Abstract
Patients with brain arteriovenous malformation (bAVM) are at risk of intracranial hemorrhage (ICH). Overall, bAVM accounts for 25% of hemorrhagic strokes in adults <50 years of age. The treatment of unruptured bAVMs has become controversial, because the natural history of these patients may be less morbid than invasive therapies. Available treatments include observation, surgical resection, endovascular embolization, stereotactic radiosurgery, or combination thereof. Knowing the risk factors for bAVM hemorrhage is crucial for selecting appropriate therapeutic strategies. In this review, we discussed several biological risk factors, which may contribute to bAVM hemorrhage.
Keywords: brain arteriovenous malformation, hemodynamic, intracranial hemorrhage, vascular endothelial growth factor, vascular integrity
1. INTRODUCTION
Brain arteriovenous malformations (bAVMs) are abnormal vessels that are prone to rupture causing life‐threatening intracranial hemorrhage (ICH) and long‐term disability, especially in young adults.1 About 45% of bAVM cases present with hemorrhage. However, as many as 88% of bAVM patients are asymptomatic. Currently, there is no specific and safe medical therapy available to prevent bAVM hemorrhage. Risk factors for hemorrhage have not been consistent across longitudinal studies, primarily due to small sample sizes or selection biases of cases. A continuous identification of risk factors is important as the risk of hemorrhage can vary widely from 0.9% to 34.3%, depending on the number of overlapping risk factors carried by a patient.2 Furthermore, findings from a randomized trial of unruptured bAVM (ARUBA) showed that stroke and mortality were lower in unruptured bAVM patients randomized to conservative management than patients that received any interventional therapy.3 Therefore, there is an urgent need to identify risk factors to stratify patients who would benefit most from the treatment.
Molecular characterization of resected bAVM tissue has provided evidence for involvement of angiogenic and inflammatory pathways, but a complete understanding of pathogenic pathways and determinants of disease progression remain obscure. Recent works have shown that elevations of vascular endothelial growth factor (VEGF) or alterations in the vascular wall, such as a loss of pericytes, may contribute to bAVM rupture.4, 5, 6 Abnormally high blood flow through arterial‐venous shunting has also been suggested to contribute to bAVM rupture.
This review discussed the roles of VEGF, signaling pathways involved in vascular integrity, and hemodynamic changes in bAVM hemorrhage. We have also discussed current therapeutic options and the potential direction for development of specific medical treatment in the future (Figure 1).
Figure 1.
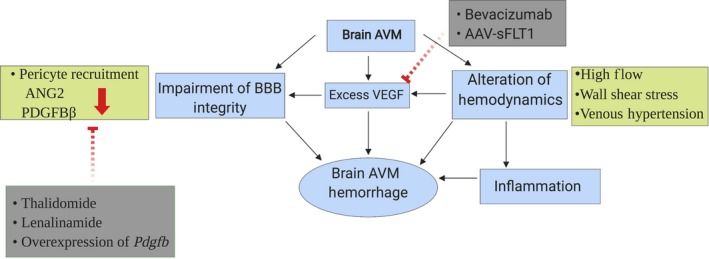
The risk factors for brain AVM hemorrhage. Brain AVMs have increased level of VEGF, reduced mural cell coverage, and altered hemodynamics. All of these increase the risk of brain AVM hemorrhage. Alteration in hemodynamics including high flow, increased wall shear stress, and VH can also induce inflammation, BBB leakage, and elevation of VEGF levels, which further increases the risk of hemorrhage. Thalidomide and lenalidomide treatment increase EC PDGFB production and pericyte recruitment. Overexpression of Pdgfb could be another therapeutic strategy to improve BBB integrity. Bevacizumab treatment and intravenous injection of AAV‐sFLT1 vectors that express the extracellular domain of VEGF receptor 1 blocks excess VEGF and inhibits the bAVM formation and progression
2. ELEVATION OF VEGF LEVEL EXACERBATES BAVM HEMORRHAGE THROUGH IMPAIRMENT OF BLOOD‐BRAIN BARRIER (BBB) INTEGRITY
The VEGF family comprises in mammals five members: VEGF‐A, placenta growth factor (PGF), VEGF‐B, VEGF‐C, and VEGF‐D. The VEGF discussed in this review is VEGF‐A.
Vascular endothelial growth factor plays a crucial role in vascular remodeling and angiogenesis. VEGF receptor 1 (VEGFR‐1) and VEGF receptor 2 (VEGFR‐2) are the two main receptors mediating VEGF angiogenesis function. The downstream players of VEGFR‐2 signaling include (a) Ras/Raf/MEK, (b) PI3K‐AKT/PKB, and (c) p38/MAPK‐HSP27 pathways.7 Signaling through VEGFR‐2, VEGF increases vessel permeability in ischemic condition8 by disruption of endothelium tight junctions via downregulation of zonula occludens‐1 and disorganization of the actin cytoskeleton.9 Tight junction proteins, for example, occludin and claudin‐5, are also downregulated when VEGF level is correlated with BBB breakdown.10
2.1. Role of VEGF in bAVM hemorrhage
Brain AVM represents a rare but important source of neurological morbidity in young adults.11 The abnormally high levels of VEGF and VEGF receptors in endothelial cells (ECs) of surgically resected bAVM tissue have been reported.12, 13, 14 Animal models suggest that VEGF may contribute to the hemorrhagic tendency.4, 15 The high VEGF levels are associated with increased permeability of BBB and bAVM hemorrhage.16, 17, 18 The imbalanced expression of VEGF, angiopoietin‐1, and angiopoietin‐2 contributes to abnormal vascular remodeling causing impaired wall structure in bAVM vessels. We recently demonstrated a direct link between the elevated VEGF level and bAVM hemorrhage in a mouse bAVM model.4
Furthermore, it has been shown that plasma VEGF levels are elevated in bAVM patients.19 Contrastingly, a decreased serum VEGF level in patients with AVM was suggested to be through pooling of circulating VEGF within and around the nidus and concomitant depletion of systemic VEGF secretion due to negative feedback loop.20
2.2. Interplay of VEGF and hemodynamic changes
The abnormal vessels in human bAVMs are exposed to variable degrees of increased intraluminal flow and venous hypertension (VH). A positive correlation between VH and angiogenic activity was first proposed by Lawton et al.21 It has also been shown that VH upregulates the expression of VEGF and hypoxia‐inducible factor 1‐alpha (HIF‐1α).22, 23 Upregulation of the nuclear factor erythroid 2‐related factor 2 (Nrf2) and its downstream targets HIF‐1α and VEGF have been detected in human AVM samples and a rat VH model.24 Nrf2, a transcriptional factor, regulates antioxidant genes and influences angiogenesis. Therefore, the interplay between Nrf2 and VEGF might contribute to VH‐induced angiogenesis in bAVM pathogenesis.
Recently, we reported that creation of VH in mice with bAVMs caused severe hemorrhage in the bAVM lesions and high mortality.4 Therefore, elevation of VEGF induced by VH might be one of the responsible factors of bAVM hemorrhage.
2.3. Inhibition of VEGF
Vascular endothelial growth factor expression in the brain is high during embryonic development and is reduced in the adult brain. VEGF stimulation is necessary for induction of bAVM formation in adult mice.25, 26, 27 VEGF neutralization prevented and normalized AVM in an animal model for hereditary hemorrhagic telangiectasia 2 (HHT2), an autosomal‐dominant disorder characterized by telangiectasia and AVMs in multiple organs.28 Bevacizumab (an antihuman VEGF antibody) treatment inhibits the bAVM formation and progression.29 Furthermore, intravenous injection of an adeno‐associated viral vector expressing sFLT1 (the extracellular domain of VEGF receptor 1) attenuated the phenotype severity of bAVMs in mice.30
These data indicated that VEGF plays a crucial role in bAVM hemorrhage and can be a therapeutic target.
3. ROLES OF PLATELET‐DERIVED GROWTH FACTOR‐B (PDGFB) SIGNALING AND PERICYTES IN BAVM HEMORRHAGE
Brain ECs form a one cell thick lining of the vascular lumen. Adjacent cells are tightly connected via tight and adherens junctional proteins, including claudins, occludins, and vascular endothelial cadherin, and form the BBB.31, 32 This EC barrier excludes large, nonlipophilic molecules (>40 Da), such as circulating bloodborne cells or plasma proteins, from the brain unless specific transport proteins are present.31, 32, 33, 34 Although the ECs are the anatomic site of the BBB, much of the structural integrity of the vascular wall comes from extracellular matrix and other surrounding cells—such as vascular smooth muscle cells or pericytes. ECs are embedded within a vascular basement and serves as a vital structural scaffolding comprised of laminins, fibronectins, collagens, and heparin sulfate proteoglycans.35 Differential expression of basement membranes, for example, perlecan, or adhesion molecules, such as integrins, responsible for anchoring vascular cells to the basement membrane has been reported and associated with AVM formation and rupture.36, 37, 38
The identity of the mural cells (vascular smooth muscle cells and pericytes) is dependent on the location along the arterial‐venous axis.39 In larger arteries, concentric rings of vascular smooth muscle cells are formed. As they branch into more distal arterioles, vascular smooth muscle cells become less numerous and no longer form concentric rings.40 In capillaries, vascular smooth muscle cells are replaced by the pericyte as the principle cell component of the vascular wall, which extend finger‐like cell processes covering much of the ECs.33, 34, 41 Crosstalk between mural cells promotes EC barrier properties.32, 42, 43, 44 Mural cells express both contractile proteins and proteins of the extracellular matrix, which help regulate vascular diameter.45, 46, 47, 48, 49 Loss of vascular smooth muscle cells and pericytes are associated with ectasia, aneurysm formation, and either leakage or rupture of arteries and capillaries, respectively.33, 42, 43, 44, 47, 50, 51, 52
3.1. PDGFB/PDGF Receptor β (PDGFRβ) Signaling Pathway
One important pathway for EC‐mural cell crosstalk is the PDGFB/PDGFRβ pathway.32, 33, 41 PDGFB is secreted from the endothelium as a disulfide‐linked homodimer and retained within the extracellular matrix as the result of electrostatic interactions.53, 54 This creates a steep perivascular concentration gradient of PDGF‐BB shown to be essential for the recruitment of mural cells—including migration, attachment and proliferation.42, 54, 55 Both pericytes and vascular smooth muscle cells express PDGFRβ—a tyrosine kinase receptor.35 PDGF‐BB binding to PDGFRβ triggers receptor dimerization, autophosphorylation, and activation of multiple downstream signal transduction pathways—including multiple Src homology 2 binding proteins, GTPase activating protein, SH2 tyrosine phosphatase, and phospholipase Cγ0.32 Deletion or genetic manipulation of Pdgfb or Pdgfrβ results in deficiency of vascular smooth muscle cells and pericytes.35, 42, 43, 44, 56, 57 A common consequence of reductions of pericytes or vascular smooth muscle is breakdown of the BBB, leakage of circulating plasma proteins into the brain and hemorrhage,35, 42, 43, 44, 56, 57 and homozygous deletion of Pdgfb or Pdgfrβ in rodents results in utero death due to widespread hemorrhage.58
3.2. BBB integrity and mural cell recruitment in bAVM pathogenesis
Abnormal expression of PGDF‐B and PDGFRβ has been described in bAVMs in rodent models and patients.6, 59, 60 Recent works showed that both human and mouse bAVM vessels have less mural cell coverage compared to normal brain vessels,5, 6, 59 suggesting an abnormal vascular remodeling in bAVMs. The number of pericytes is inversely correlated with the degree of overt symptomatic hemorrhage or clinically occult microhemorrhage.5, 59 However, vascular smooth muscle cells have yet to be fully characterized in human AVMs, and the functional consequences of other described abnormalities—such as cytoskeleton and contractile proteins—remain unclear.61, 62, 63
Despite these uncertainties, vascular smooth muscle cells and pericytes are an emerging therapeutic target through pharmacological manipulation of PDGFB.6, 41, 64 HHT patients are characterized by systemic mucosal capillary dilations, which are prone to bleeding resulting in epistaxis or gastrointestinal bleeding. Approximately, 5%‐23% of patients with HHT develop brain vascular malformations—including AVMs.65, 66 In HHT patients, thalidomide treatment was shown to reduce epistaxis. Increases in EC PDGFB production and enhanced pericyte recruitment were demonstrated to exert thalidomide's vascular stabilizing effect in Eng+/− rodents.64 More recently, treatment with thalidomide or its less toxic analog—lenalidomide—was shown to increase recruitment of both pericytes and vascular smooth muscle cells in mouse bAVMs.6 Enhanced mural recruitment was associated with reductions in vascular dysplasia and hemorrhage. Mechanistic experiments confirmed that this effect was the result of increased EC Pdgfb expression. Overexpression of Pdgfb recapitulated the therapeutic benefit of thalidomide.6 These studies provided first proof‐of‐principle evidence that vascular smooth muscle and pericytes may represent novel therapeutic targets in bAVMs. Whether these results may be translated into human patients to stabilize bAVMs remains to be seen.
4. ALTERATION OF HEMODYNAMICS EXACERBATES BAVM HEMORRHAGE
On functional level, an AVM is a collection of vessels that transmit a disproportionately higher per unit volume of blood relative to its surrounding vasculature. This phenomenon is important in understanding the pathophysiology of AVMs in that these nonphysiological hemodynamics manifest forces that can affect the molecular and structural composition of a vessel wall. We described flow in dimensions of pressure, velocity, and organization (eg, laminar or turbulent) as functions of time, geometry, and surface area. The collective study of these metrics, namely computational fluid dynamics (CFD), is expansive and complex. When applied in relatively simple biological models (eg, flow chamber), meaningful relationships between cell biology and hemodynamics crystalized. Brain AVMs, however, are considerably more complicated and challenging to study.67
4.1. Hemodynamics in bAVMs
A key aspect in discussing the role of fluid dynamics in AVMs is to understand its relevance to stroke risk as part of disease natural history or therapeutic intervention. Currently, there is no reliable method to predict AVM‐related ICH due to inconsistency in clinical and angioarchitectural risk factors across series. More flow metrics might improve identification of patients at risk. Alternatively, we also have uncertainty of posttherapeutic ICH, such as after microsurgical, embolization, or gamma knife treatments.68 Demonstrating flow parameters preintervention that elevate posttreatment risk could help direct patients to alternative therapies and/or different postsurgical management.69, 70, 71, 72, 73 Research into both of these clinical scenarios has enlisted hemodynamic metrics to help explain such hemorrhagic episodes with varying degrees of success.
4.2. Flow in brain hemorrhage
With the exception of aneurysm‐related subarachnoid hemorrhage, the precise vascular locus of AVM‐related ICH is unknown. There is varying evidence for potential location(s), which could be at an arterial, nidal, and/or venous sites. Knowing precisely which vascular compartment was at risk would be helpful in targeting fluid dynamics. Even in the absence of this clarity, much has been learned on how hemodynamics may affect ICH risk. There are many studies characterizing fluid dynamics within aneurysms74, 75, 76 with or without ruptured. However, these studies are inconsistent in defining which and in what direction fluid dynamic parameters lead to aneurysm rupture. Many groups have focused on wall shear stress and its association with aneurysmal wall inflammation.77, 78, 79, 80 However, there are few longitudinal studies detailing this question and lack of consistency between groups on how to generate such CFD values. There is evidence that high‐flow arteries carry a greater likelihood of aneurysm formation than those anatomically matched, low flow vessels. For example, Shakur et al, using MRA‐based flow methods, noted increased flow rates and relative wall shear stress in AVM arterial afferents harboring aneurysms than those that did not.81 However, there is again limited information about which hemodynamic parameters can predict when and where an AVM‐related aneurysm may form. Ultimately, 15%‐50% of AVMs have aneurysms.82, 83, 84, 85, 86, 87, 88 Whether the presence of aneurysms is a risk factor of ICH is controversy.82, 83, 86, 89, 90 As such, more effort in the evaluation of the role of hemodynamics on AVM natural history has gone into its relation to the nidus rather than any specific prenidal arterial segment.
AV shunting is pathological not only in that it reduces or even eliminates the physiological exchange of blood gases, nutrients, and waste products a tissue bed requires, but it also exposes veins to supraphysiological pressures. Veins of the brain are thinned walled, valveless vessels designed for passive return of blood to the cardiopulmonary circuit. In the setting of an AV shunt, the vein(s) walls will thicken in response to the increased pressure. Over time such neointimal hyperplasia can cause the vein(s) to narrow and/or occlude, evidenced commonly in the surgical dialysis arteriovenous fistula population as well as in AVM patients.91, 92, 93, 94, 95, 96 Based on this rationale, many groups have thus studied flow parameters to the nidus to determine relationships with ICH and other clinical and treatment outcomes. Bolstering this rationale, even if inconsistent, is an observation that smaller AVMs with a single and/or stenosed draining vein are more likely to have hemorrhagic clinical presentation.97
4.3. Methods for flow assessment
Technically, capturing such flow information has been difficult over the past 40 years. Qualitative assessments of flow are helpful, although the reproducibility and nuance of such measures are poor. For quantitative methods, radiotracers and transcranial ultrasound were used initially,98, 99 the use of MR has become more common. MR is significantly more sensitive, accurate, and comprehensive tool in capturing the flow metrics in feeding arteries and draining veins. There are a few studies discussed contradictions in the use of MR to analyze the role of hemodynamics in bAVM hemorrhage. For example, Illies et al, in 72 patients undergoing 4D MRA, noted no association between historical AVM ICH risk factors and hemodynamic metrics save that prior ICH was associated with increased transit time by 2.4 seconds (95% CI, 1.2‐3.6 seconds, P < 0.001).100 Similarly, Shakur et al,101 using quantitative MRA, noted no relationship between indices of arterial afferent pulsatility or resistance and historical angioarchitectural ICH risk factors. Conversely, Raoult et al, using 4D MRA, noted that the draining‐vein‐to‐arterial‐feeder time‐to‐peak ratio was significantly lower in the hemorrhagic compared with nonhemorrhagic patients (1.50 vs 2.1; P = 0.001),102 and Todaka et al103 demonstrated a significant difference in the mean number of draining veins (1.50 vs 2.3; P = 0.006) and the mean transit times (MTT) of the feeding artery (1.10 vs 1.62; P = 0.03). There are additional series further supporting both positions that MR‐based flow metrics do and do not104 help predict ICH events.
Despite these efforts, MR‐based methods are more complicated105 and require experts during MR scanning to focus regions of interest and provide the real‐time feedback during catheter angiography. In the setting of endovascular treatment, angiography is always performed providing the most sensitive and specific information of vascular anatomy and blood flow, though the efforts to quantitate such flow have been limited. Norris et al106 used digital subtraction angiograph (DSA) to measure arterial time‐to‐peak and nidal MTT within 31 bAVM patients noting an association between prolongation of time to peak (TTP) and shorter MTT and hemorrhagic presentation. This is congruent with some of the above MR‐based studies implicate increased upstream resistance, nidal, and/or venous as an ICH risk factor. More recently, groups have used parametric color coding, a technique that instantaneously converts a 2D X‐ray angiogram into a color‐map image where the time dimension is color‐encoded.107 The power of the technique is its simplicity and immediacy to the operator. The outputs enable production of time‐contrast density curves that then can be visually inspected and quantified. For example, Chen et al108 noted that patients with clinically occult nidal microhemorrhage demonstrated shorter nidal MTT. Some of these studies have been helpful as it relates to determine intranidal flow and detailed hemodynamic measurements within any given nidal vessel can be difficult given their complex anatomy. This technique is limited in that it is motion‐sensitive, and vessels of interest may overlap other vascular anatomy reducing signal clarity. Most importantly, it does not provide a true measure of flow as MR does. There is evidence of agreement between the modalities on this measure; for example, Brunozzi et al and Shakur et al reported correlation between MR‐based flow analysis and DSA‐based contrast time‐density curves, with the former also noting that ICH presentation was more common in those with decreased venous transit times and the latter decreased MTT and seizure presentation.109, 110 These studies are promising, and more recent work has expanded the technology to 3D datasets111 and treatment assesments.73 More work needs to be done validating the parametric color‐coding method in addition to bAVM hemodynamics and natural history in general.
5. CURRENT TREATMENTS AND FUTURE DIRECTIONS/DEVELOPMENTS PROSPECTION
Despite recent advances in bAVM molecular biology, no established medical therapy presently exists. Available treatment options include observation, surgical resection, endovascular embolization, stereotactic radiosurgery, or combination thereof.112 The advantages and disadvantages of these therapies are summarized in Table 1. Recent completed two randomized clinical trials or prospective registries suggested that risks of treatment may outweigh risks of future hemorrhage and favor observation.3, 113 The ARUBA is a randomized trial aimed to compare the risk of death and symptomatic stroke in patients with an unruptured bAVM who were allocated to either medical management alone or medical management with interventional therapy. The data obtained from ARUBA trial showed that medical management alone is superior to medical management with interventional therapy for the prevention of death or stroke in patients with unrupture bAVMs.3 The Scottish Audit of Intracranial Vascular Malformations (SAIVM) compared the long‐term outcomes of conservative management vs intervention for unruptured bAVM. This study found that among patients aged 16 years or older, the conservative management was associated with better clinical outcomes for up to 12 years compared with intervention.113 Numerous centers have since reported superior safety profiles with treatment.114, 115, 116, 117, 118, 119, 120 The optimal treatment remains controversial.
Table 1.
The advantages and disadvantages of current bAVM treatments
| Treatment | Advantages | Disadvantages | Usage |
|---|---|---|---|
| Microsurgery |
|
|
|
| Radiosurgery |
|
|
|
| Embolization |
|
|
|
| Combination therapy (M + E) |
|
|
|
| Combination therapy (M + R) |
|
|
|
| Combination therapy (R + E) |
|
|
|
| Combination therapy (R + E + M) |
|
|
|
5.1. Neurosurgical approaches
For AVMs located near the brain surface or easily accessible through open neurosurgical approaches, surgery has the highest rates of complete cure with an acceptable safety profile.121 Nuances for the surgical approach for bAVMs have been reviewed elsewhere.121 Candidacy for surgery is assigned by grading rubrics—such as the Spetzler‐Martin and Lawton‐Young supplementary scores.122, 123, 124 These scoring systems took into account size or morphology of the nidus, pattern of venous drainage, lesion location, and whether the location fulfills an eloquent brain function, age, and rupture status. A combined score of ≤6 are generally thought to have a risk‐benefit profile favorable for surgery, whereas those ≥7 are often evaluated for other therapies.121
5.2. Stereotactic radiosurgery
Stereotactic radiosurgery is another less, invasive treatment strategy in which targeted radiation induces vascular damage and gradually leads to occlusion of the AVM.121 Radiation results in endothelial degeneration and vascular smooth muscle proliferation, which occludes or compresses the vascular lumen.125, 126 Radiation also decreases circulating levels of multiple proangiogenic factors within three months, including VEGF, TGF‐β, angiopoietin‐2, and basic fibroblast growth factor.127 However, radiation takes several years to have its effect during which bleeding may occur and also effects the adjacent brain resulting in nonspecific radiation induced changes or rarely radiation induced malignancies or other vascular malformations which may cause neurological symptoms.121
5.3. Endovascular embolization
Endovascular embolization is an alternative treatment method, though is largely used as a presurgical adjuvant to reduce microsurgical risk. The evidence supporting the use of embolization of bAVMs, whether as a primary treatment or adjuvant, is limited and controversial. The rarity and heterogeneity of bAVMs, along with the variation in embolization techniques and materials, make formal study of its role in treatment difficult to assess. Nonetheless, there are individual and collective series that provide guidance for how to best use embolization. In the most common scenario, where embolization is performed prior to microsurgery, there are a few series noting its impact on reducing blood loss relative to comparatively surgical grade lesions.128, 129, 130, 131, 132, 133 More recently, however, Donzelli et al noted no such effect, with their analysis also including operative time and the use of microclips as representative variables of surgical complexity. It is noteworthy that the majority of embolizations were performed using n‐cyanobutyl acrylate (nBCA) and there is evidence that ethylene vinyl alcohol (EVOH) copolymer may be a more effective material for nidal penetration, which may in turn have impacted the results.
Alternative strategies for embolization include targeted embolization of discrete high‐risk features (eg, aneurysm). Alexander et al demonstrated that embolization of nidal or perinidal aneurysmal AVM bleeding sites can reduce rebleeding within the first year following initial hemorrhage when compared to matched patients not undergoing such treatment.134 Such a practice has been advocated by others too.128 As it relates to attempted curative embolization, using transarterial or transvenous methods, there are a collection of small series noting high angiographic cure rates for bAVMs using embolization alone, though many of these series note higher rates of clinical complication relative to microsurgery and with limited clinical outcomes or long‐term follow‐up data.135 Finally, as it relates to the use of adjunctive embolization in radiosurgical practice, there is evidence of reduced obliteration rates relative to nonembolized lesions undergoing radiosurgery.136
5.4. Future development
As mentioned, there is no medical therapy for bAVMs at this time, although with the expansion of molecular understanding of these lesions, and their various genetic subgroups, treatments may be available. The use of bevacizumab, mitogen‐activated protein kinase enzyme (MEK) inhibitors, rapamycin, and thalidomide has all been used with varying degrees of efficacy for patients with non‐CNS AVMs. For example, the anti‐VEGF monoclonal antibody, bevacizumab, has been used to treat HHT‐related AVMs 137, 138 and radiation‐related injuries.139 Anti‐angiogenic drugs such as thalidomide have also been used to reduce bleeding of AVMs in HHT patients and GI syndrome patients.140, 141 In addition, other studies identified the role of RAS/MAPK and PI3K/mTOR pathways, which are involved in regulation of vascular growth and organization, in pathogenesis of AVMs. It has been shown that delivery of MEK inhibitors to AVM ECs may lead to reduced ERK activity and decreased vessel abnormalities,142, 143 while delivery of rapamycin, an mTOR inhibitor, has demonstrated positive effects on patients with varying vascular anomalies including AVMs.144, 145
Since the most devastating symptom of bAVM is ICH, unlike cancer‐related chemotherapy that aims to shrink abnormal tumor tissue, the concept for the treatment of bAVM should be to stabilize vascular tissue and thereby decrease the risk of spontaneous ICH. Therefore, all of aforementioned pathways and agents might be used to develop strategies to reduce bAVM size or hemorrhage.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
ACKNOWLEDGEMENTS
This study was supported by grants to H. Su from the National Institutes of Health (R01 NS027713, R01 HL122774) and from the Michael Ryan Zodda Foundation.
Shaligram SS, Winkler E, Cooke D, Su H. Risk factors for hemorrhage of brain arteriovenous malformation. CNS Neurosci Ther. 2019;25:1085–1095. 10.1111/cns.13200
REFERENCES
- 1. Kim H, Su H, Weinsheimer S, Pawlikowska L, Young WL. Brain arteriovenous malformation pathogenesis: a response‐to‐injury paradigm. Acta Neurochir Suppl. 2011;111:83‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66(9):1350‐1355. [DOI] [PubMed] [Google Scholar]
- 3. Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non‐blinded, randomised trial. Lancet. 2014;383(9917):614‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng P, Ma LI, Shaligram S, et al. Effect of elevation of vascular endothelial growth factor level on exacerbation of hemorrhage in mouse brain arteriovenous malformation. J Neurosurg. 2019;26:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen W, Guo Y, Walker EJ, et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1‐deficient brain. Arterioscler Thromb Vasc Biol. 2013;33(2):305‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu W, Chen W, Zou D, et al. Thalidomide reduces hemorrhage of brain arteriovenous malformations in a mouse model. Stroke. 2018;49(5):1232‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood‐brain barrier. Nat Med. 2013;19(12):1584‐1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown LF, Yeo KT, Berse B, et al. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176(5):1375‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia‐induced hyperpermeability in brain microvessel endothelial cells involves VEGF‐mediated changes in the expression of zonula occludens‐1. Microvasc Res. 2002;63(1):70‐80. [DOI] [PubMed] [Google Scholar]
- 10. Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF‐mediated disruption of endothelial CLN‐5 promotes blood‐brain barrier breakdown. Proc Natl Acad Sci USA. 2009;106(6):1977‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arteriovenous Malformation Study Group . Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340(23):1812‐1818. [DOI] [PubMed] [Google Scholar]
- 12. Hashimoto T, Emala CW, Joshi S, et al. Abnormal pattern of Tie‐2 and vascular endothelial growth factor receptor expression in human cerebral arteriovenous malformations. Neurosurgery. 2000;47(4):910‐919; discussion 918–919. [DOI] [PubMed] [Google Scholar]
- 13. Hashimoto T, Wu Y, Lawton MT, Barbaro N, Young WL. Co‐expression of angiogenic factors in brain arteriovenous malformations [Abstract]. J Neurosurg Anesthesiol. 2004;16(4):334. [Google Scholar]
- 14. Koizumi T, Shiraishi T, Hagihara N, Tabuchi K, Hayashi T, Kawano T. Expression of vascular endothelial growth factors and their receptors in and around intracranial arteriovenous malformations. Neurosurgery. 2002;50(1):117‐126; discussion 124–116. [DOI] [PubMed] [Google Scholar]
- 15. Lee CZ, Xue Z, Zhu Y, Yang GY, Young WL. Matrix metalloproteinase‐9 inhibition attenuates vascular endothelial growth factor‐induced intracranial hemorrhage. Stroke. 2007;38(9):2563‐2568. [DOI] [PubMed] [Google Scholar]
- 16. Hashimoto T, Wu Y, Lawton MT, Yang GY, Barbaro NM, Young WL. Co‐expression of angiogenic factors in brain arteriovenous malformations. Neurosurgery. 2005;56(5):1058‐1065; discussion 1058–1065. [PubMed] [Google Scholar]
- 17. Shen F, Su H, Liu W, Kan YW, Young WL, Yang GY. Recombinant adeno‐associated viral vector encoding human VEGF165 induces neomicrovessel formation in the adult mouse brain. Front Biosci. 2006;11:3190‐3198. [DOI] [PubMed] [Google Scholar]
- 18. Zhang ZG, Zhang LI, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood‐brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106(7):829‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sandalcioglu IE, Wende D, Eggert A, et al. Vascular endothelial growth factor plasma levels are significantly elevated in patients with cerebral arteriovenous malformations. Cerebrovasc Dis. 2006;21(3):154‐158. [DOI] [PubMed] [Google Scholar]
- 20. Ding D. Role of vascular endothelial growth factor in the pathophysiology of intracranial arteriovenous malformations. Br J Neurosurg. 2014;28(3):428‐429. [DOI] [PubMed] [Google Scholar]
- 21. Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87(2):267‐274. [DOI] [PubMed] [Google Scholar]
- 22. Zhu Y, Lawton MT, Du R, et al. Expression of hypoxia‐inducible factor‐1 and vascular endothelial growth factor in response to venous hypertension. Neurosurgery. 2006;59(3):687‐696; discussion 687–696. [DOI] [PubMed] [Google Scholar]
- 23. Gao P, Zhu Y, Ling F, et al. Nonischemic cerebral venous hypertension promotes a pro‐angiogenic state through HIF‐1 downstream genes and leukocyte‐derived MMP‐9. J Cereb Blood Flow Metab. 2009;29(8):1482‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li L, Pan H, Wang H, et al. Interplay between VEGF and Nrf2 regulates angiogenesis due to intracranial venous hypertension. Sci Rep. 2016;6:37338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen W, Sun Z, Han Z, et al. De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke. 2014;45(3):900‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi EJ, Chen W, Jun K, Arthur HM, Young WL, Su H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS ONE. 2014;9(2):e88511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walker EJ, Su H, Shen F, et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69(6):954‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han C, Choe S‐W, Kim YH, et al. VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis. 2014;17(4):823‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walker EJ, Su H, Shen F, et al. Bevacizumab attenuates VEGF‐induced angiogenesis and vascular malformations in the adult mouse brain. Stroke. 2012;43(7):1925‐1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu W, Shen F, Mao L, et al. Soluble FLT1 gene therapy alleviates brain arteriovenous malformation severity. Stroke. 2017;48(5):1420‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zlokovic BV. The blood‐brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178‐201. [DOI] [PubMed] [Google Scholar]
- 32. Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19(6):771‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14(11):1398‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer's disease? Brain Pathol. 2014;24(4):371‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tallquist MD, French WJ, Soriano P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 2003;1(2):E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kahle MP, Lee B, Pourmohamad T, et al. Perlecan domain V is upregulated in human brain arteriovenous malformation and could mediate the vascular endothelial growth factor effect in lesional tissue. NeuroReport. 2012;23(10):627‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seker A, Yildirim Ö, Kurtkaya Ö, et al. Expression of integrins in cerebral arteriovenous and cavernous malformations. Neurosurgery. 2006;58(1):159‐168; discussion 159–168. [DOI] [PubMed] [Google Scholar]
- 38. Su H, Kim H, Pawlikowska L, et al. Reduced expression of integrin alphavbeta8 is associated with brain arteriovenous malformation pathogenesis. Am J Pathol. 2010;176(2):1018‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vanlandewijck M, He L, Mäe MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554(7693):475‐480. [DOI] [PubMed] [Google Scholar]
- 40. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Winkler EA, Lu AY, Raygor KP, et al. Defective vascular signaling & prospective therapeutic targets in brain arteriovenous malformations. Neurochem Int. 2019;126:126‐138. [DOI] [PubMed] [Google Scholar]
- 42. Armulik A, Genové G, Mäe M, et al. Pericytes regulate the blood‐brain barrier. Nature. 2010;468(7323):557‐561. [DOI] [PubMed] [Google Scholar]
- 43. Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood‐brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87(1):95‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fernandez‐Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA. 2010;107(51):22290‐22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gautam J, Zhang X, Yao Y. The role of pericytic laminin in blood brain barrier integrity maintenance. Sci Rep. 2016;6:36450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mishra A, O'Farrell FM, Reynell C, Hamilton NB, Hall CN, Attwell D. Imaging pericytes and capillary diameter in brain slices and isolated retinae. Nat Protoc. 2014;9(2):323‐336. [DOI] [PubMed] [Google Scholar]
- 49. Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443(7112):700‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Henshall TL, Keller A, He L, et al. Notch3 Is necessary for blood vessel integrity in the central nervous system. Arterioscler Thromb Vasc Biol. 2015;35(2):409‐420. [DOI] [PubMed] [Google Scholar]
- 51. Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF‐B‐deficient mice. Science. 1997;277(5323):242‐245. [DOI] [PubMed] [Google Scholar]
- 52. Chalouhi N, Ali MS, Jabbour PM, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32(9):1659‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Andrae J, Gallini R, Betsholtz C. Role of platelet‐derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abramsson A, Kurup S, Busse M, et al. Defective N‐sulfation of heparan sulfate proteoglycans limits PDGF‐BB binding and pericyte recruitment in vascular development. Genes Dev. 2007;21(3):316‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Enge M, Bjarnegard M, Gerhardt H, et al. Endothelium‐specific platelet‐derived growth factor‐B ablation mimics diabetic retinopathy. Embo J. 2002;21(16):4307‐4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF‐B and PDGFR‐beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126(14):3047‐3055. [DOI] [PubMed] [Google Scholar]
- 57. Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV. Blood‐spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab. 2012;32(10):1841‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hellström M, Gerhardt H, Kalén M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153(3):543‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Winkler EA, Birk H, Burkhardt J‐K, et al. Reductions in brain pericytes are associated with arteriovenous malformation vascular instability. J Neurosurg. 2018;129(6):1464‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yildirim O, Bicer A, Ozkan A, Kurtkaya O, Cirakoglu B, Kilic T. Expression of platelet‐derived growth factor ligand and receptor in cerebral arteriovenous and cavernous malformations. J Clin Neurosci. 2010;17(12):1557‐1562. [DOI] [PubMed] [Google Scholar]
- 61. Uranishi R, Baev NI, Kim JH, Awad IA. Vascular smooth muscle cell differentiation in human cerebral vascular malformations. Neurosurgery. 2001;49(3):671‐680; discussion 679–680. [DOI] [PubMed] [Google Scholar]
- 62. Wong JH, Awad IA, Kim JH. Ultrastructural pathological features of cerebrovascular malformations: a preliminary report. Neurosurgery. 2000;46(6):1454‐1459. [DOI] [PubMed] [Google Scholar]
- 63. Kim YH, Choe SW, Chae MY, Hong S, Oh SP. SMAD4 deficiency leads to development of arteriovenous malformations in neonatal and adult mice. J Am Heart Assoc. 2018;7(21):e009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lebrin F, Srun S, Raymond K, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med. 2010;16(4):420‐428. [DOI] [PubMed] [Google Scholar]
- 65. Fulbright RK, Chaloupka JC, Putman CM, et al. MR of hereditary hemorrhagic telangiectasia: prevalence and spectrum of cerebrovascular malformations. AJNR Am J Neuroradiol. 1998;19(3):477‐484. [PMC free article] [PubMed] [Google Scholar]
- 66. Meybodi AT, Kim H, Nelson J, et al. Surgical treatment vs nonsurgical treatment for brain arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia: a retrospective multicenter consortium study. Neurosurgery. 2018;82(1):35‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guglielmi G. Analysis of the hemodynamic characteristics of brain arteriovenous malformations using electrical models: baseline settings, surgical extirpation, endovascular embolization, and surgical bypass. Neurosurgery. 2008;63(1):1‐11; discussion 11. [DOI] [PubMed] [Google Scholar]
- 68. Fennell VS, Martirosyan NL, Atwal GS, et al. Hemodynamics associated with intracerebral arteriovenous malformations: the effects of treatment modalities. Neurosurgery. 2018;83(4):611‐621. [DOI] [PubMed] [Google Scholar]
- 69. Li CQ, Hsiao A, Hattangadi‐Gluth J, Handwerker J, Farid N. Early hemodynamic response assessment of stereotactic radiosurgery for a cerebral arteriovenous malformation using 4D flow MRI. AJNR Am J Neuroradiol. 2018;39(4):678‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alaraj A, Shakur SF, Amin‐Hanjani S, et al. Changes in wall shear stress of cerebral arteriovenous malformation feeder arteries after embolization and surgery. Stroke. 2015;46(5):1216‐1220. [DOI] [PubMed] [Google Scholar]
- 71. Donzelli GF, Nelson J, McCoy D, et al. The effect of preoperative embolization and flow dynamics on resection of brain arteriovenous malformations. J Neurosurg. 2019;17:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ansari SA, Schnell S, Carroll T, et al. Intracranial 4D flow MRI: toward individualized assessment of arteriovenous malformation hemodynamics and treatment‐Induced changes. AJNR Am J Neuroradiol. 2013;34(10):1922‐1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rivera R, Sordo JG, Echeverria D, Badilla L, Pinto C, Merino‐Osorio C. Quantitative evaluation of arteriovenous malformation hemodynamic changes after endovascular treatment using parametric color coding: A case series study. Interv Neuroradiol. 2017;23(6):650‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Detmer FJ, Chung BJ, Mut F, et al. Development and internal validation of an aneurysm rupture probability model based on patient characteristics and aneurysm location, morphology, and hemodynamics. Int J Comput Assist Radiol Surg. 2018;13(11):1767‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cebral JR, Duan X, Chung BJ, Putman C, Aziz K, Robertson AM. Wall mechanical properties and hemodynamics of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2015;36(9):1695‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sforza DM, Kono K, Tateshima S, Vinuela F, Putman C, Cebral JR. Hemodynamics in growing and stable cerebral aneurysms. J Neurointerv Surg. 2016;8(4):407‐412. [DOI] [PubMed] [Google Scholar]
- 77. Cebral J, Ollikainen E, Chung BJ, et al. Flow conditions in the intracranial aneurysm lumen are associated with inflammation and degenerative changes of the aneurysm wall. AJNR Am J Neuroradiol. 2017;38(1):119‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Varble N, Rajabzadeh‐Oghaz H, Wang J, Siddiqui A, Meng H, Mowla A. Differences in morphologic and hemodynamic characteristics for "PHASES‐Based" intracranial aneurysm locations. AJNR Am J Neuroradiol. 2017;38(11):2105‐2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xiang J, Yu J, Choi H, et al. Rupture Resemblance Score (RRS): toward risk stratification of unruptured intracranial aneurysms using hemodynamic‐morphological discriminants. J Neurointerv Surg. 2015;7(7):490‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol. 2014;35(7):1254‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shakur SF, Amin‐Hanjani S, Mostafa H, Charbel FT, Alaraj A. Hemodynamic characteristics of cerebral arteriovenous malformation feeder vessels with and without aneurysms. Stroke. 2015;46(7):1997‐1999. [DOI] [PubMed] [Google Scholar]
- 82. Stein K‐P, Wanke I, Forsting M, et al. Associated aneurysms in supratentorial arteriovenous malformations: impact of aneurysm size on haemorrhage. Cerebrovasc Dis. 2015;39(2):122‐129. [DOI] [PubMed] [Google Scholar]
- 83. Platz J, Berkefeld J, Singer OC, et al. Frequency, risk of hemorrhage and treatment considerations for cerebral arteriovenous malformations with associated aneurysms. Acta Neurochir (Wien). 2014;156(11):2025‐2034. [DOI] [PubMed] [Google Scholar]
- 84. Schmidt NO, Reitz M, Raimund F, et al. Clinical relevance of associated aneurysms with arteriovenous malformations of the posterior fossa. Acta Neurochir Suppl (Wien). 2011;112:131‐135. [DOI] [PubMed] [Google Scholar]
- 85. Mpotsaris A, Loehr C, Harati A, Lohmann F, Puchner M, Weber W. Interdisciplinary clinical management of high grade arteriovenous malformations and ruptured flow‐related aneurysms in the posterior fossa. Interv Neuroradiol. 2010;16(4):400‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kim EJ, Halim AX, Dowd CF, et al. The relationship of coexisting extranidal aneurysms to intracranial hemorrhage in patients harboring brain arteriovenous malformations. Neurosurgery. 2004;54(6):1349‐1358. discussion 1357–1348. [DOI] [PubMed] [Google Scholar]
- 87. Stapf C, Mohr JP, Pile‐Spellman J, et al. Concurrent arterial aneurysms in brain arteriovenous malformations with haemorrhagic presentation. J Neurol Neurosurg Psychiatry. 2002;73(3):294‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kremer PHC, Koeleman BPC, Pawlikowska L, et al. Evaluation of genetic risk loci for intracranial aneurysms in sporadic arteriovenous malformations of the brain. J Neurol Neurosurg Psychiatry. 2015;86(5):524‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lv X, Wu Z, Jiang C, et al. Angioarchitectural characteristics of brain arteriovenous malformations with and without hemorrhage. World Neurosurg. 2011;76(1–2):95‐99. [DOI] [PubMed] [Google Scholar]
- 90. Meisel HJ, Mansmann U, Alvarez H, Rodesch G, Brock M, Lasjaunias P. Cerebral arteriovenous malformations and associated aneurysms: analysis of 305 cases from a series of 662 patients. Neurosurgery. 2000;46(4):793‐802; discussion 800–792. [DOI] [PubMed] [Google Scholar]
- 91. Campos B, Lee T, Roy‐Chaudhury P. Arteriovenous fistula failure: is there a role for epigenetic regulation? Semin Nephrol. 2013;33(4):400‐406. [DOI] [PubMed] [Google Scholar]
- 92. Lee T, Wadehra D. Genetic causation of neointimal hyperplasia in hemodialysis vascular access dysfunction. Semin Dial. 2012;25(1):65‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Roy‐Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17(4):1112‐1127. [DOI] [PubMed] [Google Scholar]
- 94. Fry DL. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ Res. 1968;22(2):165‐197. [DOI] [PubMed] [Google Scholar]
- 95. Vinuela F, Nombela L, Roach MR, Fox AJ, Pelz DM. Stenotic and occlusive disease of the venous drainage system of deep brain AVM's. J Neurosurg. 1985;63(2):180‐184. [DOI] [PubMed] [Google Scholar]
- 96. Shakur SF, Hussein AE, Amin‐Hanjani S, Valyi‐Nagy T, Charbel FT, Alaraj A. Cerebral arteriovenous malformation flow is associated with venous intimal hyperplasia. Stroke. 2017;48(4):1088‐1091. [DOI] [PubMed] [Google Scholar]
- 97. Kim H, Al‐Shahi Salman R, McCulloch CE, Stapf C, Young WL, Coinvestigators M. Untreated brain arteriovenous malformation: patient‐level meta‐analysis of hemorrhage predictors. Neurology. 2014;83(7):590‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jo KI, Kim JS, Hong SC, Lee JI. Hemodynamic changes in arteriovenous malformations after radiosurgery: transcranial Doppler evaluation. World Neurosurg. 2012;77(2):316‐321. [DOI] [PubMed] [Google Scholar]
- 99. Kader A, Young WL, Pile‐Spellman J, et al. The influence of hemodynamic and anatomic factors on hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1994;34(5):801‐808; discussion 807–808. [DOI] [PubMed] [Google Scholar]
- 100. Illies T, Forkert ND, Saering D, et al. Persistent hemodynamic changes in ruptured brain arteriovenous malformations. Stroke. 2012;43(11):2910‐2915. [DOI] [PubMed] [Google Scholar]
- 101. Shakur SF, Amin‐Hanjani S, Mostafa H, Aletich VA, Charbel FT, Alaraj A. Relationship of pulsatility and resistance indices to cerebral arteriovenous malformation angioarchitectural features and hemorrhage. J Clin Neurosci. 2016;33:119‐123. [DOI] [PubMed] [Google Scholar]
- 102. Raoult H, Bannier E, Maurel P, et al. Hemodynamic quantification in brain arteriovenous malformations with time‐resolved spin‐labeled magnetic resonance angiography. Stroke. 2014;45(8):2461‐2464. [DOI] [PubMed] [Google Scholar]
- 103. Todaka T, Hamada J, Kai Y, Morioka M, Ushio Y. Analysis of mean transit time of contrast medium in ruptured and unruptured arteriovenous malformations: a digital subtraction angiographic study. Stroke. 2003;34(10):2410‐2414. [DOI] [PubMed] [Google Scholar]
- 104. Wu C, Ansari SA, Honarmand AR, et al. Evaluation of 4D vascular flow and tissue perfusion in cerebral arteriovenous malformations: influence of Spetzler‐Martin grade, clinical presentation, and AVM risk factors. AJNR Am J Neuroradiol. 2015;36(6):1142‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shao X, Zhao Z, Russin J, et al. Quantification of intracranial arterial blood flow using noncontrast enhanced 4D dynamic MR angiography. Magn Reson Med. 2019;82(1):449‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Norris JS, Valiante TA, Wallace MC, et al. A simple relationship between radiological arteriovenous malformation hemodynamics and clinical presentation: a prospective, blinded analysis of 31 cases. J Neurosurg. 1999;90(4):673‐679. [DOI] [PubMed] [Google Scholar]
- 107. Strother CM, Bender F, Deuerling‐Zheng Y, et al. Parametric color coding of digital subtraction angiography. AJNR Am J Neuroradiol. 2010;31(5):919‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chen X, Cooke DL, Saloner D, et al. Higher flow is present in unruptured arteriovenous malformations with silent intralesional microhemorrhages. Stroke. 2017;48(10):2881‐2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Brunozzi D, Hussein AE, Shakur SF, et al. Contrast time‐density time on digital subtraction angiography correlates with cerebral arteriovenous malformation flow measured by quantitative magnetic resonance angiography, angioarchitecture, and hemorrhage. Neurosurgery. 2018;83(2):210‐216. [DOI] [PubMed] [Google Scholar]
- 110. Shakur SF, Brunozzi D, Hussein AE, et al. Validation of cerebral arteriovenous malformation hemodynamics assessed by DSA using quantitative magnetic resonance angiography: preliminary study. J Neurointerv Surg. 2018;10(2):156‐161. [DOI] [PubMed] [Google Scholar]
- 111. Lin CJ, Yang HC, Chien AC, et al. In‐room assessment of intravascular velocity from time‐resolved rotational angiography in patients with arteriovenous malformation: a pilot study. J Neurointerv Surg. 2018;10(6):580‐586. [DOI] [PubMed] [Google Scholar]
- 112. Lawton MT, Rutledge WC, Kim H, et al. Brain arteriovenous malformations. Nat Rev Dis Prim. 2015;1:15008. [DOI] [PubMed] [Google Scholar]
- 113. Al‐Shahi Salman R, White PM, Counsell CE, et al. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA. 2014;311(16):1661‐1669. [DOI] [PubMed] [Google Scholar]
- 114. Lang M, Moore NZ, Rasmussen PA, Bain MD. Treatment outcomes of a randomized trial of unruptured brain arteriovenous malformation‐eligible unruptured brain arteriovenous malformation patients. Neurosurgery. 2018;83(3):548‐555. [DOI] [PubMed] [Google Scholar]
- 115. Tonetti DA, Gross BA, Atcheson KM, et al. The benefit of radiosurgery for ARUBA‐eligible arteriovenous malformations: a practical analysis over an appropriate follow‐up period. J Neurosurg. 2018;128(6):1850‐1854. [DOI] [PubMed] [Google Scholar]
- 116. Wong J, Slomovic A, Ibrahim G, Radovanovic I, Tymianski M. Microsurgery for ARUBA trial (A randomized trial of unruptured brain arteriovenous malformation)‐eligible unruptured brain arteriovenous malformations. Stroke. 2017;48(1):136‐144. [DOI] [PubMed] [Google Scholar]
- 117. Hong CS, Peterson EC, Ding D, et al. Intervention for A randomized trial of unruptured brain arteriovenous malformations (ARUBA) ‐ Eligible patients: an evidence‐based review. Clin Neurol Neurosurg. 2016;150:133‐138. [DOI] [PubMed] [Google Scholar]
- 118. Ding D, Starke RM, Kano H, et al. Radiosurgery for cerebral arteriovenous malformations in a randomized trial of unruptured brain arteriovenous malformations (ARUBA)‐eligible patients: a multicenter study. Stroke. 2016;47(2):342‐349. [DOI] [PubMed] [Google Scholar]
- 119. Nerva JD, Mantovani A, Barber J, et al. Treatment outcomes of unruptured arteriovenous malformations with a subgroup analysis of ARUBA (A randomized trial of unruptured brain arteriovenous malformations)‐eligible patients. Neurosurgery. 2015;76(5):563‐570; discussion570; quiz 570. [DOI] [PubMed] [Google Scholar]
- 120. Rutledge WC, Abla AA, Nelson J, Halbach VV, Kim H, Lawton MT. Treatment and outcomes of ARUBA‐eligible patients with unruptured brain arteriovenous malformations at a single institution. Neurosurg Focus. 2014;37(3):E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Potts MB, Lau D, Abla AA, Kim H, Young WL, Lawton MT. Current surgical results with low‐grade brain arteriovenous malformations. J Neurosurg. 2015;122(4):912‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kim H, Abla AA, Nelson J, et al. Validation of the supplemented Spetzler‐Martin grading system for brain arteriovenous malformations in a multicenter cohort of 1009 surgical patients. Neurosurgery. 2015;76(1):25‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL. Mikhak B, Young WL. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery. 2010;66(4):702‐713; discussion 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65(4):476‐483. [DOI] [PubMed] [Google Scholar]
- 125. Tu J, Stoodley MA, Morgan MK, Storer KP. Storer KP. Responses of arteriovenous malformations to radiosurgery: ultrastructural changes. Neurosurgery. 2006;58(4):749‐758; discussion 749–758. [DOI] [PubMed] [Google Scholar]
- 126. Friedman WA, Bova FJ. Radiosurgery for arteriovenous malformations. Neurol Res. 2011;33(8):803‐819. [DOI] [PubMed] [Google Scholar]
- 127. Xu M, Liu X, Mei G, Zhang J, Wang W, Xu H. Radiosurgery reduces plasma levels of angiogenic factors in brain arteriovenous malformation patients. Brain Res Bull. 2018;140:220‐225. [DOI] [PubMed] [Google Scholar]
- 128. Bradac O, Charvat F, Benes V. Treatment for brain arteriovenous malformation in the 1998–2011 period and review of the literature. Acta Neurochir (Wien). 2013;155(2):199‐209. [DOI] [PubMed] [Google Scholar]
- 129. Loh Y, Duckwiler GR. A prospective, multicenter, randomized trial of the Onyx liquid embolic system and N‐butyl cyanoacrylate embolization of cerebral arteriovenous malformations. J Neurosurg. 2010;113(4):733‐741. [DOI] [PubMed] [Google Scholar]
- 130. Natarajan SK, Ghodke B, Britz GW, Born DE, Sekhar LN. Multimodality treatment of brain arteriovenous malformations with microsurgery after embolization with onyx. Neurosurgery. 2008;62(6):1213‐1226; discussion 1225–1216. [DOI] [PubMed] [Google Scholar]
- 131. Weber W, Kis B, Siekmann R, Jans P, Laumer R, Kuhne D. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery. 2007;61(2):244‐254; discussion 252–244. [DOI] [PubMed] [Google Scholar]
- 132. n BCATI . N‐butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi‐center trial. AJNR Am J Neuroradiol. 2002;23(5):748‐755. [PMC free article] [PubMed] [Google Scholar]
- 133. Jafar JJ, Davis AJ, Berenstein A, Choi IS, Kupersmith MJ. The effect of embolization with N‐butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg. 1993;78(1):60‐69. [DOI] [PubMed] [Google Scholar]
- 134. Alexander MD, Hippe DS, Cooke DL, et al. Targeted embolization of aneurysms associated with brain arteriovenous malformations at high risk for surgical resection: a case‐control study. Neurosurgery. 2018;82(3):343‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wu EM, El Ahmadieh TY, McDougall CM, et al. Embolization of brain arteriovenous malformations with intent to cure: a systematic review. J Neurosurg. 2019;1:1‐12. [DOI] [PubMed] [Google Scholar]
- 136. Russell D, Peck T, Ding D, et al. Stereotactic radiosurgery alone or combined with embolization for brain arteriovenous malformations: a systematic review and meta‐analysis. J Neurosurg. 2018;128(5):1338‐1348. [DOI] [PubMed] [Google Scholar]
- 137. Dupuis‐Girod S, Ambrun A, Decullier E, et al. ELLIPSE Study: a Phase 1 study evaluating the tolerance of bevacizumab nasal spray in the treatment of epistaxis in hereditary hemorrhagic telangiectasia. MAbs. 2014;6(3):794‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Riss D, Burian M, Wolf A, Kranebitter V, Kaider A, Arnoldner C. Intranasal submucosal bevacizumab for epistaxis in hereditary hemorrhagic telangiectasia: a double‐blind, randomized, placebo‐controlled trial. Head Neck. 2015;37(6):783‐787. [DOI] [PubMed] [Google Scholar]
- 139. Deibert CP, Ahluwalia MS, Sheehan JP, et al. Bevacizumab for refractory adverse radiation effects after stereotactic radiosurgery. J Neurooncol. 2013;115(2):217‐223. [DOI] [PubMed] [Google Scholar]
- 140. Amanzada A, Toppler GJ, Cameron S, Schworer H, Ramadori G. A case report of a patient with hereditary hemorrhagic telangiectasia treated successively with thalidomide and bevacizumab. Case Rep Oncol. 2010;3(3):463‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Harrison L, Kundra A, Jervis P. The use of thalidomide therapy for refractory epistaxis in hereditary haemorrhagic telangiectasia: systematic review. J Laryngol Otol. 2018;132(10):866‐871. [DOI] [PubMed] [Google Scholar]
- 142. Nikolaev SI, Vetiska S, Bonilla X, et al. Somatic activating KRAS mutations in arteriovenous malformations of the brain. N Engl J Med. 2018;378(3):250‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Al‐Olabi L, Polubothu S, Dowsett K, et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest. 2018;128(4):1496‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Adams DM, Ricci KW. Vascular anomalies: diagnosis of complicated anomalies and new medical treatment options. Hematol Oncol Clin North Am. 2019;33(3):455‐470. [DOI] [PubMed] [Google Scholar]
- 145. Adams DM, Trenor CC, Hammill AM, et al. Efficacy and Safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016;137(2):e20153257. [DOI] [PMC free article] [PubMed] [Google Scholar]


