Summary
Aims
Previous studies indicated that intraventricular injection of thrombin would induce hydrocephalus. But how thrombin works in this process remains unclear. Since cadherin plays a critical role in hydrocephalus, we aimed to explore the mechanisms of how thrombin acted on choroid plexus vascular endothelium and how thrombin interacted with vascular endothelial‐cadherin (VE‐cadherin) during hydrocephalus.
Methods
There were two parts in this study. Firstly, rats received an injection of saline or thrombin into the right lateral ventricle. Magnetic resonance imaging was applied to measure the lateral ventricle volumes. Albumin leakage and Evans blue content were assessed to test the blood‐brain barrier function. Immunofluorescence and Western blot were applied to detect the location and the expression of VE‐cadherin. Secondly, we observed the roles of protease‐activated receptors‐1 (PAR1) inhibitor (SCH79797), Src inhibitor (PP2), p21‐activated kinase‐1 (PAK1) inhibitor (IPA3) in the thrombin‐induced hydrocephalus, and their effects on the regulation of VE‐cadherin.
Results
Our study demonstrated that intraventricular injection of thrombin caused significant downregulation of VE‐cadherin in choroid plexus and dilation of ventricles. In addition, the inhibition of PAR1/p‐Src/p‐PAK1 pathway reversed the decrease of VE‐cadherin and attenuated thrombin‐induced hydrocephalus.
Conclusions
Our results suggested that the thrombin‐induced hydrocephalus was associated with the inhibition of VE‐cadherin via the PAR1/p‐Src/p‐PAK1 pathway.
Keywords: choroid plexuses, hydrocephalus, PAR1 pathway, thrombin, vascular endothelial‐cadherin
1. INTRODUCTION
Hydrocephalus is an independent predictor of poor outcome for intraventricular hemorrhage (IVH), which occurs in nearly half of patients of spontaneous intracerebral hemorrhage (ICH)1 However, neither the mechanisms of IVH‐induced hydrocephalus nor efficacy treatments have been suggested.1, 2, 3, 4 Heparinized blood injection resulted in less hydrocephalus compared with the whole blood injection, indicating that coagulation cascade played a critical role in the IVH‐induced hydrocephalus.5, 6, 7, 8, 9 Our previous study revealed that thrombin, a critical factor of the coagulation cascade, contributed to the hydrocephalus development after IVH and this thrombin‐induced hydrocephalus was dependent on protease‐activated receptors‐1 (PAR1).8, 10 However, the downstream signaling pathways of PAR1 in thrombin‐related hydrocephalus are scarcely explored.
Cerebrospinal fluid (CSF) is mainly produced in the choroid plexuses, which consist of plenty of capillaries constructing the blood‐CSF barrier.11 Choroid plexuses dysfunction would be expected to cause an increase in the production of CSF.12, 13 The transmembrane protein vascular endothelial‐cadherin (VE‐cadherin) exists in the central nervous system endothelium.14 VE‐cadherin belongs to the cadherin superfamily which is involved in cell‐cell adhesion and signaling.15 Moreover, it has been recognized that cadherin plays a critical role in the formation of hydrocephalus.16, 17, 18 It is also found that thrombin could lead to the degradation of VE‐cadherin as well as the breakdown of the blood‐brain barrier (BBB) integrity and thus induce the brain edema after ICH.19 Furthermore, PAR1 is generally acknowledged as the main thrombin receptor on the endothelial cells.20, 21 Previous studies suggested that thrombin increased the microvascular permeability by activating PAR1 pathway, which increased the activity of downstream protein kinases, such as p21‐activated kinase‐1 (PAK1).19 Several lines of evidence also indicated that the phosphorylation of Src or PAK1 was involved in the degradation and loss of VE‐cadherin.22, 23, 24, 25
In this study, we hypothesized that thrombin‐induced hydrocephalus was related to the PAR1 signaling pathway which resulted in the decline of VE‐cadherin in choroid plexuses. And this process may shed light on the formation of hydrocephalus after IVH.
2. METHODS
2.1. Animals model
Animal protocols were approved by and conducted in accordance with the ethical guidelines of the Zhejiang University Animal Experimentation Committee and were in complete compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A total of 44 male Sprague Dawley rats (3 months old, Zhejiang University Laboratories), at the weight of 280‐320 g, were used in this study. Animals were anesthetized with pentobarbital (50 mg/kg intraperitoneally (IP)) and were positioned in a stereotaxic frame. A cranial burr hole (1 mm) was drilled, and a 26‐gauge needle was inserted stereotaxically into the right lateral ventricle (coordinates: 0.6 mm posterior, 4.5 mm ventral, and 1.6 mm lateral to the bregma). Saline or thrombin (3U in 50 μL) was infused using a microinfusion pump (World Precision Instruments). The needle was removed after injection, the burr hole was filled with bone wax, and the skin incision was sutured.
2.2. Experimental groups
In brief, the rats were randomly divided into two parts. First, rats received an intracerebroventricular (ICV) injection of three units rat thrombin (Sigma‐Aldrich, St Louis, MO, USA) in saline or saline alone (50 μL) in 7 minutes and were anesthetized at 24 hours for magnetic resonance imaging (MRI) scanning. And then, rats were euthanized for intracardiac perfusion and the brains were used for Western blot (n = 4 for each group), brain histology (n = 4 for each group), and Evans blue assay (n = 4 for each group). Second, rats were treated with vehicle (1% dimethyl sulfoxide in saline) or PAR1 inhibitor (SCH79797, Abcam, USA, 25 μg/kg, IP), Src inhibitor (PP2, Abcam, USA, 1 mg/kg, IP), PAK1 inhibitor (IPA3, Abcam, USA, 1 mg/kg, IP) immediately while thrombin infusion. Serial MRI scanning was performed 24 hours after injection, and the brains were used for Western blot (n = 4 for each group, Figure 1).
Figure 1.
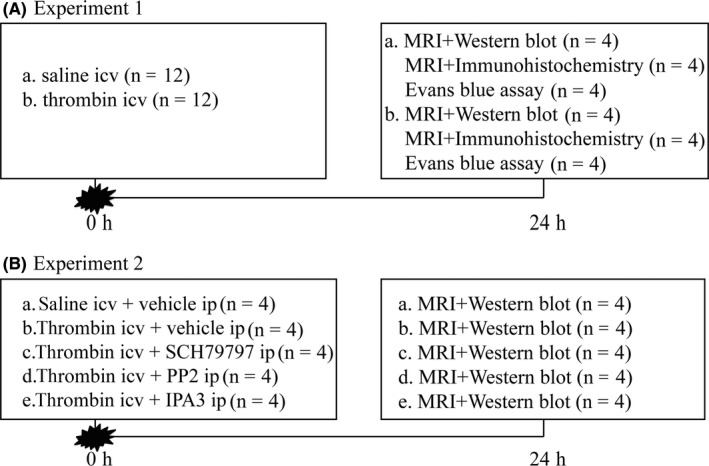
Experimental designs. (A) Experiment 1 was designed to investigate the effect of thrombin on dilation of ventricles and the VE‐cadherin expression in choroid plexus. (B) Experiment 2 was designed to evaluate the effect of PAR1/p‐Src/p‐PAK1 pathway on thrombin‐induced hydrocephalus
2.3. MRI scanning and ventricle volume measurement
Under chloral hydrate anesthesia, the rats were placed in a rodent coil. Magnetic resonance imaging was performed in a 3.0‐T Varian MR scanner (GE Signa HDxt) with a T2 sequence using a view field of 50 mm × 50 mm, a matrix of 128 × 128, and 0.8 mm slice thickness. Ventricular volumes were measured and calculated as described previously.8 Bilateral ventricles from frontal horns to the fourth ventricle were outlined and measured. Ventricular volume was calculated by summing the ventricle areas over all slices and multiplying by section thickness. All image analysis was performed using ImageJ program by a masked observer.
2.4. Immunohistochemistry and immunofluorescence
Rats were anesthetized with pentobarbital (100 mg/kg, IP) and perfused with 4% paraformaldehyde in 0.1 mol/L phosphate‐buffered saline (pH 7.4). The brains were removed and kept in 4% paraformaldehyde for 24 hours and then immersed in 30% sucrose for 2‐3 days at 4°C. Brains were embedded in optimal cutting temperature compound and 18‐µm thick slices cut using a cryostat (LEICA, CM3050S). DAB staining and immunofluorescence studies were performed as previously described.5 The primary antibodies were sheep anti‐rat albumin (1:500 dilution; Bethyl Laboratories, Montgomery, TX, USA) and mouse anti‐VE‐cadherin (1:50 dilution; Santa Cruz, USA).
2.5. Western blot analysis
Briefly, brain tissue was immersed in western sample buffer and lysed. The lysate was centrifuged at 14 000 g for 30 minutes at 4°C, and the supernatant was collected. Protein concentration was determined by the BCA protein assay kit (Pierce, Thermo Scientific, USA). Protein samples were separated using SDS‐polyacrylamide gel electrophoresis and transferred to the nitrocellulose membrane. After blocking with 5% nonfat milk, the membranes were incubated with primary antibodies anti‐VE‐cadherin(1:200 dilution; Santa Cruz, USA), anti‐p‐Src(1:1000 dilution; CST, USA), anti‐p‐PAK1(1:5000 dilution; Abcam, USA), and anti‐GAPDH (1:3000; KangChen, Shanghai, China) at 4°C overnight. After repeated washes, the membranes were reacted with antibodies against rabbit IgG (1:3000; Multi Sciences, China) or mouse IgG (1:3000; Multi Sciences, China) for 2 hours at room temperature. The relative densities of bands were analyzed with ImageJ software.
2.6. Evans blue extravasation assay
Evans blue dye (2%; 5 mL/kg) was injected intraperitoneally and allowed to circulate for 3 hours. Rats were then euthanized by intracardiac perfusion with 0.01 mol/L ice‐cold phosphate‐buffered saline, and brains were divided into six regions. Tissues were homogenized in 50% trichloroacetic. After centrifugation (4°C, 20 000 g for 20 minutes), the amount of extravascular Evans blue dye was measured by spectrofluorophotometer. Evans blue extravasation was calculated as thrombin/saline ratios according to different regions.
2.7. Statistical analysis
Values are given as means ± SD. T tests were used to compare the data between saline and thrombin group. One‐way ANOVA with Tukey's post hoc test was used to analyze the data between multiple groups. Differences were considered significant at P < 0.05.
3. RESULTS
3.1. Intraventricular injection of thrombin caused hydrocephalus
This current study confirmed that injection of thrombin resulted in obvious ventricles dilation on MRI (Figure 2A). Lateral ventricular volumes in thrombin‐injected rats were significantly larger than in rats receiving the saline injection at 24 hours (21.82 ± 1.54 vs 8.73 ± 0.56 mm3 in saline control, P < 0.01, Figure 2B).
Figure 2.
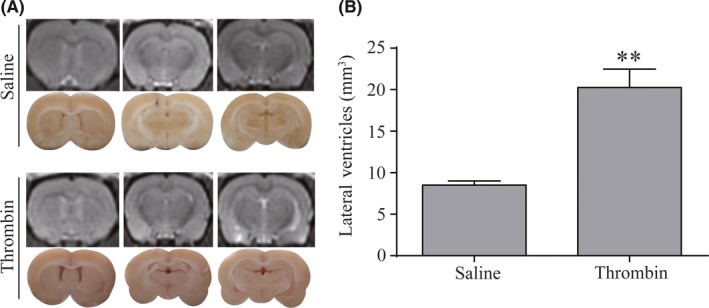
Thrombin‐induced lateral ventricles dilation. (A) T2‐weighted MRI images and frozen coronal brain sections at 24 h after injection of 50 μL of saline or thrombin (3 units) into the right lateral ventricle. (B) Quantification of lateral ventricle volume from magnetic resonance imaging (MRI) images. Values are means ± SD, n = 8, **P < 0.01 vs saline group
3.2. Intraventricular injection of thrombin‐induced impairment of blood‐brain barrier
Compared with the saline injection group, we observed more albumin deposit and Evans blue leakage around the brain ventricles after thrombin injection (Figure 3A, B). Evans blue binds to albumin, and it can be quantified in the extravascular compartment if there is a breakdown of BBB, which possibly suggested the impaired VE‐cadherin. These results strongly inferred that thrombin also caused the dysfunction of VE‐cadherin in choroid plexus and ultimately led to hydrocephalus.
Figure 3.
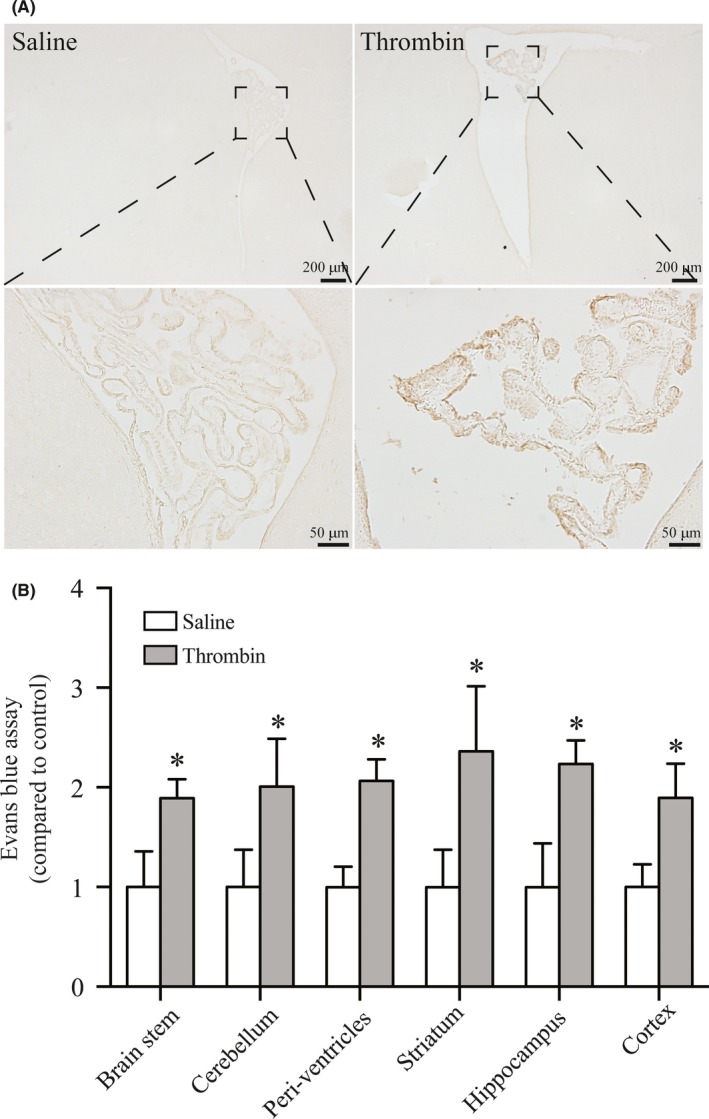
The effect of thrombin on BBB was assessed by albumin staining and Evans blue leakage. (A) DAB staining of coronal sections showed albumin deposited at choroid plexus and surrounding ventricles. Scale bar = 200 μm (low magnification) and 50 μm (high magnification). (B) Rats showed more Evans blue leakage after intraventricular injection of thrombin than the saline group. Values are means ± SD, n = 4, *P < 0.05 vs saline group
3.3. Intraventricular injection of thrombin resulted in downregulation of VE‐cadherin in choroid plexus
We observed the expression of VE‐cadherin in choroid plexus during thrombin‐induced hydrocephalus. At 24 hours after thrombin injection, immunofluorescence staining of choroid plexus was performed. In the saline injection group, VE‐cadherin abundantly distributed in the borders of choroid plexus endothelial cells. After thrombin injection, the expression of VE‐cadherin was remarkably decreased (Figure 4A) in the choroid plexus compared to the saline‐injected group, which was assessed by Western blot (0.54 ± 0.06 vs 1.05 ± 0.07 in the saline group, P < 0.01, Figure 4B, C).
Figure 4.
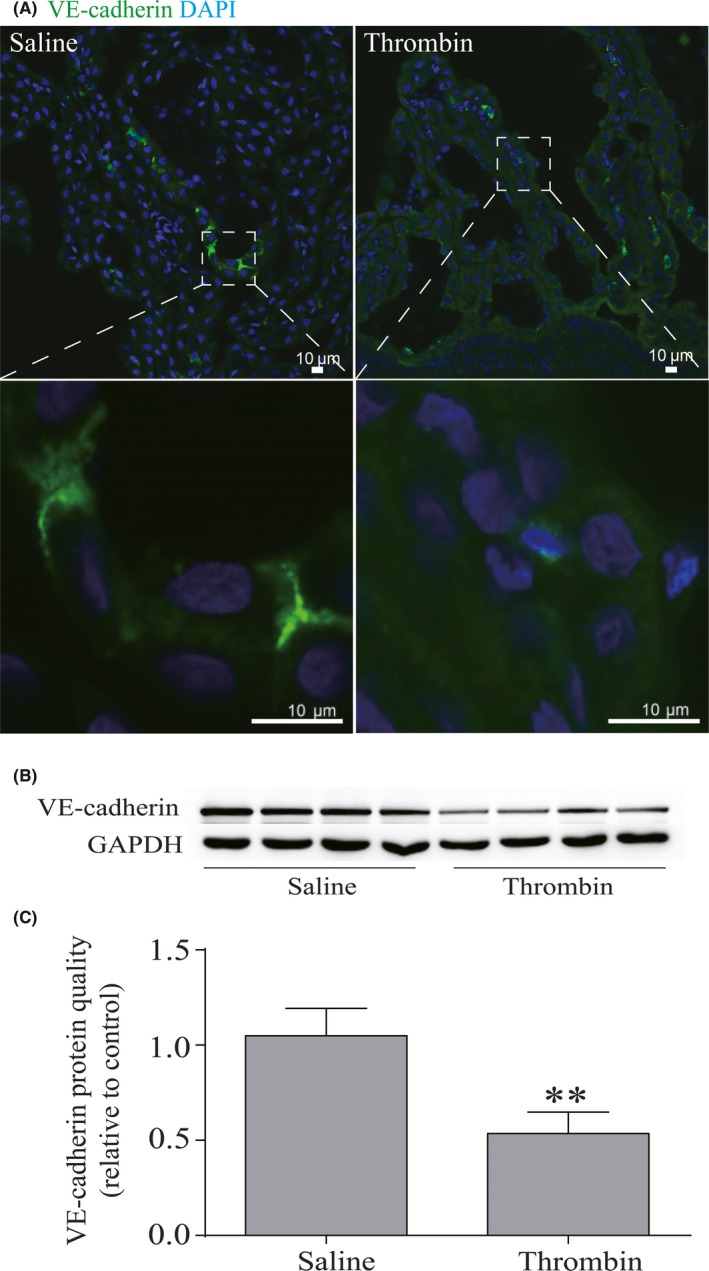
Thrombin reduced the level of VE‐cadherin in the choroid plexus. (A) immuno fluorescence staining of coronal sections showed the downregulation of VE‐cadherin after thrombin injection, n = 4. Scale bar = 10 μm. (B) VE‐cadherin expression level was assessed by Western blot. (C) Quantitative analysis of VE‐cadherin. Values are means ± SD, n = 4, **P < 0.01 vs saline group
3.4. Inhibition of PAR1, p‐Src, or p‐PAK1 attenuated thrombin‐induced hydrocephalus
Rats were treated with either vehicle or PAR1 inhibitor (SCH79797, 25 μg/kg, IP), Src inhibitor (PP2, 1 mg/kg, IP), PAK1 inhibitor (IPA3, 1 mg/kg, IP) immediately when they got thrombin infusion. MRI scanner was performed 24 hours after thrombin injection. Results demonstrated that blocking PAR1, p‐Src, or p‐PAK1 all alleviated ventricles dilation after thrombin injection (P < 0.05, Figure 5).
Figure 5.
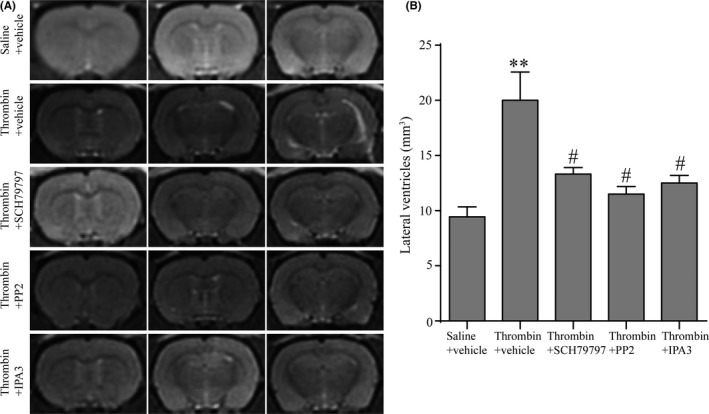
Inhibition of PAR1, p‐Src or p‐PAK1 attenuated thrombin‐induced hydrocephalus. (A) T2‐weighted magnetic resonance imaging (MRI) images at 24 h after injection of 50 μL of thrombin (3 units) into the right lateral ventricle with a treatment of vehicles/SCH79797/PP2/IPA3. (B) Quantification of lateral ventricle volume from MRI images. Values are means ± SD, n = 4, **P < 0.01 vs saline group, #P < 0.05 vs thrombin + vehicle group
3.5. Thrombin downregulated VE‐cadherin in choroid plexus via activating PAR1/p‐Src/p‐PAK1 pathway
We used the PAR1 inhibitor (SCH79797), Src inhibitor (PP2), and PAK1 inhibitor (IPA3) to explore the downstream mechanism in the thrombin‐induced hydrocephalus. We found that PAR1, p‐Src, and p‐PAK1 were significantly increased in the thrombin group (P < 0.05; Figure 6A‐C). The increase of PAR1 was reversed by SCH79797, but not PP2 or IPA3 treatment (P < 0.05; Figure 6A), it's reasonable to conclude that PAR1 is upstream of p‐Src and p‐PAK1. Meanwhile, the increase of p‐Src was reversed by SCH79797 and PP2 treatment, but not IPA3 (P < 0.05; Figure 6B), this indicated p‐Src is an upstream regulator of p‐PAK1 in the PAR1 signaling pathway. In further, the increase of p‐PAK1 was reversed by SCH79797, PP2, or IPA3 treatment (P < 0.05; Figure 6C). In addition, administration of SCH79797, PP2, or IPA3 led to improved outcome in the level of VE‐cadherin (P < 0.05; Figure 6D). Overall, it indicated that PAR1/p‐Src/p‐PAK1 pathway played an important role in thrombin‐induced downregulation of VE‐cadherin.
Figure 6.
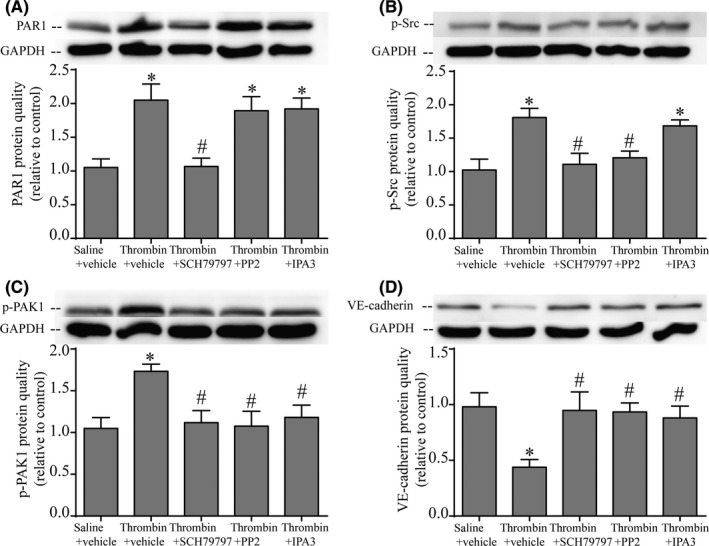
Thrombin reduced VE‐cadherin via activating PAR1/p‐Src/p‐PAK1 pathway. (A) SCH79797, as a PAR‐1 antagonist, suppressed PAR‐1 expression. (B) SCH79797 decreased the phosphorylated Src level. (C) The increase of p‐PAK1 was reversed by SCH79797, PP2, or IPA‐3 treatment. (D) SCH79797, PP2, and IPA‐3 all alleviated the loss of VE‐cadherin via blocking the PAR‐1‐p‐Src‐pPAK1 signaling pathway. Protein level was assessed by Western blot with quantification. Values are means ± SD, n = 4, *P < 0.05 vs saline group, #P < 0.05 vs thrombin + vehicle group
4. DISCUSSIONS
The major findings of this study are as follows: (a) thrombin contributed to hydrocephalus development after IVH; (b) thrombin resulted in the downregulation of VE‐cadherin in the choroid plexus; (c) inhibition of PAR1/p‐Src/p‐PAK1 pathway reversed the dampened VE‐cadherin and alleviated thrombin‐induced hydrocephalus.
The mechanisms of brain damage caused by IVH have been poorly illustrated. Dilatation of the ventricles is a specific character of severe IVH or subarachnoid hemorrhage (SAH) and has been reported as an unfavorable predictor of the patients’ survival and neurological outcome.26, 27, 28 Previous theory supported that the mass effect of blood clots may be one of the major factors accounting for hydrocephalus following IVH, but these mechanical factors could not explain all aspects of clinical situations.29, 30 IVH secondary to ICH is a medical emergency, and the deterioration of neurological functions occurs within the first 24‐48 hours.1, 31, 32 Thrombin is an essential component of the coagulation cascade and can be produced immediately in the brain after IVH. The increased plasma thrombin after IVH can result from the disruption of arteries and the endogenous thrombin production.19 Thrombin production is related to early brain injury (within the first 2 days) after hemorrhage stroke.6, 33 Our current study focused on the time point of 24 hours after thrombin injection to explore the role of thrombin in IVH. In the previous study, we found that heparin could alleviate IVH‐induced hydrocephalus and intraventricular injection of thrombin alone could induce hydrocephalus8; all these studies suggested that thrombin should be responsible for the IVH‐induced hydrocephalus. Our present study aimed to identify how thrombin causes hydrocephalus.
Cerebrospinal fluid is primarily produced in the choroid plexuses, which consist of many capillaries and are important components of the blood‐CSF barrier.11 VE‐cadherin is a crucial component of adherent junctions (AJs) in vascular endothelial cells, which regulates water and solutes in and out vessels34 and forms the restrictive endothelial barrier.34, 35 VE‐cadherin possesses a general modular structure of five extracellular repeats, a transmembrane domain, and a cytoplasmic tail.36 The external domain of VE‐cadherin mediates homophilic binding,36, 37 while the cytoplasmic domain of VE‐cadherin interacts with catenin proteins which prevent VE‐cadherin proteolysis.36 Previous evidence demonstrated that thrombin downregulated VE‐cadherin leading to the BBB disruption and brain edema by breaking off vascular integrity.19, 38 In the present study, we actually found the hyperpermeability in choroid plexus and/or around the brain ventricles. The downregulation of VE‐cadherin in choroid plexuses was also observed, which impaired cellular adhesion and resulted in an irreversible blood‐CSF barrier disruption. Finally, this change contributed to an increased vessel permeability and the extra‐leaking of CSF, which was shown as hydrocephalus.
Moreover, we explored the mechanisms of how the thrombin influences the function of VE‐cadherin during hydrocephalus. PARs are G‐protein‐coupled receptors specific for thrombin signaling.21 Activating PAR1 could generate increased microvascular permeability, which is confirmed in the SAH model.19 Our study also observed a significant increase of PAR1 in the hydrocephalus model, and we hypothesized that thrombin increased the choroid plexus permeability by activating PAR1 and degrading VE‐cadherin. Activation of the PARs accelerated the activation of Src kinases and PAK1.19 Src contributed to increased vascular permeability, brain edema, and mortality after intracerebral hemorrhage.39, 40 And PAK1 is a downstream Rac effector and a major cyclin‐dependent kinase 5 (Cdk5) substrate which is related to neurons apoptosis following human stroke.41 In this study, the level of phosphorylated Src and PAK1 was increased after thrombin injection, and SCH79797 treatment markedly suppressed the phosphorylation of Src and PAK1. This is consistent with the mechanism that thrombin leads to brain edema in the SAH model.19 What is more important, inhibition of PAR1, Src, or PAK1 can all attenuate the thrombin‐induced hydrocephalus with the reversion of the VE‐cadherin expression. This is in line with the hypothesis that activation of PAR1/p‐Src/p‐PAK1 would accelerate the degradation of VE‐cadherin and ultimately give rise to hydrocephalus.
However, there is also considerable evidence on other roles of thrombin in brain damage. For example, thrombin triggered platelet activation which could release inflammatory cytokines42 and disrupt the integrity of endothelial cell monolayers.43 Claudin‐5, ZO‐1, and other tight junction proteins also contributed to the thrombin‐induced increase in vascular permeability.38, 44, 45 But their roles in hydrocephalus still remain to be understood. Future studies will examine more detailed scenarios of hydrocephalus after IVH.
5. CONCLUSION
In summary, the present study demonstrated that thrombin‐induced hydrocephalus can be partly attributed to the increased permeability of blood‐CSF barrier, which may be due to the degradation of VE‐cadherin via the activation of PAR1/ p‐Src/ p‐PAK1 pathway.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China (491020‐N11453, F. Gao) and National Natural Science Foundation of China (NSFC) (81500991) to L.S. Tong. XH, CL, and HZ performed the experiments, analyzed the data. XH prepared the figures and wrote the manuscript. DS performed the MRI scanning. LT and FG designed the experiments, interpreted the data, and edited the manuscript.
Hao X‐D, Le C‐S, Zhang H‐M, Shang D‐S, Tong L‐S, Gao F. Thrombin disrupts vascular endothelial‐cadherin and leads to hydrocephalus via protease‐activated receptors‐1 pathway. CNS Neurosci Ther. 2019;25:1142–1150. 10.1111/cns.13129
Hao and Le contributed equally to this work.
Contributor Information
Lu‐Sha Tong, Email: 2310040@zju.edu.cn.
Feng Gao, Email: 2202012@zju.edu.cn.
REFERENCES
- 1. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46(7):2032‐2060. [DOI] [PubMed] [Google Scholar]
- 2. Yilmazlar S, Abas F, Korfali E. Comparison of ventricular drainage in poor grade patients after intracranial hemorrhage. Neurol Res. 2005;27(6):653‐656. [DOI] [PubMed] [Google Scholar]
- 3. Yadav YR, Mukerji G, Shenoy R, Basoor A, Jain G, Nelson A. Endoscopic management of hypertensive intraventricular haemorrhage with obstructive hydrocephalus. BMC Neurol. 2007;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Del Bigio MR, Di Curzio DL. Nonsurgical therapy for hydrocephalus: a comprehensive and critical review. Fluids Barriers CNS. 2015;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao C, Du H, Hua Y, Keep RF, Strahle J, Xi G. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab. 2014;34(6):1070‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38(2 Suppl):759‐762. [DOI] [PubMed] [Google Scholar]
- 7. Gao F, Zheng M, Hua Y, Keep RF, Xi G. Acetazolamide attenuates thrombin‐induced hydrocephalus. Acta Neurochir Suppl. 2016;121:373‐377. [DOI] [PubMed] [Google Scholar]
- 8. Gao F, Liu F, Chen Z, Hua Y, Keep RF, Xi G. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab. 2014;34(3):489‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garton T, Keep RF, Wilkinson DA, et al. Intraventricular hemorrhage: the role of blood components in secondary injury and hydrocephalus. Transl Stroke Res. 2016;7(6):447‐451. [DOI] [PubMed] [Google Scholar]
- 10. Klebe D, Flores JJ, McBride DW, et al. Dabigatran ameliorates post‐haemorrhagic hydrocephalus development after germinal matrix haemorrhage in neonatal rat pups. J Cereb Blood Flow Metab. 2017;37(9):3135‐3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiang J, Routhe LJ, Wilkinson DA, et al. The choroid plexus as a site of damage in hemorrhagic and ischemic stroke and its role in responding to injury. Fluids Barriers CNS. 2017;14(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simard PF, Tosun C, Melnichenko L, Ivanova S, Gerzanich V, Simard JM. Inflammation of the choroid plexus and ependymal layer of the ventricle following intraventricular hemorrhage. Transl Stroke Res. 2011;2(2):227‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karimy JK, Zhang J, Kurland DB, et al. Inflammation‐dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 2017;23(8):997‐1003. [DOI] [PubMed] [Google Scholar]
- 14. Tietz S, Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol. 2015;209(4):493‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gul IS, Hulpiau P, Saeys Y, van Roy F. Evolution and diversity of cadherins and catenins. Exp Cell Res. 2017;358(1):3‐9. [DOI] [PubMed] [Google Scholar]
- 16. Kaji C, Tomooka M, Kato Y, Kojima H, Sawa Y. The expression of podoplanin and classic cadherins in the mouse brain. J Anat. 2012;220(5):435‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oliver C, González CA, Alvial G, Flores CA, Rodríguez EM, Bátiz LF. Disruption of CDH2/N‐Cadherin‐based adherens junctions leads to apoptosis of ependymal cells and denudation of brain ventricular walls. J Neuropathol Exp Neurol. 2013;72(9):846‐860. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto H, Maruo T, Majima T, et al. Genetic deletion of afadin causes hydrocephalus by destruction of adherens junctions in radial glial and ependymal cells in the midbrain. PLoS ONE. 2013;8(11):e80356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan J, Manaenko A, Chen S, et al. Role of SCH79797 in maintaining vascular integrity in rat model of subarachnoid hemorrhage. Stroke. 2013;44(5):1410‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sokolova E, Reiser G. Prothrombin/thrombin and the thrombin receptors PAR‐1 and PAR‐4 in the brain: Localization, expression and participation in neurodegenerative diseases. Thromb Haemost. 2008;100(4):576‐581. [PubMed] [Google Scholar]
- 21. Coughlin SR. Thrombin signalling and protease‐activated receptors. Nature. 2000;407(6801):258‐264. [DOI] [PubMed] [Google Scholar]
- 22. Lei X, Chen M, Huang M, et al. Desacetylvinblastine monohydrazide disrupts tumor vessels by promoting VE‐cadherin internalization. Theranostics. 2018;8(2):384‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang F, Flavahan S, Flavahan N. Superoxide inhibition restores endothelium‐dependent dilatation in aging arteries by enhancing impaired adherens junctions. Am J Physiol Heart Circ Physiol. 2018;314(4):H805–H811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nwariaku F, Liu Z, Zhu X, et al. NADPH oxidase mediates vascular endothelial cadherin phosphorylation and endothelial dysfunction. Blood. 2004;104(10):3214‐3220. [DOI] [PubMed] [Google Scholar]
- 25. Gavard J, Gutkind J. VEGF controls endothelial‐cell permeability by promoting the beta‐arrestin‐dependent endocytosis of VE‐cadherin. Nat Cell Biol. 2006;8(11):1223‐1234. [DOI] [PubMed] [Google Scholar]
- 26. Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke. 1998;29(7):1352‐1357. [DOI] [PubMed] [Google Scholar]
- 27. Bhattathiri P, Gregson B, Prasad K, Mendelow A. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. In: Brain Edema XIII. Springer. Acta Neurochir Suppl. 2006;96:65‐68. [DOI] [PubMed] [Google Scholar]
- 28. Dupont S, Rabinstein AA. Extent of acute hydrocephalus after subarachnoid hemorrhage as a risk factor for poor functional outcome. Neurol Res. 2013;35(2):107‐110. [DOI] [PubMed] [Google Scholar]
- 29. Mayfrank L, Kim Y, Kissler J, et al. Morphological changes following experimental intraventricular haemorrhage and intraventricular fibrinolytic treatment with recombinant tissue plasminogen activator. Acta Neuropathol. 2000;100(5):561‐567. [DOI] [PubMed] [Google Scholar]
- 30. Mayfrank L, Kissler J, Raoofi R, et al. Ventricular dilatation in experimental intraventricular hemorrhage in pigs: characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28(1):141‐148. [DOI] [PubMed] [Google Scholar]
- 31. Leira R, Davalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63(3):461‐467. [DOI] [PubMed] [Google Scholar]
- 32. Fan J‐S, Huang H‐H, Chen Y‐C, et al. Emergency department neurologic deterioration in patients with spontaneous intracerebral hemorrhage: incidence, predictors, and prognostic significance. Acad Emerg Med. 2012;19(2):133‐138. [DOI] [PubMed] [Google Scholar]
- 33. Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5(1):53‐63. [DOI] [PubMed] [Google Scholar]
- 34. Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol. 2010;119(1):55‐73. [DOI] [PubMed] [Google Scholar]
- 35. Miyamoto Y, Sakane F, Hashimoto K. N‐cadherin‐based adherens junction regulates the maintenance, proliferation, and differentiation of neural progenitor cells during development. Cell Adh Migr. 2015;9(3):183‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463‐493. [DOI] [PubMed] [Google Scholar]
- 37. Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84(3):345‐357. [DOI] [PubMed] [Google Scholar]
- 38. Keep RF, Andjelkovic AV, Xiang J, et al. Brain endothelial cell junctions after cerebral hemorrhage: changes, mechanisms and therapeutic targets. J Cereb Blood Flow Metab. 2018;38(8):1255‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu DZ, Waldau B, Ander BP, et al. Inhibition of Src family kinases improves cognitive function after intraventricular hemorrhage or intraventricular thrombin. J Cereb Blood Flow Metab. 2017;37(7):2359‐2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ardizzone TD, Zhan X, Ander BP, Sharp FR. SRC kinase inhibition improves acute outcomes after experimental intracerebral hemorrhage. Stroke. 2007;38(5):1621‐1625. [DOI] [PubMed] [Google Scholar]
- 41. Mitsios N, Saka M, Krupinski J, et al. A microarray study of gene and protein regulation in human and rat brain following middle cerebral artery occlusion. BMC Neurosci. 2007;8(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaestner S, Dimitriou I. TGF beta1 and TGF beta2 and their role in posthemorrhagic hydrocephalus following SAH and IVH. J Neurol Surg A Cent Eur Neurosurg. 2013;74(05):279‐284. [DOI] [PubMed] [Google Scholar]
- 43. Madhavan Nair J, Maria M, Agudelo M, Yndart A, Vargas‐Rivera ME. Platelets contribute to BBB disruption induced by HIV and alcohol. J Alcohol Drug Depend. 2015;3(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kondo N, Ogawa M, Wada H, Nishikawa S‐I. Thrombin induces rapid disassembly of claudin‐5 from the tight junction of endothelial cells. Exp Cell Res. 2009;315(17):2879‐2887. [DOI] [PubMed] [Google Scholar]
- 45. Brailoiu E, Shipsky MM, Yan G, Abood ME, Brailoiu GC. Mechanisms of modulation of brain microvascular endothelial cells function by thrombin. Brain Res. 2017;1657:167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]


