Abstract
Intracerebral hemorrhage (ICH) is a cerebrovascular disorder with high mortality and disability rates. Although a lot of effort has been put in ICH, there is still no effective treatment for this devastating disease. Recent studies suggest that oligodendrocytes play an important role in brain repair after ICH and thus may be targeted for the therapies of ICH. Here in this review, we first introduce the origin, migration, proliferation, differentiation, and myelination of oligodendrocytes under physiological condition. Second, recent findings on how ICH affects oligodendrocyte biology and function are reviewed. Third, potential crosstalk between oligodendrocytes and other cells in the brain is also summarized. Last, we discuss the therapeutic potential of oligodendrocyte‐based treatments in ICH. Our goal is to provide a comprehensive review on the biology and function of oligodendrocytes under both physiological and ICH conditions.
Keywords: intracerebral hemorrhage, myelination, oligodendrocyte precursor cells, oligodendrocytes
1. INTRODUCTION
Stroke is the 5th leading cause of death and the leading cause of long‐term disability in the United States.1, 2 Based on the pathology, stroke is categorized into two types: ischemic stroke that occurs when blood supply to the brain is impeded, and hemorrhagic stroke that occurs when blood vessel ruptures in or around the brain. Depending on the site of bleeding, hemorrhagic stroke can be further divided into intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH).
ICH, which accounts for 10%‐15% of all stroke subtypes,3 is the most common type of hemorrhagic stroke. Its major risk factors include aging and hypertension, which are usually difficult to control. When ICH occurs, blood leaks into brain parenchyma resulting in primary and secondary brain damage. The former is predominantly caused by mass effect and tissue disruption. The latter is mainly due to inflammatory reaction and erythrocyte lysis. Although there is not much we can do about the primary damage, a lot of effort has been made to attenuate secondary damage. Unfortunately, there is still no effective treatment for this devastating disorder. Recent studies suggest that a better understanding of remyelination and white matter injury may shed new light on the treatment of ICH.4 The cell type that actively regulates remyelination and white matter injury is oligodendrocytes.5, 6 Here in this review, we first introduce the origin, migration, proliferation, differentiation, and myelination of oligodendrocytes under physiological condition. Second, recent findings on how ICH affects oligodendrocyte biology and function are reviewed. Third, potential crosstalk between oligodendrocytes and other cells in the brain is summarized. Last, we discuss the therapeutic potential of oligodendrocyte‐based treatments in ICH. The search criteria include a combination of “intracerebral hemorrhage” or “hemorrhagic stroke” and oligodendrocyte‐related keywords, such as “oligodendrocyte,” “OPC,” “OPC proliferation,” “oligodendrocyte differentiation,” “oligodendrocyte myelination,” “oligodendrocyte development,” “oligodendrocyte heterogeneity,” “oligodendrocyte morphology,” and “oligodendrocyte function.”
2. OLIGODENDROCYTE PHYSIOLOGY
2.1. Oligodendrocyte Origin
In the brain, oligodendrocytes are derived from oligodendrocyte progenitor cells (OPCs), which originate from neuroepithelial progenitor cells (NPCs) of neuroepithelium in the embryonic neural tube and forebrain.7, 8 In mice, NPCs become radial glial cells at about embryonic day (E) 9, which differentiate into OPCs at a later time.7, 8, 9
A study by Kessaris et al. Elegantly demonstrated three waves of OPC generation in the forebrain.10 The first wave arises at the medial ganglionic eminence (MGE) and anterior entopeduncular area (AEP) in the ventral forebrain at E11.5‐E12.5.10 These OPCs migrate across the telencephalon from ventral to dorsal area and account for most part of the embryonic forebrain until the next wave emanates. OPCs originated from the first wave, however, show significantly decreased number in most parts of the adult forebrain. The second wave emanates from the lateral and/or caudal ganglionic eminences (LGE and/or CGE) at around E15.10 These cells populate the cortical intermediate zone in embryonic brain and comprise most of the postnatal telencephalon. The third wave originates from the postnatal cortex at around the day of birth.10 OPCs generated from this wave are found only at dorsal telencephalon and mostly remain in the cortex until adulthood.
Interestingly, functional redundancy exists among different OPC populations. For example, ablation of one population leads to the substitution of the excised population by another population and normal survival and behavior of the resulting mice.10 In addition, it has been shown that OPCs from the diencephalon repopulate the telencephalon when all telencephalic OPCs are removed from the origins.11, 12 These findings suggest great plasticity of OPCs during development.12
2.2. Oligodendrocyte migration
Once OPCs are specified, multiple signaling cues guide them to their destination in the brain. It has been reported that the direction of OPC migration is largely determined by spatial gradients of BMPs (bone morphogenic proteins), Shh (sonic hedgehog), and Wnt proteins.13 In addition, growth factors, such as PDGF (platelet‐derived growth factor),14 VEGF (vascular endothelial growth factor),15 and FGF (fibroblast growth factor),16 are known to augment OPC migration. Furthermore, there is also evidence suggesting that brain vascularization regulates OPC migration.17 It has been shown that OPCs migrate (crawl along and jump between blood vessels) by physically contacting blood vessels in the brain.17 It should be noted that the exact molecular mechanisms underlying OPC migration remain largely unclear and need further investigation.
2.3. Oligodendrocyte proliferation
Unlike neurons, which have very limited capacity of proliferation, OPCs remain highly proliferative in adult brain.18 As the major population of proliferating cells in the adult central nervous system (CNS), OPCs maintain their density and number until later in life.19 Their ability of self‐renewal is closely related to stimulation of cell cycle and inhibition of differentiation. Mitogens and growth factors play critical roles in regulating cell cycle. For instance, PDGF, by binding to its receptor PDGFRα, enhances OPC proliferation and survival in vivo.20, 21 Similarly, FGF2, BDNF (brain‐derived neurotrophic factor), and NT‐3 (neurotrophin‐3) have also been shown to enhance OPC proliferation in vitro.22, 23, 24 Additionally, translocation of transcription factor Id2 (inhibitor of DNA binding 2) into nucleus has been demonstrated to induce OPC proliferation and inhibit their differentiation.25 Initiation of differentiation, on the contrary, slows down cell cycle and reduces OPC proliferation.18
2.4. OPC differentiation
Molecules that inhibit OPC proliferation usually act as inducers of OPC differentiation. For example, by inhibiting PDGF‐driven OPC proliferation, TGF‐β (transforming growth factor‐β) functions as a possible inducer of OPC differentiation.26 Similarly, translocation of Id2 from the nucleus to the cytoplasm precedes OPC differentiation.25 In addition, thyroid hormones have been shown to promote OPC differentiation.27, 28 Recently, there is evidence showing that microRNA also regulates OPC differentiation.29
OPC differentiation involves two continuous steps: the differentiation of OPCs into immature postmitotic pre‐oligodendrocytes (pre‐OLs) and subsequent maturation of these pre‐OLs into myelinating oligodendrocytes (mature‐OLs).13, 18 This differentiation process involves striking changes at both morphological and biochemical levels (Figure 1). Structurally, the highly proliferative OPCs usually take a bipolar or oval shape.30, 31 Compared to OPCs, pre‐OLs have various processes, the number of which correlates with the extent of differentiation.31 Unlike pre‐OLs, mature‐OLs develop processes that enwrap neuronal axons, forming myelin sheaths.31
Figure 1.
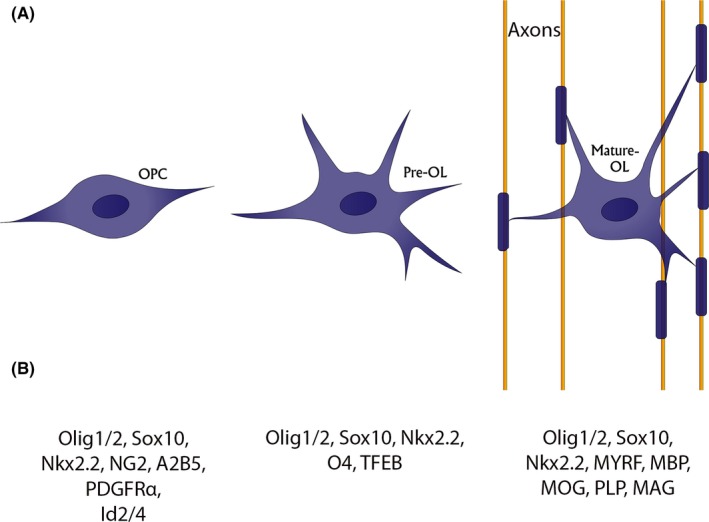
Diagram of oligodendrocyte differentiation. Morphological (A) and biochemical (B) features that characterize each differentiation stage
Biochemically, oligodendrocyte‐lineage cells express a variety of molecular markers at distinct differentiation stages. Upon lineage specification, Olig1/2, Sox10 (SRY‐Box transcription factor 10), and Nkx2.2 are substantially upregulated and persist throughout the life of oligodendrocyte‐lineage cells.7, 13, 31, 32, 33, 34, 35, 36, 37, 38, 39 Thus, these molecules are used as markers for oligodendrocyte‐lineage cells. Classical OPC markers include NG2 (neuron‐glial antigen 2), PDGFRα, and Id2/4.34, 40, 41, 42, 43, 44, 45 It should be noted that these markers are not OPC‐specific. For example, NG2 is also expressed in mural cells and PDGFRα is also found in fibroblasts.46, 47 Therefore, multiple markers should be used to identify OPCs. Markers for pre‐OLs are relatively less well characterized. A recent study reported that TFEB (transcription factor EB) is highly enriched in pre‐OLs and can be used as a pre‐OL marker.48 In addition, O4 has been also suggested as a pre‐OL marker,40, 44, 49 although there is also evidence showing that it is also expressed in mature‐OLs.50, 51 Mature‐OLs form myelin sheaths and express a group of unique proteins, including MYRF (myelin regulatory factor),52 MBP (myelin basic protein),38, 44, 53 MOG (myelin oligodendrocyte glycoprotein),54, 55, 56 PLP (proteolipid protein),44, 53, 57 and MAG (myelin‐associated glycoprotein).58, 59 These proteins are widely used as molecular markers for mature‐OLs. For a summary of the expression of these markers in oligodendrocyte‐lineage cells, please refer to Table 1.
Table 1.
Markers of oligodendrocyte‐lineage cells
| Markers | Stages | ||
|---|---|---|---|
| OPCs | Pre‐OLs | Mature‐OLs | |
| Olig1/2 | + | + | + |
| Sox10 | + | + | + |
| Nkx2.2 | + | + | + |
| NG2 | + | ||
| PDGFRa | + | ||
| Id2/4 | + | ||
| TFEB | + | ||
| O4 | + | ||
| MYRF | + | ||
| MBP | + | ||
| MOG | + | ||
| PLP | + | ||
| MAG | + | ||
While OPCs and mature‐OLs are extensively studied, pre‐OLs are in general understudied. For example, fewer molecular markers are available for pre‐OLs compared to OPCs and mature‐OLs. The functions of pre‐OLs in physiological and pathological conditions remain largely unknown. Therefore, future studies should focus on elucidating the markers and functions of pre‐OLs.
2.5. Myelination
The major function of oligodendrocytes in the CNS is to myelinate axons. Myelinated axons contain myelin sheaths and gaps known as nodes of Ranvier. This unique structure allows faster action potential propagation via saltatory conduction,60 in which current flows and jumps from one node of Ranvier to the next. Without myelin sheaths, action potentials show lower amplitudes, longer latencies, decreased conduction velocity, and gradual dispersion,61 suggesting a crucial role of myelination in the propagation of electrical signal along axons. Consistent with this important function, myelination has been found to be indispensable for various neurological functions, including motor learning62, 63 and social behavior.64
Myelination is tightly coupled with OPC differentiation and is orchestrated by diverse cellular mechanisms. Upon differentiation, OPCs develop processes to wrap neuronal axons. Once a process first contacts an axon, that contact can be stabilized, creating an axoglial communication.65 Next, the myelin membrane establishes polarized domains after molecular translocation in the cell.66 This polarized tip of myelin then expands on the axon both laterally toward the nodes of Ranvier and radially via growing underneath the previously formed membrane.67 After lateral and radial expansion of myelin sheath follows myelin compaction, which is composed of intracellular and extracellular leaflet compaction.68 The intracellular compaction is done by neutralization of negatively charged myelin membrane.65 In this process, MBP neutralizes the membrane and pulls the inner leaflets of myelin sheaths close to each other, forming the major dense line (MDL).69, 70 The extracellular compaction, on the other hand, is a process where the outer surfaces of myelin membranes are brought together, creating intraperiod line (IPL).71 Compared to intracellular compaction, extracellular compaction is relatively less well understood.
Since myelin sheaths are in direct contact with axons, it has been speculated that axons play an important role in oligodendrocyte maturation and myelination. Various studies support this hypothesis. For instance, upon neuronal stimulation or activation in the brain, the thickness72 and length73 of myelin sheath as well as the number of myelin sheath generated by an oligodendrocyte74 are increased. In sharp contrast to these findings, however, there is also evidence showing that the presence of axons is unnecessary for myelination. It has been shown that oligodendrocytes are able to myelinate electron‐spun nanofibers in a neuron‐free culture system,75 suggesting that oligodendrocytes have an intrinsic propensity to wrap axons independent of neuronal activity. These results suggest that neuronal activity is not absolutely required for myelination, but it can enhance the extent of myelination. In addition, a recent study reported different myelination patterns toward distinct populations of axons from different brain regions,76 suggesting functional heterogeneity of oligodendrocytes. Consistent with this hypothesis, 13 distinct oligodendrocyte populations have been identified based on single‐cell RNA sequencing.62 What causes oligodendrocyte heterogeneity and how oligodendrocyte heterogeneity affects myelination remain unknown. These important questions need future investigation.
2.6. Signaling pathways regulating myelination
Myelination is a delicate process that requires tight regulation. Multiple signaling pathways have been reported to be involved in myelination. First, extracellular cellular matrix (ECM) molecules have been shown to actively regulate the myelination process in the CNS. For example, by binding to integrins, laminin and fibronectin lengthen the OPC processes.77, 78, 79 In addition, loss of laminin‐α2β1γ1 leads to reduced mature‐OLs and dysmyelinated axons in multiple brain regions.80 Furthermore, laminin‐α2β1γ1, by binding to integrin β1, has also been shown to regulate the thickness of myelin sheath.81, 82 Apart from integrins, dystroglycan can also mediate laminin's effect on myelination.83 It has been shown that dystroglycan function‐blocking antibody significantly decreases the complexity of myelin morphology.83 These results suggest a crucial role of ECM‐integrin/dystroglycan signaling pathway in myelination.
Next, there is also evidence showing that Fyn is a promyelinating factor. It has been shown that upregulation of Fyn in OPCs promotes the production of highly branched processes in vitro. 79 Consistent with this finding, the tyrosine kinase activity of Fyn, which is activated at the early stage of OPC differentiation, is required for the branching of processes and formation of myelin sheaths.84 In addition, the regulatory mechanism of Fyn on cytoskeletal rearrangement also contributes to myelination.84 For example, Fyn‐Tau‐tubulin cascade has been shown to be important for the outgrowth of oligodendrocyte processes.85, 86 Less and shorter oligodendrocyte processes are observed when the interaction of Fyn and Tau is inhibited.86 Together, these results suggest a crucial role of Fyn signaling in myelination.
In addition, many other signaling pathways have also been reported to participate in the regulation of myelination. First, mice lacking ERK1/2 (extracellular signal‐regulated protein kinases 1/2) in oligodendrocyte‐lineage cells fail to produce myelin sheaths with proper thickness and show a reduced expression of major myelin genes, including PLP and MBP,87 suggesting a promyelinating effect of ERK/MAPK signaling pathway. Next, overexpression of active AKT in oligodendrocytes leads to hypermyelination without affecting the survival of oligodendrocytes or OPCs,88 while mTOR (mammalian target of rapamycin) inhibitor rapamycin inhibits hypermyelination,89 suggesting that AKT/mTOR signaling pathway positively regulates myelination. Interestingly, although the presence of axons is not required for myelination,75 neuronal expression of type III neuregulin‐1 promotes myelination and positively regulates myelin thickness in the forebrain,90 indicating a critical role of neuronal signal in myelination. Additionally, there is also evidence supporting that Wnt91 and BDNF92 promote myelination. For a summary of the factors that affect oligodendrocyte biology, please refer to Table 2.
Table 2.
Factors that affect oligodendrocyte biology
| Migration | Proliferation | Differentiation | Maturation (Myelination) |
|---|---|---|---|
|
BMP Shh Wnt PDGF VEGF FGF Brain vasculature |
PDGF FGF2 BDNF NT‐3 Id2 (nucleus) |
TGF‐β Id2 (cytoplasm) Thyroid hormones Micro RNA |
Neuronal activity Type III neuregulin‐1 Integrin Dystroglycan Fyn ERK/MAPK AKT/mTOR Wnt BDNF |
2.7. Age‐ and sex‐related differences in ICH and oligodendrocyte biology
Accumulating evidence suggests that age is a major risk factor for ICH.93, 94 It has been shown that aging increases the incidence of ICH due to worse chronic conditions, such as hypertension, atrial fibrillation, and diabetes.3 In addition, age‐related changes in brain vasculature, including increased blood‐brain barrier permeability, endothelial dysfunction, and decreased vascular density, also contribute to the pathogenesis of ICH.3 Apart from age, sex is another risk factor for ICH. It has been reported that ICH occurs more frequently in males (52.4%) than in females (47.6%) based on a study conducted on 515 acute primary ICH patients.95 The study also showed that women were older than men, although hematoma volume was similar in both sexes,95 suggesting that males are generally more susceptible to ICH than females.95 Similarly, a higher incidence (53.5%) of ICH was found in males in another study containing 2212 patients.96 Interestingly, this study identified male sex as a risk factor for hematoma expansion, which was defined as hematoma growth >33% or >6 mL from baseline ICH volume after 24 hours of onset.96
Whether age‐ and sex‐related differences exist in oligodendrocyte biology remains largely unknown. A recent study reported that OPCs have distinct molecular signatures at different ages.97 Specifically, OPCs display migrating molecular signature at E16 and proliferating molecular signature at P12.97 In addition, molecular signatures of both proliferation and differentiation decrease from P12 to P80.97 In another study, it has been demonstrated that the number of OPCs in both dorsal and ventral Ammon's horns decreases with age and that the number of OLs reduces in the ventral but not dorsal Ammon's horn with age,98 indicating region‐specific age‐related differences in the biology of oligodendrocyte‐lineage cells. Similarly, there is evidence supporting that sex may affect OL biology. For example, it has been shown that: (a) In young (6‐8 months) rats, males have significantly larger volumes of white matter, myelinated nerve fibers, and myelin sheaths than females99; and (b) in middle‐aged (18 months) rats, these volumes are much larger in females than males.99 In addition, OPC proliferation and maturation are regulated by sex hormones.100 First, OPCs isolated from female pups generated significantly more OLs than those isolated from male ones.100 Second, 17β‐estradiol, the major female sex hormone, prevented OPCs from exiting cell cycle in response to mitogen withdrawal, whereas progesterone and testosterone failed to do so.100 Third, progesterone‐treated cells demonstrated more complex (matured) morphology, while 17β‐estradiol‐ and testosterone‐treated cells showed less complex morphology.100 Further studies are needed to uncover the mechanisms underlying age‐ and sex‐related differences in oligodendrocyte biology.
3. EFFECTS OF ICH ON OLIGODENDROCYTE‐LINEAGE CELLS
3.1. Oligodendrocyte death after ICH
When ICH occurs, blood leaks into brain parenchyma, causing a series of changes, including iron toxicity and cell death.101, 102, 103 As the cell type that contains a high level of iron in the CNS, oligodendrocytes are very sensitive to iron overload104 and thus particularly susceptible to ICH injury. It has been reported that ICH induces oligodendrocyte death and demyelination in white matter,105, 106 where functional and morphological maintenance is highly dependent on oligodendrocytes and their myelin sheaths.107
How do oligodendrocytes die after ICH? On the one hand, there is evidence suggesting that apoptosis is responsible for oligodendrocyte death after ICH. For example, it was reported that oligodendrocytes expressed a significantly higher level of caspase‐3 at the injury site after internal capsule hemorrhage, compared to noninjured controls.105 Further mechanistic study revealed that ER stress and mitochondrial dysfunction contributed to oligodendrocyte apoptosis.105 Like in ICH, oligodendrocyte apoptosis also occurs in ischemia.108 Necrosis, on the other hand, has also been proposed to contribute to oligodendrocyte death after ICH, especially at the acute phase. For instance, it has been demonstrated that more than half of injured cells are necrotic 48 hours after injury in the collagenase‐induced ICH model.109
3.2. OPC proliferation after ICH
Based on that OPCs remain proliferative throughout life, it has been speculated that OPCs contribute to cell repopulation in the brain after injury.19 Consistent with this hypothesis, OPCs are activated and become highly proliferative after demyelinating injury.110, 111 In addition, it has been shown that Olig2+ and NG2+Olig2+ cells increase dramatically in the perihematoma region after ICH4 and that this increase is not due to migration of oligodendrocyte‐lineage cells from the subventricular zone (SVZ)—the site of oligodendrogenesis.4, 112 Together, these findings strongly indicate that ICH induces OPC proliferation.
3.3. OPC differentiation after ICH
After injury, OPCs proliferate and differentiate into mature‐OLs, which remyelinate damaged axons through a series of processes, including contact with demyelinated axons, myelin membrane production, and ensheathment of target axons.111 It should be noted that our understanding of the remyelination process mainly comes from demyelinating diseases, such as multiple sclerosis. How ICH affects OPC differentiation and remyelination, however, remains largely unknown. One recent study showed that the density of mature‐OLs increased and peaked at day 7 after ICH at the perihematoma region.4 Although this finding suggests that OPCs are able to differentiate into mature‐OLs after ICH, it remains unclear whether these mature‐OLs are able to remyelinate damaged axons at the functional level. Thus, future studies should focus on characterizing OPC differentiation and remyelination after ICH. Understanding the time course of these changes will substantially deepen our knowledge in ICH pathogenesis and promote the development of effective treatments for ICH.
4. CROSSTALK BETWEEN OLIGODENDROCYTES AND OTHER CELLS
Oligodendrocytes function to myelinate axons during development and remyelinate damaged axons after injury. These processes are tightly regulated by a variety of signaling pathways from different cell types. It is important to understand the interactions between oligodendrocytes and other brain cells, taking into account the crucial functions of oligodendrocyte‐lineage cells in white matter damage repair.113 Here, we summarize crosstalk between oligodendrocytes and other cells in the brain. For a summary of the factors from other brain cells and their functions in oligodendrocyte biology, please refer to Table 3.
Table 3.
Factors from other brain cells and their functions in oligodendrocyte biology
| Cell type | Factors | Function |
|---|---|---|
| Neurons | Neuregulin‐1 | OPC proliferation |
| CNTF | OPC proliferation, differentiation, myelination, OL apoptosis | |
| Astrocytes | pStat3 | OPC activation, myelination |
| TNFα‐TNFR2 | OPC proliferation, differentiation, remyelination, OL maturation | |
| BDNF | Oligodendrogenesis | |
| Endothelial cells | FGF | OPC proliferation |
| BDNF | OPC proliferation | |
| Microglia | Activin‐A | OL differentiation, remyelination |
4.1. Crosstalk between oligodendrocytes and neurons
As the target of myelination/remyelination, neurons are well‐positioned to talk with oligodendrocytes. Although not absolutely required for myelination,75 neurons have been shown to actively regulate myelination via neuregulin‐1. For example, glial growth factor, the soluble neuregulin‐1 isoform, has been found to promote OPC proliferation.114 In addition, neuronal expression of neuregulin increases significantly in the penumbra at day 3 after permanent middle cerebral artery occlusion (MCAO).115 Furthermore, neuregulin‐1 has been found to inhibit cortical damage, apoptosis, and inflammatory responses in an MCAO model.116 Based on these results, we hypothesize that neuronal neuregulin‐1 induces OPC proliferation and regulates injury pathology/outcome in ICH.
Another neuronal factor that affects oligodendrocyte function is CNTF (ciliary neurotrophic factor).117 A strong promyelinating effect of CNTF has been proposed based on the enzymatic index of myelination.118 Using transgenic mice without CNTF, it has been shown that CNTF enhances OPC proliferation, reduces oligodendrocyte apoptosis, and protects myelin integrity and function,119 again suggesting a promyelinating effect of CNTF. It is thus speculated that CNTF induces oligodendrocyte differentiation and promotes remyelination after ICH. Future studies should focus on investigating how exactly neurons crosstalk with oligodendrocytes and how this interaction affects disease progression.
4.2. Crosstalk between oligodendrocytes and astrocytes
Astrocyte‐oligodendrocyte crosstalk has been well documented. Accumulating evidence suggests that astrocytes actively regulate OPC differentiation and myelination via secreted growth factors and chemokines.120, 121 First, oligodendrocyte‐mediated remyelination occurs in the areas with astrocytes.122 Next, OPC activation and maturation as well as myelin formation are significantly attenuated in transgenic mice lacking phosphorylated STAT3 (signal transducer and activator of transcription 3) specifically in astrocytes (GFAP‐STAT3‐CKO mice),122 suggesting that pStat3 signaling in astrocytes contributes to OPC activation and subsequent myelination. Third, it has been shown that TNFα (tumor necrosis factor α)‐TNFR2 signaling in astrocytes induces the expression of CXCL12 (C‐X‐C motif chemokine 12), which regulates OPC proliferation, differentiation, and remyelination by binding to its receptor CXCR4.121 In addition, there is also evidence showing that TNFR2 signaling promotes oligodendrocyte maturation via LIF (leukemia inhibitory factor).123 Furthermore, astrocyte‐derived BDNF has also been demonstrated to positively regulate oligodendrogenesis after white matter damage.124 Although none of these studies were performed in ICH condition, it is logical to hypothesize astrocyte‐oligodendrocyte crosstalk induces OPC differentiation and remyelination after ICH in a similar way. This hypothesis will be tested in future studies.
4.3. Crosstalk between oligodendrocytes and endothelial cells
Endothelium‐oligodendrocyte interaction has been reported in previous studies. For example, it has been shown that OPCs make physical contact with brain endothelial cells and migrate along the vasculature during development.17 This correlation between oligodendrocytes and brain endothelial cells has also been found in pathological conditions. It has been reported that oligodendrocytes facilitate angiogenesis partially via upregulating matrix metalloproteinase‐9 (MMP9) after white matter injury.125 Similarly, vessel density partially correlates with the number of Olig2+ cells and oligodendrocytes in an ischemic stroke model,126 and strong angiogenic activity is found in the SVZ, the main neural stem cell niche that produces OPCs after demyelinating injury.127 In addition, OPCs have been shown to enhance the blood‐brain barrier integrity by secreting TGF‐β1 and upregulating tight junction proteins.128 Furthermore, cerebral endothelial cells have been speculated to stimulate OPC proliferation by secreting FGF and BDNF via Src and AKT signaling pathways.24 It is worth noting that the above‐mentioned studies were not done in ICH condition. Future studies should investigate how exactly endothelial cells and oligodendrocytes talk to each other after ICH.
4.4. Crosstalk between oligodendrocytes and microglia
Microglia are one of the first cell types activated after ICH. Activated microglia are classified into two main states: proinflammatory M1 state and antiinflammatory M2 state.129 These two states have different dynamics after ICH. For example, M1 polarization peaks as early as 4 hours after ICH, whereas M2 polarization peaks 24 hours after ICH.130, 131 Although how exactly microglia communicate with oligodendrocytes remains unknown, it is believed that microglia‐derived cytokines are able to regulate oligodendrocyte differentiation and myelination. For example, M2 microglia have been shown to promote oligodendrocyte differentiation and remyelination via activin‐A in demyelinating injury.132 Further studies should address whether and to what extent M2 microglia‐derived activin‐A contributes to oligodendrocyte differentiation and remyelination after ICH.
5. OLIGODENDROCYTE‐BASED THERAPIES
Due to the important functions of oligodendrocytes in myelination/remyelination, oligodendrocyte‐based therapies have attracted a lot of attention. It has been shown that OPC transplantation following spinal cord injury significantly improves the percentage of myelinated axons133 and stimulates functional recovery.134, 135 In addition, OPC transplantation has also been shown to induce myelin sheath formation, stimulate neural stem cell proliferation, facilitate spatial learning and memory recovery, promote BDNF and Bcl‐2 expression, and inhibit neuronal apoptosis in a rat model of periventricular leukomalacia.136 Based on these findings, it is speculated that oligodendrocytes may play a neuroprotective role in ICH by promoting remyelination and aiding in the repair process after injury. This hypothesis will be tested in future research. If a beneficial role of oligodendrocyte‐lineage cells is observed in ICH, these cells may be targeted to develop novel therapies for ICH. Specifically, minimizing oligodendrocyte death/enhancing oligodendrocyte survival and promoting oligodendrocyte differentiation/maturation should be able to improve ICH outcome. A thorough understanding of the biology of oligodendrocyte‐lineage cells and their functions in ICH will open doors for novel and effective treatments for this devastating disease.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This work was partially supported by the American Heart Association Scientist Development Grant (16SDG29320001) to YY.
Kang M, Yao Y. Oligodendrocytes in intracerebral hemorrhage. CNS Neurosci Ther. 2019;25:1075–1084. 10.1111/cns.13193
REFERENCES
- 1. Caceres JA, Goldstein JN. Intracranial hemorrhage. Emerg Med Clin North Am. 2012;30(3):771‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu J, Murphy SL, Kochanek KD, Bastian B, Arias E. Deaths: final data for 2016. Natl Vital Stat Rep. 2018;67(5):1‐76. [PubMed] [Google Scholar]
- 3. Camacho E, LoPresti MA, Bruce S, et al. The role of age in intracerebral hemorrhages. J Clin Neurosci. 2015;22(12):1867‐1870. [DOI] [PubMed] [Google Scholar]
- 4. Joseph M, Caliaperumal J, Schlichter LC. After intracerebral hemorrhage, oligodendrocyte precursors proliferate and differentiate inside white‐matter tracts in the rat striatum. Transl Stroke Res. 2016;7(3):192‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19(1):197‐203. [DOI] [PubMed] [Google Scholar]
- 6. Fancy S, Zhao C, Franklin R. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci. 2004;27(3):247‐254. [DOI] [PubMed] [Google Scholar]
- 7. Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial‐cell specification. Nature. 2010;468(7321):214‐222. [DOI] [PubMed] [Google Scholar]
- 8. Zuchero JB, Barres BA. Glia in mammalian development and disease. Development. 2015;142(22):3805‐3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kessaris N, Pringle N, Richardson WD. Specification of CNS glia from neural stem cells in the embryonic neuroepithelium. Philos Trans R Soc B Biol Sci. 2008;363(1489):71‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wegner M, Kessaris N, Grist M, Richardson WD, Fogarty M, Iannarelli P. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9(2):173‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oligodendrocyte IP. Population Dynamics and Plasticity Probed by Genetic Manipulation in Mice. PhD thesis. London: University College London; 2014. [Google Scholar]
- 12. Bergles DE, Richardson WD. Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol. 2016;8(2):1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rowitch DH, Wagenaar N, de Vries LS, et al. Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia. 2017;66(2):221‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh J, Sharma K, Frost EE, Pillai,. Role of PDGF‐A‐activated ERK signaling mediated FAK‐Paxillin interaction in oligodendrocyte progenitor cell migration. J Mol Neurosci. 2019;67(4):564–573. [DOI] [PubMed] [Google Scholar]
- 15. Hayakawa K, Seo JH, Pham L‐D, et al. Cerebral endothelial derived vascular endothelial growth factor promotes the migration but not the proliferation of oligodendrocyte precursor cells in vitro. Neurosci Lett. 2012;513(1):42‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bribián A, Barallobre J, Soussi‐yanicostas N, De CF. Anosmin‐1 modulates the FGF‐2‐dependent migration of oligodendrocyte precursors in the developing optic nerve. Mol Cell Neurosci. 2006;33:2‐14. [DOI] [PubMed] [Google Scholar]
- 17. Fancy S, Daneman R, Zhang H, et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science. 2016;351(6271):379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kazanis I. Oligodendrocyte progenitor cells the ever mitotic cells of the CNS. Front Biosci. 2016;8(1):29‐43. [DOI] [PubMed] [Google Scholar]
- 19. Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self‐repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16(6):668‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Calver AR, Hall AC, Yu W‐P, et al. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20(5):869‐882. [DOI] [PubMed] [Google Scholar]
- 21. Barres BA, Hart IK, Coles H, et al. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70(1):31‐46. [DOI] [PubMed] [Google Scholar]
- 22. Chandran S. FGF‐dependent generation of oligodendrocytes by a hedgehog‐independent pathway. Development. 2003;130(26):6599‐6609. [DOI] [PubMed] [Google Scholar]
- 23. McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin‐3 and brain‐derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 2018;18(14):5354‐5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arai K, Lo EH. An Oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29(14):4351‐4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang S, Sdrulla A, Johnson JE, Yokota Y, Barres BA. A role for the helix‐loop‐helix protein Id2 in the control of oligodendrocyte development. Neuron. 2001;29(3):603‐614. [DOI] [PubMed] [Google Scholar]
- 26. McKinnon RD, Piras G, Ida JA, Dubois‐Dalcq M. A role for TGF‐β in oligodendrocyte differentiation. J Cell Biol. 1993;121(6):1397‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120(5):1097‐1108. [DOI] [PubMed] [Google Scholar]
- 28. Giuliani A, Aloe L, Calza L, Fernandez M, Giardino L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin‐forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc Natl Acad Sci. 2002;99(5):3258‐3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao X, He X, Han X, et al. MicroRNA‐mediated control of oligodendrocyte differentiation. Neuron. 2010;65(5):612‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arai K, Lo EH. Oligovascular signaling in white matter stroke. Biol Pharm Bull. 2009;32(10):1639‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olude MA, Bello ST, Mustapha OA, et al. Oligodendrocyte morphology in the developing brain of the African giant rat (Cricetomys gambianus, Waterhouse): Histology, immunohistochemistry and electron microscopy. J Vet Med Ser C Anat Histol Embryol. 2018;47(3):231‐238. [DOI] [PubMed] [Google Scholar]
- 32. Lappe‐Siefke C, Goebbels S, Gravel M, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33(3):366‐374. [DOI] [PubMed] [Google Scholar]
- 33. Gong X, Lin T, Sun Z, Fu M, Zuo H, Xie Z. Olig1 is downregulated in oligodendrocyte progenitor cell differentiation. NeuroReport. 2008;19(12):1203‐1207. [DOI] [PubMed] [Google Scholar]
- 34. Traiffort E, Zakaria M, Laouarem Y, Ferent J. Hedgehog: a key signaling in the development of the oligodendrocyte lineage. J Dev Biol. 2016;4(3):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xin M. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1‐Null mice. J Neurosci. 2005;25(6):1354‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuhlmann T, Miron V, Cuo Q, Wegner C, Antel J, Brück W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131(7):1749‐1758. [DOI] [PubMed] [Google Scholar]
- 37. Ligon KL, Alberta JA, Kho AT, et al. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63(5):499‐509. [DOI] [PubMed] [Google Scholar]
- 38. Snaidero N, Velte C, Myllykoski M, et al. Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep. 2017;18(2):314‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scherer SS, Braun PE, Grinspan J, Collarini E, Wang DY, Kamholz J. Differential regulation of the 2′,3′‐cyclic nucleotide 3′‐phosphodiesterase gene during oligodendrocyte development. Neuron. 1994;12(6):1363‐1375. [DOI] [PubMed] [Google Scholar]
- 40. Tse KH, Herrup K. DNA damage in the oligodendrocyte lineage and its role in brain aging. Mech Ageing Dev. 2017;161:37‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilson HC, Scolding NJ, Raine CS. Co‐expression of PDGF α receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;176(1–2):162‐173. [DOI] [PubMed] [Google Scholar]
- 42. Chen X‐S, Zhang Y‐H, Cai Q‐Y, Yao Z‐X. ID2: A negative transcription factor regulating oligodendroglia differentiation. J Neurosci Res. 2012;90(5):925‐932. [DOI] [PubMed] [Google Scholar]
- 43. Kondo T. The Id4 HLH protein and the timing of oligodendrocyte differentiation. EMBO J. 2002;19(9):1998‐2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jessen KR. Glial cells. Int J Biochem Cell Biol. 2004;36(10):1861‐1867. [DOI] [PubMed] [Google Scholar]
- 45. De Castro F, Bribián A. The molecular orchestra of the migration of oligodendrocyte precursors during development. Brain Res Rev. 2005;49(2):227‐241. [DOI] [PubMed] [Google Scholar]
- 46. Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193‐215. [DOI] [PubMed] [Google Scholar]
- 47. Vanlandewijck M, He L, Mäe MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554(7693):475‐480. [DOI] [PubMed] [Google Scholar]
- 48. Sun LO, Mulinyawe SB, Collins HY, et al. Spatiotemporal control of CNS myelination by oligodendrocyte programmed cell death through the TFEB‐PUMA axis. Cell. 2018;175(7):1811‐1826.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21(4):1302‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Warrington AE, Pfeiffer SE. Proliferation and differentiation of O4+oligodendrocytes in postnatal rat cerebellum: analysis in unfixed tissue slices using anti‐glycolipid antibodies. J Neurosci Res. 1992;33(2):338‐353. [DOI] [PubMed] [Google Scholar]
- 51. Butts BD, Houde C, Mehmet H. Maturation‐dependent sensitivity of oligodendrocyte lineage cells to apoptosis: Implications for normal development and disease. Cell Death Differ. 2008;15(7):1178‐1186. [DOI] [PubMed] [Google Scholar]
- 52. Bujalka H, Koenning M, Jackson S, et al. Is a membrane‐associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS Biol. 2013;11(8):e1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Philips T, Rothstein JD. Oligodendroglia: metabolic supporters of neurons. J Clin Investig. 2017;127(9):3271‐3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peschl P, Bradl M, Höftberger R, Berger T, Reindl M. Myelin oligodendrocyte glycoprotein: Deciphering a target in inflammatory demyelinating diseases. Front Immunol. 2017;8:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jiang W, Yang W, Yang W, et al. Identification of Tmem10 as a novel late‐stage oligodendrocytes marker for detecting hypomyelination. Int J Biol Sci. 2013;10(1):33‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brunner C, Lassmann H, Waehneldt TV, Matthieu J‐M, Linington C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2′,3′‐cyclic nucleotide 3′‐phosphodiesterase in the CNS of adult rats. J Neurochem. 1989;52(1):296‐304. [DOI] [PubMed] [Google Scholar]
- 57. Yin X, Kidd GJ, Ohno N, et al. Proteolipid protein‐deficient myelin promotes axonal mitochondrial dysfunction via altered metabolic coupling. J Cell Biol. 2016;215(4):531‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Han H, Myllykoski M, Ruskamo S, Wang C, Kursula P. Myelin‐specific proteins: a structurally diverse group of membrane‐interacting molecules. BioFactors. 2013;39(3):233‐241. [DOI] [PubMed] [Google Scholar]
- 59. Quarles RH. Myelin‐associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007;100(6):1431‐1448. [DOI] [PubMed] [Google Scholar]
- 60. Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol. 2007;17(1):29‐35. [DOI] [PubMed] [Google Scholar]
- 61. Roncagliolo M, Schlageter C, León C, Couve E, Bonansco C, Eguibar JR. Developmental impairment of compound action potential in the optic nerve of myelin mutant taiep rats. Brain Res. 2006;1067(1):78‐84. [DOI] [PubMed] [Google Scholar]
- 62. Gyllborg D, Hochgerner H, Li H, et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science. 2016;352(6291):1326–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McKenzie IA, Ohayon D, Li H, et al. Motor skill learning requires active central myelination. Science. 2014;346(6207):318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu J, Dupree JL, Gacias M, et al. Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J Neurosci. 2016;36(3):957‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nave K‐A, Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30(1):503‐533. [DOI] [PubMed] [Google Scholar]
- 66. Baron W, Hoekstra D. On the biogenesis of myelin membranes: Sorting, trafficking and cell polarity. FEBS Lett. 2010;584(9):1760‐1770. [DOI] [PubMed] [Google Scholar]
- 67. Snaidero N, Möbius W, Czopka T, et al. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3‐dependent polarized growth at the inner tongue. Cell. 2014;156(1–2):277‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. García‐Mateo N, Pascua‐Maestro R, Pérez‐Castellanos A, Lillo C, Sanchez D, Ganfornina MD. Myelin extracellular leaflet compaction requires apolipoprotein D membrane management to optimize lysosomal‐dependent recycling and glycocalyx removal. Glia. 2018;66(3):670‐687. [DOI] [PubMed] [Google Scholar]
- 69. Aggarwal S, Snaidero N, Pähler G, et al. Myelin membrane assembly is driven by a phase transition of myelin basic proteins into a cohesive protein meshwork. PLoS Biol. 2013;11(6):e1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Raasakka A, Ruskamo S, Kowal J, et al. Membrane association landscape of myelin basic protein portrays formation of the myelin major dense line. Sci Rep. 2017;7(1):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McLaurin J, Ackerley CA, Moscarello MA. Localization of basic proteins in human myelin. J Neurosci Res. 1993;35(6):618‐628. [DOI] [PubMed] [Google Scholar]
- 72. Bechler ME, Ffrench‐Constant C. A new wrap for neuronal activity? Science. 2014;344(6183):480–481. [DOI] [PubMed] [Google Scholar]
- 73. Ford MC, Alexandrova O, Cossell L, et al. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat Commun. 2015;6:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mensch S, Baraban M, Almeida R, et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci. 2015;18(5):628‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lee S, Leach MK, Redmond SA, et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods. 2012;9(9):917‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Osanai Y, Shimizu T, Mori T, et al. Rabies virus‐mediated oligodendrocyte labeling reveals a single oligodendrocyte myelinates axons from distinct brain regions. Glia. 2017;65(1):93‐105. [DOI] [PubMed] [Google Scholar]
- 77. Hu J, Deng L, Wang X, Xu XM. Effects of extracellular matrix molecules on the growth properties of oligodendrocyte progenitor cells in vitro. J Neurosci Res. 2009;87(13):2854‐2862. [DOI] [PubMed] [Google Scholar]
- 78. Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience. 2014;276:29‐47. [DOI] [PubMed] [Google Scholar]
- 79. Liang X, Draghi NA, Resh MD. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J Neurosci. 2004;24(32):7140‐7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chun SJ, Rasband MN, Sidman RL, Habib AA, Vartanian T. Integrin‐linked kinase is required for laminin‐2‐induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol. 2003;163(2):397‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Buttery PC, Ffrench‐Constant C. Laminin‐2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14(3):199‐212. [DOI] [PubMed] [Google Scholar]
- 82. Barros CS, Nguyen T, Spencer K, Nishiyama A, Colognato H, Muller U. β1 integrins are required for normal CNS myelination and promote AKT‐dependent myelin outgrowth. Development. 2009;136(16):2717‐2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Colognato H, Galvin J, Wang Z, et al. Identification of dystroglycan as a second laminin receptor in oligodendrocytes, with a role in myelination. Development. 2007;134(9):1723‐1736. [DOI] [PubMed] [Google Scholar]
- 84. Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol. 1999;145(6):1209‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Richter‐Landsberg C. The cytoskeleton in oligodendrocytes: Microtubule dynamics in health and disease. J Mol Neurosci. 2008;35(1):55‐63. [DOI] [PubMed] [Google Scholar]
- 86. Krämer E‐M, Klein C, Trotter J, Schraven B, Cardine A‐M, Brandt R. Process outgrowth of oligodendrocytes is promoted by interaction of Fyn kinase with the cytoskeletal protein tau. J Neurosci. 2002;22(3):698‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bansal R, Furusho M, Miller RH, Fyffe‐Maricich SL, Ishii A. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J Neurosci. 2012;32(26):8855‐8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Flores AI, Narayanan SP, Morse EN, et al. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28(28):7174‐7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci. 2009;29(21):6860‐6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Taveggia C, Thaker P, Petrylak A, et al. Type III neuregulin‐1 promotes oligodendrocyte myelination. Glia. 2008;56(3):284‐293. [DOI] [PubMed] [Google Scholar]
- 91. Fonte C, Schumacher M, Makoukji J, et al. Wnt/β‐Catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J Neurosci. 2011;31(10):3729‐3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xiao J, Wong AW, Willingham MM, Van Den Buuse M, Kilpatrick TJ, Murray SS. Brain‐derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals. 2011;18(3):186‐202. [DOI] [PubMed] [Google Scholar]
- 93. An SJ, Kim TJ, Yoon B‐W. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J stroke. 2017;19(1):3‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ariesen MJ, Claus SP, Rinkel G, Algra A. Risk factors for intracerebral hemorrhage in the general population: A systematic review. Stroke. 2003;34(8):2060‐2065. [DOI] [PubMed] [Google Scholar]
- 95. Roquer J, Rodríguez‐Campello A, Jiménez‐Conde J, et al. Sex‐related differences in primary intracerebral hemorrhage. Neurology. 2016;87(3):257‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Marini S, Morotti A, Ayres AM, et al. Sex differences in intracerebral hemorrhage expansion and mortality. J Neurol Sci. 2017;379(617):112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Spitzer SO, Sitnikov S, Kamen Y, et al. Oligodendrocyte progenitor cells become regionally diverse and heterogeneous with age. Neuron. 2019;101(3):459‐471.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yamada J, Jinno S. Age‐related differences in oligodendrogenesis across the dorsal‐ventral axis of the mouse hippocampus. Hippocampus. 2014;24(8):1017‐1029. [DOI] [PubMed] [Google Scholar]
- 99. Yang S, Li C, Zhang W, Wang W, Tang Y. Sex differences in the white matter and myelinated nerve fibers of Long‐Evans rats. Brain Res. 2008;1216:16‐23. [DOI] [PubMed] [Google Scholar]
- 100. Marin‐Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia‐Bonnefil P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci. 2004;26(2–4):245‐254. [DOI] [PubMed] [Google Scholar]
- 101. Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. The Lancet. 2009;373(9675):1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wu H, Wu T, Xu X, Wang J, Wang J. Iron toxicity in mice with collagenase‐induced intracerebral hemorrhage. J Cereb Blood Flow Metab. 2011;31(5):1243‐1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hua YA, Nakamura T, Keep RF, et al. Long‐term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104(2):305‐312. [DOI] [PubMed] [Google Scholar]
- 104. Goldstein EZ, Church JS, Pukos N, Gottipati MK, Popovich PG, McTigue DM. Intraspinal TLR4 activation promotes iron storage but does not protect neurons or oligodendrocytes from progressive iron‐mediated damage. Exp Neurol. 2017;298:42‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhuo F, Qiu GuoPing, Xu J, et al. Both endoplasmic reticulum and mitochondrial pathways are involved in oligodendrocyte apoptosis induced by capsular hemorrhage. Mol Cell Neurosci. 2016;72:64‐71. [DOI] [PubMed] [Google Scholar]
- 106. Wasserman JK, Schlichter LC. White matter injury in young and aged rats after intracerebral hemorrhage. Exp Neurol. 2008;214(2):266‐275. [DOI] [PubMed] [Google Scholar]
- 107. Zuo S, Pan P, Li Q, Chen Y, Feng H. White matter injury and recovery after hypertensive intracerebral hemorrhage. Biomed Res Int. 2017;2017:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23(3):263‐274. [DOI] [PubMed] [Google Scholar]
- 109. Zhu X, Tao L, Tejima‐Mandeville E, et al. Plasmalemma permeability and necrotic cell death phenotypes after intracerebral hemorrhage in mice. Stroke. 2012;43(2):524‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Franklin R, Kotter MR. The biology of CNS remyelination: The key to therapeutic advances. J Neurol. 2008;255(Suppl. 1):19‐25. [DOI] [PubMed] [Google Scholar]
- 111. Franklin R, Ffrench‐Constant C. Remyelination in the CNS: From biology to therapy. Nat Rev Neurosci. 2008;9(11):839‐855. [DOI] [PubMed] [Google Scholar]
- 112. Menn B, Garcia‐Verdugo JM, Yaschine C, Gonzalez‐Perez O, Rowitch D, Alvarez‐Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26(30):7907‐7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shindo A, Liang AC, Maki T, et al. Subcortical ischemic vascular disease: roles of oligodendrocyte function in experimental models of subcortical white‐matter injury. J Cereb Blood Flow Metab. 2016;36(1):187‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17(2):229‐243. [DOI] [PubMed] [Google Scholar]
- 115. Parker MW, Chen Y, Hallenbeck JM, Ford BD. Neuregulin expression after focal stroke in the rat. Neurosci Lett. 2002;334(3):169‐172. [DOI] [PubMed] [Google Scholar]
- 116. Xu Z, Jiang J, Ford G, Ford BD. Neuregulin‐1 is neuroprotective and attenuates inflammatory responses induced by ischemic stroke. Biochem Biophys Res Commun. 2004;322(2):440‐446. [DOI] [PubMed] [Google Scholar]
- 117. Itoh K, Maki T, Lok J, Arai K. Mechanisms of cell‐cell interaction in oligodendrogenesis and remyelination after stroke. Brain Res. 2015;1623(21):135‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stankoff B, Aigrot M‐S, Noël F, Wattilliaux A, Zalc B, Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF‐related molecules. J Neurosci. 2002;22(21):9221‐9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Linker RA, Mäurer M, Gaupp S, et al. CNTF is a major protective factor in demyelinating CNS disease: A neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002;8(6):620‐624. [DOI] [PubMed] [Google Scholar]
- 120. Biancotti JC, Kumar S, De Vellis J. Activation of inflammatory response by a combination of growth factors in cuprizone‐induced demyelinated brain leads to myelin repair. Neurochem Res. 2008;33(12):2615‐2628. [DOI] [PubMed] [Google Scholar]
- 121. Patel JR, Williams JL, Muccigrosso MM, et al. Astrocyte TNFR2 is required for CXCL12‐mediated regulation of oligodendrocyte progenitor proliferation and differentiation within the adult CNS. Acta Neuropathol. 2012;124(6):847‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Monteiro De Castro G, Deja NA, Ma D, Zhao C, Franklin R. Astrocyte activation via Stat3 signaling determines the balance of oligodendrocyte versus schwann cell remyelination. Am J Pathol. 2015;185(9):2431‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Fischer R, Wajant H, Kontermann R, Pfizenmaier K, Maier O. Astrocyte‐specific activation of TNFR2 promotes oligodendrocyte maturation by secretion of leukemia inhibitory factor. Glia. 2014;62(2):272‐283. [DOI] [PubMed] [Google Scholar]
- 124. Miyamoto N, Maki T, Shindo A, et al. Astrocytes promote oligodendrogenesis after white matter damage via brain‐derived neurotrophic factor. J Neurosci. 2015;35(41):14002‐14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pham L‐D, Hayakawa K, Seo JH, et al. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60(6):875‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jiang L, Shen F, Degos V, et al. Oligogenesis and oligodendrocyte progenitor maturation vary in different brain regions and partially correlate with local angiogenesis after ischemic stroke. Transl Stroke Res. 2011;2(3):366‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cayre M, Courtes S, Martineau F, et al. Netrin 1 contributes to vascular remodeling in the subventricular zone and promotes progenitor emigration after demyelination. Development. 2013;140(15):3107‐3117. [DOI] [PubMed] [Google Scholar]
- 128. Seo JH, Maki T, Maeda M, et al. Oligodendrocyte precursor cells support blood‐brain barrier integrity via TGF‐β signaling. PLoS ONE. 2014;9(7):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang Z, Zhang Z, Lu H, Yang Q, Wu H, Wang J. Microglial Polarization and inflammatory mediators after intracerebral hemorrhage. Mol Neurobiol. 2017;54(3):1874‐1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wan S, Cheng Y, Jin H, et al. Microglia activation and polarization after intracerebral hemorrhage in mice: the role of protease‐activated receptor‐1. Transl Stroke Res. 2016;7(6):478‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhao X, Sun G, Ting S‐M, et al. Cleaning up after ICH: The role of Nrf2 in modulating microglia function and hematoma clearance. J Neurochem. 2015;133(1):144‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Miron VE, Boyd A, Zhao J‐W, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Fu H, Hu D, Zhang L, Shen X, Tang P. Efficacy of oligodendrocyte progenitor cell transplantation in rat models with traumatic thoracic spinal cord injury: a systematic review and meta‐analysis. J Neurotrauma. 2018;35(21):2507‐2518. [DOI] [PubMed] [Google Scholar]
- 134. Yang J, Xiong LL, Wang YC, et al. Oligodendrocyte precursor cell transplantation promotes functional recovery following contusive spinal cord injury in rats and is associated with altered MicroRNA expression. Mol Med Rep. 2018;17(1):771‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sun Y, Xu C‐C, Li J, et al. Transplantation of oligodendrocyte precursor cells improves locomotion deficits in rats with spinal cord irradiation injury. PLoS ONE. 2013;8(2):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chen L‐X, Ma S‐M, Zhang P, et al. Neuroprotective effects of oligodendrocyte progenitor cell transplantation in premature rat brain following hypoxic‐ischemic injury. PLoS ONE. 2015;10(3):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]


