Abstract
Background
Nivolumab has recently become available for third-line chemotherapy of advanced gastric cancer in Japan. The drug is expected to provide long-term survival in some patients. However, not all patients receive third-line therapy. In this study, we investigated the frequency of prescribing and the predictive factors for prescribing of third-line chemotherapy for patients with advanced gastric cancer.
Methods
We retrospectively analyzed the medical records of 271 patients with unresected advanced gastric cancer who had started chemotherapy between January 2006 and June 2017 at Kurashiki Central Hospital. Patients’ median age was 68 years, and 190 patients were male. We compared baseline characteristics of patients who did or did not receive third-line chemotherapy and, through multivariate logistic-regression analysis, identified potential predictive factors for receiving third-line chemotherapy.
Results
Among the 271 patients, 71 (26.2%) received third-line chemotherapy. In the univariate analysis, the rate of receiving this care was significantly related to patients’ performance status, cancer histology, and several laboratory variables at baseline. Multivariate analysis revealed that performance status 0 and serum C-reactive protein levels ≤0.6 mg/dL were independent and significant predictive factors for administration of the third-line chemotherapy; adjusted odds ratios of the two factors were 4.17 (95% confidence interval, 2.13–8.15) and 2.46 (1.19–5.08), respectively.
Conclusions
In this real-world study, only 26.2% of patients received third-line chemotherapy. Poor performance status and high serum C-reactive protein value at the start of first-line chemotherapy were significantly associated with lower frequency of administration of third-line chemotherapy.
Keywords: Gastric cancer, third-line chemotherapy, performance status (PS), C-reactive protein (CRP)
Introduction
Gastric cancer is the fifth most common cancer and the third most common cause of cancer mortality worldwide (1). In Japan, nearly 45,000 people die from gastric cancer annually (2). Efforts to improve the outcome of gastric cancer remain a major medical issue. Since ipilimumab, an anti-CTLA-4 antibody, improved overall survival of patients with metastatic melanoma (3), immune checkpoint inhibitors (ICIs) have been spotlighted for treatment of various types of cancers (3-8).
A recent study has found a significant survival benefit of nivolumab, an anti-PD-1 ICI, for patients with advanced gastric cancer (AGC) who had received two or more previous chemotherapy regimens (9). The results of that study led to approval of nivolumab for treatment of third-line chemotherapy for AGC in some countries, including Japan. Before that, irinotecan or other cytotoxic agents had been used for third-line treatment, but long-term survival was difficult to achieve with those drugs (10-14). Nivolumab has provided longer survival time in some patients with AGC (9), thus increasing expectations of benefits with this third-line chemotherapy. However, no reports have focused on the rate of AGC patients receiving this drug or on predictors for the possibility of their receiving it in the real-world setting. Thus, we addressed these issues in the present study conducted in a community hospital in Japan.
Methods
We retrospectively reviewed medical records of patients with locally unresectable gastric cancer or with distant metastasis who had been treated in Kurashiki Central Hospital. Eligible patients had histologically diagnosed gastric cancer, including adenocarcinoma at the esophagogastric junction. They had started chemotherapy between January 2006 and June 2017. Patients with recurrent gastric cancer after radical surgery and those who were still receiving chemotherapy on March 2018 were excluded.
Chemotherapy regimens were decided according to the latest national guidelines in Japan at the time of treatment (15-18). The following changes of regimen were not considered changes of the treatment line: (I) addition of trastuzumab at identification of HER2 overexpression, and (II) withdrawal of one of the agents in combined chemotherapy due to adverse events, e.g., from S-1 (tegafur, gimeracil and oteracil potassium) plus cisplatin to S-1 monotherapy.
Baseline patients’ clinicopathologic and laboratory data were compared between the two groups: those who had proceeded to the third-line chemotherapy and those had not. In this study, the term “baseline” means the moment of the starting of the first-line chemotherapy. Continuous variables, expressed as median (interquartile range) or means ± standard deviation, were compared using the Mann-Whitney U test or Student’s t-test. Categorical variables, expressed as numbers (percentages), were compared using Chi-square test. Univariate analysis was conducted to identify potential predictive factors for the delivery of the third-line treatment. Factors with P values <0.05 were then taken to a multivariate logistic-regression analysis. All statistical analyses were performed with the EZR software program (Saitama Medical Center, Saitama, Japan) (19).
Results
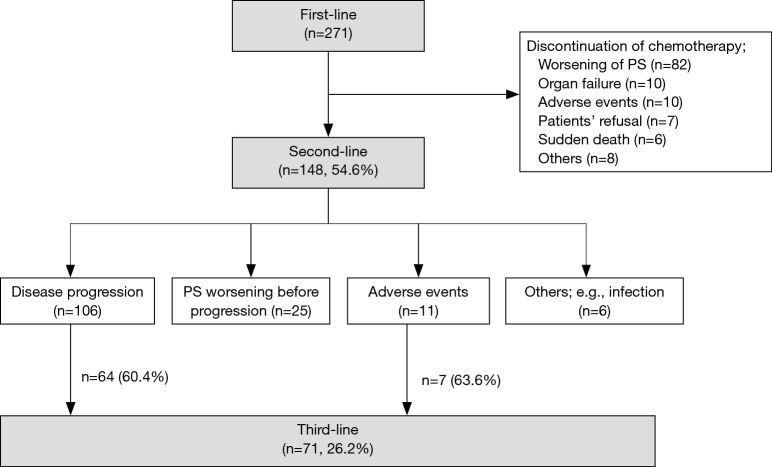
Two hundred seventy-one patients were included in this study. Their baseline clinicopathologic characteristics are listed in Table 1. One hundred forty-eight patients (54.6%) received second-line chemotherapy. Among them, 71 (26.2%) in total proceeded to third-line chemotherapy; 64 patients (60.4%) of 106 patients with disease progression and 7 (63.6%) of 11 patients with adverse events (Figure 1). The reasons for not proceeding to third-line chemotherapy after disease progression were performance status (PS) deterioration (37 patients), organ function inadequate for further chemotherapy (3 patients), infection (1 patient), and patient’s refusal (1 patient).
Table 1. Patients’ baseline characteristics.
| Characteristics | Total, n=271 |
|---|---|
| Age, median [IQR], years | 68 [62–74] |
| Sex, n (%) | |
| Male | 190 (70.1) |
| Female | 81 (29.9) |
| BMI, median (IQR), kg/m2 | 21.3 (18.9–23.6) |
| ECOG PS, median [IQR] | 1 [0–1] |
| Tumor site, n (%) | |
| Esophagogastric junction | 22 (8.1) |
| Stomach | 249 (91.9) |
| Histology, n (%) | |
| Intestinal | 83 (30.6) |
| Diffuse | 179 (66.1) |
| Indeterminable | 9 (3.3) |
| HER2 status, n (%) | |
| Positive | 17 (6.3) |
| Negative | 92 (33.9) |
| Unknown | 162 (59.8) |
| Ascites, n (%) | |
| Presence | 142 (52.4) |
| Absence | 124 (45.8) |
| Unknown | 5 (1.8) |
| Number of organs with metastases, median [IQR] | 2 [1–2] |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; PS, performance status; HER2, human epidermal growth factor receptor type 2; IQR, interquartile range.
Figure 1.
Patients flow. PS, performance status.
The chemotherapy regimens and median survival time from the start of each line of chemotherapy are listed in Table 2. S-1 plus cisplatin, paclitaxel monotherapy, and irinotecan monotherapy were the most frequently used regimen for the first, second, and third-line treatment, respectively. The median survival time from the start of third-line treatment was approximately 5 months.
Table 2. Chemotherapy regimens and median survival time from the start of each line of chemotherapy.
| Variables | Total, n=271 |
|---|---|
| First-line regimen, n (%) | |
| S-1 + cisplatin | 144 (53.1) |
| S-1 + oxaliplatin | 24 (8.9) |
| S-1 monotherapy | 73 (26.9) |
| Trastuzumab containing regimen | 7 (2.6) |
| Others | 23 (8.5) |
| Second-line regimen, n (%) | |
| Paclitaxel + ramucirumab | 12 (8.1) |
| Paclitaxel or docetaxel monotherapy | 77 (52.0) |
| Irinotecan monotherapy | 35 (23.6) |
| Others | 24 (16.2) |
| Third-line regimen, n (%) | |
| Irinotecan monotherapy | 35 (49.3) |
| Paclitaxel or docetaxel monotherapy | 23 (32.4) |
| Nivolumab | 1 (1.4) |
| Others | 12 (16.9) |
| Survival time from the start of the first line, median [IQR], days | 234 [133–486] |
| Survival time from the start of the second line, median [IQR], days | 166 [102–323] |
| Survival time from the start of the third line, median [IQR], days | 151 [87–239] |
IQR, interquartile range.
Characteristics of patients who received the third-line chemotherapy (n=71) and those who did not (n=200) were compared (Table 3). There were significant differences between the two populations in Eastern Cooperative Oncology Group (ECOG) PS, tumor histology, and these laboratory markers: hemoglobin, percentages of peripheral blood lymphocytes, total protein, albumin, blood urea nitrogen, and C-reactive protein (CRP). The two populations were not significantly different in age, gender, body mass index, tumor site, HER2 status, ascites status, numbers of organs with metastases, the serum tumor markers carcinoembryonic antigen and carbohydrate antigen 19-9, and several routine laboratory blood tests (white blood cell count, platelet count, serum bilirubin, alanine aminotransferase, aspartate aminotransferase, lactic dehydrogenase, alkaline phosphatase, blood urea nitrogen, and serum creatinine).
Table 3. Baseline characteristics of patients who did or did not receive third-line chemotherapy.
| Characteristics | 3rd-line therapy (n=71) | No 3rd-line (n=200) | P value |
|---|---|---|---|
| Age, median (IQR), years | 66.0 (62.0–73.5) | 69.0 (62.8–74.3) | 0.151 |
| Sex, n (%) | 1 | ||
| Male | 50 (70.4) | 140 (70.0) | |
| Female | 21 (29.6) | 60 (30.0) | |
| BMI, median (IQR), kg/m2 | 21.5 (19.3–23.7) | 21.1 (18.6–23.5) | 0.462 |
| ECOG PS, median [IQR] | 0 [0–1] | 1 [0–1] | <0.001 |
| Tumor site, n (%) | 1 | ||
| Esophagogastric junction | 6 (8.5) | 16 (8.0) | |
| Stomach | 65 (91.5) | 184 (92.0) | |
| Histology, n (%) | 0.02 | ||
| Intestinal | 29 (40.8) | 54 (27.0) | |
| Diffuse | 37 (52.1) | 142 (71.0) | |
| Indeterminable | 5 (7.0) | 4 (2.0) | |
| HER2 status, n (%) | 0.428 | ||
| Positive | 2 (2.8) | 15 (7.5) | |
| Negative | 22 (31.0) | 70 (35.0) | |
| Unknown | 47 (66.2) | 115 (57.5) | |
| Ascites, n (%) | 0.101 | ||
| Presence | 31 (43.7) | 111 (55.5) | |
| Absence | 39 (54.9) | 85 (42.5) | |
| Unknown | 1 (1.4) | 4 (2.0) | |
| Number of metastatic organs, median [IQR] | 2 [1–2] | 2 [1–2] | 0.204 |
| Hb, mean ± SD (g/dL) | 11.4±2.3 | 10.7±2.3 | 0.022 |
| WBC, median (IQR) (/ìL) | 6,400 (5,600–8,100) | 7,100 (5,600–8,900) | 0.117 |
| Lym, median (IQR) (%) | 19.8 (16.2–27.2) | 18.0 (12.9–25.1) | 0.013 |
| TP, mean ± SD (g/dL) | 6.4±0.6 | 6.1±0.7 | 0.002 |
| ALB, median (IQR) (g/dL) | 3.5 (3.1–3.8) | 3.1 (2.7–3.5) | <0.001 |
| BUN, median [IQR] (mg/dL) | 13 [11–15] | 14 [11–19] | 0.03 |
| CRP, median (IQR) (mg/dL) | 0.40 (0.12–1.37) | 1.09 (0.34–3.97) | <0.001 |
| CEA, median (IQR) (ng/mL) | 5.5 (2.8–40.3) | 5.4 (2.4–42.4) | 0.945 |
| CA19-9, median (IQR) (U/mL) | 31.7 (11.4–807) | 55.4 (7.7–607) | 0.788 |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; PS, performance status; HER2, human epidermal growth factor receptor type2; Hb, hemoglobin; WBC, white blood cells; Lym, lymphocytes; TP, total protein; ALB, albumin; BUN, blood urea nitrogen; CRP, C-reactive protein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
The factors mentioned above with significant differences between the two patient groups were analyzed to determine predictive variables for the administration of third-line chemotherapy; cut-off values of continuous variables were determined by creating receiver operating characteristics curves (Table 4). Hemoglobin, which had no appropriate cut-off value, was not included in the analysis. In the univariate analysis, ECOG PS 0, intestinal-type tumor histology, peripheral blood lymphocytes ≥19%, total serum protein ≥6.4 g/dL, serum albumin ≥3.3 g/dL, blood urea nitrogen ≤14 mg/dL, and CRP ≤0.6 mg/dL were significantly associated with the administration of third-line chemotherapy. In the multivariate logistic-regression analysis, ECOG PS 0 (odds ratio, 4.17; 95% CI, 2.13–8.15) and CRP ≤0.6 mg/dL (odds ratio, 2.46; 95% CI, 1.19–5.08) were the significant and independent predictors for the administration of the third-line chemotherapy.
Table 4. Univariate and multivariate analyses of predictors for treatment with third-line chemotherapy.
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| ECOG PS (0 vs. ≥1) | 6.39 (3.48–11.70) | <0.001 | 4.17 (2.13–8.15) | <0.001 | |
| Histology (intestinal vs. diffuse) | 2.06 (1.16–3.68) | 0.014 | 1.88 (0.96–3.68) | 0.066 | |
| Hb, g/dL (≥11 vs. <11) | 1.57 (0.91–2.72) | 0.105 | – | – | |
| Lym, % (≥19 vs. <19) | 1.87 (1.08–3.24) | 0.026 | 0.84 (0.42–1.70) | 0.633 | |
| TP, g/dL (≥6.4 vs. <6.4) | 2.20 (1.27–3.81) | 0.005 | 1.84 (0.90–3.74) | 0.093 | |
| Alb, g/dL (≥3.3 vs. <3.3) | 3.88 (2.18–6.89) | <0.001 | 1.83 (0.84–3.99) | 0.130 | |
| BUN, mg/dL (≤13 vs. >13) | 1.85 (1.05–3.27) | 0.034 | 1.84 (0.94–3.63) | 0.076 | |
| CRP, mg/dL (≤0.6 vs. >0.6) | 3.65 (2.07–6.46) | <0.001 | 2.46 (1.19–5.08) | 0.015 | |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PS, performance status; Hb, hemoglobin; Lym, lymphocytes; TP, total protein; Alb, albumin; BUN, blood urea nitrogen; CRP, C-reactive protein.
The number and percentages of patients who received third-line chemotherapy, according to ECOG PS and serum CRP values, are illustrated in Figure 2. The percentage of third-line chemotherapy was 55.0% when patients had good PS (0) and serum CRP values ≤0.6 mg/dL, whereas the rate was only 5.1% when patients had poor PS (≥1) and CRP values >0.6 mg/dL.
Figure 2.
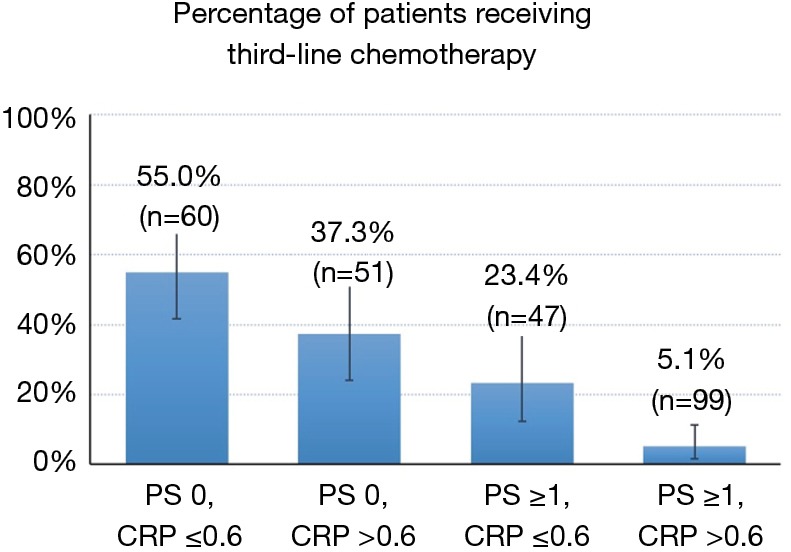
The number and percentage of patients who received third-line chemotherapy, according to ECOG PS and serum CRP values. The bars represent the percentages of third-line chemotherapy received, and the whiskers represent 95% confidence intervals. ECOG, Eastern Cooperative Oncology Group; PS, performance status; CRP, C-reactive protein.
Discussion
In this study, we found that only a quarter of AGC patients received third-line chemotherapy in a real-world practice in Japan. Most reported studies on third-line chemotherapy in AGC have been in trial settings. We found also that patients’ baseline ECOG PS and serum CRP values at the start of first-line chemotherapy were significantly associated with the administration of third-line chemotherapy.
To the best of our knowledge, this is the first study that focused on the frequency of AGC patients receiving third-line chemotherapy. In clinical trials, rates of advancing chemotherapy from first-line to second-line therapy were 38–75% (20-22) and from second to third-line 45–48% (23). Corresponding rates in our study were similar—54.6% and 48.0%, which means that only 26.2% of our AGC patients received third-line chemotherapy. This relatively low rate has prompted us to consider ways in which the frequency of treating AGC patients with third-line therapy can be improved, e.g., starting third-line therapy earlier in the progression of chemotherapy regimens. However, studies with pembrolizumab, an anti-PD-1 ICI, for second-line treatment (24) and with avelumab, an anti-PD-L1 ICI for third-line treatment (25), did not find superiority of these ICIs over conventional cytotoxic agents; thus, earlier administration of these drugs may not be advantageous. Nivolumab, though, has been reported to have survival benefits as well as other important features not seen with other cytotoxic agents, such as long-lasting effects and excellent safety profile in heavily pretreated AGC patients. Thus, earlier start of nivolumab and related drugs may be considered in some situations.
We found that patients’ ECOG PS and serum CRP levels at baseline were significantly associated with the administration of third-line chemotherapy. ECOG PS and CRP are reported to predict prognosis of patients with metastatic gastric cancer (26,27). In our study, the delivery of third-line therapy was well stratified according to the two factors. Validation of this simple approach to predict the possibility for AGC patients to proceed to third-line chemotherapy is warranted in future study. Tumor histology, known as another prognostic factor for AGC patients (28), tended to be associated with the administration of third-line therapy (P=0.066), but did not reach statistical significance.
It is also important to determine whether nivolumab should be used earlier in lines of therapy, i.e., in second or even in first-line therapy. Currently, several phase III randomized controlled trials are evaluating the efficacy of ICIs in first-line therapy for AGC. If those trials meet the primary endpoints, the chance to use this ICI for AGC patients will increase.
Limitations of this study are: first, it is a retrospective, single-center study. Second, the study did not include patients with recurrent gastric cancer after radical surgery. Third, the study covered more than ten years, and treatment strategies for AGC changed significantly during the period. Only 8.1% of AGC patients had received ramucirumab plus paclitaxel for second-line therapy, which is the most-recommended regimen in the current guidelines in Japan (18); such changes could have influenced our findings.
In conclusion, the present study showed that only 26.2% of patients received third-line chemotherapy and that their baseline PS and serum CRP levels were associated with the delivery to the therapy. How we raise the chance for AGC patients to receive therapy with expected effect such as nivolumab needs further study.
Acknowledgments
None.
Ethical Statements: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Kurashiki Central Hospital Ethics Committee reviewed and approved this study (No. 2872). In this study, patient data were retrieved from hospital medical record system. Therefore, informed consent was obtained in an opt-out system. The authors expect that the study outcomes will affect the future management of the patients and they declare that the patients’ personal data have been secured.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Cancer information service, National Cancer Center, Japan. Cancer registry and statistics. Accessed 31 December 2018. Available online: https://ganjoho.jp/en/index.html
- 3.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803-13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856-67. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 2016;17:1283-94. 10.1016/S1470-2045(16)30167-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Angelo SP, Russell J, Lebbé C, et al. Efficacy and Safety of First-line Avelumab Treatment in Patients With Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol 2018;4:e180077. 10.1001/jamaoncol.2018.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71. 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 10.Kawakami T, Machida N, Yasui H, et al. Efficacy and safety of irinotecan monotherapy as third-line treatment for advanced gastric cancer. Cancer Chemother Pharmacol 2016;78:809-14. 10.1007/s00280-016-3138-z [DOI] [PubMed] [Google Scholar]
- 11.Nishimura T, Iwasa S, Nagashima K, et al. Irinotecan monotherapy as third-line treatment for advanced gastric cancer refractory to fluoropyrimidines, platinum, and taxanes. Gastric Cancer 2017;20:655-62. 10.1007/s10120-016-0670-9 [DOI] [PubMed] [Google Scholar]
- 12.Makiyama A, Arimizu K, Hirano G, et al. Irinotecan monotherapy as third-line or later treatment in advanced gastric cancer. Gastric Cancer 2018;21:464-72. 10.1007/s10120-017-0759-9 [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y, Zhu XQ, Ren XG. Third-line chemotherapy in advanced gastric cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e6884. 10.1097/MD.0000000000006884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan WL, Yuen KK, Siu SW, et al. Third-line systemic treatment versus best supportive care for advanced/metastatic gastric cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2017;116:68-81. 10.1016/j.critrevonc.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 15.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2004 (ver. 2). Tokyo: Kanehara & Co., Ltd.; 2004:1-38. [Google Scholar]
- 16.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. 10.1007/s10120-011-0042-4 [DOI] [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. 10.1007/s10120-016-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (ver. 5). Tokyo: Kanehara & Co., Ltd.; 2018:1-91. [Google Scholar]
- 19.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215-21. 10.1016/S1470-2045(08)70035-4 [DOI] [PubMed] [Google Scholar]
- 21.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 22.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. 10.1200/JCO.2011.36.2236 [DOI] [PubMed] [Google Scholar]
- 23.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 24.Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123-33. 10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- 25.Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase 3, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment for patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 2018;29:2052-60. 10.1093/annonc/mdy264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 2015;26:141-8. 10.1093/annonc/mdu472 [DOI] [PubMed] [Google Scholar]
- 27.Yu Q, Yu XF, Zhang SD, et al. Prognostic role of C-reactive protein in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev 2013;14:5735-40. 10.7314/APJCP.2013.14.10.5735 [DOI] [PubMed] [Google Scholar]
- 28.Chen YC, Fang WL, Wang RF, et al. Clinicopathological Variation of Lauren Classification in Gastric Cancer. Pathol Oncol Res 2016;22:197-202. 10.1007/s12253-015-9996-6 [DOI] [PubMed] [Google Scholar]