Abstract
As pediatric patients with sickle cell anemia (SCA) have impaired growth and puberty patterns, we studied the effect of disease modifying therapies on growth and puberty patterns for patients with SCA receiving hydroxyurea (HU), transfusions, or no therapy. We performed a retrospective study of children with SCA in whom anthropometric measurements and therapy type were recorded. Penalized smoothing splines were fitted to estimate growth curves and growth velocity, and linear mixed models were used to examine differences across treatment groups. Across group analyses were divided into early childhood (4.0–7.9 years) and peri-pubertal (8.0–12.0 years). We analyzed growth data on 157 SCA patients. From 8.0–12.0 years, girls on transfusion therapy were significantly taller than girls on HU (range 5.7–7.2 cm; p-value range 0.002–0.01). From 10.0–12.0 years, boys on transfusion therapy were significantly taller than boys on HU (range 4.1–9.4 cm; p-value range <0.0001–0.04). In addition, boys on transfusion therapy had an earlier peak height velocity as compared to boys on either HU or no therapy. In conclusion, children receiving transfusions tended to be taller than children on HU or no therapy. Children on HU did not demonstrate superior growth pattern when compared to children on no therapy in the peri-pubertal years.
Keywords: Sickle cell disease, Growth pattern, Transfusion therapy, Hydroxyurea, Height velocity
Introduction:
Patients with sickle cell anemia (SCA: HbSS and HbSβ0 thalassemia) suffer from significant clinical complications and end-organ dysfunction in part due chronic hemolysis and vaso-occlusion. Growth in children with SCA may also be impaired; however, few large studies provide evidence for this growth impairment.1–5 One three-year retrospective study evaluating height and weight suggested that children with SCA have reduced height and weight as compared to National Health Statistics norms.1 In addition to comparing growth outcomes to non-sickle cell control patients, the Cooperative Study of Sickle Cell Disease (CSSCD) suggested that children with SCA had impaired growth outcomes as compared to patients with milder forms of sickle cell disease (HbSC and HbSβ+ thalassemia).2 As patients with SCA may not follow traditional pediatric growth curves, sickle cell specific growth curves have been developed in two cohorts.5,6 Finally, a few studies have suggested that sickle cell patients experience delayed puberty as compared to control patients, and patients with HbSS have a significant delay in menarche as compared to patients with HbSC.2–5,7
To reduce complications from SCA or as standard of care, patients may receive disease modifying therapies including hydroxyurea (HU) or chronic transfusions therapy, while other patients (or their parents) may decline any sickle cell disease modifying therapies. These SCA interventions have proven clinical benefits in pediatrics; however, the longitudinal data on improvements in growth outcomes for SCA patients on disease modifying therapies have rarely been reported.8–11 In a two-year randomized trial of blood transfusion or placebo for children with SCA and abnormal cerebral blood flow, 53 children randomized to transfusion therapy displayed significantly improved height velocity, weight gain, and body mass index as compared to 41 control children.12 A retrospective cohort study on 36 patients receiving chronic erythrocytapheresis suggested that males achieved an earlier peak height velocity than matched controls from CSSCD study, but females did not experience a difference in age of peak height velocity.13 Finally, the HUG-KIDS study of 68 children and adolescents with SCA showed no adverse effect of HU for at least two years on height, weight gain or pubertal development as compared to CSSCD controls.14
The large longitudinal cohort CSSCD could not account for the impact of current sickle cell disease modifying therapies on growth outcomes and the more recent studies that evaluated transfusion therapy or HU are limited by a smaller sample size and were not longitudinal cohort studies. Therefore, it is difficult for clinicians to counsel patients about the effect of sickle cell disease modifying therapy on growth patterns. To address this limitation in the literature, we performed a large, 18-year retrospective longitudinal cohort study of growth patterns in pediatric patients with SCA. We hypothesized that pediatric patients with SCA receiving either transfusion or HU would have improved growth and pubertal patterns as compared to children that were not receiving any therapy.
Materials and Methods:
Subjects:
We performed an institutional review board at University of Alabama at Birmingham approved retrospective cohort study of children (0–18 year-olds) with SCA (HbSS and HbSβ0 thalassemia) who were cared at the University of Alabama at Birmingham Pediatrics Sickle Cell Clinics from 1999–2017. We confirmed the diagnosis of HbSS and HbSβ0 thalassemia by hemoglobin (Hb) electrophoresis records in all participants. Study data were collected and managed using REDCap electronic data capture tools hosted at University of Alabama at Birmingham.15
Objectives and definition of variables
The primary objective was to study overall height in children with SCA based on the different sickle cell disease modifying therapy arms (chronic transfusion, hydroxyurea, and no sickle cell disease modifying therapy). The secondary objectives were to study height velocity by age and the correlation of Hb to height and height velocity. To study the growth patterns in early childhood and peri-pubertal periods, we abstracted height, weight, Hb, and type of sickle cell disease modifying therapy (including no disease modifying therapy) for each subject from each annual clinic visit from birth to 18 years of age, the traditional age of transition to an adult care provider. The goal was to obtain anthropometric measurements every 12 months. If these measurements were not available at exactly 12 months from the prior visit, we recorded the date and months from the nearest recorded clinic visit. To ensure appropriate longitudinal data for our analysis, we excluded patients if they had fewer than 7 observations overall, if they are currently less than 10.0 years of age, or if the minimum age for their first measurement was over 7.0 years. We obtained the Hb concentration from the complete blood count; patients on transfusion therapy had their pre-transfusion Hb recorded, as post-transfusion Hb levels are only obtained prior to surgery or with red cell exchange. As standard of care, clinicians do not always document staging of children’s sexual maturation staging or obtain pubertal hormone concentrations; therefore, these data were excluded from our data analysis. Recorded height measurements were abstracted to estimate height velocity and to calculate peak height velocity (PHV). Given that in healthy girls, PHV occurs at around 11.5 years (Sexual Maturation Rating 2–3) and in healthy boys, PHV occurs near 13.5 years (Sexual Maturation Rating 4)16, we used the age at which peak height velocity occurred as a surrogate for pubertal development.
For each clinic visit, we abstracted the sickle cell disease modifying therapy the patient received (red blood cell transfusion therapy, HU, or no therapy). We did not differentiate transfusion therapy between patients receiving simple chronic transfusion therapy or erythrocytapheresis. We categorized participants as receiving hydroxyurea based on prescription refill as compared to analyzing hydroxyurea response based on dose or fetal hemoglobin level. Growth curve rely on longitudinal analysis, yet our patients changed therapy over an eight year time period; thus, we analyzed growth outcomes for two age ranges to account for therapy received during the early childhood and peri-pubertal ages. We evaluated growth for patients based on the majority therapy received from 4.0–7.9 years (early childhood) and then re-evaluated patients based on the majority therapy received from 8.0–12.0 years (peri-pubertal). The majority therapy received in each cohort was defined as the therapy a patient received for >50% of the clinic visits during years 4.0–7.9 and 8.0–12.0.
Statistical analysis:
Demographic data were analyzed using descriptive statistics. Since growth curves are known not to conform to typical functional forms, e.g. quadratic or cubic, penalized smoothing splines (P-splines) were used to model individual growth curves using the fda package in R (https://CRAN.R-project.org/package=fda); the smoothing parameter was chosen by generalized cross validation.17,18 We extracted height estimates from the p-spline fits; this can be seen as an imputation approach to allow comparison at particular ages of interest; only estimates within a subject’s observed age were included, i.e. if the maximum age observed was 14.3 years then we would not include an estimate at age 15. These were then included in linear mixed version 9.4 (Cary, NC); in all models the random effects consisted of only the intercept (Figure 1, 2) while age, treatment and age by treatment interaction were assigned fixed effects. Polynomial of at most order 3 (i.e., linear-cubic) were considered in modeling the mean height over time. Derivative estimates were obtained from the penalized spline fits for height to estimate peak height velocity during peri-pubertal years. These estimates were used in a linear mixed model as described above to model height velocity during peri-pubertal years and compare differences among treatment groups. Hypothesis tests were conducted globally to determine whether there were treatment differences represented in the model by the treatment by age interaction term. When the global test was significant at the 0.05 level, estimates were extracted by year and compared with t-tests to get a coarse idea of where the differences occurred. Models were stratified by age group (4.0–7.9 and 8.0–12.0) for height analysis. For height velocity and Hb correlation, data were analyzed for children in the peri-pubertal cohort till 14.0 years of age. Hypothesis tests for differences at each year were capped at 14.0 years due to low sample sizes at later years. No adjustments for multiple testing was done as this is an exploratory study. Graphics were produced with the aid of the ggplot2 package in R.19
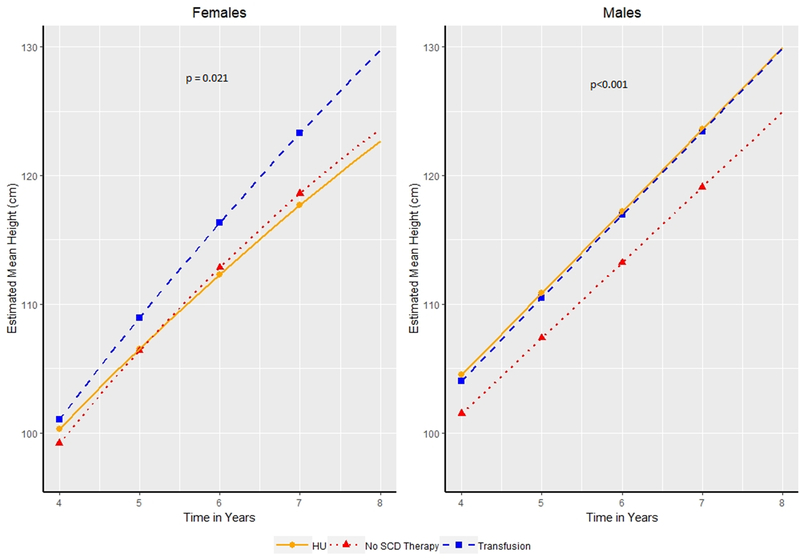
Figure 1:
Estimated Mean Height in centimeters by age of patients (4.0–7.9 years of age) for males and females. Patients categorized as receiving transfusion therapy, hydroxyurea (HU) or no Sickle Cell Disease (SCD) modifying therapy based on the therapy a patient received for the majority of patients. Symbols indicate years at which hypothesis tests for differences in mean were conducted.
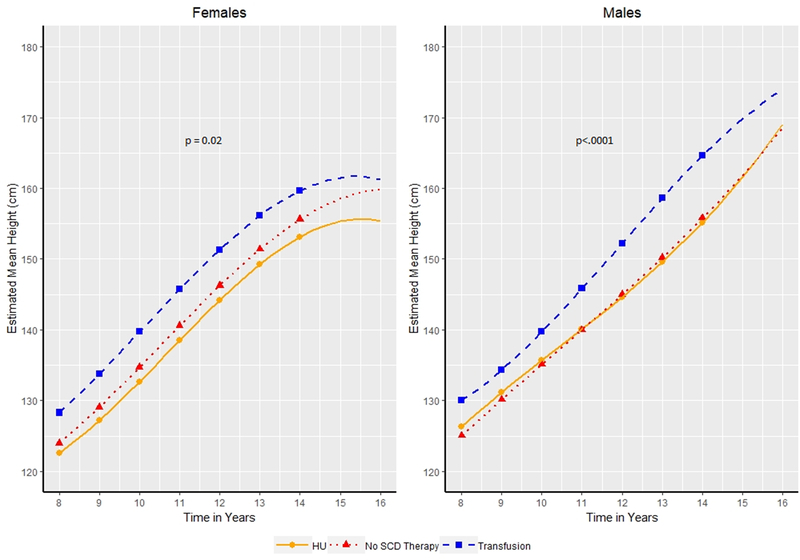
Figure 2:
Estimated Mean Height in centimeters by age of patients (8.0–14.0 years of age) for males and females. Patients categorized as receiving transfusion therapy, hydroxyurea (HU) or no Sickle Cell Disease (SCD) modifying therapy based on the therapy a patient received for the majority of patients. Symbols indicate years at which hypothesis tests for differences in mean were conducted.
Results:
Study population:
We identified 453 subjects with HbSS and HbSβ0 and extracted data from 3374 annual visits. The mean number of annual visits per patient was 7.4 visits. After eliminating subjects based on the exclusion criteria, we identified 157 evaluable subjects (Table 1). Seventy-six (48%) patients were female and 81 (52%) were male. For our analysis of effect of therapy in early childhood (4.0–7.9 years), 17 patients were categorized as receiving transfusion therapy, 35 receiving HU, and 105 receiving no therapy. For our analysis of therapy in the peri-pubertal period (8.0—12.0 years), 28 patients were categorized as receiving transfusion therapy, 62 receiving HU, and 67 receiving no therapy.
TABLE 1.
Treatment by Gender and Age Category
| Treatment Category | Gender Frequency (Column %) | ||
|---|---|---|---|
| Female | Male | Total | |
| Ages: 4.0–7.9 years | |||
| HU | 16 (21.0%) | 19 (23.5%) | 35 (22.2%) |
| No therapy | 52 (68.4%) | 53 (65.4%) | 105 (66.9%) |
| Transfusion | 8 (10.5%) | 9 (11.1%) | 17 (10.8%) |
| Total | 76 | 81 | 157 |
| Ages 8.0–12.0 | |||
| HU | 28 (36.8%) | 34 (42.0%) | 62 (39.5%) |
| No therapy | 35 (46.0%) | 32 (39.5%) | 67 (42.7%) |
| Transfusion | 13 (17.1%) | 15 (18.5%) | 28 (17.8%) |
| Total | 76 | 81 | 157 |
HU: Hydroxyurea
Height:
We analyzed heights at each specific age (4.0, 5.0, 6.0, 7.0 years for 4.0–7.9 year-olds and at 8.0, 9.0, 10.0, 11.0, and 12.0 years for 8.0–12.0 year-olds) to evaluate if and when a particular sickle cell disease modifying therapy resulted in a difference in linear growth.
Height during early childhood (4.0–7.9 years):
The overall model of growth curves for girls and boys from 4.0–7.9 years based on therapy was significant (p = 0.021 for girls, p<0.001 for boys). In girls, the growth curve model did not identify a statistically significant difference in height at 4.0–6.0 years based on sickle cell therapy (transfusion, hydroxyurea, or no therapy) but by 7 years, girls receiving transfusion therapy became significantly taller than girls on either no therapy (123.29 vs 118.6 cm, 4.68 cm taller, p = 0.0155) or HU (123.29 vs 117.7 cm, 5.6 cm taller, p = 0.0113). At 7.0 years, we still did not identify a significant difference in height for girls receiving HU as compared to no therapy (117.7 vs 118.6 cm, p=0.53).
At 4.0 years of age (Figure 1), boys receiving transfusion therapy were not significantly taller than boys on HU (104 vs 104.4 cm, p=0.80) or no therapy (104 vs 101.4 cm, p=0.11). Starting at 6.0 years, boys receiving transfusion therapy became significantly taller than boys on no therapy (116.96 vs 113.23 cm, 3.73 cm at 6.0 years and 123.43 vs 119.1 cm, 4.32 cm taller at 7.0 years, p=0.02 and p=0.007, respectively). We identified no difference in height from 4.0–7.9 years for boys on transfusion as compared to hydroxyurea. Four year old boys receiving HU were significantly taller than boys receiving no therapy (104.5 vs 101.5 cm, p=0.01) and remained taller at age 7.0 (123.58 vs 119.1 cm, 4.47 cm taller, p=0.0002).
Height during peri-pubertal ages (8.0–12.0 years):
In modelling the growth curves during the peri-pubertal years, we noted a significant impact of SCD modifying therapy on height among girls (p=0.02) and boys (p<.0001). At 8.0 years of age (Figure 2), girls on transfusion therapy were already significantly taller than girls on HU (128.29 vs 122.58 cm, 5.7 cm, p=0.0143) and remained significantly taller every year from ages 9.0–12.0 (range 6.53–7.21 cm; p-value range 0.0018–0.0047). Girls on transfusion at 8.0 years of age were 4.2 cm taller compared to no therapy, but this did not meet the specified statistical significance threshold (128.29 vs 124 cm, p=0.05); however, girls on transfusion were significantly taller than girls on therapy every year from ages 9.0–12.0 (range 4.69–5.13 cm; p-value range 0.022–0.036). We identified no difference in height between girls on HU as compared to no therapy at either 8.0 years (122.58 vs 124 cm, p=0.42) or between 9.0 and 12.0 years of age (smallest p-value=0.24).
At 8.0 years of age (Figure 2), boys on transfusion therapy were not significantly taller than boys on HU (130.01 vs 126.35 cm, 3.66 cm, p=0.073), but became significantly taller every year between 10.0 and 12.0 years of age (range 4.06–9.43 cm; p-value range <0.0001–0.0456). At 8.0 years, boys on transfusion therapy as compared to no therapy were significantly taller (130.01 vs 125.04 cm, 4.97 cm, p=0.016) and remained significantly taller from every year from 9.0 to 12.0 years of age (range 4.19–8.80 cm; p-value range <0.0001–0.0410). We identified no significant difference in height among boys on HU or no therapy at 8.0 (126.35 vs 125.04 cm, p=0.42) or between 9.0 and 12.0 years of age (smallest p-value=0.53).
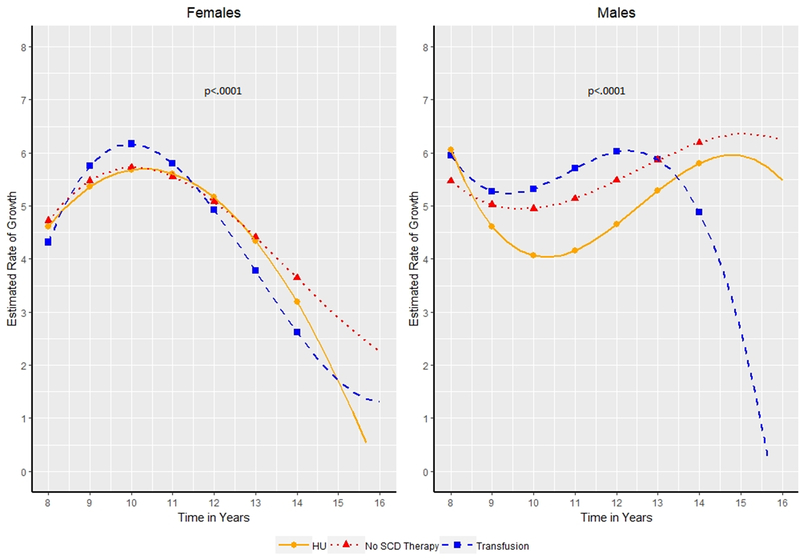
Height velocity (ages 8.0–14.0):
Recorded heights were abstracted to calculate PHV as a surrogate of puberty. We identified a significant impact of treatment on the peak height velocity during the peri-pubertal years (p<.0001 for girls and boys). Girls on transfusion, HU, and no therapy all experienced their highest peak height velocity at 10.0 years of age (Figure 3). Girls on transfusion had a significantly higher peak velocity than girls on no therapy (6.2 vs 5. 7 cm/year p = 0.02) and non-significantly higher than girls on HU (6.2 vs 5.7 cm/year, p=0.06). No difference was noted in the peak height velocity between girls on HU as compared to no therapy.
Figure 3:
Estimated Height velocity in centimeters/year by age of patients (8.0–14.0 years of age) for males and females. Patients categorized as receiving transfusion therapy, hydroxyurea (HU) or no Sickle Cell Disease (SCD) modifying therapy based on the therapy a patient received for the majority of patients. Symbols indicate years at which hypothesis tests for differences in mean were conducted.
Boys on transfusion therapy and HU experienced a peak height velocity at 12 years of age and boys on no therapy experienced peak height velocity at 14 years of age (Figure 3). Boys on transfusion had a significantly higher peak velocity than boys on HU (6.0 vs 4.64 cm/year, p<0.0001).
Correlation of hemoglobin to height and height velocity among children on no sickle cell disease modifying therapy:
As hemoglobin concentration could be marker of overall health status, we wanted to analyze the hemoglobin concentration and growth patterns of children not receiving sickle cell modifying therapy. In the 4.0–7.9 years of age cohort, we identified no impact of hemoglobin concentration and height or height velocity, with the exception of hemoglobin and height for females at age 6.0 (Pearson Correlation (95% CL) 0.35 [0.04, 0.60] and 7.0 (0.43 [0.13, 0.66]). Among the 8.0–12.0 year girl cohort, we identified a significant correlation between height and hemoglobin at each of ages 8.0–13.0: 9.0 years (0.43 [0.13, 0.65]), 10.0 years (0.52 [0.25, 0.72]), 11.0 years (0.52 [0.22, 0.74]), and 12.0 years (0.45 [0.10, 0.70]) and 13.0 years (Correlation 0.45[0.07–0.72]). Among the 8.0–12.0 year boy cohort, we identified a significant correlation between height velocity and hemoglobin level at 12.0 years (Correlation 0.43 [0.09, 0.67]) and 13.0 years (Correlation 0.56 [0.22, 0.77]) and significant correlation between height and hemoglobin concentration at 13.0 years (Correlation 0.41[0.25–0.69]).
Discussion:
SCA has been reported to be associated with impairment of growth and development in children, but sickle cell modifying therapies may alter these patterns. Our study is first to show that children receiving chronic transfusion therapy are taller during early childhood and during the peri-pubertal period. We did not identify a significant impact of HU as compared to no therapy on growth in the peri-pubertal period which mirrors the HUG-KIDS study14 and the Baby HUG randomized trial20; however, we did observe a statistical difference in growth patterns in boys between 4.0 – 7.9 years of age between the HU group and no therapy.21 While we do not have adult data, we postulate that patients on chronic transfusion therapy attained taller adult heights as the difference in height SD was maintained prior to puberty and through the peri-pubertal years.
Children with SCA face a number of complications due to the associated anemia. In our study, we show that boys with SCA on transfusion therapy attained an earlier peak height velocity than boys on HU and no therapy. Even among patients on no therapy, our data shows that a higher hemoglobin level is associated with better growth. In children with chronic anemia, chronic hypoxemia may lead to decreased production and action of insulin like growth factor 1 (IGF-1) and decreased secretion of growth hormone and gonadotropins from the pituitary gland resulting in poor linear growth and delayed pubertal onset.22 However, the higher hemoglobin, especially in boys, may also be a reflection of normal pubertal production of testosterone which promotes erythropoiesis.23
While this study adds to the literature on growth outcomes, some limitations are worth noting. Based on our exclusion criteria, the smaller sample size of older adolescent patients in our study limited our analysis of growth data beyond 14.0 years of age; thus, we may have missed the true peak height velocity in boys if they experienced a delay in pubertal onset especially among the boys on HU and no sickle cell disease modifying therapy. Second, some patients were not adherent to all sickle cell clinic annual visits; therefore, complete growth data were not available for all patient visits each year, and thus growth charts were estimated by statistical analysis. Third, consistent recording of sexual maturation rating were not recorded at each visit so peak height velocity based on retrospective analysis of growth data during pubertal years served as a surrogate for pubertal progression.24 Fourth, we used the sickle cell disease modifying therapy a patient received for the majority during the pre-puberty and peri-pubertal analysis as not all patients remained on the same therapy during that time period. Due to sample size limitations, patients initiated on chronic transfusion therapy and then transitioned to red cell exchange were continued to be categorized as the transfusion therapy group. Finally, details related to HU exposure (dose, duration, adherence, maximum tolerated dose, fetal hemoglobin and mean corpuscular volume) and transfusion therapy (frequency, iron status and type of transfusion therapy) were not recorded.
This study provides evidence that patients initiated on transfusion therapy are taller and likely have a more normal pubertal onset as compared to patients on HU or no therapy. The majority of patients in our practice under the age of 14 years that receive chronic transfusion therapy are initiated on this treatment for CNS protection. While the evidence from the STOP12,25, SIT9, and SWiTCH11 studies provide evidence for CNS protection, the additional growth benefit of chronic transfusion on growth pattern could be discussed with families. Future prospective studies should incorporate sexual maturation rating and hormone levels to determine their impact on growth and pubertal development in these children with SCA.
Acknowledgments:
The authors acknowledge the other members of the Sickle Cell Team that contribute to the daily care of our patients including Thomas Howard MD, Lee Hilliard, MD Christina Bemrich Stolz MD, Lindsey Evans CRNP, Kristen Osborn DNP, Susan McGee CRNP, Heather Carlton CRNP, and Brandi Pernell DNP.
Abbreviations:
- Hb
Hemoglobin
- SCD
Sickle Cell Disease
- SCA
Sickle Cell Anemia
- HU
Hydroxyurea
- PHV
Peak Height Velocity
Footnotes
Conflicts of Interest: Dr. Lebensburger is funded by the National Institutes of Health for grant work related to sickle cell kidney disease and served as a consultant for Novartis related to sickle cell kidney disease.
References:
- 1.Luban NL, Leikin SL, August GA. Growth and development in sickle cell anemia. Preliminary report. Am J Pediatr Hematol Oncol. 1982;4(1):61–65. [PubMed] [Google Scholar]
- 2.Platt OS, Rosenstock W, Espeland MA. Influence of sickle hemoglobinopathies on growth and development. N Engl J Med. 1984;311(1):7–12. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes M, Akohoue SA, Shankar SM, et al. Growth patterns in children with sickle cell anemia during puberty. Pediatric blood & cancer. 2009;53(4):635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serjeant GR, Singhal A, Hambleton IR. Sickle cell disease and age at menarche in Jamaican girls: observations from a cohort study. Archives of disease in childhood. 2001;85(5):375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas PW, Singhal A, Hemmings-Kelly M, Serjeant GR. Height and weight reference curves for homozygous sickle cell disease. Archives of disease in childhood. 2000;82(3):204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf RB, Saville BR, Roberts DO, et al. Factors associated with growth and blood pressure patterns in children with sickle cell anemia: Silent Cerebral Infarct Multi-Center Clinical Trial cohort. Am J Hematol. 2015;90(1):2–7. [DOI] [PubMed] [Google Scholar]
- 7.Singhal A, Thomas P, Cook R, Wierenga K, Serjeant G. Delayed adolescent growth in homozygous sickle cell disease. Archives of disease in childhood. 1994;71(5):404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornburg CD, Files BA, Luo Z, et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood. 2012;120(22):4304–4310; quiz 4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. The New England journal of medicine. 2014;371(8):699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet (London, England). 2016;387(10019):661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware RE, Helms RW. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH). Blood. 2012;119(17):3925–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang WC, Morales KH, Scher CD, et al. Effect of Long-term Transfusion on Growth in Children with Sickle Cell Anemia: Results of the Stop Trial. The Journal of pediatrics. 2005;147(2):244–247. [DOI] [PubMed] [Google Scholar]
- 13.Bavle A, Raj A, Kong M, Bertolone S. Impact of long-term erythrocytapheresis on growth and peak height velocity of children with sickle cell disease. Pediatric blood & cancer. 2014;61(11):2024–2030. [DOI] [PubMed] [Google Scholar]
- 14.Wang WC, Helms RW, Lynn HS, et al. Effect of hydroxyurea on growth in children with sickle cell anemia: results of the HUG-KIDS Study. The Journal of pediatrics. 2002;140(2):225–229. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Archives of disease in childhood. 1976;51(3):170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao J, Cai J, Wang L. Estimating curves and derivatives with parametric penalized spline smoothing. Stat Comput Statistics and Computing. 2012;22(5):1059–1067. [Google Scholar]
- 18.Craven P, Wahba G. Smoothing noisy data with spline functions Estimating the correct degree of smoothing by the method of generalized cross-validation. Numer Math Numerische Mathematik. 1978;31(4):377–403. [Google Scholar]
- 19.Wickham H, Sievert C Ggplot2 : elegant graphics for data analysis. 2016.
- 20.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet. 2011;377(9778):1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rana S, Houston PE, Wang WC, et al. Hydroxyurea and growth in young children with sickle cell disease. Pediatrics. 2014;134(3):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soliman AT, De Sanctis V, Yassin M, Adel A. Growth and Growth hormone - Insulin Like Growth Factor -I (GH-IGF-I) Axis in Chronic Anemias. Acta Biomed. 2017;88(1):101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomsen K, Riis B, Krabbe S, Christiansen C. Testosterone regulates the haemoglobin concentration in male puberty. Acta Paediatr Scand. 1986;75(5):793–796. [DOI] [PubMed] [Google Scholar]
- 24.Harstad EB, Weaver AL, Katusic SK, et al. ADHD, stimulant treatment, and growth: a longitudinal study. Pediatrics. 2014;134(4):e935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood. 2006;108(3):847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]