Abstract
Background & Aims:
Gastric emptying (GE) is involved in regulation of appetite. We compared times of GE after different bariatric endoscopic and surgical interventions and associations with weight loss.
Methods:
We performed a comprehensive search of publication databases, through September 14, 2018, for randomized and non-randomized studies reporting outcomes of weight-loss surgeries. Two independent reviewers selected and appraised studies. Outcome of interest was GE T1/2 (min), measured before and after the procedure. A random effects model was used to pool the mean change in T1/2 (min) after the intervention. We performed meta-regression analysis to find associations between GE and weight loss. Heterogeneity was calculated using the I2 statistic. Methodological quality was assessed.
Results:
From 762 citations, the following studies were included in our analysis: 9 of sleeve gastrectomy, 5 of intragastric balloons, and 5 of antral botulinum toxin. After sleeve gastrectomy, the pooled mean reduction in GE T1/2 at 3 months was a 29.2 minutes (95% CI, reductions of 40.9 to 17.5 min; I2=91%). Fluid-filled balloons increased GE T1/2 by 116 minutes (95% CI, 29.4 to 203.4 min; I2=58.6%). Air-filled balloons did not produce a statistically significant difference in GE T1/2. Antral botulinum injections increased GE T1/2 by 9.6 min (95% CI, 2.8–16.4 min; I2=13.3%). Placebo interventions reduced GE T1/2 by 6.3 min (95% CI, reductions of 10 to 2.6 min). Changes in GE were associated with weight loss after sleeve gastrectomy and intragastric balloons but not botulinum toxin injections.
Conclusions:
In a systematic review and meta-analysis, we found that sleeve gastrectomy reduces GE T1/2 whereas fluid-filled balloons significantly increase GE T1/2. Air-filled balloons do not significantly change the time of GE, which could account for their low efficacy. Antral botulinum toxin injections produced small temporary increases in GE time, which were not associated with weight loss. Changes in GE time after surgical and endoscopic bariatric interventions correlate with weight loss and might be used to select interventions, based on patients’ physiology.
Keywords: IGB, motility, stomach, food
INTRODUCTION:
Obesity has reached epidemic proportions with close to 60% of Americans being overweight or obese. (1, 2) This rise has led to a similar increase in excess weight-related comorbidities such has hypertension, type 2 diabetes, non-alcoholic fatty liver disease (NAFLD) and cancer.(3, 4) While the cornerstones of obesity treatment remain lifestyle and behavioral interventions, many individuals fail to reach the levels of weight loss necessary for effective reduction of comorbidity with these approaches.(5) Bariatric surgery has been shown to be the most effective treatment for initial and long term weight loss with improvements in comorbidities, quality of life and incidence of cancer.(6-9) The mechanisms involved in the success of bariatric surgery are multifactorial, including restrictive, partially malabsorptive and hormonal changes that synergistically lead to weight loss.(9-11) Endoscopic bariatric therapies, such as intragastric balloons, small intestine bypass liners and stomach remodeling techniques have been developed to reduce net caloric intake by mimicking the perturbations generated by bariatric surgery.(12, 13)
The regulation of food intake however remains complex, involving both appetite and orexigenic signals that arise from multiple areas in the central nervous system and gastrointestinal tract. Central to appetite regulation are two important gastrointestinal traits, satiation and satiety, which are in part regulated by rates of gastric emptying and the stomach accommodation volume. (14, 15) Abnormalities in these traits, such as accelerated gastric emptying, have been implicated in obesity, but the effects of bariatric surgery and endoscopic bariatric procedures on gastric emptying are variable in the published literature. Our general hypothesis is that each bariatric surgery and endoscopic bariatric intervention has a specific effect on gastric emptying that in turn regulates post-interventional appetite, tolerance and weight loss. Our specific aim was to perform a systematic review and meta-analysis of the effects on gastric emptying of sleeve gastrectomy, Roux-en-Y gastric bypass, adjustable gastric banding and endoscopic bariatric therapies such as intragastric balloons, botulinum toxin injections, implantable small bowel bypass liners and stomach remodeling techniques and investigate their associations with weight loss.
METHODS:
This systematic review and meta-analysis was reported following the PRISMA statement guidelines with a priori-developed protocol. PRISMA CHECKLIST is available in the appendix
Data Sources and Search Strategy
A comprehensive search of several databases from each database’s inception date to September 14th, 2018 was conducted. The databases included Ovid MEDLINE Epub Ahead of Print, Ovid Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian (L.J.P) with input from the study’s investigators (E.J.V, F.B., B.A.D.) Controlled vocabulary supplemented with keywords was used to search for the effects of bariatric surgery and endoscopic bariatric therapies on gastric emptying in English language. The actual search strategy included the following key words gastric emptying, obesity, bariatric surgery, intragastric balloon, botulinum toxin, endoscopy, sleeve, bypass, adjustable gastric band is included in the appendix.
Study Selection
This systematic review and meta-analysis included randomized clinical trials and observational studies that investigated patients 18 years or older who underwent bariatric surgery (Roux-en y gastric bypass, adjustable gastric band and sleeve gastrectomy), intragastric balloons, endoscopic botulinum toxin antral injections, small bowel bypass liner placement or stomach remodeling techniques for the treatment of obesity. Studies involving patients who underwent bariatric surgery for reasons other than obesity were excluded. Only studies that assessed both pre- and post-intervention gastric emptying of solids were included to allow for quantitative analysis. Placebo/sham arms from interventional studies were also pooled for analysis to represent a control arm. Any abstracts found on the initial search were re-searched for full published text during the analysis phase until September 18, 2018.
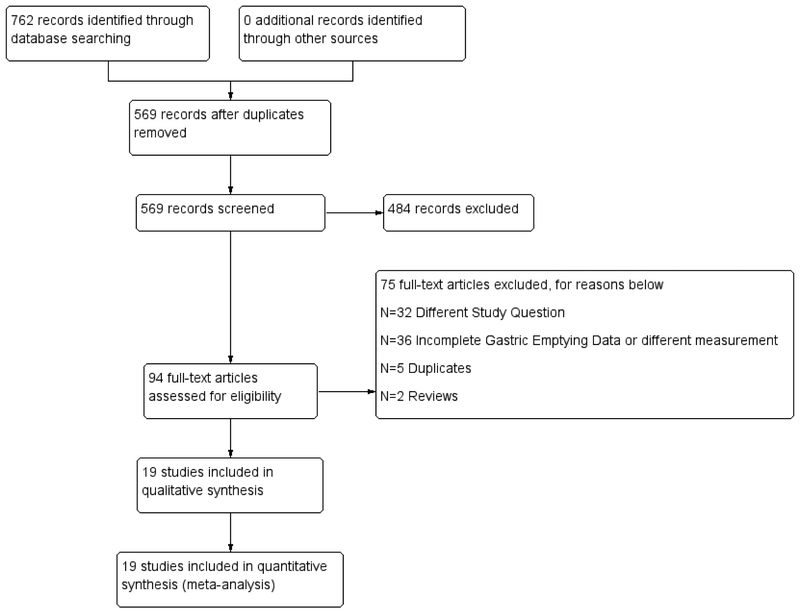
Two independent reviewers (E.J.V and G.C) working in pairs screened the titles and abstracts for initial eligibility criteria and final eligible references. Any discrepancies during the process between the independent reviewers were discussed. If no consensus was reached, an independent third reviewer was asked to settle the discrepancy. Kappa statistic was calculated to assess agreement beyond chance.(16) The study selection flow diagram appears in figure 1.
Figure 1:
Study Selection Flow Diagram
Measurement of Gastric Emptying
For each study, the gastric emptying methodology was compared to the consensus guidelines that set to standardize the measurement of gastric emptying(17). Details regarding the type of meal (solid vs. semisolid), total calories, fat content (% of calories), scintigraphy vs. breath testing, and duration of testing were collected. Gastric emptying studies were graded optimal vs suboptimal as recently recommended in the literature, based on the use of a solid meal, at least 30% fat, monitoring for at least 3 hours (18), absence of confounding medications as well as documented pre and post T1/2 (minutes) with standard deviation.
Data Extraction
A priori data collection form was developed prior to data abstraction. The following information was abstracted from each study: author, year, study design, patient characteristics, type of gastric emptying assessment, time of pre- and post- intervention gastric emptying assessment, and mean/median T1/2 (minutes), as the measures of gastric emptying. The information was collected in duplicate. Any missing data was handled by contacting the first and/or corresponding author via electronic means with limited responses(19) For the endoscopic bariatric techniques, we collected the type of endoscopic procedure and device indwelling time, and gastric emptying data as above. Data expressed in median and interquartile range were converted using the Cochrane handbook guidelines. Weight loss after each intervention in percent excess body weight lost (%EWL) and %percent total body weight lost (%TBWL) was collected for surgical and endoscopic interventions, respectively
Methodological Quality Assessment
The methodological quality of randomized trials was assessed using the Cochrane Collaboration risk assessment tool. This assessment focused on how the randomization sequence was generated; how allocation was concealed, whether there were important imbalances at baseline; which groups were masked (patients, care givers, data collectors, outcome assessors, data analysts); attrition rate; whether the analyses was by intention to treat; how was missing outcome data dealt with. The methodological quality of non-randomized studies (which applied to the majority of studies) was done using the Newcastle-Ottawa quality assessment tool as per the Cochrane Handbook.(20) This tool includes assessment of how the participants represented the population of interest, how the comparative group was selected, and how outcome was assessed, as well as length and adequacy of follow-up when applicable. Any discrepancy was resolved by a third independent reviewer. Results were compiled into a single bias assessment graph.
Main Outcome Measure
The main outcome of interest for this systematic review and analysis was a priori defined mean difference in T1/2 (expressed in minutes) after the intervention, which is the amount of time it takes for 50% of solid contents to empty from the stomach. Liquid emptying was not assessed as the emptying of solids or semi-solid represents the typical food ingested day to day and since it is conceivable that the interventions might enhance delivery of food from the proximal stomach to the distal stomach, or the interventions might interfere with the trituration of solid food. Other measures of gastric emptying, such as percent emptied or retained at 2 and 4 hours were inconsistently reported in surgical studies; therefore, T1/2 was selected as the main outcome measure. A negative mean difference in (post minus pre-procedure) GE T1/2 indicates acceleration, whereas a positive mean difference in GE T1/2 indicates a gastric emptying delay following the intervention.
STATISTICAL ANALYSES
We conducted a meta-analysis to pool the mean difference in T1/2 (minutes) after the interventions using the DerSimonian and Laird random effects model to account for heterogeneity between and within studies.(21, 22) The I-squared statistic was used to estimate the percentage of total between-study variation due to heterogeneity rather than chance(23). The I-square ranges from 0-100%, with values of ≤25%, 25-50% and ≥50% indicating low, moderate and high heterogeneity, respectively. A priori determined sensitivity analysis based on the timing of post intervention gastric emptying assessment (≤1 months or > 1 months) was conducted to explore sources of heterogeneity. For the endoscopic bariatric therapies, a priori determined sensitivity analysis was performed by type of endoscopic device (fluid vs. gas filled balloon). Meta-regressions to assess the role of baseline gastric emptying rate and optimal vs. suboptimal gastric emptying measurement methods on the final mean difference after the intervention were also performed. In order to explore the association between changes in gastric emptying and weight loss, a meta-regression was performed for each intervention separately. Statistical evaluation of publication bias and construction of funnel plots was not feasible due to the heterogeneity and small number of included studies.(24, 25) Thus, the impact of publication bias on certainty in the evidence remains unclear. All meta-analyses were performed using STATA 15 software. All values are two-tailed with an alpha level set at 0.05.
RESULTS
Description of Included Studies
A total of 762 citations were identified through database searches. After removing duplicates, 569 citations were reviewed. Only 94 conference abstracts and full manuscripts went to full review after excluding animal studies, reviews, studies involving non-obesity interventions such as cancer. Data abstraction was performed in a total of 19 studies (Figure 1). Chance adjusted agreement (Kappa statistic) for final inclusion in the list of manuscripts to be reviewed was 0.974.
Methodology of Gastric Emptying Measurement
Out of the 19 studies, only 8 studies collected gastric emptying measurements for at least 3 hours. Of these, 1 used a semi-solid meal. The remaining 11 studies collected measurements anywhere from 1 through 2.5 hours, with 2 studies using a semi-solid meal. Overall, the caloric content of the meals, and the percent derived from fat varied between studies. The majority (15/19) studies reported used scintigraphy and the remainder used breath-based testing. (Table 1-2)
Table 1:
Characteristics of included Sleeve Gastrectomy and Intragastric Balloon Studies
| Study (Year ) | Study Type |
Interventions | Sample size |
Age, years |
Sex, female (%) |
BMI, kg/m2 (meant± s.d.) |
Pre- T1/2, min (mean ±s.d.) |
Time of 2nd |
Post- T1/2, min (mean ± s.d.) |
Length | Type of Test | Type of Meal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melissas (2007) | Single arm | LSG | 11 | 41.5±10.7 | 72.7% | 48±5.9 | 94.3 ± 15.4 | 6 months | 47.6 ± 23.2 | 1.5 | Scintigraphy | Solid 390 calories (52% fat, 32% protein, 16% CHO) |
| Bernstine (2009) | Single arm | LSG | 21 | 35(Range 19-58) | 85.7% | 45 | 62.4 ± 19.8 | 3 months | 56.8±18.7 | 4 | Scintigraphy | Solid 230 kcal (35% fat, 18% protein 47% CHO |
| Michalsky (2013) | Single arm | LSG | 4 | N/A | N/A | N/A | 57.5±12.7 | 3 months | 32.3±17.3 | 1.5 | Scintigraphy | Solid Unknown kcal ( 2 slices of bread, 2 eggs, 250 ml black tea) |
| Pilone (2013) | Single arm | LSG | 45 | N/A | N/A | N/A | 80.4±33.1 | 3 months | 64.3±40 | 4 | Scintigraphy | Semi-solid 230 kcal (35% fat 18% protein 47% CHO) |
| Melissas (2013) | Single arm | LSG | 21 | 38 | N/A | 46.8 (Range 35.8-62.5) | 63.7±16.5 | 4 months | 53.2±20.1 | 4 | Scintigraphy | Solid 358 kcal (50% fat, 20% protein, 30% CHO) |
| Kandeel (2015) | Single arm | LSG | 45 | 32.5±8.1 | 80% | 49.1±7.1 | 74.9±7.1 | 1 month | 28.4±8.3 | 2 | Scintigraphy | Solid 2 meals, pre 2 egg sandwich, post LSG 1 egg |
| Vigneshwaran (2016) | Single arm | LSG | 20 | 41.6±9.5 | 45% | 33.4±1.2 | 38.4±13 | 3 - 6 months | 20.3±7.6 | N/A | Scintigraphy | Semi-solid Unknown Kcal. Grounded rice |
| Vives (2017) 3cma | RCT | LSG | 30 | 51.3±12.1 | 73.3% | 51±5.2 | 96.5±78.9 | 6 months | 44.3±21.1 | 1 | Scintigraphy | Semi-solid 400 kcal ( 30% fat, 15% protein 55% CHO) |
| Vives (2017) 8cma | RCT | LSG | 30 | 50.5±10.6 | 70% | 51.3±7.2 | 99.9±71.4 | 6 months | 48.1±21.6 | 1 | Scintigraphy | Same as above |
| Sista (2017) | Single arm | LSG | 26 | 40.9±12.5 | 69.2% | 46.7±4.2 | 68.7±25 | 3 months | 33.5±18 | 2 | Scintigraphy | Solid 80-82 calorie boiled egg |
| Barkin (1988) | Single arm | IGB- Air | 26 | 37 | 58% | 100±25 | 0.5 month | 90 ± 10 | 2.5 | Scintigraphy | Solid 2 eggs. 2 pieces of white toast, followed by 300 ml of clear juice. | |
| Velchik(1989) | Single arm | IGB- Air | 10 | 36 | 100% | 57± 27.8 | 1 month | 67 ± 27.8 | 2 | Scintigraphy | Solid 300 kcal egg white sandwich (20% fat) | |
| Mion (2005) | Single arm | IGB- Fluid | 15 | 34.9 | 86% | 34.4 ±0.7 | 92±45 | 1 month | 157 ± 70 | 4 | Breath | Solid 250 kcal 1 egg 2 slices bread 5g margarine [13C]-octanoic acid |
| Su (2013) | Single arm | IGB-Fluid | 3 | 36.8±9.2 | 66.7% | 40.9±15.2 | 114±18.5 | 3 months | 375.3 ± 207 | 2 | Scintigraphy | Solid 260 kcal egg white sandwich |
| Gomez (2016) | RCT | IGB-Fluid/Placebt | 13 | 38.1±8.8 | 87% | 34.7±3.4 | 64.7±39.79 | 4 months | 212.4±167.6 | 2 | Scintigraphy | Solid 255 kcal egg white sandwich with jam and 120 ml of water |
Table 2:
Characteristics of Included Botulinum Toxin Injection Studies
| Study (Year) | Study type | N | Age, years |
Sex, female (%) |
BMI, kg/m2 (mean ± s.d.) |
Botox Dose |
Injection Location |
Pre- T1/2, min (mean ±s.d.) |
Time of 2nd |
Post- T1/2, min (mean± s.d.) |
Length | Type of Test |
Type of Meal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Junior(2006) | Prospective | 12 | N/A | 67% | 46.2 ± 4.3 | Mix | 140.9 ± 18.8 | 144.2 ± 15.1 | 4 | Breath | Solid ~270 kcal (35% fat) 1 egg, two slices white bread 5g margarine (13C Octanoic Acid) | ||
| I -200 | 3 | 52.5±6.9 | 200 | Antrum 8 | 147.3±23.9 | 3 months | 144.7 ± 26 | ||||||
| II-200 | 3 | 46.7 ± 2.8 | 200 | Antrum 16 | 144.3 ± 5.5 | 3 months | 154.3± 16 | ||||||
| III-300 | 3 | 43.8 ± 1.8 | 300 | Antrum 16 | 134.7 ± 23.2 | 3 months | 145.7 ± 8.7 | ||||||
| IV-300 | 3 | 44.5 ± 1.1 | 300 | Antrum 24 | 148.3 ± 31 | 3 months | 140.7 ± 16.8 | ||||||
| Gui (2006) | RCT | 14 | Mix | 2 | Scintigraph | Solid 420 kcal (28% fat) 2 eggs, 100 g bread and water | |||||||
| I – 133 | 6 | 46 ± 12 | 50% | 49.4±7 | 133 | Antrum 8 | 71±16.8 | 0.5 months | 71.8±18.3 | ||||
| II – 200 | 4 | 43 ± 8 | 25% | 49.4±7 | 200 | Antrum 8 | 82.8±13.1 | 0.5 months | 75.3±6.4 | ||||
| III- Placebo | 4 | 34±11 | 50% | 44.5±7.3 | placebo | Antrum | 70.8±12.8 | 0.5 months | 62.5±13.7 | ||||
| Foschi(2008) | RCT | 18/30 | 40.6±3.5 | 83% | 44.6±1.4 | 200 | Antrum and Fundus | 83.4±3.9 | 2 months | 101.6 ± 9.9 | 3 | Breath | Solid 378 kcal standard bun(33.3% fat) (13C Octanoic Acid) |
| II- Placebo | 12/30 | 45.2±3.7 | 75% | 44.5±7.3 | Placebo | 103.4±4.5 | 2 months | 96.6 ± 5.6 | |||||
| Li (2012) | RCT | 19 | 35.7±10.7 | 52% | 34.9±5.9 | 147.2±34.6 | 1 month | 177.8±47.2 | 4 | Breath | Solid 260 kcal (18% fat) 50g flour, 1 egg 150 ml of water (13C OctanoicAcid) | ||
| I - 200 | 9 | 33.6±6 | 200 | Antrum Body and Fundus | 153.2±42.2 | 1 month | 188.4±48.3 | ||||||
| II - 300 | 10 | 36.1±6 | 300 | Antrum Body and Fundus | 141.8±27.2 | 1 month | 168.5±46.6 | ||||||
| Topazian (2013) | RCT | 45/60 | 49 | 87% | 37.9 | 4 | Scintigraphy | Solid 296 kcal 35% fat egg, toast, and milk | |||||
| I - 100 | 15 | 100 | Antrum | 0.5 months | MD: 14±29 | ||||||||
| II-300 | 15 | 300 | Antrum | 0.5 months | MD: 24±39 | ||||||||
| III- 500 | 15 | 500 | Antrum | 0.5 months | MD: 14±27 | ||||||||
| IV- Placebo | 15 | Placebo | Antrum | 0.5 months | MD: 0.8±24 |
Using the recommendations in the literature, only 8/19 studies were initially judged to be of optimal quality based on the duration of testing (>3 hours) with 7 using a solid meal. Overall, studies with optimal methodology and solid meal testing, involved 2 sleeve gastrectomy, 2 intragastric balloon, and 4 with antral botulinum toxin injections.
Methodological Quality Assessment:
The 19 studies in this meta-analysis were assessed for bias using the Newcastle Ottawa scale for cohort studies and the Cochrane Collaboration risk assessment tool for randomized studies.(20, 26)
Sleeve Gastrectomy
For sleeve gastrectomy, 8 out of 9 studies were non-randomized trials; therefore, the risk of bias was high for randomization, allocation, and masking (blinding) for these studies, whereas the single randomized clinical trial was still biased on assessment of the outcome due to the lack of masking (blinding). The risk of bias graph and summary are available in Supplementary Figure 8. The non-randomized studies were considered to be of medium quality on the Newcastle Ottawa Scale Assessment.
Intragastric Balloons
All 5 intragastric balloon studies were judged to be of low randomized quality except the one randomized clinical trial which was considered to be of medium quality. The RCT was downgraded from high to medium quality due to lack of allocation concealment, masking (blinding) and masking during the outcome assessment (Supplementary Figure 9). On the Newcastle Ottawa Assessment scale, they were judged to be high to medium quality.
Endoscopic Botulinum Toxin Antral Injections
Among the studies using botulinum toxin injections, the majorities (80%) were double-blind randomized clinical trials and were judged to be of medium to high quality. One study was non-randomized and was considered poor quality on the Cochrane risk assessment tool, but medium to high quality in the Newcastle Ottawa Assessment Scale. (Supplementary Figure 10)
Studied Interventions
Sleeve Gastrectomy
A total of 25 laparoscopic sleeve gastrectomy studies, with only 9 meeting full inclusion criteria comparing pre- and post-surgery T1/2 outcomes were found (n=233 patients) (19, 27-34). For the 1 randomized clinical trial, each of the two arms of the study was treated as a separate study in the metaanalysis phase as opposed to pooling their values (27). The remaining studies were single arm pre- and post- intervention gastric emptying assessments. Only 2 studies had optimal (≥3 hour) and solid meal testing.
Roux-en-Y Gastric Bypass
There were no studies reporting both pre- and post-Roux-en-Y changes in gastric emptying of solids, and 2 only reported post-surgical changes (35, 36). Therefore, changes after RYGB were excluded from the meta-analysis.
Laparoscopic Adjusted Gastric Banding
Two individual adult studies with the laparoscopic adjustable gastric band (LAGB) and solid gastric emptying measurements were found. Only 1 study had pre-and post-surgery gastric emptying assessments and was therefore not included in the meta-analysis.(37)
Intragastric Balloons
A total of 11 studies using an intragastric balloon for obesity were identified. Only 5 reported pre and post intervention GE T1/2 for solid meals (38-42). The studies included 3 fluid-filled balloons and 2 gas-filled balloons. All studies performed a gastric emptying assessment within 3 months of balloon placement. Only 1 study with a fluid-filled balloon utilized optimal gastric emptying methodology. No studies with present day available gas-filled balloons or dual fluid-filled balloons were found. The placebo arm from 1 intragastric balloon studies was included in the meta-analysis phase.
Endoscopic Botulinum Toxin Antral Injections
Only 4 studies of endoscopic botulinum toxin injections for obesity reported pre- and post-intervention GE T1/2 and one study reported mean difference with standard deviation. Therefore, a total of 5 studies were included in the meta-analysis phase (43-47), with 4/5 using optimal gastric emptying measurement methodology. Three studies reported changes in gastric emptying for the sham group (control injection) and were studied in the analysis phase with the 2 placebo arms to represent the control group.
Other Endoscopic Interventions
A total of 3 studies using the small bowel implantable device were found. One of the studies reported changes in T1/2, %2 hour and %4 hour retention (48). The remaining two studies only reported either %2hr retention or %4hr retention and thus were excluded from the meta-analysis. In 2 studies involving differing stomach remodeling techniques, (1 Endoscopic Sleeve Gastroplasty using Apollo Endosurgery’s OverStitch Device and 1 Primary Obesity Surgery Endolumenal procedure using the USGI’s Incisionless Operating Platform), changes in gastric emptying were found (49, 50). Given the differences in technique and device, these studies were excluded from the meta-analysis. The final studies included in the meta-analysis along with details by intervention are included in Tables 1-2.
Change in Gastric Emptying (T1/2) and Association with Weight Loss
Sleeve Gastrectomy
There were a total of 9 studies included in the meta-analysis (n=233 patients). Using a random effects model, the pooled change in gastric emptying 3 months after sleeve gastrectomy was a mean acceleration of −29.2 minutes (95% CI −40.9 to −17.5) (Supplementary Figure 1). There was high heterogeneity in the results (I2=91.4%). Sensitivity analysis excluding studies in which the gastric emptying assessment was performed less than 1 month post intervention, heterogeneity decreased to 77.8%, with a mean acceleration of −25.5 minutes ( 95% CI: −34.8 to −16.3). Subgroup analysis by optimal (n=2 studies) vs suboptimal gastric emptying (n=4 studies) methodology using only pure solid-meal testing (6/8 studies), resulted in decrease in heterogeneity from 91.4% to 0% and 50.5%, respectively. In the optimal testing group, sleeve gastrectomy accelerated gastric emptying by −8.3 minutes (95% CI: −16.5 to −0.05 minutes) and in the suboptimal testing group, by −41.1 minutes (95% CI: −49.6 to −32.6 minutes) Supplementary Figure 2)
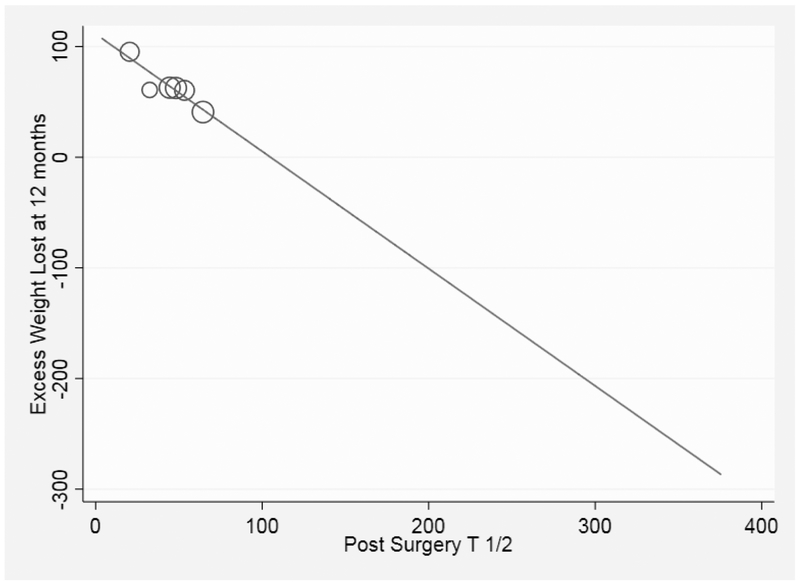
On meta-regression examining the relationship between change in GE and %EWL at 12 months (n=6 studies), no significant association was found (p=0.986). However, when examining the post sleeve gastrectomy T 1/2 association with weight loss, lower (faster) post-LSG T1/2 was associated with more %EWL at 12 months (p=0.009) (Figure 2). We then performed a meta regression assessing the relationship between baseline gastric emptying and final mean difference in gastric emptying T1/2 after the intervention, suggesting slower baseline GE was associated with faster post SG (coefficient −0.61;p=0.051) (Supplementary Figure 3).
Figure 2:
Association between Post SG T 1/2 and Excess Weight Loss at 12 months
Intragastric Balloons
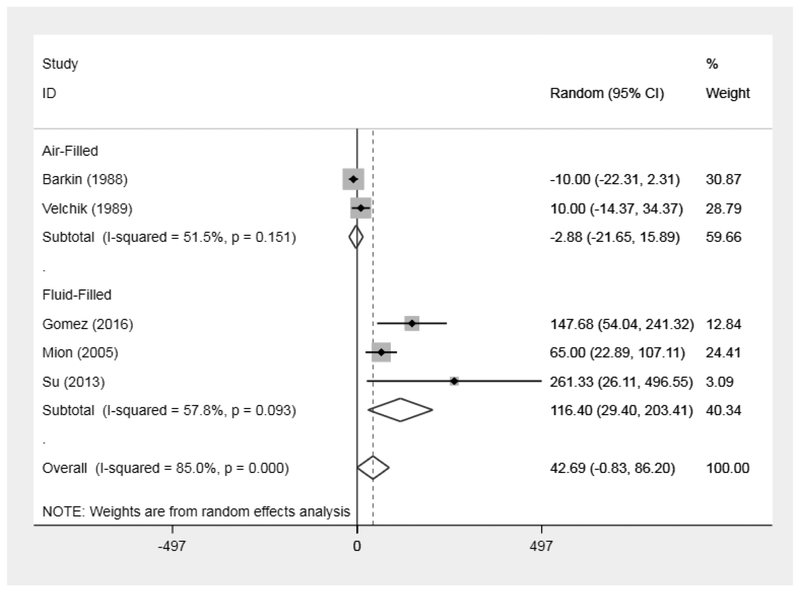
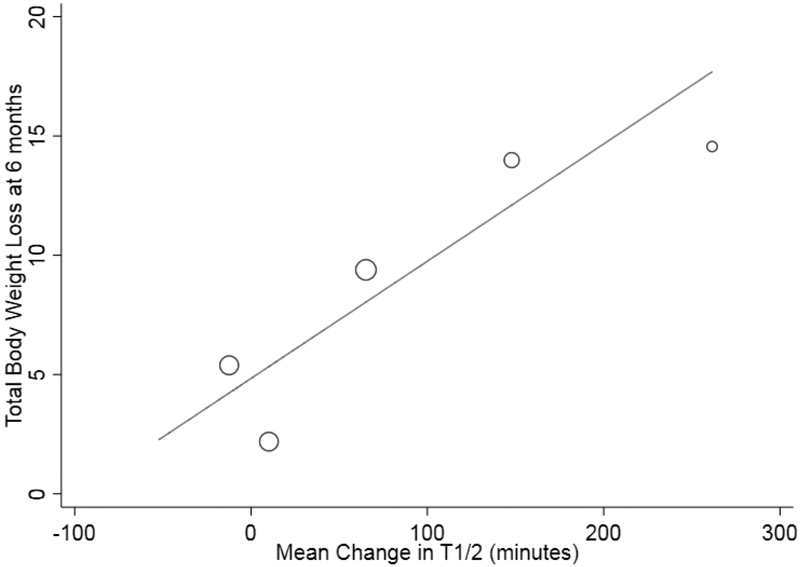
A total of 5 studies were included in the initial meta-analysis (n=66). The pooled mean change in gastric emptying was a non-significant delay of 42.7 minutes (95% CI: −0.83 to 86.2). Heterogeneity was high (I2=85%) (Figure 3). In the subgroup analysis comparing gas vs. fluid-filled balloons, fluid-filled balloons delayed gastric emptying by a mean 116.4 minutes (95% CI: 29.4 to 203.4) whereas air-filled balloons non-significantly altered gastric emptying by a mean −2.9 minutes (95% CI: −21.7 – 15.9). Heterogeneity decreased from the overall 85% for all 5 studies to 51.5% (I2=51.5%), and 57.8% (I2=57.8%), in the air and fluid-filled balloon analyses, respectively (Figure 3). When examining the association between GE and weight loss (n=5 studies), meta-regression revealed greater changes (produced delays) in gastric emptying was associated with more %TBWL at 6 months (p=0.05). Additionally, slower post-IGB GE was also associated with more weight loss at 6 months (p=0.04). (Figure 4)
Figure 3:
Forest Plot with All Intragastric Balloon Studies
Figure 4:
Association between post IGB T1/2 and %TBWL at 6 months
Endoscopic Botulinum Toxin Injections for Weight Loss
5 studies were included in the meta-analysis separated in a total 12 arms by study dose. The mean change in gastric emptying post intervention was a mean 9.6 minute delay (95% CI: 2.8-16.4) Heterogeneity was low at 13.3% (I2=13.3%). (Supplementary Figure 4) On sensitivity analysis by study dose (<300 units or ≥ 300 units Botulinum Toxin), low doses were associated with a non-significant delay of 5.6 minutes (95% CI: −3.5 to 14.6), and higher doses were associated with a statistically significant delay of 9.6 minutes (95% CI: 2.8-16.4). Heterogeneity remained low at 17.3% and 13.3%, respectively. On subgroup analysis using only optimal gastric emptying testing, antral botulinum toxin injections <300 units delayed emptying by 13.2 minutes ( 95% CI 2.7-23.7 minutes) and doses ≥300 units delayed emptying by 14.6 minutes (95% CI: 7.5-21.7 minutes) with low heterogeneity (I2=0.0%). (Supplementary Figure 5) On meta regression, changes in GE were not associated with absolute weight loss at an average 4 months after the botulinum toxin injections.
Placebo
A total of 4 placebo/sham arms from botulinum toxin and intragastric balloon studies were pooled to investigate the changes in gastric emptying. The mean change in gastric emptying was a mean acceleration of −6.1 minutes (95% CI: −9.8 to −2.4). Heterogeneity was low at 0%.(Supplementary Figure 6). Supplementary Figure 7 contains all the studies by intervention in the meta-analysis.
DISCUSSION
In this systematic review and meta-analysis on the effects of bariatric surgery and endoscopic treatments on gastric emptying, there were several important findings. First, in bariatric surgery, a high level of heterogeneity across sleeve gastrectomy studies was attributable to differences in gastric emptying measurement methodology, with a tendency for sleeve gastrectomy to accelerate gastric emptying T1/2 of solids. No more than 1 study for either the adjustable gastric band, or Roux-en-Y gastric bypass was found, limiting the assessment of gastric emptying changes in a meta-analysis approach. Second, in regard to endoscopic bariatric therapies, fluid-filled balloons delayed emptying of stomach contents by almost 2 hours, whereas air-filled balloons did not statistically change gastric emptying, potentially explaining the difference in efficacy and tolerance found across air vs. fluid-filled balloons in a recent network meta-analysis(51). For botulinum injections to the antrum, small temporary delays in gastric emptying were found, with lower doses failing to delay gastric emptying. Other endoscopic approaches, such as the endoscopic sleeve gastroplasty (ESG), primary obesity surgery endoluminal (POSE), and the small bowel implantable device (EndoBarrier) procedures, the low number of studies with gastric emptying assessments limited the ability to perform a meta-analysis despite their global tendency to delay or alter emptying in their individual studies(50, 52, 53). Third, and more importantly, we revealed that significant alterations in gastric emptying were associated with weight loss, with faster gastric emptying time after sleeve gastrectomy, and larger delays after intragastric balloon placement being associated with greater weight loss at 12 and 6 months, respectively. However, no associations with botulinum toxin injections to the antrum and weight loss were found, likely due to the short-lived effect of the toxin on GE compared to sleeve gastrectomy and implantable devices. These botulinum toxin findings are consistent with the recent systematic review and meta-analysis, that found antral injections to be ineffective for weight loss despite reports of additional applications to the fundus aiding in short term weight loss likely mediated through changes in accommodating volume, that could be measured through validated SPECT imaging(54). However, the need to repeat botulinum toxins every 4 weeks and minimal physiological effects limits its appeal as a cost-effective bariatric intervention, when compared to sleeve gastrectomy and longer-lasting endoscopic approaches. (55)
The relationship between gastric motility and regulation of food intake has several components. During the early phases of meal consumption, the fundus relaxes to accommodate incoming food, minimizing increases in intragastric pressure that stimulate stretch mechanoreceptors that in turn stimulate vagal afferents to the brain.(56) Not surprisingly, impaired accommodation has been shown to be associated with early post-prandial fullness, bloating and weight loss, with targeted pharmacotherapy improving accommodation and producing weight gain.(57) For gastric emptying, studies have shown associations between physiological or induced gastric emptying delays with early meal termination, with gastroparesis serving as an extreme example of this phenomenon. This effect is thought to be mediated through a variety of factors, including a prolonged duration of increased intragastric pressure stimulating vagal afferents, with the return to normotension promoting a return of hunger and finally, through a delayed but constant intestinal nutrient exposure simulating a high caloric meal.(58) In adults with obesity, fast gastric emptying of solids has been postulated as one of the key drivers of increased hunger and appetite between meals in the absence of disordered eating behaviors such as binge eating, nighttime eating and emotional eating disorders. By targeting gastric emptying, endoscopic and bariatric interventions are modifying the body’s existing food regulation pathways facilitating weight loss with greater perturbations associated with more weight loss per our analysis.
The observed accelerated gastric emptying after sleeve gastrectomy and association with weight loss is likely multifactorial. After sleeve gastrectomy, the proximal portion of the stomach and the majority of the greater curvature is resected, reducing Ghrelin production, reducing stomach accommodation, increasing intraluminal pressure, and promoting content delivery to the antrum. However, by leaving the lesser curvature intact, antral innervation remains functional, promoting faster gastric emptying through the pylorus, promoting early nutrient delivery to the intestine. As a result, through the increased intragastric pressure, reduced Ghrelin production and changes in bile acid metabolism through earlier nutrient delivery, weight loss ensues (59). In contrast, fluid-filled balloons markedly delay gastric emptying through an outlet obstruction mechanism, while also promoting a sense of fullness by stimulating stretch receptors by the food and the balloon itself without any increases in Ghrelin.(41) Early studies investigating fluid-filled balloons found a reduction in food intake with increasing fluid volume, but were unable to identify delays in gastric emptying.(60, 61) Subsequent studies with air-filled balloons showed initial promise for weight loss, but were abandoned due to the high rates of distal intestinal obstruction and poor efficacy in weight loss.(39, 42) Our results suggest air-filled balloons do not alter gastric emptying, providing a possible explanation for the decreased efficacy in induction of weight loss, though there is improved tolerability of the newer commercially available air-filled balloons.(51, 62) Air-filled balloons likely fail to delay gastric emptying as they tend to float in the stomach, whereas the fluid-filled balloons delay gastric emptying through an obstructive physiology and stronger stretch receptor stimulation. This obstructive mechanism with fluid-filled IGBs is likely responsible for the increased nausea, vomiting and reflux experienced early on during device residency, which improves over time. Predictably, this improvement parallels the weight loss plateau seen with fluid-filled balloons around 3 months, when patient’s accommodating volume has likely increased/improved, behavioral change fatigue ensues, or hormonal adaptations begin to occur. However, changes in stomach accommodating volume during IGB use have not been investigated. Indeed, early studies have shown that impaired accommodation leads to earlier meal-induced satiation in the presence of an obstruction, providing a putative factor contributing to weight regain.(63) With botulinum toxin injections to the antrum, the transient delays in GE are likely a consequence of inhibiting the cholinergic neurons that control antral contractility and the interstitial cells of Cajal, which likely increase intragastric pressure and antrum distention leading to weight loss. However, the short duration of botulinum toxin’s action on cholinergic neurons likely explains the failure to produce sustained weight loss in the absence of repeated injections, thus the lack of association between changes in GE with weight loss in our analysis.
Our study has many limitations, including the small number of studies available for all interventions, low methodological quality of the trials, and absence of direct comparisons on gastric emptying between the interventions. Additionally, we were limited by the significant amount of clinical and statistical heterogeneity across the studies largely due to their non-randomized nature and different methods of gastric emptying assessment. Varying meal size, fat content, duration of testing and type of assessment (breath vs. scintigraphy) likely explain the heterogeneity between studies as determined in our subgroup analyses. with different surgical techniques in the laparoscopic sleeve gastrectomy (bougie size, distance from the antrum) likely explaining the remaining heterogeneity.
Despite these limitations, this is the first study aimed at evaluating the effects of different bariatric interventions on gastric emptying and its relationship with weight loss, showing larger perturbations in gastric emptying being associated with more weight loss and opposite effects of fluid-filled IGBs and sleeve gastrectomy surgery on gastric emptying suggesting a different mechanism of action targeting different gastric and small intestinal physiology.
The obesity management tools box is expanding to include multiple medical, endoscopic, and surgical interventions. Individual patients’ response to these interventions, however, varies and follows a traditional Bells curve distribution with low, average, and high responders, given the heterogeneity of obesity as chronic multifactorial disease with varying pathophysiologic and behavioral etiologies. This meta-analysis synthesizes the available data revealing an association between gastric emptying and weight loss after sleeve gastrectomy and fluid-filled IGBs, suggesting gastric emptying can be utilized to tailor obesity management across interventions and measure response to such. Overall, patients with slower gastric emptying at baseline may not tolerate fluid-filled IGBs, and maybe better candidates for sleeve gastrectomy surgery or air-filled gastric balloon. On the other hand, patients with accelerated gastric emptying at baseline might be more responsive to an intervention that significantly delays gastric emptying, such as fluid-filled IGBs. This physiologically tailored and personalized approach to bariatric intervention, have shown promise in proof-of-concept prospective trials both with obesity pharmacotherapies and IGBs (55, 64, 65) Therefore, we recommend patients with obesity should undergo a standardized gastric emptying of solids assessment in order to personalize their approach with medications, endoscopic devices and bariatric surgery, reducing the number of non-responders, maximizing device efficacy and health care resources.
Supplementary Material
Supplementary Figure 1: Forest Plot of Sleeve Gastrectomy Studies
Supplementary Figure 2: Forest Plot of Sleeve Gastrectomy Studies by Optimal Methodology
Supplementary Figure 3: Slower baseline GE associated with faster GE after Sleeve Gastrectomy
Supplementary Figure 4: Forest Plot of Botox Studies
Supplementary Figure 5: Forest Plot of Botox Studies by Dose
Supplementary Figure 6: Forest Plot Placebo Arms
Supplementary Figure 7: Overall Forest Plot All Interventions
Supplementary Figure 8: Bias Assessment Sleeve Gastrectomy Studies
Supplementary Figure 9: Bias Assessment Intragastric Balloon Studies
Supplementary Figure 10: Bias Assessment Botox Studies
Need to Know.
Background:
We performed a systematic review and meta-analysis of times of gastric emptying (GE) after different bariatric endoscopic and surgical interventions and associations with weight loss.
Findings:
In a systematic review and meta-analysis, we found that sleeve gastrectomy reduces GE T1/2 whereas fluid-filled balloons significantly increase GE T1/2. Air-filled balloons do not significantly change the time of GE, and antral botulinum toxin injections produced small temporary increases in GE time. Changes in GE time after surgical and endoscopic bariatric interventions correlate with weight loss.
Implications for Patient Care:
Changes in GE time after bariatric procedures might be used to select interventions for weight loss, based on patients’ physiology.
Acknowledgments
FUNDING: UL1 TR002377 and Dr. M. Camilleri is funded by RO1 DK67071 for studies in obesity.
prisma 2009 Checklist
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 3 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 4 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 4 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 4 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 4 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 4 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 5 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 5 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 6 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 6 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 7 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 7 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 7 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 8 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 8 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 8 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 9 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 11 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 9- |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | 11 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 13 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 14 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 14 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 17 |
Footnotes
DISCLOSURES:
Drs. Vargas, Bazerbachi, Calderon, Gomez, Murad, Acosta and Camilleri as well as Mr. Larry J. Prokop have no relevant disclosures.
Dr. Abu Dayyeh: research support from Apollo Endosurgery and Spatz3
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. Jama. 2010;303(3):235–41. Epub 2010/01/15. doi: 10.1001/jama.2009.2014. PubMed PMID: 20071471. [DOI] [PubMed] [Google Scholar]
- 2.Organization WH. Global Health Observatory (GHO) data: overweight and obesity 2016 [11/11/2017]. Available from: http://www.who.int/gho/ncd/risk_factors/overweight_text/en/(2016).
- 3.Collaborators TGO. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. New England Journal of Medicine. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. PubMed PMID: 28604169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Releases CN. Cancers Associated with Overweight and Obesity Make up 40 percent of Cancers Diagnosed in the United States 2017. [11/11/2017]. Available from: https://www.cdc.gov/media/releases/2017/p1003-vs-cancer-obesity.html.
- 5.Acosta A, Abu Dayyeh BK, Port JD, Camilleri M. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut. 2014;63(4):687–95. doi: 10.1136/gutjnl-2013-306235. PubMed PMID: 24402654; PubMed Central PMCID: PMCPMC4170188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–61. Epub 2007/08/24. doi: 10.1056/NEJMoa066603. PubMed PMID: 17715409. [DOI] [PubMed] [Google Scholar]
- 7.Schauer DP, Feigelson HS, Koebnick C, Caan B, Weinmann S, Leonard AC, et al. Bariatric Surgery and the Risk of Cancer in a Large Multisite Cohort. Ann Surg. 2017. Epub 2017/09/25. doi: 10.1097/SLA.0000000000002525. PubMed PMID: 28938270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376(7):641–51. doi: 10.1056/NEJMoa1600869. PubMed PMID: 28199805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salminen P, Helmio M, Ovaska J, Juuti A, Leivonen M, Peromaa-Haavisto P, et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. Jama. 2018;319(3):241–54. Epub 2018/01/18. doi: 10.1001/jama.2017.20313. PubMed PMID: 29340676; PubMed Central PMCID: PMCPMC5833550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–8. doi: 10.1007/s11695-012-0622-3. PubMed PMID: 22354457; PubMed Central PMCID: PMCPMC3319900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–30. doi: 10.1056/NEJMoa012908. PubMed PMID: 12023994. [DOI] [PubMed] [Google Scholar]
- 12.Vargas EJ, Rizk M, Bazerbachi F, Abu Dayyeh BK. Medical Devices for Obesity Treatment: Endoscopic Bariatric Therapies. Med Clin North Am. 2018;102(1):149–63. Epub 2017/11/21. doi: 10.1016/j.mcna.2017.08.013. PubMed PMID: 29156183. [DOI] [PubMed] [Google Scholar]
- 13.Jirapinyo P, Thompson CC. Endoscopic Bariatric and Metabolic Therapies: Surgical Analogues and Mechanisms of Action. Clin Gastroenterol Hepatol. 2017;15(5):619–30. Epub 2016/12/19. doi: 10.1016/j.cgh.2016.10.021. PubMed PMID: 27989851; PubMed Central PMCID: PMCPMC5444453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta A, Camilleri M, Shin A, Vazquez-Roque MI, Iturrino J, Burton D, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology. 2015;148(3):537–46 e4. Epub 2014/12/09. doi: 10.1053/j.gastro.2014.11.020. PubMed PMID: 25486131; PubMed Central PMCID: PMCPMC4339485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen P, Berghe PV, Verschueren S, Lehmann A, Depoortere I, Tack J. Review article: the role of gastric motility in the control of food intake. Alimentary Pharmacology & Therapeutics. 2011;33(8):880–94. doi: doi: 10.1111/j.1365-2036.2011.04609.x. [DOI] [PubMed] [Google Scholar]
- 16.McGinn T, Wyer PC, Newman TB, Keitz S, Leipzig R, For GG, et al. Tips for learners of evidence-based medicine: 3. Measures of observer variability (kappa statistic). CMAJ. 2004;171(11):1369–73. Epub 2004/11/24. doi: 10.1503/cmaj.1031981. PubMed PMID: 15557592; PubMed Central PMCID: PMCPMC527344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol. 2008;36(1):44–54. Epub 2008/02/22. doi: 10.2967/jnmt.107.048116. PubMed PMID: 18287197. [DOI] [PubMed] [Google Scholar]
- 18.Pathikonda M, Sachdeva P, Malhotra N, Fisher RS, Maurer AH, Parkman HP. Gastric emptying scintigraphy: is four hours necessary? J Clin Gastroenterol. 2012;46(3):209–15. Epub 2011/10/01. doi: 10.1097/MCG.0b013e31822f3ad2. PubMed PMID: 21959322. [DOI] [PubMed] [Google Scholar]
- 19.Vigneshwaran B, Wahal A, Aggarwal S, Priyadarshini P, Bhattacharjee H, Khadgawat R, et al. Impact of Sleeve Gastrectomy on Type 2 Diabetes Mellitus, Gastric Emptying Time, Glucagon-Like Peptide 1 (GLP-1), Ghrelin and Leptin in Non-morbidly Obese Subjects with BMI 30-35.0 kg/m2: a Prospective Study. Obesity Surgery. 2016;26(12):2817–23. doi: 10.1007/s11695-016-2226-9. PubMed PMID: 27185177. [DOI] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. Epub 2010/07/24. doi: 10.1007/s10654-010-9491-z. PubMed PMID: 20652370. [DOI] [PubMed] [Google Scholar]
- 21.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14. Epub 2006/06/30. doi: 10.1016/j.cct.2006.04.004. PubMed PMID: 16807131. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. Epub 1986/09/01. PubMed PMID: 3802833. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–58. doi: doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ : British Medical Journal. 2006;333(7568):597–600. PubMed PMID: PMC1570006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murad MH, Chu H, Lin L, Wang Z. The effect of publication bias magnitude and direction on the certainty in evidence. BMJ Evidence-Based Medicine. 2018;23(3):84–6. doi: 10.1136/bmjebm-2018-110891. PubMed PMID: PMC5969367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–3. Epub 2018/02/09. doi: 10.1136/bmjebm-2017-110853. PubMed PMID: 29420178; PubMed Central PMCID: PMCPMC6234235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vives M, Molina A, Danus M, Rebenaque E, Blanco S, Paris M, et al. Analysis of Gastric Physiology After Laparoscopic Sleeve Gastrectomy (LSG) With or Without Antral Preservation in Relation to Metabolic Response: a Randomised Study. Obesity Surgery. 2017;27(11):2836–44. doi: 10.1007/s11695-017-2700-z. PubMed PMID: 615977376. [DOI] [PubMed] [Google Scholar]
- 28.Sista F, Abruzzese V, Clementi M, Carandina S, Cecilia M, Amicucci G. The effect of sleeve gastrectomy on GLP-1 secretion and gastric emptying: a prospective study. Surg. 2017;13(1):7–14. doi: 10.1016/j.soard.2016.08.004. PubMed PMID: 27692912. [DOI] [PubMed] [Google Scholar]
- 29.Michalsky D, Dvorak P, Belacek J, Kasalicky M. Radical resection of the pyloric antrum and its effect on gastric emptying after sleeve gastrectomy. Obesity Surgery. 2013;23(4):567–73. doi: 10.1007/s11695-012-0850-6. PubMed PMID: 23306796. [DOI] [PubMed] [Google Scholar]
- 30.Melissas J, Koukouraki S, Askoxylakis J, Stathaki M, Daskalakis M, Perisinakis K, et al. Sleeve gastrectomy: a restrictive procedure? Obesity Surgery. 2007;17(1):57–62. doi: 10.1007/s11695-007-9006-5. PubMed PMID: 17355769. [DOI] [PubMed] [Google Scholar]
- 31.Melissas J, Leventi A, Klinaki I, Perisinakis K, Koukouraki S, de Bree E, et al. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Annals of Surgery. 2013;258(6):976–82. doi: 10.1097/SLA.0b013e3182774522. PubMed PMID: 23160151. [DOI] [PubMed] [Google Scholar]
- 32.Bernstine H, Tzioni-Yehoshua R, Groshar D, Beglaibter N, Shikora S, Rosenthal RJ, et al. Gastric emptying is not affected by sleeve gastrectomy--scintigraphic evaluation of gastric emptying after sleeve gastrectomy without removal of the gastric antrum. Obesity Surgery. 2009;19(3):293–8. doi: 10.1007/s11695-008-9791-5. PubMed PMID: 19089519. [DOI] [PubMed] [Google Scholar]
- 33.Pilone V, Tramontano S, Di Micco R, Monda A, Hasani A, Izzo G, et al. Gastric emptying after sleeve gastrectomy: statistical evidence of a controlled prospective study with gastric scintigraphy. Minerva Chir. 2013;68(4):385–92. PubMed PMID: 24019046. [PubMed] [Google Scholar]
- 34.Kandeel AA, Sarhan MD, Hegazy T, Mahmoud MM, Ali MH. Comparative assessment of gastric emptying in obese patients before and after laparoscopic sleeve gastrectomy using radionuclide scintigraphy. Nucl Med Commun. 2015;36(8):854–62. doi: 10.1097/MNM.0000000000000337. PubMed PMID: 25932537. [DOI] [PubMed] [Google Scholar]
- 35.Suarez-Pinera M, Prat L, Fuertes J, Gras B, Delgado-Aros S. Gastric emptying and small boweltransit assessed by scintigraphy in obese patients after bariatric surgery: Sleeve gastrectomy and Gastric Bypass. (preliminary results). European Journal of Nuclear Medicine and Molecular Imaging. 2011;2):S403. doi: 10.1007/s00259-011-1911-0. PubMed PMID: 70581098. [DOI] [Google Scholar]
- 36.Naslund I, Beckman KW. Gastric emptying rate after gastric bypass and gastroplasty. Scand J Gastroenterol. 1987;22(2):193–201. PubMed PMID: 3554493. [DOI] [PubMed] [Google Scholar]
- 37.Burton PR, Yap K, Brown WA, Laurie C, O'Donnell M, Hebbard G, et al. Effects of adjustable gastric bands on gastric emptying, supra- and infraband transit and satiety: a randomized double-blind crossover trial using a new technique of band visualization. Obesity Surgery. 2010;20(12):1690–7. doi: 10.1007/s11695-010-0278-9. PubMed PMID: 20835896. [DOI] [PubMed] [Google Scholar]
- 38.Gomez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: Results of a prospective study. Obesity (Silver Spring). 2016;24(9):1849–53. doi: 10.1002/oby.21555. PubMed PMID: 27465076. [DOI] [PubMed] [Google Scholar]
- 39.Velchik MG, Kramer FM, Stunkard AJ, Alavi A. Effect of the Garren-Edwards gastric bubble on gastric emptying. J Nucl Med. 1989;30(5):692–6. PubMed PMID: 2715831. [PubMed] [Google Scholar]
- 40.Su H-J, Kao C-H, Chen W-C, Chang T-T, Lin C-Y. Effect of intragastric balloon on gastric emptying time in humans for weight control. Clin Nucl Med. 2013;38(11):863–8. doi: 10.1097/RLU.0000000000000224. PubMed PMID: 24089058. [DOI] [PubMed] [Google Scholar]
- 41.Mion F, Napoleon B, Roman S, Malvoisin E, Trepo F, Pujol B, et al. Effects of intragastric balloon on gastric emptying and plasma ghrelin levels in non-morbid obese patients. Obesity Surgery. 2005;15(4):510–6. doi: 10.1381/0960892053723411. PubMed PMID: 15946431. [DOI] [PubMed] [Google Scholar]
- 42.Barkin JS, Reiner DK, Goldberg RI, Phillips RS, Janowitz WR. The effects of morbid obesity and the Garren-Edwards gastric bubble on solid phase gastric emptying. Am J Gastroenterol. 1988;83(12):1364–7. PubMed PMID: 3195541. [PubMed] [Google Scholar]
- 43.Li L, Liu Q-S, Liu W-H, Yang Y-S, Yan D, Peng L-H, et al. Treatment of obesity by endoscopic gastric intramural injection of botulinum toxin A: a randomized clinical trial. Hepatogastroenterology. 2012;59(118):2003–7. doi: 10.5754/hge11755. PubMed PMID: 22193433. [DOI] [PubMed] [Google Scholar]
- 44.Topazian M, Camilleri M, Enders FT, Clain JE, Gleeson FC, Levy MJ, et al. Gastric antral injections of botulinum toxin delay gastric emptying but do not reduce body weight. Clinical Gastroenterology & Hepatology. 2013;11(2):145–50.e1. doi: 10.1016/j.cgh.2012.09.029. PubMed PMID: 23063681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foschi D, Lazzaroni M, Sangaletti O, Corsi F, Trabucchi E, Bianchi Porro G. Effects of intramural administration of Botulinum Toxin A on gastric emptying and eating capacity in obese patients. Dig Liver Dis. 2008;40(8):667–72. doi: 10.1016/j.dld.2008.02.040. PubMed PMID: 18420471. [DOI] [PubMed] [Google Scholar]
- 46.Gui D, Mingrone G, Valenza V, Spada PL, Mutignani M, Runfola M, et al. Effect of botulinum toxin antral injection on gastric emptying and weight reduction in obese patients: a pilot study. Alimentary Pharmacology & Therapeutics. 2006;23(5):675–80. PubMed PMID: 16480407. [DOI] [PubMed] [Google Scholar]
- 47.Junior AC, Savassi-Rocha PR, Coelho LGV, Sposito MMdM, Albuquerque W, Diniz MTC, et al. Botulinum A toxin injected into the gastric wall for the treatment of class III obesity: a pilot study. Obesity Surgery. 2006;16(3):335–43. PubMed PMID: 16545166. [DOI] [PubMed] [Google Scholar]
- 48.Escalona A, Yanez R, Pimentel F, Galvao M, Ramos AC, Turiel D, et al. Initial human experience with restrictive duodenal-jejunal bypass liner for treatment of morbid obesity. Surg. 2010;6(2):126–31. doi: 10.1016/j.soard.2009.12.009. PubMed PMID: 20359665. [DOI] [PubMed] [Google Scholar]
- 49.Espinos JC, Turro R, Moragas G, Bronstone A, Buchwald JN, Mearin F, et al. Gastrointestinal Physiological Changes and Their Relationship to Weight Loss Following the POSE Procedure. Obesity Surgery. 2016;26(5):1081–9. doi: 10.1007/s11695-015-1863-8. PubMed PMID: 605877692. [DOI] [PubMed] [Google Scholar]
- 50.Abu Dayyeh BK, Acosta A, Camilleri M, Mundi MS, Rajan E, Topazian MD, et al. Endoscopic Sleeve Gastroplasty Alters Gastric Physiology and Induces Loss of Body Weight in Obese Individuals. Clinical Gastroenterology & Hepatology. 2017;15(1):37–43.e1. doi: 10.1016/j.cgh.2015.12.030. PubMed PMID: 26748219. [DOI] [PubMed] [Google Scholar]
- 51.Bazerbachi F, Haffar S, Sawas T, Vargas EJ, Kaur RJ, Wang Z, et al. Fluid-Filled Versus Gas-Filled Intragastric Balloons as Obesity Interventions: a Network Meta-analysis of Randomized Trials. Obes Surg. 2018;28(9):2617–25. Epub 2018/04/18. doi: 10.1007/s11695-018-3227-7. PubMed PMID: 29663250. [DOI] [PubMed] [Google Scholar]
- 52.Turro R, Espinos JP, Mata A, Turro J, Delgado SA, Mearin F. Primary obesity surgery endolumenal (POSE): Weight, satiation, and metabolic changes in a prospective cohort of obese patients. United European Gastroenterology Journal. 2013;1):A281. doi: 10.1177/2050640613502900. PubMed PMID: 72263738. [DOI] [Google Scholar]
- 53.de Moura EGH, Lopes GS, Martins BC, Orso IRB, Coutinho AMN, de Oliveira SL, et al. Effects of Duodenal-Jejunal Bypass Liner (EndoBarrier) on Gastric Emptying in Obese and Type 2 Diabetic Patients. Obesity Surgery. 2015;25(9):1618–25. doi: 10.1007/s11695-015-1594-x. PubMed PMID: 25691349. [DOI] [PubMed] [Google Scholar]
- 54.Breen M, Camilleri M, Burton D, Zinsmeister AR. Performance characteristics of the measurement of gastric volume using single photon emission computed tomography. Neurogastroenterol Motil. 2011;23(4):308–15. Epub 2011/01/08. doi: 10.1111/j.1365-2982.2010.01660.x. PubMed PMID: 21210894; PubMed Central PMCID: PMCPMC3105214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halawi H, Khemani D, Eckert D, O'Neill J, Kadouh H, Grothe K, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol. 2017;2(12):890–9. Epub 2017/09/30. doi: 10.1016/S2468-1253(17)30285-6. PubMed PMID: 28958851. [DOI] [PubMed] [Google Scholar]
- 56.Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51 Suppl 1:i2–5. Epub 2002/06/22. PubMed PMID: 12077054; PubMed Central PMCID: PMCPMC1867719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tack J, Janssen P, Masaoka T, Farre R, Van Oudenhove L. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2012;10(11):1239–45. Epub 2012/07/21. doi: 10.1016/j.cgh.2012.06.036. PubMed PMID: 22813445. [DOI] [PubMed] [Google Scholar]
- 58.Geliebter A, Westreich S, Gage D. Gastric distention by balloon and test-meal intake in obese and lean subjects. Am J Clin Nutr. 1988;48(3):592–4. Epub 1988/09/01. doi: 10.1093/ajcn/48.3.592. PubMed PMID: 3414573. [DOI] [PubMed] [Google Scholar]
- 59.Mans E, Serra-Prat M, Palomera E, Sunol X, Clave P. Sleeve gastrectomy effects on hunger, satiation, and gastrointestinal hormone and motility responses after a liquid meal test. Am J Clin Nutr. 2015;102(3):540–7. doi: 10.3945/ajcn.114.104307. PubMed PMID: 26201818. [DOI] [PubMed] [Google Scholar]
- 60.Geliebter A, Westreich S, Hashim SA. Intragastric balloon induced reduction of test meal intake. FED PROC. 1985;44(5). [Google Scholar]
- 61.Geliebter A, Westreich S, Gage D. Gastric distention by balloon and test-meal intake in obese and lean subjects. Am J Clin Nutr. 1988;48(3):592–4. PubMed PMID: 3414573. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan S, Swain J, Woodman G, Edmundowicz S, Hassanein T, Shayani V, et al. Randomized Sham-Controlled Trial of the Six-Month Swallowable Gas-Filled Intragastric Balloon System for Weight Loss. Surgery for Obesity and Related Diseases. 2018. doi: 10.1016/j.soard.2018.09.486. [DOI] [PubMed] [Google Scholar]
- 63.Vanden Berghe P, Janssen P, Kindt S, Vos R, Tack J. Contribution of different triggers to the gastric accommodation reflex in humans. Am J Physiol Gastrointest Liver Physiol. 2009;297(5):G902–6. Epub 2009/10/23. doi: 10.1152/ajpgi.00046.2009. PubMed PMID: 19846891. [DOI] [PubMed] [Google Scholar]
- 64.Camilleri M, Acosta A. Gastrointestinal traits: individualizing therapy for obesity with drugs and devices. Gastrointestinal Endoscopy. 2016;83(1):48–56. doi: 10.1016/j.gie.2015.08.007. PubMed PMID: 26271184; PubMed Central PMCID: PMCSource: NLM. NIHMS715303 [Available on 01/01/17]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abu Dayyeh BK, Lopez-Nava G, Bautista-Castano I, Vargas EJ, Bazerbachi F, Rizk M, et al. Personalization of bariatric and metabolic endoscopy therapies based on physiology: A prospective feasibility trial with the single fluid-filled intragastric balloon. Gastrointestinal Endoscopy. 2018;87 (6 Supplement 1):AB65. PubMed PMID: 622899747. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Forest Plot of Sleeve Gastrectomy Studies
Supplementary Figure 2: Forest Plot of Sleeve Gastrectomy Studies by Optimal Methodology
Supplementary Figure 3: Slower baseline GE associated with faster GE after Sleeve Gastrectomy
Supplementary Figure 4: Forest Plot of Botox Studies
Supplementary Figure 5: Forest Plot of Botox Studies by Dose
Supplementary Figure 6: Forest Plot Placebo Arms
Supplementary Figure 7: Overall Forest Plot All Interventions
Supplementary Figure 8: Bias Assessment Sleeve Gastrectomy Studies
Supplementary Figure 9: Bias Assessment Intragastric Balloon Studies
Supplementary Figure 10: Bias Assessment Botox Studies