Abstract
The genus Aspergillus is ubiquitous in the environment and contains a number of species, primarily A. fumigatus, that cause mold-associated disease in humans. Humans inhale several hundred to several thousand Aspergillus conidia (i.e., vegetative spores) daily and typically clear these in an asymptomatic manner. In immunocompromised individuals, Aspergillus conidia can germinate into tissue-invasive hyphae, disseminate, and cause invasive aspergillosis. In this review, we first discuss novel concepts in host defense against Aspergillus infections, and emphasize new insights in fungal recognition and signaling, innate immune activation and fungal killing. Second, the review focuses on novel concepts of Aspergillus pathogenesis and highlights emerging knowledge regarding fungal strain heterogeneity, stress responses, and metabolic adaptations on infectious outcomes. Mechanistic insight into the host-pathogen interplay is thus critical to define novel druggable fungal targets and to exploit novel immune-based strategies to improve clinical outcomes associated with aspergillosis in vulnerable patient populations.
Introduction
Fungal pathogens represent a substantial public health risk with more than 1 million attributable fatalities worldwide annually [1]. The limited number and diversity of contemporary antifungal drugs (i.e., three major drug classes that target two biosynthetic pathways), the emergence of drug resistance in human pathogenic fungi, the lack of vaccines to prevent fungal disease, and the growing number of humans that live in immune compromised states have all contributed to the emergence of a broad range of invasive mycoses. The latter factor is most critical to understanding fungal diseases attributable to filamentous molds in general, and to the genus Aspergillus in particular.
There are more than 200 Aspergillus species on Earth, though only a handful are associated with human disease (see Table I). Like other Aspergillus species, A. fumigatus, the most common agent of invasive aspergillosis (IA), releases asexual spores called conidia into the air. Humans inhale hundreds to thousands of these infectious propagules on a daily basis. Due to their small size (2–3 μm diameter), A. fumigatus conidia can bypass mucociliary clearance and lodge in the lower respiratory tract. In immunocompetent individuals, the coordinated action of the respiratory epithelium, lung-resident macrophages, as well as recruited neutrophils and monocytes clear conidia efficiently.
Table I:
Common human disease states associated with Aspergillus.
| Disease: | Clinical syndromes: | Common patient populations and notes: |
|---|---|---|
| Invasive aspergillosis (tissue-invasive hyphae ± angioinvasion) | Pneumonia and systemic disease; CNS most common site of dissemination | Patients with acute leukemia, lymphoma, myelodysplastic syndrome, aplastic anemia, and allogeneic hematopoietic cell transplant recipients; lung and heart transplant recipients Most common species: A. fumigatus (~60–70%), A. flavus, A. niger, A. terreus, A. versicolor, A. ustus, A. lentulus, A. nidulans (only in chronic granulomatous disease patients) |
| Aspergillus keratitis | Ocular disease caused by hyphal growth in humans with corneal damage | Agricultural workers in resource-limited countries |
| Chronic pulmonary aspergillosis (hyphae in pre-existing lung cavity) | Pneumonia, typically observed with structural lung disease | Chronic obstructive lung disease, bronchiectasis, sarcoidosis, previously treated tuberculosis |
| Aspergillus-associated allergic disease | Allergic bronchopulmonary aspergillosis (ABPA), extrinsic allergic alveolitis | ABPA occurs primarily in patients with atopic asthma and cystic fibrosis |
The hallmark of IA is the germination of inhaled conidia and the ensuing growth of highly destructive filamentous hyphae that can disseminate hematogenously to remote sites. The term IA does not refer to a specific site of disease, though the respiratory tree is the most common site of infection and the primary focus of this review [2]. However, innate immune suppression or injury is a major risk factor associated with the development of IA.
Specifically, profound and prolonged neutropenia and monocytopenia, common side effects of myelotoxic chemotherapy regimens and hematopoietic cell transplantation, predispose to IA in human cohorts and in high-inoculum mouse models of disease [3]. Chronic granulatomous disease (CGD), caused by a defect in NADPH oxidase, represents a primary immune deficiency with a functional rather than numeric defect in neutrophil function and is also associated with IA susceptibility. Similarly, severe pharmacologic impairment of myeloid cell function, for example by prolonged corticosteroid exposure, renders patients vulnerable to IA [3]. In most instances, the combination of multiple simultaneous insults to the innate immune system (e.g., myelotoxic chemotherapy, corticosteroids, and small molecule inhibitors of myeloid function) defines patients at high risk of IA.
In these vulnerable patient groups, failure of the respiratory innate immune system to prevent conidial germination sets the stage for invasive disease [4]. Despite contemporary antifungal drugs, an estimated 200,000 cases of IA occur globally [5], with fungus-attributable mortality rates that exceed 20% in patients with hematologic malignancies and in allogeneic hematopoietic cell transplants [6]. Patients that receive ibrutinib [7] for B cell malignancies, COPD patients, ICU patients, in particular critically ill patients with influenza, are increasingly recognized as being vulnerable to IA [8,9]. In this review, we summarize recent developments in antifungal immunity and fungal pathogenesis, with an emphasis on studies conducted since 2016. For a review of Aspergillus-associated allergenic and toxin-mediated disease states, the reader is referred to the following publications [10–12]. Beyond the common pathogenic species of A. fumigatus, A. flavus, A. terreus, A. niger, and A. nidulans, the following publication highlights rare and emerging Aspergillus species, primarily seen in patients with primary immune deficiency diseases [13].
A. The Host Response to Aspergillus
Novel Advances in Aspergillus Recognition
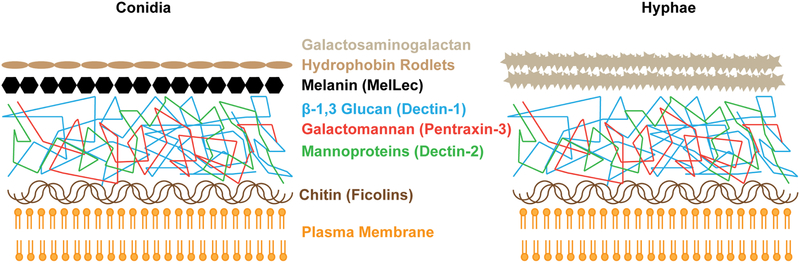
The A. fumigatus cell surface, like that of other fungal pathogens, consists of a multilayered, polysaccharide-rich cell wall [14]. The conidial cell wall is covered by a proteinaceous, highly hydrophobic layer of rodlets called hydrophobins, which conceals an underlying fungal pigment, melanin. The 1,8-dihydroxynapthalene (DHN) pathway produces the conidial pigment in A. fumigatus and A. terreus, while 3,4-dihydroxy phenylalanine (DOPA) pathway operates in A. niger and A. flavus [15]. These outer layers are shed during conidial germination, exposing a core layer of β−1,3-linked glucan polysaccharides, mannoproteins, and galactomannan, all of which activate pattern recognition receptors of the innate immune system. The inner layer of the polysaccharide-rich cell wall consists of chitin, a polymer of N-acetylglucosamine; this layer resides immediately above the plasma membrane [16]. Hyphae lack the hydrophobin and melanin layer and synthesize an external layer of galactosaminogalactan which is absent from conidia (Figure 1) [17].
Figure 1: Schematic of the A. fumigatus cell wall.
The composition of the A. fumigatus cell wall depends on the fungal morphotype. In conidia, a hydrophobin and melanin layer shields the immunogenic core of the cell well, consisting of carbohydrates and glycoproteins. Germlings and hyphae lose the outer conidial layer, leading to exposure of immunoreactive polysaccharides and the eventual synthesis of an outer galactosaminogalactan layer. The indicated receptors trigger innate immune activation in response to exposure of defined polysaccharide and melanin ligands.
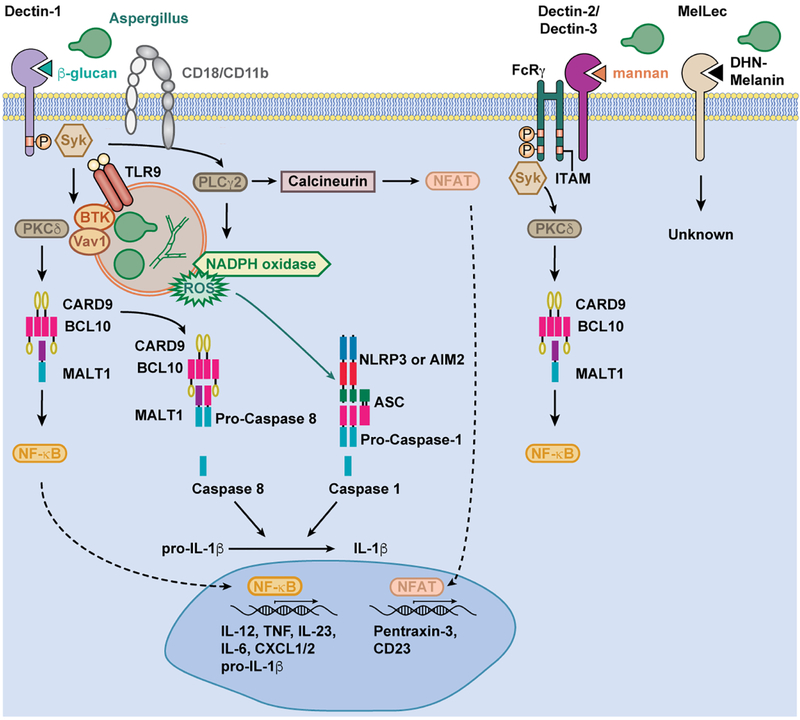
The C-type lectin family of receptors (CLR) has emerged as a critical point of response to fungal pathogens, and specifically A. fumigatus. Conidial germination is the major trigger for innate immune activation, with a dominant role of Dectin-1/CLEC7A signaling in response to A. fumigatus β−1,3-glucan exposure [18,19]. The carbohydrate-binding domain of Dectin-1 recognizes particulate β−1,3-glucans, a process that leads to Src kinase-dependent phosphorylation of its intracellular ITAM-like motif [1,20] and to the activation of spleen tyrosine kinase (Syk). Syk activation then promotes the assembly of a trimeric complex of CARD9, BCL10, and MALT1 which is essential for the production of NF-κB-dependent cytokines by myeloid cells in Aspergillus-infected mice, including pro-IL1β, TNF, IL-17A, and IL-22 [21–24]. Dectin-1/Syk activation also activates NFAT transcription factors that regulate additional receptors, e.g., FcεRI/CD23/CLEC4J, implicated in fungal control (see below).
Dectin-1/Syk signaling also leads to the assembly of inflammasomes, multiprotein complexes that include a NOD-like receptor family member (i.e., NLRP3 or AIM2), a common structural component (ASC), and a caspase moiety [25–27] (Figure 2). In the case of A. fumigatus, NLRP3 inflammasomes relevant for host defense consist of a ternary complex of NLRP3, ASC, and caspase-1; the resulting complexes lead to the caspase-1-dependent proteolytic processing of pro-IL-1β into bioactive IL-1β. The non-canonical caspase-8 can be recruited to the CARD9/BCL10/MALT1 complex with ASC, resulting in an alternate IL-1β processing pathway [28]. A recent study suggested that caspase-11 may activate caspase-1 and thus regulate IL-1β release during A. fumigatus infection [29]. Consistent with a role for inflammasome activity in anti-Aspergillus defense, corticosteroid-treated NLRP3-, AIM2-, or caspase-1 deficient mice are more susceptible to A. fumigatus challenge compared to corticosteroid-treated controls [27,29,30]. However, in otherwise immune competent mice, IL-1β production appears dispensable for host defense against respiratory A. fumigatus challenge [31]. Instead, the inflammasome-independent production and release of IL-1α appears critical for host defense in this setting [31].
Fig 2. Aspergillus ligands activate C-type lectin receptor signaling in myeloid cells.
A. fumigatus β-glucans activate Dectin-1 and CR3 signaling, resulting in Syk phosphorylation. A. fumigatus can activate the FcRy-coupled receptors Dectin-2 and Dectin-3 via mannan moieties, and MelLec via DHN-melanin exposure. Dectin-1/Syk and Dectin-2/Syk activation is transduced via PKCδ and CARD9 for assembly of the ternary CARD9/BCL10/MALT1 complex, NF-κB activation, and the transcription of NFκB-dependent genes, including pro-IL-1β and other cytokines. The assembly of NLRP3 and AIM2 inflammasomes results in proteolytic activation of caspase-1 and the production of bioactive IL-1β. Alternatively, ASC and caspase-8 recruitment to the CAD9/BCL10/MALT1 complex gives rise to active caspase-8 and bioactive IL-1β. CR3/Syk-dependent PLCγ2 activation is linked to NADPH oxidase assembly, the production of reactive oxygen species, and calcineurin-dependent NFAT activation. NFAT-dependent genes include pentraxin-3 and CD23. Dectin-1 can interact with VAV1 and BTK to facilitate macrophage fungal phagocytosis. BTK may also promote calcineurin activation and the production of NFAT-regulated cytokines. The model depicts fungal killing in the phagosome.
IL-1α and IL-1β trigger IL-1 receptor/MyD88 signaling in pulmonary epithelial cells to promote the initial -CXC- chemokine-mediated influx of neutrophils into the A. fumigatus-infected lung [21]. Sustained neutrophil recruitment relies on CARD9 signaling in hematopoietic cells; this amplifies the IL-1R/MyD88-dependent and CARD9-independent production of neutrophil chemotactic mediators by the lung epithelium. CARD9 function is dispensable for neutrophil-intrinsic fungal killing in mice and humans [21,32,33]. Humans with germline mutations in CARD9 do not develop IA at pulmonary sites, consistent with the idea that CARD9-dependent neutrophil recruitment can be functionally compensated by CARD9-independent mechanisms in the lung. However, outside the lung, a gross absence of neutrophil recruitment has been reported in case of abdominal aspergillosis, suggesting that neutrophil recruitment depends heavily on CARD9 signaling at extrapulmonary sites [21], similar to findings reported for neutrophil chemotaxis to the Candida-infected central nervous system [34].
A. fumigatus triggers Dectin-2/CLEC4N activation, though the precise fungal ligand on swollen conidia and germlings that binds to the Dectin-2 carbohydrate recognition domain remains unknown [34]. In other fungal organisms, cell wall mannan structures trigger Dectin-2 activation [35]. Dectin-2 signals via an adaptor protein FcRγ and activates CARD9-dependent signals [34]. In a fungal keratitis model, systemic exposure to Aspergillus antigens gives rise to Dectin-2+Rorγt+IL-17RC+ neutrophils in an IL-6- and IL-23-dependent manner; this activated neutrophil subset produces the effector cytokine IL-17A, leading to autocrine activation and heightened fungicidal responses [36]. Similarly, Aspergillus stimulation leads to increased macrophage Dectin-2 expression in a pulmonary infection model [37]. However, the precise contribution of Dectin-2 to in vivo host defense against A. fumigatus remains poorly defined and may, at least part, explain the more severe human and murine phenotype of CARD9 deficiency compared to Dectin-1 deficiency [32]. The potential contributions of the FcRγ-coupled CLRs Mincle/CLEC4E and Dectin-3/MCL/CLECSF8/CLEC4D to in vivo host defense against Aspergillus remain undefined.
CD23/FcεRI/CLEC4J, the low affinity receptor for IgE, is a FcRγ-coupled C-type lectin that binds to fungal β-glucan and α-mannan moieties and regulates NF-κB-dependent macrophage cytokine and nitric oxide production. Notably, CD23 deficiency results in impaired survival in an intravenous A. fumigatus murine infection model, although it is likely dispensable for host survival and fungal clearance during pulmonary infection [38].
Melanin-sensing C-type lectin receptor (MelLec/CLEC1A) is expressed on endothelial, but not myeloid, cells in mice and on myeloid cells in humans. MelLec recognizes A. fumigatus conidial DHN-melanin and its downstream signaling cascade remains to be characterized. In the absence of MelLec, otherwise immunocompetent mice are susceptible to systemic, but not pulmonary, infection [39]. Beyond MelLec, surfactant protein D (SP-D), a soluble C-type lectin receptor, may recognize melanin. Opsonization of conidia by SP-D stimulates phagocytosis and inflammation ex vivo. Moreover, in vivo, SP-D deficient mice have impaired cytokine responses, specifically in TNF, IL-6, IL-8, IL-1α, IL-1β, and TGF-β production, following A. fumigatus challenge. Collectively, these findings emphasize the role of melanin recognition in host defense against A. fumigatus [40].
Complement receptor 3 (CR3), a myeloid cell integrin receptor consisting of CD11b and CD18 subunits, binds β-glucan moieties in addition to its well-defined role in complement recognition [41]. In vivo, CD18 knockout mice, a model of human leukocyte adhesion deficiency, fail to recruit neutrophils to infected tissues in an Aspergillus keratitis model and develop more severe disease compared to CD18-sufficient controls [42]. In a pulmonary infection model, CD18-dependent neutrophil recruitment is not grossly impaired and CD18-deficient mice do not exhibit an overt susceptibility defect, highlighting tissue-specific requirements for CD18 in neutrophil trafficking and fungal killing during A. fumigatus infection [32]. This is not specific to A. fumigatus lung infections, as pulmonary neutrophil recruitment in response to Escherichia coli pneumonia also does not require CD18 [43]. CR3 has also been implicated in the A. fumigatus-triggered neutrophil respiratory burst and hyphal killing [42,44], as discussed below.
The soluble pattern recognition receptors Pentraxin-3 and M-ficolin recognize newly exposed carbohydrate layers during conidial germination. Pentraxin-3 binds to galactomannan, enhances human neutrophil conidial engulfment [45], and is a component of neutrophil extracellular traps (NETs) [46]. Ficolin-deficient, but otherwise immunocompetent mice display normal neutrophil recruitment as measured by lung myeloperoxidase levels, as well as normal survival, yet exhibit delayed fungal clearance and reduced IL-1β, IL-6, and TNF responses in infected lung tissues [47]. The C-type lectin receptor DC-SIGN, expressed primarily on human alveolar macrophages, recognizes galactomannan as well and likely contributes to fungal cell phagocytosis. Soluble receptors, including the ficolin family of receptors, also recognize the inner chitin layer once exposed [48]. Lung epithelial cells express the membrane bound protein fibrinogen C domain-containing protein 1 (FIBCD1). FIBCD1 recognizes chitin on the fungal cell wall and can suppress the production of IL-8, mucin, and other pro-inflammatory mediators [49]. The cell wall component galactosaminogalactan also maintains immunomodulatory functions by concealing immunogenic cell surface components from their receptors, such as shielding β-glucans from Dectin-1 [50].
Fucosylated host glycoproteins have recently emerged as important components in Aspergillus recognition, by virtue of their binding to the fungal lectin FleA. FleA acts as a ligand for fucosylated glycoproteins in purified lung mucin and regulates fucose-mediated conidial binding and uptake by macrophages. In the absence of FleA, macrophages are defective at internalizing conidia in vitro and mice infected with FleA-deficient conidia develop more severe lung infection than mice infected with FleA-sufficient conidia, establishing fucosylated structures as critical for Aspergillus recognition and clearance [51].
Importantly, human polymorphisms in the CLEC7A (~20% IA incidence versus ~15% in the control group at 24 months post-transplant), PTX3 (~20% IA incidence versus ~15% in the control group at 24 months post-transplant) [52], and CLEC1A (39% IA incidence versus 17% in the control group at 24 months post-transplant) [39] increase the risk of IA in allogeneic hematopoietic cell transplant recipients, though these genetic variants are not associated with the spontaneous development of IA in otherwise immune competent individuals. Humans with genetic defects in Toll-like receptor signaling similarly do not develop fungal disease in the absence of other risk factors [53]. In murine models, deficiency of TLR2 and TLR4 have been associated with murine susceptibility to A. fumigatus challenge in the context of pharmacologic immune suppression [54,55] In humans, a TLR4 S4 haplotype influences susceptibility to IA in allogeneic HCT patients (22% IA incidence with S4 haplotype versus ~1–10% in the control group, depending on the presence of CMV disease), but it remains unclear whether TLR4 binds and responds to A. fumigatus directly or, more likely, that this TLR4 haplotype is linked to a general alteration in innate immune reactivity to inflammatory stimuli [56].
Bruton’s tyrosine kinase, ibrutinib, and the risk of invasive aspergillosis.
Bruton’s tyrosine kinase (BTK) transduces signals from the B cell receptor (BCR) and this kinase has been targeted successfully with ibrutinib to inhibit tonic BCR signaling required for the maintenance of malignant B cells. Beyond this established role in BCR signaling, BTK acts downstream of Dectin-1- and TLR-dependent signaling in myeloid cells during interactions with fungi (Figure 2). In A. fumigatus-infected macrophages, endosomal TLR9 activates calcineurin signaling in a MyD88-independent and BTK-dependent manner to regulate NFAT-dependent TNF production [57]. Since Pentraxin-3 is an NFAT-dependent gene, this finding may explain why impaired calcineurin signaling in DCs predisposes to IA in a murine allogeneic hematopoietic cell transplant model of graft versus host disease [58]. Beyond its function in macrophage cytokine production, BTK also plays a role in Dectin-1-dependent macrophage C. albicans phagocytosis [59].
Recently, several reports observed that lymphoma patients treated with ibrutinib, a BTK inhibitor, exhibit susceptibility to IA, particularly in the central nervous system, even in the general absence of well-defined concurrent clinical risk factors such as neutropenia or corticosteroid exposure [7,60,61]. Though the studies examined heterogenous patient groups, ibrutinib treatment resulted in an IA incidence rate of 0.5%−39%, depending on the presence of concurrent chemotherapy, with most reported incidence rates below 5% [62]. These clinical findings support the concept that BTK signaling, and inhibition by ibrutinib, cause a defect in fungal immune surveillance. Consistent with this model, BTK-deficient mice are more vulnerable to A. fumigatus challenge compared to controls [7]. In addition, ibrutinib impairs NFκB/NFAT signaling during macrophage A. fumigatus phagocytosis [63]. While it remains unclear whether ibrutinib has BTK-independent effects on fungal immune surveillance, these data supports the idea that BTK signaling in myeloid cells contributes to anti-Aspergillus host defense [59].
Innate Immune Cells in Anti-Aspergillus Immunity
As outlined above, neutrophils and monocytes are essential cellular components of anti-Aspergillus immunity in humans and mice [64–67], highlighting the essential role of the innate rather than adaptive immune system in mediating sterilizing immunity. Adaptive immune responses to A. fumigatus are reviewed elsewhere [68]. In mouse models, macrophages kill conidia and contribute to fungal clearance as well [32,69], although pulmonary macrophage ablation experiments reveal that loss of their antifungal activity can be functionally compensated [64]. Monocytes and monocyte-derived dendritic (Mo-DCs) cells both kill conidia and, via intercellular cross-talk, enhance the fungicidal properties of recruited neutrophils [65]. Type I and III interferon signaling has emerged as central to this process. Lung-infiltrating CCR2+ inflammatory monocytes regulate the local and rapid production of interferon (IFN)-α which in turn stimulates IFN-λ production. Neutrophils express type I and type III IFN receptors that activate STAT1 signaling and promote the fungicidal respiratory burst [70]. Exogenous stimulation of type I IFN production leads to improved survival of CGD mice upon A. fumigatus challenge [71], suggesting that type I IFN may be beneficial even in the context of an impaired neutrophil respiratory burst.
Granulocyte-macrophage colony stimulating factor (GM-CSF) plays an important role in intercellular crosstalk and neutrophil activation as well. Neutrophils that cannot sense GM-CSF exhibit an impaired oxidative burst, and therapy with recombinant GM-CSF accelerates Aspergillus clearance in an NADPH oxidase-dependent manner [72]. Another hematopoietic growth factor, macrophage colony stimulating factor (M-CSF), accelerates myeloid cell differentiation after allo-HCT in mice and improves survival if a mouse is infected one week post-transplant [73].
A recent paper indicated that a minor neutrophil subset expresses the integrin CD11c and the major histocompatibility complex (MHC) class II receptor during respiratory fungal infection. Termed neutrophil-DC hybrids, this rare population is more efficient at fungal cell uptake and killing compared to canonical CD11c−MHC class II− neutrophils [74]. Because tools to manipulate neutrophil-DC hybrids in vivo have not been established, it remains challenging to quantify their contributions to fungal clearance.
Plasmacytoid dendritic cells (pDCs) contribute to innate defense as well, since antibody-mediated depletion renders mice susceptible to A. fumigatus challenge [75]. pDCs express Dectin-2 and are activated in response to Aspergillus antigens, though the pDC transcriptional response to appears distinct to that elicited by microbial nucleic acids, typically observed in viral infections [76]. pDCs have been proposed to form rare extracellular traps following A. fumigatus exposure. A model that integrates pDC trafficking and function in the immune response to A. fumigatus has not yet been established [76].
In immune competent mice, T cells, B cells, innate lymphoid cells, and NK cells do not play an essential role in host defense, because RAG2- and IL-2 receptor common γ chain- deficient mice do not exhibit enhanced sensitivity to respiratory challenge [65]. However, NK cells may play beneficial roles in fungal clearance in the setting of injury to the myeloid cell compartment, as shown in a neutropenic model of IA [77]. Natural killer (NK) cells express CD56, a 140 kDa subunit of the human neural-cell adhesion molecule. CD56 appears to interact directly with A. fumigatus, leading to a reduction in surface expression. Blocking CD56 impairs NK cell activation and cytokine secretion, consistent with the idea that CD56 is a fungal pattern recognition receptor for NK cells [78]. In vitro stimulation of NK cells by A. fumigatus germlings leads to an increase in inflammatory cytokine production and granule polarization, and there is an increase in fungal DNA release which is indicative of fungal damage. However, granule release is impaired upon contact with germlings. In agreement with this observation, co-incubation of A. fumigatus with NK cells and leukemic cells results in an impaired NK response to the leukemic cells. This finding suggests that fungi may dampen NK cell cytolytic activity and induce an exhaustion-like phenotype [79]. DCs exposed to A. fumigatus antigens have the capacity to stimulate NK cells in a contact-independent manner [80].
In vitro experiments support a potential regulatory role for platelets in anti-Aspergillus host defense [81]. The addition of platelet-rich plasma to macrophage-Aspergillus co-cultures significantly increases fungal phagocytosis by macrophages. In addition, platelet-rich plasma reduces fungal metabolic activity in both DC-Aspergillus and macrophage-Aspergillus co-culture experiments compared to control conditions [82]. In an in vitro model of platelet coagulation, co-culture of A. fumigatus conidia and germlings led to the formation of conidia-platelet aggregates surrounded by neutrophils [83]. Although the in vivo role of platelets remains unclear, thrombocytopenia has been identified as a risk factor for IA outcomes in liver transplant patients [84] and in neutropenic patients undergoing induction chemotherapy for hematologic malignancies [85,86].
Trained immunity is a memory-like phenotype observed in innate immune cells upon serial exposure to fungal ligands, exemplified by β-glucans and resistance to C. albicans infection [87]. Trained monocytes exhibit metabolic adaptations, including high glucose consumption, lactate production and an increased NAD+ to NADH ratio, which is indicative of a change in metabolism towards glycolysis. This supports increased pro-inflammatory responses upon subsequent encounter with fungal cell wall antigens [88]. In vivo, an initial exposure to live or killed Aspergillus antigens protects against secondary challenge in mice that lack adaptive immune cells. This protective phenotype depends on IL-17 signaling, is associated with enhanced CXCR2 signaling-dependent recruitment of neutrophils to the site of infection, as well as heightened microbicidal activity [89].
The importance of the microbiota in anti-Aspergillus defense remains poorly understood. However, a recent study established that the composition of the intestinal microbiome appears to influence the formation of CD4+ T helper 17 cells in the lung following pulmonary Aspergillus infection [90]. Much more work will be necessary to understand how either local (pulmonary) or distant (extrapulmonary) microbial communities influence host susceptibility to inhaled Aspergillus spores and to Aspergillus-associated allergenic disease states.
Novel Insights into Fungal Killing
Myeloid cells kill Aspergillus by oxidative and non-oxidative mechanisms and employ different strategies to kill conidia and hyphae. Following phagocytic uptake, conidial killing typically occurs within phagolysosomes. Murine neutrophils employ NADPH oxidase to kill conidia in a cell-intrinsic manner. However, NADPH oxidase-deficient neutrophils have a partial conidial killing defect, consistent with of alternative, non-oxidative mechanisms [32]. In contrast, hyphal killing occurs predominately in the extracellular space and may coincide with NET formation [91], a process that extrudes genomic DNA, citrullinated histones, antimicrobial peptides, pentraxin-3, and calprotectin around Aspergillus hyphae. Dectin-1 and CR3 participate in this bifurcated response to fungal cell size by regulating the process of NET formation [44,92]. Although neutrophil contact with A. fumigatus hyphae induces NET formation, available experimental evidence does not support a direct fungicidal activity of these structures [44,93,94].
A recent study demonstrated that neutrophil-derived reactive oxygen species induce an apoptosis-like regulated cell death (A-RCD) in leukocyte-engulfed conidia [95]. In other words, leukocytes trigger a gene-dependent cell death program in A. fumigatus via the action of NADPH oxidase, consistent with the susceptibility of CGD patients to IA. This process is inhibited by the action of AfBir1, a negative fungal regulator of A-RCD. AfBir1 is a homolog of the human SURVIVIN gene, a member of the inhibitor-of-apoptosis (IAP) protein family. IAP proteins in humans and fungi encode conserved BIR domains that, in the case of the human protein XIAP, can suppress mammalian apoptosis by blocking caspase activation [96,97]. Consistent with a role as a negative regulator in fungal RCD, AfBir1 overexpression diminishes leukocyte-induced conidial A-RCD and increases virulence in a murine model of IA. In contrast, presumed pharmacologic inhibition of AfBir1 diminishes conidial viability in neutrophils and leads to more rapid fungal clearance in vivo. A major gap in knowledge relates to the identity of fungal effector genes that mediate the A-RCD response [98–100].
Like their murine counterparts, human neutrophils employ different mechanisms to kill conidia and hyphae. As stated above, CR3 recognizes conidia and can regulate neutrophil killing by phosphoinositide 3-kinase (PI3K)-dependent mechanisms at high effector to target cell ratios [93]. In this study, the authors suggest that CR3- and PI3K-dependent conidial killing does not require the oxidative burst. Human neutrophils isolated from CGD patients can employ iron sequestration as a form of nutritional immunity (see below) to inhibit fungal growth [101]. In contrast to conidial killing, the killing of A. fumigatus germlings and hyphae requires antibody opsonization and neutrophil signaling through Fcγ receptor, SYK, PI3K, and PKC to generate fungicidal ROS through NADPH oxidase [93]. Differences in experimental observations are likely reconciled by redundancies in oxidative and non-oxidative conidial killing mechanisms, potential differences in the dependency of human versus murine neutrophils on specific effectors, and in vivo versus ex vivo experimental conditions.
In macrophages and neutrophils, NADPH oxidase activity promotes non-canonical autophagy known as LC3-associated phagocytosis (LAP) [102–104]. With regard to A. fumigatus, the presence of conidial melanin inhibits LAP. Whether LAP represents a critical defense mechanism in vivo remains poorly understood; hematopoietic Atg5-deficient mice have defects in macrophage-dependent conidial killing but are not vulnerable to A. fumigatus challenge in the absence of exogenous pharmacologic immune suppression [105].
B. Aspergillus Pathogenesis
Animal Models and Aspergillus Pathogenesis.
Many animal studies on IA have examined disease outcomes in immune competent mice to avoid pleiotropic effects of pharmacologic immune suppression or in chemotherapy- or corticosteroid-treated mice to mimic classic human risk factors, related to quantitative or qualitative myeloid cell injury associated with disease development [106]. A drawback of studying immune competent mice is their resistance to disease development [107], and the typical range of infectious inocula typically varies from 107 to 108 conidia [108]. In contrast, immunocompromised mice typically receive a lower inoculum that usually ranges from 104-107 conidia [109].
Neutropenic mice develop extensive and rapid hyphal growth that is angioinvasive and results in tissue necrosis [109]. Consistent with this numeric defect, adoptive transfer of CD11b+ phagocytes ameliorated IA outcomes in the neutropenic cyclophosphamide model. Corticosteroid-treated animals develop less invasive hyphal growth than cyclophosphamide-treated mice, in part due to massive infiltration of dysfunctional neutrophils, a process that leads to pronounced tissue hypoxia (see below). Accordingly, adoptive transfer of CD11b+ leukocytes does not improve infectious outcomes in this model [110]. CGD mice lack neutrophil NADPH oxidase activity and develop immune responses to A. fumigatus challenge that more closely resemble those observed in corticosteroid-treated mice than in neutropenic mice, because the extensive hyphal growth and angioinvasion seen in neutropenic mice is generally blunted in corticosteroid-treated or CGD mice. In addition, A. fumigatus produces gliotoxin, a fungal secondary metabolite that can induce regulated cell death in host cells, more readily in corticosteroid-treated and CGD mice than in neutropenic mice [109]. Dysregulated immune function is observed in mice with defects in the cystic fibrosis transmembrane regulator. Although CF patients do not develop IA, their airways are commonly colonized with Aspergillus and allergic bronchopulmonary aspergillosis is common in this patient group[111].
In mice with qualitative immune defects, researchers have examined infectious outcomes in the context of dampening fungus-induced inflammatory responses to minimize tissue damage. For example, although epithelial IL-1 receptor signaling is essential for neutrophil recruitment and fungal clearance in immune competent mice [31], dampening IL-1 signaling with an IL-1 receptor antagonist (i.e., anakinra) slightly extends survival in corticosteroid-treated mice [112]. This result highlights a paradox: at early stages of infection, IL-1 receptor signaling is essential for orchestrating the initial neutrophil recruitment to the site of fungal infection. Although anakinra treatment is not typically associated with susceptibility to invasive fungal infections, Aspergillus pneumonia has been reported in a patient treated with this agent for Still’s disease [113]. However, the results reported in [112] support the idea that unchecked IL-1 signaling may exacerbate infectious outcomes at later stages of infection in the context of functionally defective neutrophils.
The idea that excessive IL-1 signaling may be detrimental to the host has been extended to a murine CGD model, in which the pleiotropic cytokine IFN-γ, dispensable for fungal clearance in otherwise immune competent mice [70], promotes LC3-associated autophagy (discussed above) and activates the death-associated protein kinase 1 (DAPK1) pathway. DAPK1 inhibits NLRP3 inflammasome activation by proteosomal degradation, thereby attenuating downstream IL-1β production. In a murine CGD model, DAPK1 protein levels are low and LAP is attenuated due to its dependency on NADPH oxidase activity. Administration of exogenous IFN-γ restored DAPK1 activity, reduced IL-1β production, and increased fungal clearance in this model. In allo-HCT patients, DAPK1 promoter single nucleotide polymorphisms that lead to diminished protein expression were associated with an increased IA risk (42.1% IA incidence in patients with DAPK1 polymorphism versus 17.4% IA incidence in control patients) [114]. These results support the notion that unrestrained IL-1 signaling may be harmful in specific host contexts. Along these lines, anakinra administration improves outcomes in CGD mice by inhibiting excess neutrophil recruitment and, paradoxically, fungal growth [115]. How to integrate these findings into treatment strategies remains a complex challenge, given the context-specific beneficial and detrimental effects of IL-1 signaling in IA outcomes.
Strain Variability and Virulence
In recent years, strain selection has emerged as an important variable in Aspergillus pathogenesis research in recent years. Classically, Aspergillus pathogenesis has been analyzed and understood in terms of defined pharmacologic, genetic, or numeric deficits in host immune function. Beyond host immune injury, the inoculum represents an important additional variable to determine fungal clearance, since a mathematical model predicted that low dose infections, the most common occurrence in human life, would lead to fungal persistence compared to high dose infections [116].
Recent studies that compared the virulence and pathogenesis of different clinical isolates have begun to expand this view. Large-scale genome comparisons of A. fumigatus isolates did not identify specific virulence factors shared with other human fungal pathogens. However, the identification and functional characterization of strain-specific metabolic and adaptive traits critical for growth, survival, and competition within its natural habitat (i.e, decaying organic matter) form the basis for Aspergillus pathogenesis in human hosts [117]. More recently, the concept of strain-specific virulence traits has emerged within this framework and represents an active area of investigation.
In animal models of IA, two major clinical isolates are commonly utilized: AF293 (the original reference genome strain [118] that was isolated from patient with rheumatoid arthritis, see NCPF 7367) and CEA10 (a strain isolated from a patient with acute leukemia, see FGSC A1163) (Table II). AF293 is the less virulent and CEA10 the more virulent of these strains within the murine lung environment [119]. However, following experimental challenge in the mouse lung, CEA10 is cleared more rapidly than AF293, consistent with the concept that different strains induce qualitatively and quantitatively distinct host responses [120], leading to differences in clearance kinetics or in disease development in immune competent or susceptible hosts, respectively.
Table II:
Comparison of A. fumigatus CEA10 and AF293 strains.
| Strain: | CEA10 | AF293 |
| Virulence: | High | Low |
| Germination: | Fast | Slow |
| Elimination in Lung: | More Rapid | Less Rapid |
| Lung Inflammation: | Higher Levels | Lower Levels |
| Hypoxia Fitness: | High | Low, can be increased by serial passaging in hypoxia |
A consistent phenotype of the more virulent CEA10 strain is the more rapid conidial germination and hyphal growth in vitro and in vivo, observed in zebrafish and in mice [121], compared to AF293. The more rapid reduction in fungal burden seen with the CEA10 strain [120] is accompanied by a broader induction of inflammatory mediators in vitro and in vivo, in particular mediators associated with lung damage (i.e., IL-1α) and neutrophil chemotaxis (i.e., TNF, CXCL1, CXCL2, leukotriene B4) [122,123]. Alox5 null mice are unable to metabolize arachidonic acid to leukotrienes and are thus significantly more susceptible to CEA10 than AF293 challenge, highlighting the induction of and requirement for specific chemotactic pathways in a strain-specific manner [123].
Although AF293 can induce macrophage and DC IL-1β release, this strain consistently induces higher levels of the regulatory cytokine IL-10 compared to CEA10 [122] and does not require IL-1α- or leukotriene B4 (LTB4)-dependent neutrophil recruitment for fungal clearance [121]. This finding may also reflect strain-specific induction of lung hypoxia at sites of fungal tissue invasion, since tissue hypoxia drives expression of the transcription factor HIF-1α. In turn HIF-1α controls LTB4 synthesis [123] by regulating the expression of 5-lipooxygenase activating protein, a key enzyme in the leukotriene biosynthesis pathway [124]. Thus, the strain-specific differential induction and quality of inflammatory responses demonstrates an inverse relationship between inflammation-induced host damage and the kinetics of fungal clearance.
The fungal hypoxia adaptation response will be discussed in more detail below, and host hypoxia signaling is critical for murine survival in CEA10 but not AF293 infections [123]. The idea that strain-specific hypoxia fitness impacts virulence is underscored by the differential outcomes in a corticosteroid model of IA, which results in low-oxygen lesions in the lung. In this model, CEA10-infected mice succumb rapidly and significantly more than AF293. In agreement with this model, an experimentally evolved AF293 strain, created by serially passaging an isolate in hypoxia conditions, developed increased hypoxia fitness and displayed much higher virulence than the parental AF293 strain and similar to the CEA10 strain in the corticosteroid model [125]. The genetic basis that underlies this phenotypic evolution remains undefined.
Fungal Metabolism and Aspergillus Virulence
The ability of A. fumigatus to scavenge and utilize essential nutrients in a depleted environment impacts the outcome of infection. The lung airway surface liquid contains low levels of glucose, almost 10-fold lower than in the serum. The levels of ammonium, a preferred nitrogen source for fungi, and free amino acids are very low in the lung environment, unless expanded microbial communities and dysregulated tissue environments are present, e.g. in cystic fibrosis patients [126,127]. The glycoproteins of the mucus layer may be good sources of some these macronutrients, in particular because A. fumigatus, like most filamentous molds, produces a large number of extracellular proteases to degrade polypeptides [126]. Micronutrients such as metals also are required for growth of fungi [128] and the lung microenvironment contains very low levels of magnesium, calcium, iron, zinc and copper, compared to the bloodstream [129]. The regulation of micronutrient availability at portals of fungal invasion represents a form of nutritional immunity, a process by which the host may restrict the availability of necessary nutrients or flood the pathogen with those that are toxic in excess [130].
Iron is a critical nutrient for Aspergillus growth, and mutants with defective iron assimilation pathways are hypovirulent in immune compromised mice [131]. Lung transplant recipients are vulnerable to IA, particularly at anastomotic sites, consistent with the idea that presence of lung microhemorrhages may increase iron availability. In an experimental murine model of lung transplantation, the presence and extent of allograft-associated microhemorrhage, and the topical iron application in syngeneic lung grafts increased susceptibility to infection. When an iron-intolerant A. fumigatus strain was used, the enhanced susceptibility to IA disappeared [132].
Given the limited glucose sources in the lung, carbon assimilation is critical to Aspergillus growth in this environment. G-protein coupled receptors (GPCRs) and hexose transporters have been reported to be involved in glucose sensing and uptake in yeast, and functional homologs exist in A. fumigatus [126]. The GPCR GprK was recently found to be necessary for A. fumigatus proliferation on medium in which pentose was the only carbon source. Loss of this gene impairs oxidative stress tolerance and leads to lower virulence in a Galleria infection model, suggesting that carbon accumulation is important for adapting to an infection microenvironment [133].
Carbon catabolite repression refers to the process of switching from non-preferred to preferred carbon sources. The transcriptional repressor CreA mediates a carbon catabolite repression network that is dispensable for early infection establishment. However, CreA is essential for IA progression in a corticosteroid model. Early in the course of IA, infected airways contain adequate levels of oxygen and non-glucose carbon sources for conidia to germinate and form hyphae. At this early stage of infection, fungal carbon catabolite repression is unnecessary for hyphal growth. Following the induction of hypoxia in areas of fungal growth, a change in the availability of carbon sources requires that hyphae switch to glycolysis to support further elongation [134]. Thus, the induction of carbon catabolite repression acts as a disease progression factor and highlights the role of A. fumigatus metabolic flexibility for virulence in the lung environment.
Ammonium and glutamine represent preferred sources of nitrogen that A. fumigatus can assimilate. The GATA-type transcription factor AreA is required for growth on non-preferred nitrogen sources, and its activity contributes to invasive disease [135]. The A. fumigatus genome contains predicted ammonium and amino acid permeases but their roles in disease development and progression have not been characterized [126]. In contrast, amino acid biosynthesis has emerged as an additional metabolic contributor to virulence and pathogenesis. Auxotrophic A. fumigatus strains deficient in aromatic amino acid biosynthesis have attenuated growth in vitro and greater susceptibility to various forms of stress. This coincides with reduced virulence in both systemic and pulmonary infection of neutropenic mice [136]. The biosynthesis of cysteine, methionine, and histidine is necessary for virulence as well, since auxotrophic strain showed reduced virulence in immune compromised models of IA [137,138]. Beyond carbon and amino acid biosynthetic pathways, loss of vitamin biosynthesis (i.e., pantothenic acid or riboflavin) results in impaired virulence in neutropenic models of immune suppression by both pulmonary and systemic infection [139].
The Fungal Stress Response and Virulence
A. fumigatus faces hypoxia and oxidative stress in the lung. Recent work demonstrates that heterogeneity in hypoxia fitness among different A. fumigatus isolates correlates with virulence [125]. To adapt to tissue hypoxia, A. fumigatus can switch to fermentation though the action of a fungal alcohol dehydrogenase. Although loss of fungal ethanol fermentation does not affect murine survival, the AlcC null mutant strains grows more slowly in the lung tissue environment than the complemented control strain [140]. To grow in a hypoxic environment, A. fumigatus requires expression of the sterol regulatory element binding protein SrbA, a transcription factor that requires proteolytic activation by putative rhomboid homolog (Rbd) [141]. Loss of either gene sensitizes the fungus to hypoxic and to non-hypoxic stressors, including azole drugs, cell wall stress, and low iron conditions. RbdA deficient strains exhibit abnormal hyphal tip branching and have attenuated virulence in a corticosteroid model of IA [142]. The oxidoreductase HorA, located in mitochondria, is important for electron transport chain function and cellular respiration, and is highly upregulated under hypoxic conditions. The null mutant exhibited an impaired stress response to hypoxia and reductive stress and was hypovirulent in both corticosteroid and cyclophosphamide models of IA [143].
Given the central role of NADPH oxidase in host defense against Aspergillus [144], genetic pathways that counter oxidative stress are important for virulence in immune compromised models of IA. Yap1, a ROS-sensing transcription factor controls multiple aspects of the A. fumigatus responses to oxidative stress. Upon exposure to reactive oxygen species, Yap1 regulates the production of superoxide dismutases (that convert superoxide to hydrogen peroxide) and catalases (that convert hydrogen peroxide to water). In vitro hyphal growth in the presence of human neutrophils requires the expression of Yap1 and superoxide dismutase, however catalases are not required [42].
Yap1 also regulates thioredoxin antioxidant pathway that includes Prx1 and Aspf3, both presumed thioredoxin peroxidases or peroxiredoxins. These function by reducing hydrogen peroxide to water. Blocking thioredoxins pharmacologically with the anticancer drug PX-12 sensitizes hyphae to ROS and to neutrophil killing [42]. Loss of Aspf3 loss impairs growth on H2O2 and superoxide, and its protein structure implies a direct reductive action. Impaired growth in the null mutant is concomitant with reduced virulence in a corticosteroid model of IA [145]. The actin polymerizing factor cofilin may also contribute the anti-oxidation stress response. Cofilin overexpression results in increased fungal resistance to oxidative stress by regulating the transcription of oxidative stress response genes, including yap1, catA, dprA, dprB, and skn7 [146].
Fbx15, a transcriptional repressor and member of the E3 ubiquitin ligase complex, is transcriptionally induced by oxidative stress and required for growth on H2O2 and virulence in an immunosuppressed mouse model of IA. This result is likely due to two factors: first, the interaction of Fbx15 with the ubiquitin SCF E3 ligase machinery and the interaction with the transcriptional repressor SsnF. Under normal circumstances, Fbx15 is phosphorylated and interacts with the E3 complex predominantly in the cytoplasm but also in the nucleus. Phosphorylated Fbx15 assists in SsnF nuclear transport where SsnF can interact with corepressors that target oxidative stress response and secondary metabolite genes. Upon oxidative stress, Fbx15 is dephosphorylated and causes the E3 complex to become less active and concurrently leads to less nuclear SsnF, either through the nuclear export machinery or through a change in stability. This leads to a derepression of many genes, including those responsible for gliotoxin production, and other putative stress response genes [147].
Translational regulation is also important for A. fumigatus oxidative stress resistance. The cytoplasmic messenger ribonucleoprotein granule Afpab1 is required for growth on H2O2, and loss of this protein leads to increased susceptibility to phagocyte killing that is likely due to a presumed inability to detoxify ROS in vitro. Loss of Afpab1 results in impaired virulence in a corticosteroid and cyclophosphamide model of IA [148].
A. fumigatus has evolved strategies to counter host nutritional immunity that limits the availability of essential micronutrients, such as iron, or harness their toxic properties, e.g. copper. Loss of the copper-binding transcription factor AceA reduces A. fumigatus resistance to copper and ROS in vitro and increases susceptibility to macrophage-mediated killing. Although copper can function in an antioxidant manner by regulating superoxide dismutase activity, it can also generate hydroxyl radicals from H2O2 through Fenton Chemistry. In the absence of AceA, fungi are unable to regulate exposure to host-derived copper through copper transporters. In vivo, AceA, induction of the copper exporter CrpA, or depression of ROS-mediated host defense is necessary for virulence in cortisone treated mice [149]. Other metal metabolism pathways are also important, including the SchA kinase, which is upregulated during states of iron starvation. SchA was found to play a role in iron assimilation (among other functions), and in its absence A. fumigatus is avirulent in a neutropenic mouse model of IA [150].
A. fumigatus Growth in the Lung
Beyond fungal metabolism and stress adaptation, a number of fungal genes are important for hyphal growth and pathogenesis in the respiratory tract. Myosins have critical roles in the growth of Aspergillus hyphae and the pathogenesis of IA, with a requirement for MyoE in vesicle trafficking to the Spitzenkörper (i.e., cluster of transport vesicles located at growing hyphal tips) and for MyoB in regulating normal formation of septa. Although MyoB is dispensible for growth, MyoB-deficient conidia exhibit decreased viability and the deletion mutants is hypovirulent in a cyclophosphamide/corticosteroid mouse model of IA. Similarly, hyphal extension requires MyoE function, and, as a result, MyoE deletion mutants are also hypovirulent [151].
Aspergillus hyphae are able to penetrate the epithelial barrier by tunneling into the epithelial cells without impacting tissue integrity [152]. Additionally, A. fumigatus is able to coopt membrane proteins to enable uptake by epithelial and endothelial cells. The thaumatin-like protein CalA interacts with host cell integrins to enable its invasion by inducing fungal endocytosis into non-hematopoietic cells. In immunocompromised mice, CalA deletion mutant infection results in longer survival, reduced burden and less tissue invasion. This phenotype can be recapitulated with wildtype A. fumigatus strains by blocking CalA with an antibody [153].
Examples of A. fumigatus Immune Evasion
A. fumigatus completes its life cycle outside the human host and, as stated, above virulence attributes reflect adaptations to its natural habitat (i.e., decaying organic matter). Some of these attributes enable the fungus to counter and evade host defense mechanisms in the mammalian respiratory tract.
Melanin removal and exposure of immune reactive cell wall polysaccharides during germination precede the induction of LAP. In accordance with this model, the hypovirulent phenotype of amelanotic conidia is reversed when LAP is impaired [105]. Deletion of the fungal cell wall protein, CcpA enhances the immunoreactivity of swollen conidia, resulting in attenuated virulence in a murine corticosteroid model of IA. This finding suggests that CcpA, like hydrophobins in resting conidia, conceal the surface expression of immunoreactive polysaccharides [154].
Evasive hyphal branching represents a hyphal response to noxious stimuli that may serve as an immune evasive mechanism in human tissues. In vitro, A. fumigatus hyphae generate evasive branches upon interaction with neutrophils. The in vivo implications of this growth phenotype remain unclear [155].
The A. fumigatus metalloprotease Mep1p is released upon fungal interactions with collagen and can degrade various complement components. In vitro, Mep1p reduces complement deposition on conidia and the ensuing neutrophil phagocytosis. However, in a cyclophosphamide model of IA, Mep1-deficient conidia displayed no difference in virulence than wild-type conidia, suggesting that Mep1p-dependent effects on host complement components does not alter disease outcomes [156].
Conclusion
IA has emerged as an unmet medical need due to advances in medical technologies that have increased the at-risk population. Despite improvements to the standard of care and the use of triazole drugs, IA mortality remains unacceptably high. Moreover, azole-resistant Aspergillus isolates are increasing in prevalence in the clinic, likely due to agricultural use of azole drugs [157]. Recent advances in understanding the molecular and cellular determinants of antifungal immunity and the tissue-specific and metabolic determinants of fungal pathogenesis have revealed new targets for antifungal therapeutic strategies, including small molecules which target the cell wall, the mitochondria, and pyrimidine biosynthesis, and even Dectin-1 directed CAR-T cells [158]. Future research will need to emphasize clinical translation of these results to improve IA outcomes in vulnerable patient groups.
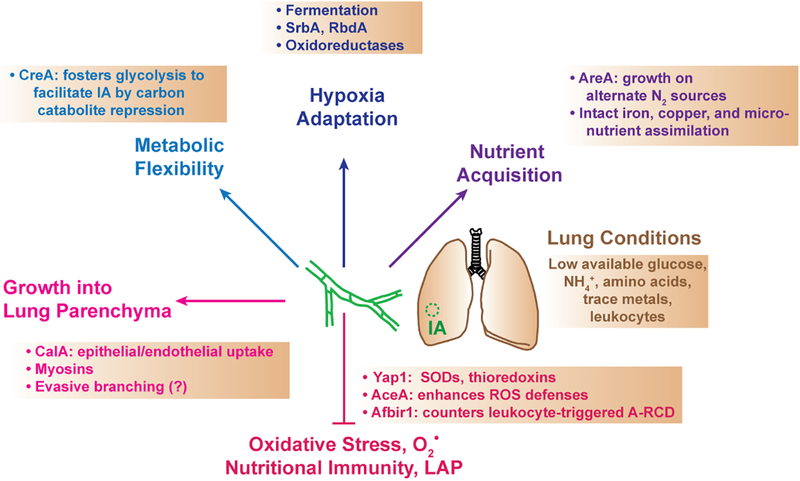
Figure 3. Aspergillus adaptations and gene programs that promote invasive pulmonary aspergillosis.
Aspergillus can establish IA under specific host conditions by overcoming scarce nutrient availability, exhibiting metabolic flexibility and adaptation of tissue hypoxia, invading the lung parenchyma, and neutralizing oxidative and non-oxidative host defense mechanisms. The panels indicate a selected set of fungal genes and fungal properties that are essential to promote invasive disease and to counter host defense mechanisms. SODs, superoxide dismutases; ROS, reactive oxygen species; A-RCD, apoptosis-like regulated cell death; LAP, LC3-associated phagocytosis.
Highlights.
Invasive aspergillosis, a mold infection, is an emerging infectious disease worldwide
Impaired innate immune function represents the major risk factor for aspergillosis
Fungal cell wall recognition initiates sterilizing immunity in the lung
The host respiratory burst triggers a gene-dependent fungal cell death pathway
Fungal metabolic flexibility and growth under hypoxic and oxidative stress promote invasive disease
Acknowledgements
TMH is supported by NIH grants RO1 AI093808, RO1 AI139632, P30 CA008748 (to MSKCC) and a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES.
- [1].Lionakis MS, Iliev ID, Hohl TM, Immunity against fungi, JCI Insight. 2 (2017). doi: 10.1172/jci.insight.93156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Segal BH, Aspergillosis N Engl. J. Med 360 (2009) 1870–1884. doi: 10.1056/NEJMra0808853. [DOI] [PubMed] [Google Scholar]
- [3].Dagenais TRT, Keller NP, Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis, Clin. Microbiol. Rev 22 (2009) 447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hohl TM, Feldmesser M, Aspergillus fumigatus: principles of pathogenesis and host defense, Eukaryotic Cell. 6 (2007) 1953–1963. doi: 10.1128/EC.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bongomin F, Gago S, Oladele RO, Denning DW, Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision, J Fungi (Basel). 3 (2017) 57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marr KA, Carter RA, Crippa F, Wald A, Corey L, Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients, Clin. Infect. Dis 34 (2002) 909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- [7].Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, et al. , Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma, Cancer Cell. 31 (2017) 833–843.e5. doi: 10.1016/j.ccell.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].van de Veerdonk FL, Kolwijck E, Lestrade PPA, Hodiamont CJ, Rijnders BJA, van Paassen J, et al. , Influenza-Associated Aspergillosis in Critically Ill Patients, Am. J. Respir. Crit. Care Med. 196 (2017) 524–527. doi: 10.1164/rccm.201612-2540LE. [DOI] [PubMed] [Google Scholar]
- [9].Baddley JW, Clinical risk factors for invasive aspergillosis, Med. Mycol 49 Suppl 1 (2011) S7–S12. doi: 10.3109/13693786.2010.505204. [DOI] [PubMed] [Google Scholar]
- [10].Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. , Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria, Clin. Exp. Allergy 43 (2013) 850–873. doi: 10.1111/cea.12141. [DOI] [PubMed] [Google Scholar]
- [11].Shah A, Panjabi C, Allergic Bronchopulmonary Aspergillosis: A Perplexing Clinical Entity, Allergy Asthma Immunol Res. 8 (2016) 282–297. doi: 10.4168/aair.2016.8.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kamei K, Watanabe A, Aspergillus mycotoxins and their effect on the host, Med. Mycol 43 Suppl 1 (2005) S95–9. [DOI] [PubMed] [Google Scholar]
- [13].Seyedmousavi S, Lionakis MS, Parta M, Peterson SW, Kwon-Chung KJ, Emerging Aspergillus Species Almost Exclusively Associated With Primary Immunodeficiencies, Open Forum Infect Dis. 5 (2018) ofy213. doi: 10.1093/ofid/ofy213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Erwig LP, Gow NAR, Interactions of fungal pathogens with phagocytes, Nat. Rev. Microbiol 14 (2016) 163–176. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- [15].Pal AK, Gajjar DU, Vasavada AR, DOPA and DHN pathway orchestrate melanin synthesis in Aspergillus species, Med. Mycol 52 (2014) 10–18. doi: 10.3109/13693786.2013.826879. [DOI] [PubMed] [Google Scholar]
- [16].Gow NAR, Latgé J-P, Munro CA, The Fungal Cell Wall: Structure, Biosynthesis, and Function, Microbiol Spectr. 5 (2017) 267–292. doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PubMed] [Google Scholar]
- [17].Latgé J-P, Beauvais A, Chamilos G, The Cell Wall of the Human Fungal Pathogen Aspergillus fumigatus: Biosynthesis, Organization, Immune Response, and Virulence, Annu. Rev. Microbiol 71 (2017) 99–116. doi: 10.1146/annurev-micro-030117-020406. [DOI] [PubMed] [Google Scholar]
- [18].Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, et al. , Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display, PLoS Pathog. 1 (2005) e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, et al. , The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus, PLoS Pathog. 1 (2005) e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brown GD, Willment JA, Whitehead L, C-type lectins in immunity and homeostasis, Nat. Rev. Immunol 18 (2018) 374–389. doi: 10.1038/s41577-018-0004-8. [DOI] [PubMed] [Google Scholar]
- [21].Jhingran A, Kasahara S, Shepardson KM, Junecko BAF, Heung LJ, Kumasaka DK, et al. , Compartment-specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection, PLoS Pathog. 11 (2015) e1004589. doi: 10.1371/journal.ppat.1004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gersuk GM, Underhill DM, Zhu L, Marr KA, Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states, J. Immunol 176 (2006) 3717–3724. [DOI] [PubMed] [Google Scholar]
- [23].Drummond RA, Saijo S, Iwakura Y, Brown GD, The role of Syk/CARD9 coupled C-type lectins in antifungal immunity, Eur. J. Immunol 41 (2011) 276–281. doi: 10.1002/eji.201041252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, et al. , Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus, Infect. Immun 80 (2012) 410–417. doi: 10.1128/IAI.05939-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tomalka J, Hise AG, Inflammasomes in aspergillosis--it takes two to tango, Cell Host Microbe. 17 (2015) 290–292. doi: 10.1016/j.chom.2015.02.017. [DOI] [PubMed] [Google Scholar]
- [26].Saïd-Sadier N, Padilla E, Langsley G, Ojcius DM, Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase, PLoS ONE. 5 (2010) e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Karki R, Man SM, Malireddi RKS, Gurung P, Vogel P, Lamkanfi M, et al. , Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection, Cell Host Microbe. 17 (2015) 357–368. doi: 10.1016/j.chom.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, et al. , Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome, Nat. Immunol 13 (2012) 246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- [29].Sun Y, Abbondante S, Karmakar M, de Jesus Carrion S, Che C, Hise AG, et al. , Neutrophil Caspase-11 Is Required for Cleavage of Caspase-1 and Secretion of IL-1β in Aspergillus fumigatus Infection, J. Immunol 201 (2018) 2767–2775. doi: 10.4049/jimmunol.1701195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Man SM, Karki R, Briard B, Burton A, Gingras S, Pelletier S, et al. , Differential roles of caspase-1 and caspase-11 in infection and inflammation, Sci Rep. 7 (2017) 45126. doi: 10.1038/srep45126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Caffrey AK, Lehmann MM, Zickovich JM, Espinosa V, Shepardson KM, Watschke CP, et al. , IL-1α signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge, PLoS Pathog. 11 (2015) e1004625. doi: 10.1371/journal.ppat.1004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jhingran A, Mar KB, Kumasaka DK, Knoblaugh SE, Ngo LY, Segal BH, et al. , Tracing conidial fate and measuring host cell antifungal activity using a reporter of microbial viability in the lung, Cell Rep. 2 (2012) 1762–1773. doi: 10.1016/j.celrep.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rieber N, Gazendam RP, Freeman AF, Hsu AP, Collar AL, Sugui JA, et al. , Extrapulmonary Aspergillus infection in patients with CARD9 deficiency, JCI Insight. 1 (2016) e89890. doi: 10.1172/jci.insight.89890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Goyal S, Castrillón-Betancur JC, Klaile E, Slevogt H, The Interaction of Human Pathogenic Fungi With C-Type Lectin Receptors, Front Immunol. 9 (2018) 1261. doi: 10.3389/fimmu.2018.01261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McGreal EP, Rosas M, Brown GD, Zamze S, Wong SYC, Gordon S, et al. , The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose, Glycobiology. 16 (2006) 422–430. doi: 10.1093/glycob/cwj077. [DOI] [PubMed] [Google Scholar]
- [36].Taylor PR, Roy S, Leal SM, Sun Y, Howell SJ, Cobb BA, et al. , Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2, Nat. Immunol 15 (2014) 143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sun H, Xu X-Y, Shao H-T, Su X, Wu X-D, Wang Q, et al. , Dectin-2 is predominately macrophage restricted and exhibits conspicuous expression during Aspergillus fumigatus invasion in human lung, Cell. Immunol 284 (2013) 60–67. doi: 10.1016/j.cellimm.2013.06.013. [DOI] [PubMed] [Google Scholar]
- [38].Guo Y, Chang Q, Cheng L, Xiong S, Jia X, Lin X, et al. , C-Type Lectin Receptor CD23 Is Required for Host Defense against Candida albicans and Aspergillus fumigatus Infection, J. Immunol 201 (2018) 2427–2440. doi: 10.4049/jimmunol.1800620. [DOI] [PubMed] [Google Scholar]
- [39].Stappers MHT, Clark AE, Aimanianda V, Bidula S, Reid DM, Asamaphan P, et al. , Recognition of DHN-melanin by a C-type lectin receptor is required for immunity to Aspergillus, Nature. 555 (2018) 382–386. doi: 10.1038/nature25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wong SSW, Rani M, Dodagatta-Marri E, Ibrahim-Granet O, Kishore U, Bayry J, et al. , Fungal melanin stimulates surfactant protein D-mediated opsonization of and host immune response to Aspergillus fumigatus spores, J. Biol. Chem 293 (2018) 4901–4912. doi: 10.1074/jbc.M117.815852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].O’Brien XM, Heflin KE, Lavigne LM, Yu K, Kim M, Salomon AR, et al. , Lectin site ligation of CR3 induces conformational changes and signaling, J. Biol. Chem 287 (2012) 3337–3348. doi: 10.1074/jbc.M111.298307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Leal SM, Vareechon C, Cowden S, Cobb BA, Latgé J-P, Momany M, et al. , Fungal antioxidant pathways promote survival against neutrophils during infection, J. Clin. Invest 122 (2012) 2482–2498. doi: 10.1172/JCI63239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mizgerd JP, Kubo H, Kutkoski GJ, Bhagwan SD, Scharffetter-Kochanek K, Beaudet AL, et al. , Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice, J. Exp. Med 186 (1997) 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Clark HL, Abbondante S, Minns MS, Greenberg EN, Sun Y, Pearlman E, Protein Deiminase 4 and CR3 Regulate Aspergillus fumigatus and β-Glucan-Induced Neutrophil Extracellular Trap Formation, but Hyphal Killing Is Dependent Only on CR3, Front Immunol. 9 (2018) 1182. doi: 10.3389/fimmu.2018.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, et al. , Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response, Nature. 420 (2002) 182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- [46].Jaillon S, Peri G, Delneste Y, Frémaux I, Doni A, Moalli F, et al. , The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps, J. Exp. Med 204 (2007) 793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Genster N, Præstekjær Cramer E, Rosbjerg A, Pilely K, Cowland JB, Garred P, Ficolins Promote Fungal Clearance in vivo and Modulate the Inflammatory Cytokine Response in Host Defense against Aspergillus fumigatus, J Innate Immun. 8 (2016) 579–588. doi: 10.1159/000447714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Snarr BD, Qureshi ST, Sheppard DC, Immune Recognition of Fungal Polysaccharides, J Fungi (Basel). 3 (2017) 47. doi: 10.3390/jof3030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jepsen CS, Dubey LK, Colmorten KB, Moeller JB, Hammond MA, Nielsen O, et al. , FIBCD1 Binds Aspergillus fumigatus and Regulates Lung Epithelial Response to Cell Wall Components, Front Immunol. 9 (2018) 1967. doi: 10.3389/fimmu.2018.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gravelat FN, Beauvais A, Liu H, Lee MJ, Snarr BD, Chen D, et al. , Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal β-glucan from the immune system, PLoS Pathog. 9 (2013) e1003575. doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kerr SC, Fischer GJ, Sinha M, McCabe O, Palmer JM, Choera T, et al. , FleA Expression in Aspergillus fumigatus Is Recognized by Fucosylated Structures on Mucins and Macrophages to Prevent Lung Infection, PLoS Pathog. 12 (2016) e1005555. doi: 10.1371/journal.ppat.1005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fisher CE, Hohl TM, Fan W, Storer BE, Levine DM, Zhao LP, et al. , Validation of single nucleotide polymorphisms in invasive aspergillosis following hematopoietic cell transplantation, Blood. 129 (2017) 2693–2701. doi: 10.1182/blood-2016-10-743294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Park SJ, Mehrad B, Innate immunity to Aspergillus species, Clin. Microbiol. Rev 22 (2009) 535–551. doi: 10.1128/CMR.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Balloy V, Si-Tahar M, Takeuchi O, Philippe B, Nahori M-A, Tanguy M, et al. , Involvement of toll-like receptor 2 in experimental invasive pulmonary aspergillosis, Infect. Immun 73 (2005) 5420–5425. doi: 10.1128/IAI.73.9.5420-5425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, et al. , The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo, J. Immunol 172 (2004) 3059–3069. [DOI] [PubMed] [Google Scholar]
- [56].Bochud P-Y, Chien JW, Marr KA, Leisenring WM, Upton A, Janer M, et al. , Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation, N. Engl. J. Med 359 (2008) 1766–1777. doi: 10.1056/NEJMoa0802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Herbst S, Shah A, Mazon Moya M, Marzola V, Jensen B, Reed A, et al. , Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus, EMBO Mol Med. 7 (2015) 240–258. doi: 10.15252/emmm.201404556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zelante T, Wong AYW, Mencarelli A, Foo S, Zolezzi F, Lee B, et al. , Impaired calcineurin signaling in myeloid cells results in downregulation of pentraxin-3 and increased susceptibility to aspergillosis, Mucosal Immunol. 10 (2017) 470–480. doi: 10.1038/mi.2016.52. [DOI] [PubMed] [Google Scholar]
- [59].Strijbis K, Tafesse FG, Fairn GD, Witte MD, Dougan SK, Watson N, et al. , Bruton’s Tyrosine Kinase (BTK) and Vav1 contribute to Dectin1-dependent phagocytosis of Candida albicans in macrophages, PLoS Pathog. 9 (2013) e1003446. doi: 10.1371/journal.ppat.1003446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ghez D, Calleja A, Protin C, Baron M, Ledoux M-P, Damaj G, et al. , Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib, Blood. 131 (2018) 1955–1959. doi: 10.1182/blood-2017-11-818286. [DOI] [PubMed] [Google Scholar]
- [61].Varughese T, Taur Y, Cohen N, Palomba ML, Seo SK, Hohl TM, et al. , Serious Infections in Patients Receiving Ibrutinib for Treatment of Lymphoid Malignancies, Clin. Infect. Dis 31 (2018) 88. doi: 10.1093/cid/ciy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chamilos G, Lionakis MS, Kontoyiannis DP, Call for Action: Invasive Fungal Infections Associated With Ibrutinib and Other Small Molecule Kinase Inhibitors Targeting Immune Signaling Pathways, Clin. Infect. Dis 66 (2018) 140–148. doi: 10.1093/cid/cix687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bercusson A, Colley T, Shah A, Warris A, Armstrong-James D, Ibrutinib blocks Btk-dependent NF-ĸB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis, Blood. 132 (2018) 1985–1988. doi: 10.1182/blood-2017-12-823393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mircescu MM, Lipuma L, van Rooijen N, Pamer EG, Hohl TM, Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection, J. Infect. Dis 200 (2009) 647–656. doi: 10.1086/600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, et al. , Inflammatory monocytes orchestrate innate antifungal immunity in the lung, PLoS Pathog. 10 (2014) e1003940. doi: 10.1371/journal.ppat.1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kontoyiannis DP, Selleslag D, Mullane K, Cornely OA, Hope W, Lortholary O, et al. , Impact of unresolved neutropenia in patients with neutropenia and invasive aspergillosis: a post hoc analysis of the SECURE trial, J. Antimicrob. Chemother 73 (2017) 757–763. doi: 10.1093/jac/dkx423. [DOI] [PubMed] [Google Scholar]
- [67].Garcia-Vidal C, Upton A, Kirby KA, Marr KA, Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation, Clin. Infect. Dis 47 (2008) 1041–1050. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Verma A, Wüthrich M, Deepe G, Klein B, Adaptive immunity to fungi, Cold Spring Harb Perspect Med. 5 (2014) a019612–a019612. doi: 10.1101/cshperspect.a019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bhatia S, Fei M, Yarlagadda M, Qi Z, Akira S, Saijo S, et al. , Rapid host defense against Aspergillus fumigatus involves alveolar macrophages with a predominance of alternatively activated phenotype, PLoS ONE. 6 (2011) e15943. doi: 10.1371/journal.pone.0015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Espinosa V, Dutta O, McElrath C, Du P, Chang Y-J, Cicciarelli B, et al. , Type III interferon is a critical regulator of innate antifungal immunity, Sci Immunol. 2 (2017) eaan5357. doi: 10.1126/sciimmunol.aan5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Seyedmousavi S, Davis MJ, Sugui JA, Pinkhasov T, Moyer S, Salazar AM, et al. , Exogenous Stimulation of Type I Interferon Protects Mice with Chronic Granulomatous Disease from Aspergillosis through Early Recruitment of Host-Protective Neutrophils into the Lung, MBio. 9 (2018) e00422–18. doi: 10.1128/mBio.00422-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kasahara S, Jhingran A, Dhingra S, Salem A, Cramer RA, Hohl TM, Role of Granulocyte-Macrophage Colony-Stimulating Factor Signaling in Regulating Neutrophil Antifungal Activity and the Oxidative Burst During Respiratory Fungal Challenge, J. Infect. Dis 213 (2016) 1289–1298. doi: 10.1093/infdis/jiw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kandalla PK, Sarrazin S, Molawi K, Berruyer C, Redelberger D, Favel A, et al. , M-CSF improves protection against bacterial and fungal infections after hematopoietic stem/progenitor cell transplantation, J. Exp. Med 213 (2016) 2269–2279. doi: 10.1084/jem.20151975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Fites JS, Gui M, Kernien JF, Negoro P, Dagher Z, Sykes DB, et al. , An unappreciated role for neutrophil-DC hybrids in immunity to invasive fungal infections, PLoS Pathog. 14 (2018) e1007073. doi: 10.1371/journal.ppat.1007073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ramirez-Ortiz ZG, Lee CK, Wang JP, Boon L, Specht CA, Levitz SM, A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus, Cell Host Microbe. 9 (2011) 415–424. doi: 10.1016/j.chom.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Loures FV, Röhm M, Lee CK, Santos E, Wang JP, Specht CA, et al. , Recognition of Aspergillus fumigatus hyphae by human plasmacytoid dendritic cells is mediated by dectin-2 and results in formation of extracellular traps, PLoS Pathog. 11 (2015) e1004643. doi: 10.1371/journal.ppat.1004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Morrison BE, Park SJ, Mooney JM, Mehrad B, Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis, J. Clin. Invest 112 (2003) 1862–1870. doi: 10.1172/JCI18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ziegler S, Weiß E, Schmitt A-L, Schlegel J, Burgert A, Terpitz U, et al. , CD56 Is a Pathogen Recognition Receptor on Human Natural Killer Cells, Sci Rep. 7 (2017) 6138. doi: 10.1038/s41598-017-06238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Santiago V, Rezvani K, Sekine T, Stebbing J, Kelleher P, Armstrong-James D, Human NK Cells Develop an Exhaustion Phenotype During Polar Degranulation at the Aspergillus fumigatus Hyphal Synapse, Front Immunol. 9 (2018) 2344. doi: 10.3389/fimmu.2018.02344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Weiß E, Ziegler S, Fliesser M, Schmitt A-L, Hünniger K, Kurzai O, et al. , First Insights in NK-DC Cross-Talk and the Importance of Soluble Factors During Infection With Aspergillus fumigatus, Front Cell Infect Microbiol. 8 (2018) 288. doi: 10.3389/fcimb.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Speth C, Rambach G, Lass-Flörl C, Platelet immunology in fungal infections, Thromb. Haemost 112 (2014) 632–639. doi: 10.1160/TH14-01-0074. [DOI] [PubMed] [Google Scholar]
- [82].Czakai K, Dittrich M, Kaltdorf M, Müller T, Krappmann S, Schedler A, et al. , Influence of Platelet-rich Plasma on the immune response of human monocyte-derived dendritic cells and macrophages stimulated with Aspergillus fumigatus, Int. J. Med. Microbiol 307 (2017) 95–107. doi: 10.1016/j.ijmm.2016.11.010. [DOI] [PubMed] [Google Scholar]
- [83].Frealle E, Gosset P, Leroy S, Delattre C, Wacrenier A, Zenzmaier C, et al. , In vitro coagulation triggers anti-Aspergillus fumigatus neutrophil response, Future Microbiol. 13 (2018) 659–669. doi: 10.2217/fmb-2017-0190. [DOI] [PubMed] [Google Scholar]
- [84].Rosenhagen M, Feldhues R, Schmidt J, Hoppe-Tichy T, Geiss HK, A risk profile for invasive aspergillosis in liver transplant recipients, Infection. 37 (2009) 313–319. doi: 10.1007/s15010-008-8124-x. [DOI] [PubMed] [Google Scholar]
- [85].Lien M-Y, Chou C-H, Lin C-C, Bai L-Y, Chiu C-F, Yeh S-P, et al. , Epidemiology and risk factors for invasive fungal infections during induction chemotherapy for newly diagnosed acute myeloid leukemia: A retrospective cohort study, PLoS ONE. 13 (2018) e0197851. doi: 10.1371/journal.pone.0197851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nouér SA, Nucci M, Kumar NS, Grazziutti M, Restrepo A, Anaissie E, Baseline platelet count and creatinine clearance rate predict the outcome of neutropenia-related invasive aspergillosis, Clin. Infect. Dis 54 (2012) e173–83. doi: 10.1093/cid/cis298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, et al. , Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes, Cell Host Microbe. 12 (2012) 223–232. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. , mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity, Science. 345 (2014) 1250684–1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Savers A, Rasid O, Parlato M, Brock M, Jouvion G, Ryffel B, et al. , Infection-Mediated Priming of Phagocytes Protects against Lethal Secondary Aspergillus fumigatus Challenge, PLoS ONE. 11 (2016) e0153829. doi: 10.1371/journal.pone.0153829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].McAleer JP, Nguyen NLH, Chen K, Kumar P, Ricks DM, Binnie M, et al. , Pulmonary Th17 Antifungal Immunity Is Regulated by the Gut Microbiome, J. Immunol 197 (2016) 97–107. doi: 10.4049/jimmunol.1502566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gazendam RP, van de Geer A, Roos D, van den Berg TK, Kuijpers TW, How neutrophils kill fungi, Immunol. Rev 273 (2016) 299–311. doi: 10.1111/imr.12454. [DOI] [PubMed] [Google Scholar]
- [92].Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. , Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens, Nat. Immunol 15 (2014) 1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Gazendam RP, van Hamme JL, Tool ATJ, Hoogenboezem M, van den Berg JM, Prins JM, et al. , Human Neutrophils Use Different Mechanisms To Kill Aspergillus fumigatus Conidia and Hyphae: Evidence from Phagocyte Defects, J. Immunol 196 (2016) 1272–1283. doi: 10.4049/jimmunol.1501811. [DOI] [PubMed] [Google Scholar]
- [94].Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A, et al. , Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA, PLoS Pathog. 6 (2010) e1000873. doi: 10.1371/journal.ppat.1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Shlezinger N, Irmer H, Dhingra S, Beattie SR, Cramer RA, Braus GH, et al. , Sterilizing immunity in the lung relies on targeting fungal apoptosis-like programmed cell death, Science. 357 (2017) 1037–1041. doi: 10.1126/science.aan0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chai J, Shiozaki E, Srinivasula SM, Wu Q, Datta P, Alnemri ES, et al. , Structural basis of caspase-7 inhibition by XIAP, Cell. 104 (2001) 769–780. [DOI] [PubMed] [Google Scholar]
- [97].Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, et al. , Mechanism of XIAP-mediated inhibition of caspase-9, Mol. Cell 11 (2003) 519–527. [DOI] [PubMed] [Google Scholar]
- [98].Shlezinger N, Goldfinger N, Sharon A, Apoptotic-like programed cell death in fungi: the benefits in filamentous species, Front Oncol. 2 (2012) 97. doi: 10.3389/fonc.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gonçalves AP, Heller J, Daskalov A, Videira A, Glass NL, Regulated Forms of Cell Death in Fungi, Front Microbiol. 8 (2017) 1837. doi: 10.3389/fmicb.2017.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Carmona-Gutierrez D, Bauer MA, Zimmermann A, Aguilera A, Austriaco N, Ayscough K, et al. , Guidelines and recommendations on yeast cell death nomenclature, Microb Cell. 5 (2018) 4–31. doi: 10.15698/mic2018.01.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zarember KA, Sugui JA, Chang YC, Kwon-Chung KJ, Gallin JI, Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion, J. Immunol 178 (2007) 6367–6373. [DOI] [PubMed] [Google Scholar]
- [102].Chamilos G, Akoumianaki T, Kyrmizi I, Brakhage A, Beauvais A, Latgé J-P, Melanin targets LC3-associated phagocytosis (LAP): A novel pathogenetic mechanism in fungal disease, Autophagy. 12 (2016) 888–889. doi: 10.1080/15548627.2016.1157242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Martinez J, Malireddi RKS, Lu Q, Cunha LD, Pelletier S, Gingras S, et al. , Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins, Nat. Cell Biol. 17 (2015) 893–906. doi: 10.1038/ncb3192. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [104].Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MAO, et al. , Activation of antibacterial autophagy by NADPH oxidases, Proc. Natl. Acad. Sci. U.S.a. 106 (2009) 6226–6231. doi: 10.1073/pnas.0811045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Akoumianaki T, Kyrmizi I, Valsecchi I, Gresnigt MS, Samonis G, Drakos E, et al. , Aspergillus Cell Wall Melanin Blocks LC3-Associated Phagocytosis to Promote Pathogenicity, Cell Host Microbe. 19 (2016) 79–90. doi: 10.1016/j.chom.2015.12.002. [DOI] [PubMed] [Google Scholar]
- [106].Desoubeaux G, Cray C, Rodent Models of Invasive Aspergillosis due to Aspergillus fumigatus: Still a Long Path toward Standardization, Front Microbiol. 8 (2017) 841. doi: 10.3389/fmicb.2017.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hohl TM, Overview of vertebrate animal models of fungal infection, J. Immunol. Methods 410 (2014) 100–112. doi: 10.1016/j.jim.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hohl TM, Immune responses to invasive aspergillosis: new understanding and therapeutic opportunities, Curr. Opin. Infect. Dis 30 (2017) 364–371. doi: 10.1097/QCO.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sugui JA, Rose SR, Nardone G, Swamydas M, Lee C-CR, Kwon-Chung KJ, et al. , Host immune status-specific production of gliotoxin and bis-methyl-gliotoxin during invasive aspergillosis in mice, Sci Rep. 7 (2017) 10977. doi: 10.1038/s41598-017-10888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kalleda N, Amich J, Arslan B, Poreddy S, Mattenheimer K, Mokhtari Z, et al. , Dynamic Immune Cell Recruitment After Murine Pulmonary Aspergillus fumigatus Infection under Different Immunosuppressive Regimens, Front Microbiol. 7 (2016) 1107. doi: 10.3389/fmicb.2016.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Iannitti RG, Napolioni V, Oikonomou V, De Luca A, Galosi C, Pariano M, et al. , IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis, Nat Commun. 7 (2016) 10791. doi: 10.1038/ncomms10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gresnigt MS, Rekiki A, Rasid O, Savers A, Jouvion G, Dannaoui E, et al. , Reducing hypoxia and inflammation during invasive pulmonary aspergillosis by targeting the Interleukin-1 receptor, Sci Rep. 6 (2016) 26490. doi: 10.1038/srep26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Bilgin E, Erden A, Kilic L, Sari A, Armagan B, Kalyoncu U, et al. , Aspergillus Pneumonia in a Patient With Adult-Onset Still Disease Successfully Treated With Anakinra, J Clin Rheumatol. 24 (2018) 156–158. doi: 10.1097/RHU.0000000000000631. [DOI] [PubMed] [Google Scholar]
- [114].Oikonomou V, Moretti S, Renga G, Galosi C, Borghi M, Pariano M, et al. , Noncanonical Fungal Autophagy Inhibits Inflammation in Response to IFN-γ via DAPK1, Cell Host Microbe. 20 (2016) 744–757. doi: 10.1016/j.chom.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].De Luca A, Smeekens SP, Casagrande A, Iannitti R, Conway KL, Gresnigt MS, et al. , IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans, Proc. Natl. Acad. Sci. U.S.a. 111 (2014) 3526–3531. doi: 10.1073/pnas.1322831111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Tanaka RJ, Boon NJ, Vrcelj K, Nguyen A, Vinci C, Armstrong-James D, et al. , In silico modeling of spore inhalation reveals fungal persistence following low dose exposure, Sci Rep. 5 (2015) 13958. doi: 10.1038/srep13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Tekaia F, Latgé J-P, Aspergillus fumigatus: saprophyte or pathogen? Curr. Opin. Microbiol 8 (2005) 385–392. doi: 10.1016/j.mib.2005.06.017. [DOI] [PubMed] [Google Scholar]
- [118].Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, et al. , Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus, Nature. 438 (2005) 1151–1156. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- [119].Keller NP, Heterogeneity Confounds Establishment of “a” Model Microbial Strain, MBio. 8 (2017) 141. doi: 10.1128/mBio.00135-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rosowski EE, Raffa N, Knox BP, Golenberg N, Keller NP, Huttenlocher A, Macrophages inhibit Aspergillus fumigatus germination and neutrophil-mediated fungal killing, PLoS Pathog. 14 (2018) e1007229. doi: 10.1371/journal.ppat.1007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Caffrey-Carr AK, Kowalski CH, Beattie SR, Blaseg NA, Upshaw CR, Thammahong A, et al. , IL-1α is Critical for Resistance Against Highly Virulent Aspergillus fumigatus Isolates, Infect. Immun 85 (2017) 1870. doi: 10.1128/IAI.00661-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Rizzetto L, Giovannini G, Bromley M, Bowyer P, Romani L, Cavalieri D, Strain dependent variation of immune responses to A. fumigatus: definition of pathogenic species, PLoS ONE. 8 (2013) e56651. doi: 10.1371/journal.pone.0056651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Caffrey-Carr AK, Hilmer KM, Kowalski CH, Shepardson KM, Temple RM, Cramer RA, et al. , Host-Derived Leukotriene B4 Is Critical for Resistance against Invasive Pulmonary Aspergillosis, Front Immunol. 8 (2017) 1984. doi: 10.3389/fimmu.2017.01984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gonsalves CS, Kalra VK, Hypoxia-mediated expression of 5-lipoxygenase-activating protein involves HIF-1alpha and NF-kappaB and microRNAs 135a and 199a-5p, J. Immunol 184 (2010) 3878–3888. doi: 10.4049/jimmunol.0902594. [DOI] [PubMed] [Google Scholar]
- [125].Kowalski CH, Beattie SR, Fuller KK, McGurk EA, Tang Y-W, Hohl TM, et al. , Heterogeneity among Isolates Reveals that Fitness in Low Oxygen Correlates with Aspergillus fumigatus Virulence, MBio. 7 (2016) 3080. doi: 10.1128/mBio.01515-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ries LNA, Beattie S, Cramer RA, Goldman GH, Overview of carbon and nitrogen catabolite metabolism in the virulence of human pathogenic fungi, Mol. Microbiol 107 (2018) 277–297. doi: 10.1111/mmi.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Barth AL, Pitt TL, The high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pseudomonas aeruginosa, J. Med. Microbiol 45 (1996) 110–119. doi: 10.1099/00222615-45-2-110. [DOI] [PubMed] [Google Scholar]
- [128].Ene IV, Brunke S, Brown AJP, Hube B, Metabolism in fungal pathogenesis, Cold Spring Harb Perspect Med. 4 (2014) a019695–a019695. doi: 10.1101/cshperspect.a019695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Smith DJ, Anderson GJ, Bell SC, Reid DW, Elevated metal concentrations in the CF airway correlate with cellular injury and disease severity, J. Cyst. Fibros 13 (2014) 289–295. doi: 10.1016/j.jcf.2013.12.001. [DOI] [PubMed] [Google Scholar]
- [130].Skaar EP, Raffatellu M, Metals in infectious diseases and nutritional immunity, Metallomics. 7 (2015) 926–928. doi: 10.1039/c5mt90021b. [DOI] [PubMed] [Google Scholar]
- [131].Schrettl M, Beckmann N, Varga J, Heinekamp T, Jacobsen ID, Jöchl C, et al. , HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus, PLoS Pathog. 6 (2010) e1001124. doi: 10.1371/journal.ppat.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Hsu JL, Manouvakhova OV, Clemons KV, Inayathullah M, Tu AB, Sobel RA, et al. , Microhemorrhage-associated tissue iron enhances the risk for Aspergillus fumigatus invasion in a mouse model of airway transplantation, Sci Transl Med. 10 (2018) eaag2616. doi: 10.1126/scitranslmed.aag2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Jung M-G, Kim SS, Yu J-H, Shin K-S, Characterization of gprK Encoding a Putative Hybrid G-Protein-Coupled Receptor in Aspergillus fumigatus, PLoS ONE. 11 (2016) e0161312. doi: 10.1371/journal.pone.0161312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Beattie SR, Mark KMK, Thammahong A, Ries LNA, Dhingra S, Caffrey-Carr AK, et al. , Filamentous fungal carbon catabolite repression supports metabolic plasticity and stress responses essential for disease progression, PLoS Pathog. 13 (2017) e1006340. doi: 10.1371/journal.ppat.1006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Hensel M, Arst HN, Aufauvre-Brown A, Holden DW, The role of the Aspergillus fumigatus areA gene in invasive pulmonary aspergillosis, Mol. Gen. Genet 258 (1998) 553–557. [DOI] [PubMed] [Google Scholar]
- [136].Sasse A, Hamer SN, Amich J, Binder J, Krappmann S, Mutant characterization and in vivo conditional repression identify aromatic amino acid biosynthesis to be essential for Aspergillus fumigatus virulence, Virulence. 7 (2016) 56–62. doi: 10.1080/21505594.2015.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Amich J, Dümig M, O’Keeffe G, Binder J, Doyle S, Beilhack A, et al. , Exploration of Sulfur Assimilation of Aspergillus fumigatus Reveals Biosynthesis of Sulfur-Containing Amino Acids as a Virulence Determinant, Infect. Immun 84 (2016) 917–929. doi: 10.1128/IAI.01124-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Dietl A-M, Amich J, Leal S, Beckmann N, Binder U, Beilhack A, et al. , Histidine biosynthesis plays a crucial role in metal homeostasis and virulence of Aspergillus fumigatus, Virulence. 7 (2016) 465–476. doi: 10.1080/21505594.2016.1146848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Dietl A-M, Meir Z, Shadkchan Y, Osherov N, Haas H, Riboflavin and pantothenic acid biosynthesis are crucial for iron homeostasis and virulence in the pathogenic mold Aspergillus fumigatus, Virulence. 9 (2018) 1036–1049. doi: 10.1080/21505594.2018.1482181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Grahl N, Puttikamonkul S, Macdonald JM, Gamcsik MP, Ngo LY, Hohl TM, et al. , In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis, PLoS Pathog. 7 (2011) e1002145. doi: 10.1371/journal.ppat.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Dhingra S, Kowlaski CH, Thammahong A, Beattie SR, Bultman KM, Cramer RA, RbdB, a Rhomboid Protease Critical for SREBP Activation and Virulence in Aspergillus fumigatus, mSphere. 1 (2016). doi: 10.1128/mSphere.00035-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Vaknin Y, Hillmann F, Iannitti R, Ben Baruch N, Sandovsky-Losica H, Shadkchan Y, et al. , Identification and Characterization of a Novel Aspergillus fumigatus Rhomboid Family Putative Protease, RbdA, Involved in Hypoxia Sensing and Virulence, Infect. Immun 84 (2016) 1866–1878. doi: 10.1128/IAI.00011-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Kroll K, Shekhova E, Mattern DJ, Thywissen A, Jacobsen ID, Strassburger M, et al. , The hypoxia-induced dehydrogenase HorA is required for coenzyme Q10 biosynthesis, azole sensitivity and virulence of Aspergillus fumigatus, Mol. Microbiol 101 (2016) 92–108. doi: 10.1111/mmi.13377. [DOI] [PubMed] [Google Scholar]
- [144].Vethanayagam RR, Almyroudis NG, Grimm MJ, Lewandowski DC, Pham CTN, Blackwell TS, et al. , Role of NADPH oxidase versus neutrophil proteases in antimicrobial host defense, PLoS ONE. 6 (2011) e28149. doi: 10.1371/journal.pone.0028149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hillmann F, Bagramyan K, Strassburger M, Heinekamp T, Hong TB, Bzymek KP, et al. , The Crystal Structure of Peroxiredoxin Asp f3 Provides Mechanistic Insight into Oxidative Stress Resistance and Virulence of Aspergillus fumigatus, Sci Rep. 6 (2016) 33396. doi: 10.1038/srep33396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Jia X, Zhang X, Hu Y, Hu M, Tian S, Han X, et al. , Role of actin depolymerizing factor cofilin in Aspergillus fumigatus oxidative stress response and pathogenesis, Curr. Genet 64 (2018) 619–634. doi: 10.1007/s00294-017-0777-5. [DOI] [PubMed] [Google Scholar]
- [147].Jöhnk B, Bayram Ö, Abelmann A, Heinekamp T, Mattern DJ, Brakhage AA, et al. , SCF Ubiquitin Ligase F-box Protein Fbx15 Controls Nuclear Co-repressor Localization, Stress Response and Virulence of the Human Pathogen Aspergillus fumigatus, PLoS Pathog. 12 (2016) e1005899. doi: 10.1371/journal.ppat.1005899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Wang D, Wang S, He D, Gao S, Xue B, Wang L, Deletion of afpab1 Causes Increased Sensitivity to Oxidative Stress and Hypovirulence in Aspergillus fumigatus, International Journal of Molecular Sciences 2015, Vol. 16, Pages 21846–21857. 17 (2016) 1811. doi: 10.3390/ijms17111811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wiemann P, Perevitsky A, Lim FY, Shadkchan Y, Knox BP, Landero Figueora JA, et al. , Aspergillus fumigatus Copper Export Machinery and Reactive Oxygen Intermediate Defense Counter Host Copper-Mediated Oxidative Antimicrobial Offense, Cell Rep. 19 (2017) 1008–1021. doi: 10.1016/j.celrep.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Alves de Castro P, Dos Reis TF, Dolan SK, Oliveira Manfiolli A, Brown NA, Jones GW, et al. , The Aspergillus fumigatus SchASCH9 kinase modulates SakAHOG1 MAP kinase activity and it is essential for virulence, Mol. Microbiol 102 (2016) 642–671. doi: 10.1111/mmi.13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Renshaw H, Vargas-Muñiz JM, Richards AD, Asfaw YG, Juvvadi PR, Steinbach WJ, Distinct Roles of Myosins in Aspergillus fumigatus Hyphal Growth and Pathogenesis, Infect. Immun 84 (2016) 1556–1564. doi: 10.1128/IAI.01190-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Fernandes J, Hamidi F, Leborgne R, Beau R, Castier Y, Mordant P, et al. , Penetration of the Human Pulmonary Epithelium by Aspergillus fumigatus Hyphae, J. Infect. Dis 218 (2018) 1306–1313. doi: 10.1093/infdis/jiy298. [DOI] [PubMed] [Google Scholar]
- [153].Liu H, Lee MJ, Solis NV, Phan QT, Swidergall M, Ralph B, et al. , Aspergillus fumigatus CalA binds to integrin α5β1 and mediates host cell invasion, Nat Microbiol. 2 (2016) 16211. doi: 10.1038/nmicrobiol.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Voltersen V, Blango MG, Herrmann S, Schmidt F, Heinekamp T, Strassburger M, et al. , Proteome Analysis Reveals the Conidial Surface Protein CcpA Essential for Virulence of the Pathogenic Fungus Aspergillus fumigatus, MBio. 9 (2018) 862. doi: 10.1128/mBio.01557-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Ellett F, Jorgensen J, Frydman GH, Jones CN, Irimia D, Neutrophil Interactions Stimulate Evasive Hyphal Branching by Aspergillus fumigatus, PLoS Pathog. 13 (2017) e1006154. doi: 10.1371/journal.ppat.1006154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Shende R, Wong SSW, Rapole S, Beau R, Ibrahim-Granet O, Monod M, et al. , Aspergillus fumigatus conidial metalloprotease Mep1p cleaves host complement proteins, J. Biol. Chem 293 (2018) 15538–15555. doi: 10.1074/jbc.RA117.001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Berger S, El Chazli Y, Babu AF, Coste AT, Azole Resistance in Aspergillus fumigatus: A Consequence of Antifungal Use in Agriculture? Front Microbiol. 8 (2017) 1024. doi: 10.3389/fmicb.2017.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Osherov N, Kontoyiannis DP, The anti-Aspergillus drug pipeline: Is the glass half full or empty? Med. Mycol 55 (2017) 118–124. doi: 10.1093/mmy/myw060. [DOI] [PubMed] [Google Scholar]