Abstract
Pediatric Obstructive Sleep Apnea (OSA) is a condition that may lead to a variety of comorbidities in adolescence and adulthood. The gold standard of diagnosing OSA is polysomnography (PSG). Over the past fifteen years numerous publications have explored how to better visualize the upper airway to further assess OSA in the pediatric population, and eventually institute personalized treatment. Lateral neck radiograph, cephalometry, computed axial tomography, and magnetic resonance imaging are all unique imaging techniques that are used in the diagnosis of OSA. Drug Induced Sleep Endoscopy is a direct visualization technique that is gathering momentum in pediatrics. Each approach has respective benefits and weaknesses. However, none of them at this time can replace PSG, They are a helpful supplement in those patients with complicated upper airway anatomy and in those with residual OSA.
Keywords: Pediatric, obstructive sleep apnea, sleep disordered breathing, magnetic resonance imaging, lateral neck radiograph, drug induced sleep endoscopy, computerized axial tomography, cephalometry, upper airway imaging
INTRODUCTION
Sleep disordered breathing (SDB) encompasses an array of sleep disorders ranging from snoring to obstructive sleep apnea (OSA). Obstructive sleep apnea is described as sleep disrupted by prolonged partial upper airway obstruction and/or intermittent complete upper airway obstruction. 1 The prevalence of OSA in the pediatric population is estimated to range from 1.2–5.7% and is highest between the ages of 2 and 8 years. 2,3 Obesity, African American race, lower socioeconomic status and tobacco exposure are all associated with increased OSA severity.4 In addition, OSA is associated with a variety of negative comorbidities such as metabolic dysfunction, neurobehavioral impairments and cardiovascular disease. 5
Pediatric obstructive sleep apnea is caused by increased upper airway collapsibility, anatomic narrowing of the upper airway, or both. 2,4Adenotonsillar hypertrophy is the most common cause of upper airway narrowing in otherwise healthy pediatric patients with OSA. 3 However, not all children who have OSA have adenotonsillar hypertrophy nor do all children with adenotonsillar hypertrophy have OSA. Patients with craniofacial abnormalities, Trisomy 21, Prader Willi, Obesity and Bronchopulmonary dysplasia are at increased risk of obstructive sleep apnea due to complex upper airway malformations that result in upper airway narrowing.6ADD. These patients are at high risk for residual OSA after an adenotonsillectomy and may benefit from upper airway imaging or visualization.6,9,12
Upper airway visualization may be a useful addition to overnight polysomnography, the gold standard for diagnosing OSA in children with a suspicion of OSA. Multiple visualization techniques are currently used to assess upper airway abnormalities. These techniques, from least to most time intensive, include lateral neck radiograph (cephalometry), Computed Tomography, Magnetic Resonance Imaging, and Drug Induced Sleep Endoscopy (DISE). We will skew focus toward MRI and DISE as these novel technologies are at the forefront of airway visualization. In this systematic review we aim to identify the role of upper airway visualization modalities in the diagnosis and management of pediatric obstructive sleep apnea.
METHODS
A literature search was performed using PubMed, World of Science and SCOPUS. The scope of the search included English language articles published within the last fifteen years including pediatric subjects ranging from 0–18 years old. We used the following search terms: pediatrics, sleep disordered breathing, obstructive sleep apnea, OSA, CT, MRI, DISE and cephalometry.
RESULTS
LATERAL NECK RADIOGRAPHY and CEPHALOMETRY
Lateral neck radiography and cephalometry are commonly used techniques to image the upper airway. The more commonly used lateral neck radiograph visualizes the upper airway from the nasopharynx to the upper thoracic trachea also allowing the evaluation of the adenoids, tonsils and prevertebral soft tissues and their effect over the airway. In cephalometry, which includes the entire skull, different craniofacial measurements and ratios are obtained from a standardized lateral radiograph of the head and neck. 14 Figures 1 and 3 illustrate normal lateral neck radiographs in both children and infants, respectively. In contrast, Figures 2 and 4 use lateral neck radiographs to demonstrate adenoidal hypertrophy in a child and micrognathia in an infant. This technique is widely available and relatively inexpensive with low radiation exposures (between 0.01 mSv and 0.11 Sievert mSv in adults,) which is equivalent to 30 hours to 13 days of natural background radiation. 15 Radiation dose is lower in children, depending on the patient’s age and/or size. Lateral dynamic radiographic images of the central airway during fluoroscopy examination are useful in identifying dynamic airway collapse, as illustrated in Figure 5. Earlier cephalometric studies in the pediatric population with OSA have identified specific craniofacial characteristics including the vertical growth pattern of the mandible, smaller cranial base angle, retrognathia of both maxilla and mandible, and the reduced antero-posterior (AP) linear dimensions of the bony nasopharynx that influence patency of the upper airway16DDI.. Recent studies have identified both the utility and limitations of using Cephalometry to describe upper airway abnormalities in patients with OSA. In Japan, Maeda 2014 studied 13 otherwise normal pediatric patients with residual OSA after adenotonsillectomy using cephalometric measurements 21 They found that in comparison to the Japanese normal value, all of the children with residual OSA had statistically smaller mandibles (p<0.05.) 21 Pirilia-Parkken 2010 used cephalometry to assess specific measurements in 70 children with SDB. They found that those with SDB had a significantly larger palatomandibular angle (p=0.0001) as well as increased total (p= 0.019) and lower (p=0.005) anterior face heights. The patients with SDB had longer and thicker soft palates and smaller airway diameter compared to normal controls without SDB. When compared to the children with sleep-disordered breathing, the patients with OSA were not significantly distinguishable by mere observation.22 Like earlier cephalometric studies, these studies identified differences among those patients with OSA, sleep-disordered breathing and residual OSA.
Figure 1:
Normal lateral radiograph of the neck soft tissues in a 6 year old boy. (A) adenoids, (T) palatine tonsils. Pharynx airway (yellow overlay). Larynx (blue overlay)
Figure 3:
Normal lateral radiograph of the skull in a 3 month old boy for comparison. Notice the extension of the hard palate, normal size of the mandible, and normal posterior outline of the tongue (black line) resulting in normal maintained air column
Figure 2:
Lateral radiograph of the neck soft tissues in a 6-year-old boy presenting with nasal obstruction shows isolated enlargement of the adenoids (A) resulting in moderate narrowing of the nasopharyngeal airway in the anteroposterior dimension
Figure 4:
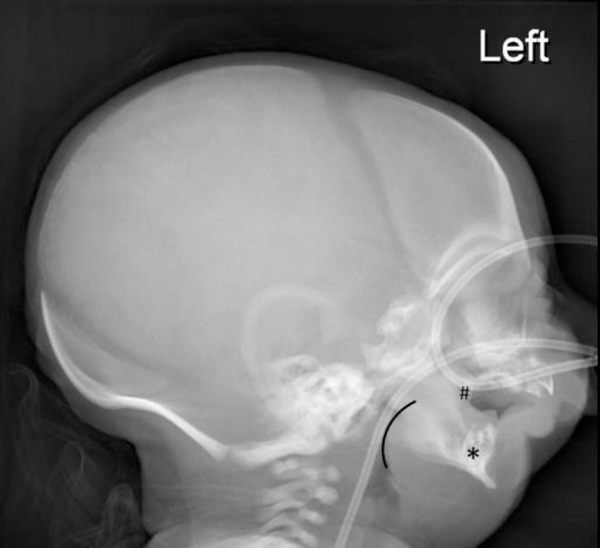
Lateral radiograph of the skull in a full term 2-day-old boy presenting with micrognathia, cleft palate, noisy breathing and poor feeding and diagnosed with Pierre Robin sequence. The radiograph shows an abnormal craniofacial ratio due to severe midface hypoplasia with partial absence of the hard palate (#) and severe micrognathia (*). Glossoptosis is also present (black outline) resulting in loss of the normal air column in the naso- and oropharynx.
Figure 5:
Lateral dynamic radiographic images of the central airway during fluorosocopy examination in a 2 month old boy with stridor show: (A) normal caliber (1.5 cm, double head arrow) with (B) Sequential narrowing (0.6 cm, double head arrow) to (C) complete collapse of the hypopharynx (supraglottic airway)
Despite the utility of cephalometry, there are limitations. Since the radiograph is taken in an upright position while the patient is awake it does not adequately represent the collapsibility of the upper airway during sleep. The low resolution of the radiograph inhibits clear visualization of soft tissue abnormalities in the upper airway. In addition, the 2-dimensional nature of a radiograph cannot accurately represent the 3-dimensional structure of the upper airway.
COMPUTED TOMOGRAPHY
Unlike Cephalometry, Computed Tomography (CT) allows providers to assess the 3 dimensional nature of the upper airway. CT imaging is a quick, commonly available mode of imaging allowing for 3-D representation of the upper airway. Unlike magnetic resonance imaging and drug induced sleep endoscopy, patients often do not need to undergo sedation. Figures 6 and 7 demonstrate the utility of CT imaging to identify soft tissue and bony abnormalities. A technique within CT, called cone beam CT (CBCT), is ultrafast and utilizes a lower amount of radiation than typical CT. CBCT uses diverging kilo voltage X-rays to visualize anatomical structures and acquire images over a much larger volume in a single scan than conventional CT scans, which use thin axial scans of a patient. 23 CBCT produces an eight- to 10- fold lower effective dose of radiation than a conventional CT. 24 Another modality within CT, Dynamic 3- dimensional CT, is a relatively new technique used for upper airway imaging. It is a cine of the airway with latest generation CT that allows constant low dose volumetric (16 cm) imaging to create a dynamic, 3-D image of the upper airway 25
Figure 6:
Axial (A) right parasagittal (B) CT images of the facial bones in a 13 year old girl demonstrate hypertrophied adenoidal (A) and tonsillar (T) tissue resulting in narrowing of the naso- and oropharyngeal airway
Figure 7:
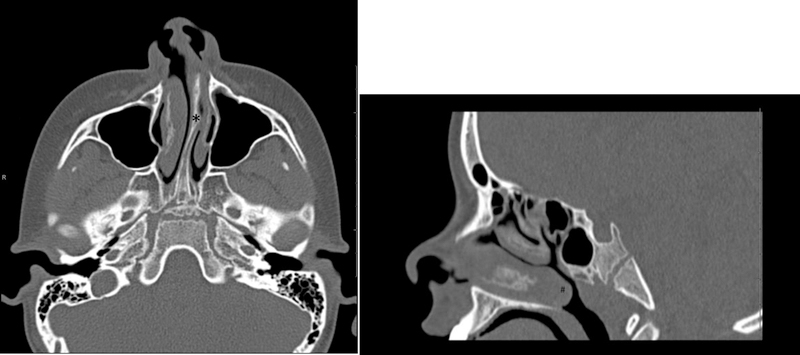
Axial (A) and Sagittal (B) CT images of the facial bones demonstrate leftward deviation of the nasal septum with a bony spur (*) and hypertrophy of the inferior turbinates (#) resulting in occlusion of the left nasal canal and nasopharyngeal airway narrowing in an 8 year old boy with sleep apnea
Recent studies have shown the value of using CT in imaging the upper airway. Van Holsbeke 2013 identified twenty-three children (mean age 6 yo) with OSA and evaluated whether anatomical and functional properties of the airway correlated with OSA severity. In the study, they found a lower mean cross sectional area between the choanae and uvula. They found a significant correlation between OSA severity, based on OAHI, and imaging parameters, specifically with mean cross sectional area of the epiglottis to the first thoracic vertebrae (r =0.45, p = 0.009). In this same cohort there was no significant correlation between the clinical scores of upper airway patency and the OSA severity, based by OAHI, by using the Brodsky and Mallampati scoring technique. 26 Alves 2011 evaluated the pharyngeal air space using cone beam CT in twenty-five nasal and twenty-five mouth breathing children without OSA aged 6–8 years. The study concluded that the pharyngeal airspace in patients who are nose breathers is significantly larger than those children who mouth breathe. 27 Although this study was not performed on patients with OSA it described the utility of cone beam CT in imaging the upper airway. Fleck 2015 utilized dynamic 3D CT to evaluate the upper airway of eight patients with OSA, four of which had previous upper airway MRI and four that had a contraindication to MRI. They compared the radiation dose to forty-one age matched control subjects that previously underwent facial CT. They found that dynamic 3D CT can be effectively performed at a dose (0.38mSv) less than that which is needed for facial CT25
Unfortunately, CT’s are not always the best imaging modality for assessing the upper airway. In addition, in most centers CT imaging uses high levels of radiation, usually around 3 mSv. 28,29. A center experienced in pediatrics may use lower doses of radiation down to 0.0531 mSv, but those centers are not as readily available26,30. Despite its advantages, CBCT is not widely available and is mostly utilized in orthodontics and craniofacial planning31.
MAGNETIC RESONANCE IMAGING
Magnetic Resonance Imaging (MRI) is an important imaging modality to localize the area of upper airway obstruction in patients with OSA. Unlike CT, MRI does not expose children to ionizing radiation.32 It creates a 3-D image of each patient’s upper airway. This 3-D reconstruction allows providers to precisely measure the bony structures and soft tissue of the upper airway, as illustrated in Figure 8. Cine MRI techniques are used in the adult population to detail airway abnormality in patients with OSA. 33DDI It is often used in patients who have residual OSA after adenotonsillectomy to pinpoint areas of upper airway obstruction. 38DDI
Figure 8:
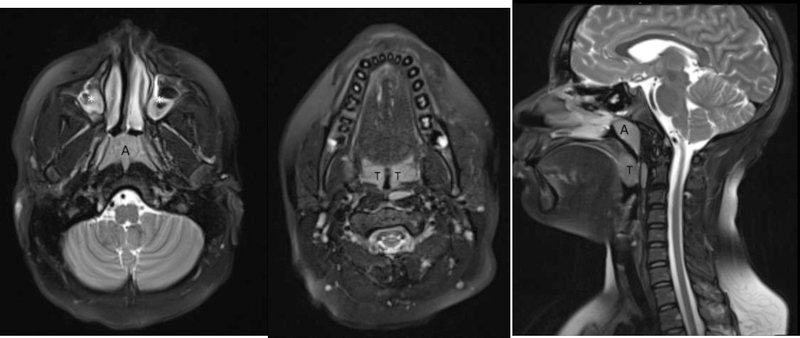
Sagittal (A) and axial (B and C) T2 weighted (TR/TE: 4500/100) MR images of the head and neck in a 12 year old boy with obstructive sleep apnea show hypertrophied adenoids (A) and palatine tonsils (T) resulting in significant narrowing of the nasopharyngeal airway. Concurrently, there is abnormal bright signal in the maxillary sinuses (white asterisks), in keeping with sinusitis.
In children, multiple studies have demonstrated the utility of MRI in patients with upper airway abnormalities and OSA. Donnely 2003 studied sixteen pediatric patients (mean age 8.7 years) with OSA using cine MRI and compared them to an age-matched control population. This group of patients with OSA was significantly more likely to demonstrate intermittent collapse of the nasopharynx and hypopharynx. The mean change in diameter of the nasopharynx and hypopharynx was also significantly greater in the OSA group than in the control group.41 Schwab 2015 studied three groups of adolescents (12–16 yo) using MRI to assess the anatomic risk factors in adolescents with OSA in comparison to those without. The three groups of adolescents included obese adolescents with OSA (n= 49), Obese adolescents without OSA (n= 38) and lean control subjects (n=50.) In this large study, they found that obese adolescents with OSAS had increased adenotonsillar tissue in comparison with obese and lean controls. In addition, obese adolescents with OSAS had a smaller nasopharyngeal airway than the controls and the sizes of the other upper airway soft tissue structures were similar between participants with OSA and those obese controls. 42 Zeng 2018 used MRI and respiratory gating technology to assess OSA severity in children. They were able to examine fifty-one children with OSA and correlated the area of the adenoids to the area of the nasopalatine pharyngeal cavity with main indices of the polysomnography. They found that through use of MRI, the area of the adenoids to the area of the nasopalatine pharyngeal cavity positively correlated with the obstructive apnea hypopnea index (r=0.922, p<0.001) and negatively correlated with the SpO2 nadir (r −0.858, P<0.001.) 43
Recent studies have evaluated the use of MRI in pediatric patients with residual OSA after adenotonsillectomy. Nandalike 2013 used MRI and PSG to compare the upper airway of twenty-seven obese children before and after adenotonsillectomy. Using MRI imaging of the upper airway this study showed that there was significant residual adenoidal tissue and an increase in the volume of the soft palate and tongue after T&A. These results may partially explain why obese patients with OSA have residual OSA after AT. 44 Isaiah 2018 used cinematic magnetic resonance imaging protocol in thirty-six patients aged 3– 18 years, 47% of whom had Down Syndrome, to evaluate persistent OSA after adenotonsillectomy and failed initiation of positive airway pressure therapy. Of the cohort, 92% had an identified site of obstruction on MRI. The most common site of obstruction in the otherwise normal children was due to lingual tonsillar hypertrophy and posterior displacement of the tongue. The base of the tongue in combination with posterior migration of the tongue was found to be the most common cause of obstruction in those children with Down syndrome. They found that through use of cine MRI alone, severity of OSA cannot be predicted.32
Unfortunately, in the pediatric population, there are limitations in using MRI. Unlike CT, MRI’s are noisy, have longer acquisition times, and are more expensive than other imaging modalities. Children under the age of 6, unlike most adults, usually necessitate sedation or anesthesia to tolerate the entirety of the MRI study. Due to the sedation, the upper airway is not representative of that when the patient is sleeping normally and might show collapse, even in patients without obstructive sleep apnea. 45
DRUG INDUCED SLEEP ENDOSCOPY
Drug induced sleep endoscopy (DISE) is a novel technique to evaluate the upper airway during periods of sleep. Unlike the other imaging modalities, DISE allows for direct visualization of the upper airway which allows the physician to directly pinpoint the area of obstruction. 46 It involves endoscopic evaluation of the upper airway that has been shown to identify and grade sites of obstruction in adults. A flexible endoscope is inserted through the nose of the sedated children and is positioned on different levels of the upper airway (nasopharynx, soft palate, tonsils, tongue base, larynx and trachea) to detect mechanisms and sites of snoring and apneas. 47,48. By elucidating the area of obstruction, surgeons are able to intraoperatively individualize their surgical management. 49,50.
Numerous studies demonstrate the utility of using DISE to image the upper airway. Boudewyns 2017 performed DISE in forty-one children less than two years old who were diagnosed with OSA. All but 3 had obstruction at the adenoids and all but 5 had obstruction at the tonsillar level. During the evaluation adenotonsillar hypertrophy was noted to be the major cause of upper airway obstruction. However, 50% of the children had multilevel obstruction. Despite the multilevel obstruction, this study found that any obstruction outside that of the adenoids and tonsils did not have a major impact of treatment outcome.49 Clark 2016 conducted a retrospective chart review that compared cine MRI with findings on DISE to evaluate multiple sites of obstruction in children with persistent OSA. Fifteen patients who had previous MRI and DISE were evaluated. In ten of the patients the same sites of upper airway obstruction were noted. In three patients DISE showed an additional site not recognized using MRI. In one patient MRI identified a site of obstruction not seen in DISE and in one patient the sites of obstruction were different. Overall, the study concluded that more information was needed to assess the sensitivity and specificity of DISE and cine MRI. 50 Alsufyani (2017) conducted a retrospective review of three hundred eighty-two patients to determine the predictors of failure in patients with sleep disordered breathing that underwent DISE directed adenoidectomy and/or tonsillectomy. They found that obesity, asthma, and age older than 7 years correlated with treatment failure. Findings on DISE that independently predicted failure include deviated nasal septum, chronic rhinitis and small tonsil size. 51 Gazzaz 2017 conducted a retrospective study on five hundred and fifty-eight patients to examine if DISE changes surgical management in patients with OSA. These patients, aged 3–17 years, underwent DISE directed surgery for snoring or sleep disordered breathing. Of these patients, 35% had changes made to their surgical plan secondary to findings on DISE. The study concluded that by using DISE patient’s surgical treatment plan may be individualized and may prevent unsuccessful surgeries. 52
Despite all of the advantages, DISE does have its drawbacks. Like MRI, it requires the patient to undergo sedation. Since the patient is pharmacologically sedated, the upper airway dynamics are not exactly representative as those during natural sleep. DISE requires a skilled pediatric provider to undergo this technique of imaging, which limits the number of hospitals in which the patient can undergo evaluation. Even with a skilled physician, airway instrumentation affects resistance and pressures in the airway and may alter the site of obstruction.53
CONCLUSION
Numerous studies demonstrate the correlation between upper airway imaging and obstructive sleep apnea. Most of these studies have focused on identifying the area and severity of the possible obstruction. There is lack of evidence that these imaging modalities may be a replacement for polysomnogram in diagnosing OSA. While cephalometry utilizes known measurements and ratios to determine areas of narrowing and possible obstruction, there are no published studies that identify the sensitivity or specificity of these ratios. Both CT and MRI offer clarity into the three dimensional structure of the upper airway. More focus has been placed on the role of MRI, rather than CT, because of the amount of radiation required for CT imaging. MRI, specifically, offers detailed analysis of the anatomy due to the ability to image the soft tissue and pharyngeal size. In young infants, no published data exists for normative values of polysomnography. However, in MRI, normative values of the upper airway are established which is especially helpful in those infant patients with craniofacial abnormalities. 54 Neither MRI nor CT has been studied as a possible replacement for PSG. DISE, the most invasive intervention, is able to directly visualize the location of obstruction.
Each of these studies is useful in the localization of the area of obstruction in patients with OSA. For children with residual OSA after adenotonsillectomy and craniofacial abnormalities requiring intervention (mandibular distraction, mid face distraction) these techniques are helpful in pinpointing the area causing the obstruction and directing further surgical intervention. Further studies are needed to identify the role of upper airway imaging in diagnosing OSA, as minimal data is currently publishing regarding the sensitivity and specificity of each test.
TABLE 1:
PROS AND CONS OF EACH IMAGING MODALITY
| PRO | CON | |
|---|---|---|
| Cephalometry | Inexpensive Readily available Fast Minimal Radiation No sedation |
Upright positioning is not representative of sleep Poor soft tissue visualization 2-dimensional imaging |
| Computerized Tomography | Readily available Fast 3D imaging No sedation 3-dimensional imaging |
High radiation Pediatric radiation dosing not universally available CBCT less available |
| Magnetic Resonance Imaging | No radiation Excellent soft tissue contrast resolution 3- dimensional imaging |
Expensive Longer studies Noisy Sedation needed |
| Drug Induced Sleep Endoscopy | No radiation Direct visualization May guide surgical management |
Expensive Sedation/General Anesthesia Invasive Requires highly skilled pediatric physician Instrumentation affects resistance and pressures in the airway and may alter the site of obstruction |
Educational Aims.
The reader will come to:
Understand common causes and pathophysiology of Pediatric Obstructive Sleep Apnea (OSA)
Recognize the multiple types of imaging and visualization modalities available to identify Pediatric OSA
Identify strengths and weaknesses of each of these modalities
Future Research:
- Cohort studies aimed at distinguishing the patient population who may benefit the most from each imaging modality are needed:
- Role of imaging in the identification of patients who may have residual OSA following surgical intervention
- Role of imaging in the diagnosis of OSA in special populations such as children with Down Syndrome
Footnotes
The authors do not have conflicts of interest.
All images are original obtained from patients of the Children’s Hospital of Philadelphia who provided consent to their utilization.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Pulmonology S on P, Syndrome S on OSA. Clinical Practice Guideline: Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome. Pediatrics 2002;109(4):704n P, S. [DOI] [PubMed] [Google Scholar]
- [2].Bodenner KA, Jambhekar SK, Com G, Ward WL. Assessment and treatment of obstructive sleep-disordered breathing. Clin Pediatr (Phila) 2014;53(6):544. [DOI] [PubMed] [Google Scholar]
- [3].Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med 2001;164(1):16t. [DOI] [PubMed] [Google Scholar]
- [4].DelRosso LM. Epidemiology and diagnosis of pediatric obstructive sleep apnea. Curr Probl Pediatr Adolesc Health Care 2016;46(1):2. [DOI] [PubMed] [Google Scholar]
- [5].Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatr Pulmonol 2009;44(5):417l. [DOI] [PubMed] [Google Scholar]
- [6].Cielo CM, Silvestre J, Paliga JT, et al. Utility of screening for obstructive sleep apnea syndrome in children with craniofacial disorders. Plast Reconstr Surg 2014;134(3):434e–41e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Silvestre J, Tahiri Y, Paliga JT, Taylor JA. Screening for obstructive sleep apnea in children with syndromic cleft lip and/or palate. J Plast Reconstr Aesthet Surg 2014;67(11):1475 Aesthet. [DOI] [PubMed] [Google Scholar]
- [8].Khayat A, Bin-Hassan S, Al-Saleh S. Polysomnographic findings in infants with Pierre Robin sequence. Ann Thorac Med 2017;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cohen M, Hamilton J, Narang I. Clinically important age-related differences in sleep related disordered breathing in infants and children with Prader-Willi syndrome. PLoS One[Internet] 2014;9(6) [cited 2018 Jul 17]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4076199/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Williams K, Scheimann A, Sutton V, Hayslett E, Glaze DG. Sleepiness and sleep disordered breathing in Prader-Willi syndrome: relationship to genotype, growth hormone therapy, and body composition. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med 2008;4(2):111. [PMC free article] [PubMed] [Google Scholar]
- [11].Breslin J, SpanSpanM Off Publ Am Acad Sleep Med, Glaze DG. Sleepiness and sleep disordered breathing in prader-willi syndrome. Dev Med Child Neurol 2014;56(7):657.24471822 [Google Scholar]
- [12].Lee C-F, Lee C-H, Hsueh W-Y, Lin M-T, Kang K-T. Prevalence of obstructive sleep apnea in children with down syndrome: a meta-analysis. J Clin Sleep Med 2018;14(5):867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ortiz LE, McGrath-Morrow SA, Sterni LM, Collaco JM. Sleep disordered breathing in bronchopulmonary dysplasia. Pediatr Pulmonol 2017;52 (12):1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Slaats MA, Van Hoorenbeeck K, Van Eyck A, et al. Upper airway imaging in pediatric obstructive sleep apnea syndrome. Sleep Med Rev 2015;21:59–71. [DOI] [PubMed] [Google Scholar]
- [15].Vilar-Palop J, Vilar J, HernHernn Eyck A, et al. Upper airway imaging in pediatric obstructive sleep apnea syndrome. J Radiol Prot Off J Soc Radiol Prot 2016;36(4):975. [Google Scholar]
- [16].Agren K, Nordlander B, Linder-Aronsson S, Zettergren-Wijk L, Svanborg E. Children with nocturnal upper airway obstruction: postoperative orthodontic and respiratory improvement. Acta Otolaryngol (Stockh) 1998;118(4):581. [DOI] [PubMed] [Google Scholar]
- [17].L. strand-Tidestr81 (Stockh)inder-Aronsson S, Zettergren-Wijk L, Svanborg E Children wiing obstruction in relation to craniofacial and dental arch morphology in 4-year-old children. Eur J Orthod 1999;21(4):3231. [DOI] [PubMed] [Google Scholar]
- [18].Zucconi M, Caprioglio A, Calori G, et al. Craniofacial modifications in children with habitual snoring and obstructive sleep apnoea: a case-control study. Eur Respir J. 1999;13(2):411. [DOI] [PubMed] [Google Scholar]
- [19].Finkelstein Y, Wexler D, Berger G, Nachmany A, Shapiro-Feinberg M, Ophir D. Anatomical basis of sleep-related breathing abnormalities in children with nasal obstruction. Arch Otolaryngol Neck Surg 2000;126(5):593. [DOI] [PubMed] [Google Scholar]
- [20].Kawashima S, PeltomltomrgBerger G, Nachmany A, Shapiro-Feinberg M, Ophir D. Anatomical basis of sleep-related breathing abnormalities in children with nasal obstruction. BoActa Paediatr 2002;91(1):71. [DOI] [PubMed] [Google Scholar]
- [21].Maeda K, Tsuiki S, Nakata S, Suzuki K, Itoh E, Inoue Y. Craniofacial contribution to residual obstructive sleep apnea after adenotonsillectomy in children: a preliminary study. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med 2014;10 (9):973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pirilä-Parkkinen K, Löppönen H, Nieminen P, Tolonen U, Pirttiniemi P. Cephalometric evaluation of children with nocturnal sleep-disordered breathing. Eur J Orthod 2010;32(6):662. [DOI] [PubMed] [Google Scholar]
- [23].Lechuga L, Weidlich GA. Cone Beam CT vs. Fan Beam CT: A comparison of image quality and dose delivered between two differing CT imaging modalities. Cureus [Internet] [cited 2018 Aug 30];8(9). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5063198/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tsiklakis K, Donta C, Gavala S, Karayianni K, Kamenopoulou V, Hourdakis CJ. Dose reduction in maxillofacial imaging using low dose Cone Beam CT. Eur J Radiol 2005;56(3):413onta. [DOI] [PubMed] [Google Scholar]
- [25].Fleck RJ, Ishman SL, Shott SR, et al. Dynamic volume computed tomography imaging of the upper airway in obstructive sleep apnea. J Clin Sleep Med 2017;13(02):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Van Holsbeke C, Vos W, Van Hoorenbeeck K, et al. Functional respiratory imaging as a tool to assess upper airway patency in children with obstructive sleep apnea. Sleep Med 2013;14(5):433. [DOI] [PubMed] [Google Scholar]
- [27].Alves M, Baratieri C, Nojima LI, Nojima MCG, Ruellas ACO. Three-dimensional assessment of pharyngeal airway in nasal- and mouth-breathing children. Int J Pediatr Otorhinolaryngol 2011;75(9):1195. [DOI] [PubMed] [Google Scholar]
- [28].Mettler FA, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254. [DOI] [PubMed] [Google Scholar]
- [29].ICRP: ICRP Publication 87 [Internet]. [cited 2018 Sep 25];Available from: http://www.icrp.org/publication.asp?id=ICRP%20Publication%2087.
- [30].Mulkens TH, Broers C, Fieuws S, Termote J-L, Bellnick P. Comparison of effective doses for low-dose MDCT and radiographic examination of sinuses in children. Am J Roentgenol 2005;184(5):1611. [DOI] [PubMed] [Google Scholar]
- [31].Hatcher DC, Dial C, Mayorga C. Cone beam CT for pre-surgical assessment of implant sites. J Calif Dent Assoc 2003;31(11):825. [PubMed] [Google Scholar]
- [32].Isaiah A, Kiss E, Olomu P, Koral K, Mitchell RB. Characterization of upper airway obstruction using cine MRI in children with residual obstructive sleep apnea after adenotonsillectomy. Sleep Med 2018;50:79. [DOI] [PubMed] [Google Scholar]
- [33].Schoenberg SO, Floemer F, Kroeger H, Hoffmann A, Bock M, Knopp MV. Combined assessment of obstructive sleep apnea syndrome with dynamic MRI and parallel EEG registration: initial results. Invest Radiol 2000;35(4):267–267. [DOI] [PubMed] [Google Scholar]
- [34].Suto Y, Matsuda E, Inoue Y, Suzuki T, Ohta Y. Sleep apnea syndrome: comparison of MR imaging of the oropharynx with physiologic indexes. Radiology 1996;201(2). 393. [DOI] [PubMed] [Google Scholar]
- [35].Shellock FG, Schatz CJ, Julien P, et al. Occlusion and narrowing of the pharyngeal airway in obstructive sleep apnea: evaluation by ultrafast spoiled GRASS MR imaging. Am J Roentgenol 1992;158(5):1019. [DOI] [PubMed] [Google Scholar]
- [36].er L J, G58(5):1019atz CJ, Julien P, et al. Occlusion and narrowing o in patients with obstructive sleep apnea. Am J Neuroradiol 1998;19(7):12059. [Google Scholar]
- [37].Suto Y, Matsuo T, Kato T, et al. Evaluation of the pharyngeal airway in patients with sleep apnea: value of ultrafast MR imaging. Am J Roentgenol 1993;160 (2):311. [DOI] [PubMed] [Google Scholar]
- [38].Donnelly LF, Shott SR, LaRose CR, Chini BA, Amin RS. Causes of persistent obstructive sleep apnea despite previous tonsillectomy and adenoidectomy in children with down syndrome as depicted on static and dynamic cine MRI. Am J Roentgenol 2004;183(1):175–81. [DOI] [PubMed] [Google Scholar]
- [39].Shott SR, Donnelly LF. Cine magnetic resonance imaging: evaluation of persistent airway obstruction after tonsil and adenoidectomy in children with down syndrome. Laryngoscope 2004;114(10):1724. [DOI] [PubMed] [Google Scholar]
- [40].Schaaf WE, Wootten CT, Donnelly LF, Ying J. Shott SR. Findings on MR sleep studies as biomarkers to predict outcome of genioglossus advancement in the treatment of obstructive sleep apnea in children and young adults. Am J Roentgenol 2010;194(5):1204. [DOI] [PubMed] [Google Scholar]
- [41].Donnelly LF, Surdulescu V, Chini BA, Casper KA, Poe SA, Amin RS. Upper airway motion depicted at cine mr imaging performed during sleep: comparison between young patients with and those without obstructive sleep apnea. Radiology 2003;227(1):239. [DOI] [PubMed] [Google Scholar]
- [42].Schwab RJ, Kim C, Bagchi S, et al. Understanding the anatomic basis for obstructive sleep apnea syndrome in adolescents. Am J Respir Crit Care Med Med 2015;191(11):1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zeng G, Teng Y, Zhu J, et al. Clinical application of MRI-respiratory gating technology in the evaluation of children with obstructive sleep apnea hypopnea syndrome. Medicine (Baltimore) 2018;97(4):e9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nandalike K, Shifteh K, Sin S, et al. Adenotonsillectomy in obese children with obstructive sleep apnea syndrome: magnetic resonance imaging findings and considerations. Sleep 2013;36(6):841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bosemani T, Hemani M, Cruz A, et al. Assessment of upper airway patency in spontaneously breathing non-intubated neonates and infants undergoing conventional MRI of head and neck. Childs Nerv Syst 2015;31(9):1521–5. [DOI] [PubMed] [Google Scholar]
- [46].Croft CB, Pringle M. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci 1991;16(5):504l. [DOI] [PubMed] [Google Scholar]
- [47].Truong MT, Woo VG, Koltai PJ. Sleep endoscopy as a diagnostic tool in pediatric obstructive sleep apnea. Int J Pediatr Otorhinolaryngol 2012;76(5):722. [DOI] [PubMed] [Google Scholar]
- [48].Durr ML, Meyer AK, Kezirian EJ, Rosbe KW. Drug-induced sleep endoscopy in persistent pediatric sleep-disordered breathing after adenotonsillectomy. Arch Otolaryngol Neck Surg 2012;138(7):638. [DOI] [PubMed] [Google Scholar]
- [49].Boudewyns A, de Heyning PV, Verhulst S. Drug-induced sedation endoscopy in children <2 years with obstructive sleep apnea syndrome: upper airway findings and treatment outcomes. Eur Arch Otorhinolaryngol 2017;274 (5):2319. [DOI] [PubMed] [Google Scholar]
- [50].Clark Christopher, Ulualp Seckin O. Multimodality assessment of upper airway obstruction in children with persistent obstructive sleep apnea after adenotonsillectomy. Laryngoscope 2016;127(5):1224. [DOI] [PubMed] [Google Scholar]
- [51].Alsufyani N, Isaac A, Witmans M, Major P, El-Hakim H. Predictors of failure of DISE-directed adenotonsillectomy in children with sleep disordered breathing. J Otolaryngol - Head Neck Surg [Internet] 2017. [cited 2018 Jul 24];46 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5420116/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gazzaz MJ, Isaac A, Anderson S, Alsufyani N, Alrajhi Y, El-Hakim H. Does drug-induced sleep endoscopy change the surgical decision in surgically nay nahing. llectomy. my. outcomes. es. without Obstructive Sleep Apnea. I. standard adenotonsillectomy? A retrospective cohort study. J Otolaryngol - Head Neck Surg [Internet] 2017. [cited 2018 Jul 24];46 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5307859/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Qubty WF, Mrelashvili A, Kotagal S, Lloyd RM. Comorbidities in Infants with Obstructive Sleep Apnea. J Clin Sleep Med 2014;10(11):1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Smitthimedhin A, Whitehead MT, Bigdeli M, Nino G, Perez G, Otero HJ. MRI determination of volumes for the upper airway and pharyngeal lymphoid tissue in preterm and term infants. Clin Imaging A 2018;50:51. [DOI] [PubMed] [Google Scholar]