friel CM, Hassinger TE, Goudreau BJ, Umapathi GA, Kron IL, Sawyer RG, Hedrick TL (2015) Standardization of Care: Impact of an Enhanced Recovery Protocol on Length of Stay, Complications, and Direct Costs after Colorectal Surgery. Journal of the American College of Surgeons [DOI] [PubMed] [Google Scholar]
- 30.Derry CJ, Derry S, Moore RA (2013) Single dose oral ibuprofen plus paracetamol (acetaminophen) for acute postoperative pain. Cochrane Database Syst Rev 6:CD010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentine AR, Carvalho B, Lazo TA, Riley ET (2015) Scheduled acetaminophen with as-needed opioids compared to as-needed acetaminophen plus opioids for post-cesarean pain management. Int J Obstet Anesth 24:210–216 [DOI] [PubMed] [Google Scholar]
- 32.Drieling RL, LaCroix AZ, Beresford SA, Boudreau DM, Kooperberg C, Heckbert SR (2016) Validity of Self-Reported Medication Use Compared With Pharmacy Records in a Cohort of Older Women: Findings From the Women’s Health Initiative. Am J Epidemiol 184:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]


