Abstract
Background
The concentration of trifluridine in tumor DNA was strongly correlated with that of white blood cells in tumor-bearing nude mice administered trifluridine-tipiracil (TAS-102). Further, a phase I study of TAS-102 in patients with advanced solid tumors showed a significant correlation between decreased neutrophil count and the area under the concentration-time curve of trifluridine. Herein, we aimed to evaluate the association of decreased neutrophil count with the efficacy of TAS-102.
Methods
We retrospectively analyzed 40 patients with pretreated metastatic colorectal cancer who received TAS-102 at Yodogawa Christian Hospital between June 2014 and May 2018. To evaluate the association between the efficacy of TAS-102 and decreased neutrophil count, patients were grouped into 4 categories according to the decrease of neutrophil count during the first cycle of TAS-102 as follows: Category A, <25%; B, 25% to <50%; C, 50% to <75%; D, ≥75%.
Results
The rate of overall survival (OS) was significantly different between Category A and B (median: 4.1 vs. 10.1 months; P=0.04), between Category A and C (median: 4.1 vs. 10.5 months; P=0.04), and between Category A and D (median: 4.1 vs. 15.6 months; P=0.04). In the multivariate analyses, a ≥25% decrease of neutrophils [hazard ratio (HR): 0.28; 95% confidence interval (CI): 0.12–0.72; P=0.01] and Eastern Cooperative Oncology Group (ECOG) performance status (PS) 2 (HR: 3.79, 95% CI: 1.04–11.2; P=0.04) were independent prognostic factors for OS.
Conclusions
Decreased neutrophil count is a predict factor for the efficacy of TAS-102. TAS-102 treatment may be ineffective in patients with a decreased neutrophil count of <25%.
Keywords: Trifluridine and tipiracil hydrochloride, neutropenia, prognostic factor, colorectal cancer, decreased neutrophil count
Introduction
Trifluridine and tipiracil hydrochloride (TAS-102) is a novel oral nucleoside antitumor agent consisting of trifluridine (FTD) and tipiracil hydrochloride at a molar ratio of 1:0.5. In a global phase III trial, TAS-102 significantly improved overall survival (OS) and progression-free survival (PFS) compared with placebo for refractory metastatic colorectal cancer (1). However, TAS-102 induced major adverse events of hematological toxicity such as neutropenia, leukopenia, and anemia. FTD, the active component of TAS-102, is rapidly converted to F3TMP by thymidine kinase, and FTD inhibits the thymidylate synthase (TS) activity. The inhibition of TS is an important mechanism for the antitumor activity of nucleoside antitumor agents such as 5-fluorouracil and fluorodeoxyuridine (FdUrd). The FTD triphosphate F3TTP is sequentially formed and incorporated into DNA, which leads DNA dysfunction and cytocidal effects (2,3). In vitro studies revealed that FTD-dependent inhibition of TS rapidly declined after washout of FTD from the medium, although FdUrd activity persisted (4). Therefore, the incorporation of FTD into DNA is the predominant mechanism for the antitumor activity of TAS-102. The amount of FTD incorporated into DNA has been hypothesized to be related to both improvement in OS and hematological toxicity of TAS-102.
A study using tumor-bearing nude mice administered TAS-102 reported a strong correlation between concentration of FTD in tumor DNA and that of white blood cells (5). Meanwhile, in a phase I study of TAS-102 in patients with advanced solid tumors, the percentage change in neutrophil count was significantly inversely correlated with the maximum plasma concentrations (Cmax) and the area under the concentration-time curve (AUC) of FTD (6). Considering these results, we hypothesized that the decrease in neutrophil count is associated with plasma concentration of FTD and effect of FTD to the tumor. The present study aimed to evaluate the association of the efficacy of TAS-102 with the decrease of neutrophil count during the first course of treatment.
Methods
Patients
This retrospective study included patients with pretreated metastatic colorectal cancer who received TAS-102 monotherapy at Yodogawa Christian Hospital between June 2014 and May 2018. The eligibility criteria were presence of histologically or cytologically proven, inoperable metastatic colorectal adenocarcinoma, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2, and adequate hematological values (neutrophil count ≥1,500/µL and platelet count ≥120,000/µL). The exclusion criteria were treatment discontinuation or dose modification in the middle of the first course, no assessment of neutrophil counts at least either one of before treatment and on days 8, 15, 22, and 29 since initiating the treatment, an active infection or seropositivity for human immunodeficiency virus, hepatitis C virus, hepatitis B surface antigen, or syphilis and serious comorbidities (severe heart disease, uncontrolled hypertension, or diabetes mellitus).
Treatment and assessment
TAS-102 (35 mg/m2) was administered at orally twice daily, after breakfast and dinner, for 5 days a week for 2 weeks, followed by a 14-day rest period (1 treatment course). This treatment cycle was repeated every 4 weeks until tumor progression (as identified on diagnostic imaging), unacceptable toxicity, or at the patient’s request. The dose was reduced if the neutrophil count was <500/µL or the platelet count was <50,000/µL. Granulocyte-colony stimulating factor was not used during treatment in this study. Toxicity was assessed via blood chemistry and complete blood cell counts, with differential counts as well as platelet counts. Blood chemistry and complete blood cell counts were performed before treatment and on days 8, 15, 22, and 29 during the first course. The decrease in neutrophil count was calculated as the percentage of nadir neutrophil count to pretreatment baseline neutrophil count during the first course. The medical records of the patients were retrospectively reviewed by investigators, and data on tumor response, PFS, OS and first imaging computed tomography (CT) scans were collected for analysis. Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors, version 1.1. PFS was defined as the interval from the start of TAS-102 treatment to either tumor progression or death, while OS was defined as the interval from the start of TAS-102 treatment to death.
Statistical analysis
To evaluate the association between TAS-102 efficacy (tumor response, PFS, and OS) and decrease in neutrophil count, patients were grouped into 4 categories according to decrease of neutrophils during the first cycle of treatment: Category A, <25%; B, 25% to <50%; C, 50% to <75%; D, ≥75%, and grouped into according to 3 categories according to neutropenia based on the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0: Grade 0, Grade 1–2, Grade 3–4. The median OS and PFS were calculated using the Kaplan-Meier method, and differences between Category A and B, C, and D were evaluated using the log-rank test. All P values were two sided, and P<0.05 was considered significant. Univariate analyses were performed using the Cox proportional hazards regression model for the following factors: decreased rate of neutrophils (≥25% vs. <25%), primary tumor location (right-sided: caecum to transverse colon vs. left-sided: splenic flexure to rectosigmoid), KRAS status (mutant type vs. wild type), age (≥70 vs. <70 years), sex, ECOG PS (2 vs. ≤1), and number of previous treatment regimens (≥3 vs. <3). Variables with P-values less than 0.1 were included in a multivariate Cox proportional hazards regression model. Hazard ratios (HRs) were calculated with corresponding 95% confidence intervals (CIs). All statistical analyses were performed using JMP v. 10 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient characteristics
Forty-six patients were initially selected after fulfilling the inclusion criteria. Six patients were excluded from the study because of temporary discontinuation of TAS-102 during the first course (n=3), no assessment of neutrophil count on day 15 or day 22 during the first course (n=3). Thus, a total of 40 patients (23 males, 17 females) were analyzed. The patient characteristics are summarized in Table 1. The median age was 68 years (range, 43–83 years). Five patients (12.5%) had an ECOG PS of 2.
Table 1. Patient characteristics.
| Factor | Value |
|---|---|
| Number of patients | 40 |
| Age (years), median [range] | 68 [43–83] |
| Sex | |
| Male | 23 |
| Female | 17 |
| Primary site of disease | |
| Colon | 24 |
| Rectum | 16 |
| Tumor location | |
| Right-sided | 8 |
| Left-sided | 32 |
| KRAS status | |
| Wild type | 22 |
| Mutant type | 18 |
| ECOG performance status | |
| 0 | 11 |
| 1 | 24 |
| 2 | 5 |
| Treatment cycle, median [range] | 4 [1–10] |
| Number of prior regimens | |
| 1 | 5 |
| 2 | 15 |
| ≥3 | 20 |
ECOG, Eastern Cooperative Oncology Group.
Efficacy
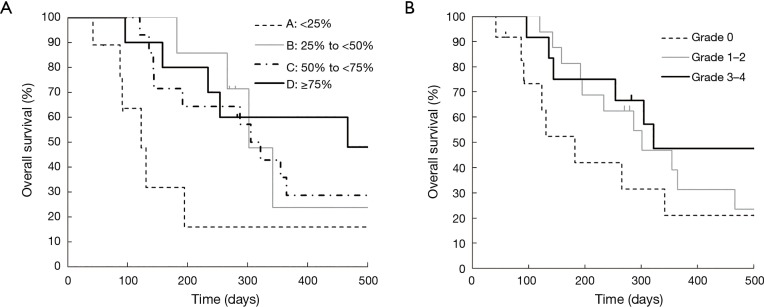
Kaplan-Meier curves for OS according to each category are shown in Figure 1. OS was significantly different between Category A and B (median: 4.1 vs. 10.1 months; P=0.04), between Category A and C (median: 4.1 vs. 10.5 months; P=0.04), and between Category A and D (median: 4.1 vs. 15.6 months; P=0.04). Although OS was also better in Grade 1–2 neutropenia than that in Grade 0 (median: 10.1 vs. 6.1 months; P=0.22), and in Grade 3–4 neutropenia than that in Grade 0 (median: 10.7 vs. 6.1 months; P=0.25), the differences were smaller than those between Category A and B, C, and D. In both Grade 0 and Grade 1–2 groups, median OS was better in patients with a ≥25% decrease in neutrophil counts than those with a <25% decrease (Table 2). Kaplan-Meier curves for PFS according to each category are shown in Figure 2. PFS was significantly different between Category A and B (median: 1.7 vs. 2.1 months; P=0.02), between Category A and C (median: 1.7 vs. 4.0 months; P<0.001), and between Category A and D (median: 1.7 vs. 4.8 months; P=0.001). The summary of baseline neutrophil count, PS, number of prior regimens, number of post treatment and response in each category is shown in Table 3. Baseline neutrophil count and ECOG PS, which may affect prognosis, had almost no difference among 4 categories. The disease control rate (DCR) tended to be higher in Categories B, C, and D than that in A. Kaplan-Meier curve for OS in patients with ECOG PS 0–1 is shown in Figure 3. OS in patients with ECOG PS 0–1 were significantly higher for patients with a ≥25% decrease in neutrophil counts than for those with a <25% decrease (median: 4.1 vs. 10.7 months; P=0.01). Univariate analyses of prognostic factors revealed that ≥25% decrease of neutrophils and ECOG PS 2 were associated with OS with a P value <0.1. In the multivariate analyses, a ≥25% decrease of neutrophils (HR: 0.28, 95% CI: 0.12–0.72; P=0.01) and ECOG PS 2 (HR: 3.79, 95% CI: 1.04–11.2; P=0.04) were independent prognostic factors for OS (Table 4).
Figure 1.
Kaplan-Meier curves for overall survival according to (A) the percentage decrease of neutrophils during the first cycle of TAS-102 treatment in the four categories: Category A (<25%), B (25% to <50%), C (50% to <75%), D (≥75%), and (B) neutropenia based on the CTCAE during the first cycle of TAS-102 treatment in the three categories: Grade 0, Grade 1–2, Grade 3–4. (A) Overall survival according to the percentage decrease of neutrophils; (B) overall survival according to neutropenia based on the CTCAE grade. CTCAE, Common Terminology Criteria for Adverse Events.
Table 2. Overall survival according to neutropenia and the percentage decrease of neutrophils.
| Percentage decrease of neutrophils (%) | n | Median OS (months) |
|---|---|---|
| Grade 0 | 12 | 6.1 |
| <25 | 8 | 3.6 |
| 25 to <50 | 3 | 8.9 |
| 50 to <75 | 1 | ≥26.1 |
| Grade 1–2 | 16 | 10.1 |
| <25 | 1 | 6.5 |
| 25 to <50 | 4 | >9.7 |
| 50 to <75 | 8 | 10.7 |
| ≥75 | 3 | ≥8.7 |
| Grade 3–4 | 12 | 10.7 |
| 50 to <75 | 5 | 10.2 |
| ≥75 | 7 | ≥17.9 |
OS, overall survival.
Figure 2.
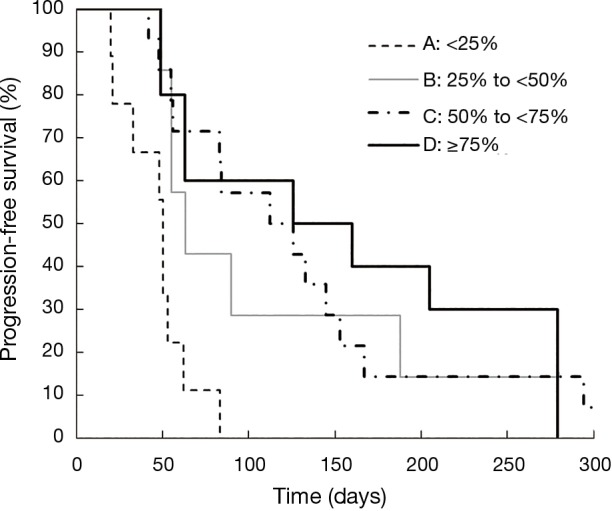
Kaplan-Meier curves for progression-free survival according to the percentage decrease of neutrophils during the first cycle of TAS-102 treatment in the four categories: Category A (<25%), B (25% to <50%), C (50% to <75%), D (≥75%).
Table 3. Summary of median OS, baseline neutrophil count, performance status, number of prior regimens, number of post treatment, response, median PFS and median OS in each category.
| Category | A (n=9) | B (n=7) | C (n=14) | D (n=10) | Total (n=40) |
|---|---|---|---|---|---|
| Percentage decrease of neutrophils (%) | <25 | 25 to <50 | 50 to <75 | ≥75 | – |
| ECOG performance status (n) | |||||
| 0–1 | 8 | 6 | 12 | 9 | 35 |
| 2 | 1 | 1 | 2 | 1 | 5 |
| Baseline neutrophil count (×109/L) | 3.89±2.39 | 3.38±1.45 | 3.40±1.21 | 4.60±1.50 | 3.99±1.83 |
| Number of prior regimens (n) | |||||
| 1 | 1 | 1 | 2 | 1 | 5 |
| 2 | 4 | 3 | 4 | 4 | 15 |
| ≥3 | 4 | 3 | 8 | 5 | 20 |
| Number of post treatment (n) | |||||
| 0 | 4 | 5 | 6 | 3 | 18 |
| 1 | 3 | 0 | 4 | 4 | 11 |
| ≥2 | 2 | 2 | 4 | 3 | 11 |
| Response | |||||
| CR (n) | 0 | 0 | 0 | 0 | 0 |
| PR (n) | 0 | 1 | 0 | 0 | 1 |
| SD (n) | 1 | 2 | 9 | 6 | 18 |
| PD (n) | 8 | 4 | 5 | 4 | 21 |
| RR (%) | 0 | 14 | 0 | 0 | 2.5 |
| DCR (%) | 11 | 43 | 64 | 60 | 45 |
| Median PFS (months) (P value) | 1.7 | 2.1 (0.02) | 4.0 (<0.001) | 4.8 (0.001) | 2.8 |
| Median OS (months) (P value) | 4.1 | 10.1 (0.04) | 10.5 (0.04) | 15.6 (0.04) | 10.1 |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; RR, response rate; DCR, disease control rate; PFS, progression free survival; OS, overall survival.
Figure 3.
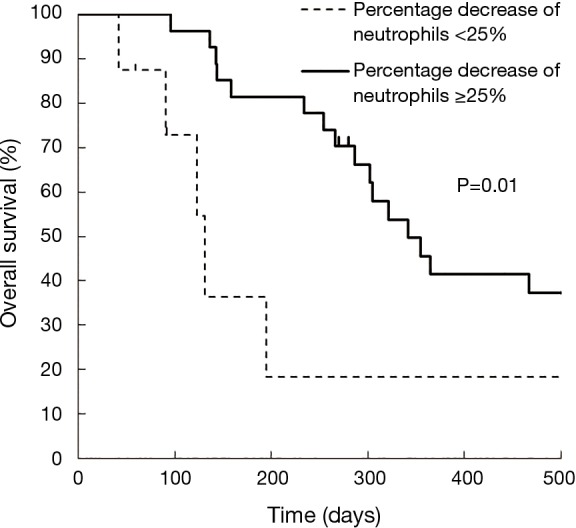
Kaplan-Meier curves for overall survival (OS) according to the percentage decrease in neutrophil counts <25% and ≥25% in patients with ECOG PS 0–1. ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Table 4. Univariate and multivariate analyses of overall survival.
| Factor | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Percentage decrease of neutrophils (≥25%) | 0.31 (0.14–0.80) | 0.02 | 0.28 (0.12–0.72) | 0.01 | |
| Primary tumor location (right-sided) | 0.99 (0.39–2.20) | 0.99 | – | – | |
| KRAS status (mutant type) | 0.14 (0.55–2.33) | 0.71 | – | – | |
| Age (≥70) | 0.69 (0.28–1.49) | 0.35 | – | – | |
| Sex (male) | 1.38 (0.67–3.00) | 0.39 | – | – | |
| ECOG performance status | 3.13 (0.87–9.02) | 0.04 | 3.79 (1.04–11.2) | 0.04 | |
| Number of prior regimens (≥3) | 1.18 (0.58–2.44) | 0.65 | – | – | |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group.
Discussion
In the present study, we evaluated whether decreased neutrophil count was a predict factor for the efficacy of TAS-102. The response rate (RR), DCR, and PFS of the patients in the present study were roughly similar to those who received TAS-102 in other randomized controlled trials such as RECOURSE (1) and J-003 (7). In the current study, the RR was 2.5%, while it was 1.6% and 0.9% in RECOURSE and J-003, respectively. The DCR was 45%, while it was 44% and 43.8% in RECOURSE and J-003, respectively. Moreover, the median PFS was 2.8 months, while it was 2.0 and 1.9 months in RECOURSE and J-003, respectively. Our results are consistent with those previously reported: worse PS tended to be associated with poor prognosis (8-11). Meanwhile, we showed that the efficacy of TAS-102 is higher in patients whose neutrophil count decreased by ≥25% than those whose neutrophil count decreased by <25% in the first cycle. Several studies reported that TAS-102-induced neutropenia is associated with its efficacy (12-14). Kimura et al. reported that grade 3–4 neutropenia (based on CTCAE version 4.0) was independently associated with survival in patients with advanced and recurrent colorectal cancer who received TAS-102 (12). Hamauchi et al. reported that TAS-102-induced grade 3–4 neutropenia during the first cycle of treatment was a significant predictive factor for PFS (13). Kasi et al. evaluated the neutrophil count within 1 month after starting TAS-102 and reported that patients who developed ≥ grade 2 neutropenia had significantly longer PFS and OS (14). Nishina et al. reported that TAS-102-induced grade 3–4 neutropenia in cycle 1 and 2 was associated with longer OS (15). However, because the evaluation of neutrophil decrease according to the CTCAE in these studies was based on the nadir value of the absolute neutrophil count, the grade of neutropenia tended to depend on pretreatment baseline neutrophil counts. Several studies have suggested that the baseline neutrophil count was a predictive factor of chemotherapy-induced severe neutropenia (16-22). Therefore, because the neutrophil decrease may have been underestimated in cases of high baseline neutrophil count, neutropenia grade could be no change during the first course. In fact, in our study, even if patients with Grade 0–2 neutropenia according to the CTCAE, OS was better tendency in patients with a ≥25% decrease in neutrophil counts than those with a <25% decrease. In our study, although patients who developed ≥ grade 1 neutropenia had longer OS than those with Grade 0, it was possible that the difference had been no significant due to small sample size. By contrast, because the level of neutropenia by using the percentage decrease of neutrophil count was accurate regardless of baseline neutrophil count, the evaluation of neutrophil decrease according to the percentage decrease of neutrophil count was greater difference than those according to the CTCAE grade in Kaplan-Meier curves for OS. In other treatment regimen, Saito et al. reported that decrease rate of neutrophils in temozolomide treatment was significantly correlated with OS in patients with glioblastoma (23). In addition, because the percentage decrease in neutrophil count was proven to be strongly associated with AUC of FTD (6), the results of our study indicate that the concentrations of FTD in the plasma and the tumor were inadequate to achieve an antitumor effect in patients whose neutrophil count decreased by <25%.
This study has some limitations, including its retrospective design and small sample size. Moreover, because this study was a retrospective analysis based on data from electronic medical records, we were unable to accurately assess the patient’s adherence to the TAS-102 regimen. Therefore, the low decrease in neutrophil count and poor efficacy may have been caused by poor adherence. Prospective studies are needed to confirm our results and examine the potential effects of adherence. The CT interval was not standardized in advance among patients because this study was retrospectively analyzed on daily clinical practice. Therefore, the data of PFS, RR and DCR calculated from the medical records are possible to be inaccurate.
In conclusion, a decrease in neutrophil count during the first course of TAS-102 treatment is a predict factor for its efficacy in patients with pretreated metastatic colorectal cancer. TAS-102 may be ineffective in patients with a percentage decrease in neutrophil count of <25%. Therefore, the percentage decrease of the neutrophil count may be used as basis for determining whether TAS-102 treatment should be discontinued and the patient should be switched to another treatment, such as regorafenib, at an early point. These findings suggest that there are interindividual differences in the pharmacokinetics of TAS-102, and that it may be necessary to increase the dosage for patients with percentage decreases in neutrophil counts of <25% for the first course.
Acknowledgments
We thank all patients and research assistants at Yodogawa Christian Hospital. We also thank Editage (www.editage.jp) for English language editing.
Ethical Statement: This study was approved by the Ethical Review Board of Yodogawa Christian Hospital (approval number: 2018-010). Given the retrospective design, the ethical review board waived the requirement for informed consent. To protect patient privacy, we removed all identifiers from our records upon completion of our analyses.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909-19. 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 2.Emura T, Nakagawa F, Fujioka A, et al. An optimal dosing schedule for a novel combination antimetabolite, TAS-102, based on its intracellular metabolism and its incorporation into DNA. Int J Mol Med 2004;13:249-55. [PubMed] [Google Scholar]
- 3.Fujiwara Y, Oki T, Heidelberger C. Fluorinated pyrimidines. XXXVII. Effects of 5-trifluoromethyl-2'-deoxyuridine on the synthesis of deoxyribonucleic acid of mammalian cells in culture. Mol Pharmacol 1970;6:273-80. [PubMed] [Google Scholar]
- 4.Tanaka N, Sakamoto K, Okabe H, et al. Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol Rep 2014;32:2319-26. 10.3892/or.2014.3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashita F, Komoto I, Oka H, et al. Exposure-dependent incorporation of trifluridine into DNA of tumors and white blood cells in tumor-bearing mouse. Cancer Chemother Pharmacol 2015;76:325-33. 10.1007/s00280-015-2805-9 [DOI] [PubMed] [Google Scholar]
- 6.Doi T, Ohtsu A, Yoshino T, et al. Phase I study of TAS-102 treatment in Japanese patients with advanced solid tumours. Br J Cancer 2012;107:429-34. 10.1038/bjc.2012.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshino T, Mizunuma N, Yamazaki K, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 2012;13:993-1001. 10.1016/S1470-2045(12)70345-5 [DOI] [PubMed] [Google Scholar]
- 8.Shitara K, Matsuo K, Yokota T, et al. Prognostic factors for metastatic colorectal cancer patients undergoing irinotecan-based second-line chemotherapy. Gastrointest Cancer Res 2011;4:168-72. [PMC free article] [PubMed] [Google Scholar]
- 9.Díaz R, Aparicio J, Gironés R, et al. Analysis of prognostic factors and applicability of Kohne's prognostic groups in patients with metastatic colorectal cancer treated with first-line irinotecan or oxaliplatin-based chemotherapy. Clin Colorectal Cancer 2005;5:197-202. 10.3816/CCC.2005.n.031 [DOI] [PubMed] [Google Scholar]
- 10.Steinberg J, Erlichman C, Gadalla T, et al. Prognostic factors in patients with metastatic colorectal cancer receiving 5-fluorouracil and folinic acid. Eur J Cancer 1992;28A:1817-20. 10.1016/0959-8049(92)90011-P [DOI] [PubMed] [Google Scholar]
- 11.Bensmaïne MA, Marty M, de Gramont A, et al. Factors predicting efficacy of oxaliplatin in combination with 5-fluorouracil (5-FU) +/- folinic acid in a compassionate-use cohort of 481 5-FU-resistant advanced colorectal cancer patients. Br J Cancer 2001;85:509-17. 10.1054/bjoc.2001.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura M, Usami E, Iwai M, et al. Severe neutropenia: a prognosticator in patients with advanced/recurrent colorectal cancer under oral trifluridine-tipiracil (TAS-102) chemotherapy. Pharmazie 2017;72:49-52. [DOI] [PubMed] [Google Scholar]
- 13.Hamauchi S, Yamazaki K, Masuishi T, et al. Neutropenia as a Predictive Factor in Metastatic Colorectal Cancer Treated With TAS-102. Clin Colorectal Cancer 2017;16:51-7. 10.1016/j.clcc.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 14.Kasi PM, Kotani D, Cecchini M, et al. Chemotherapy induced neutropenia at 1-month mark is a predictor of overall survival in patients receiving TAS-102 for refractory metastatic colorectal cancer: a cohort study. BMC Cancer 2016;16:467. 10.1186/s12885-016-2491-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishina T, Yoshino T, Shinozaki E, et al. Onset of neutropenia as an indicator of treatment response in the randomized phase II of TAS-102 vs placebo in Japanese patients with metastatic colorectal cancer (Study J003-10040030). J Clin Oncol 2016;34:abstr 3557.
- 16.Hirasawa Y, Nakashima J, Sugihara T, et al. Development of a Nomogram for Predicting Severe Neutropenia Associated With Docetaxel-Based Chemotherapy in Patients With Castration-Resistant Prostate Cancer. Clin Genitourin Cancer 2017;15:176-81. 10.1016/j.clgc.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 17.Kwon WA, Oh TH, Lee JW, et al. Factors that Predict Neutropenia in Korean Patients with Advanced Urothelial Cancer after Cisplatin-Based Systemic Chemotherapy. Urol J 2018;15:168-72. [DOI] [PubMed] [Google Scholar]
- 18.Ikesue H, Watanabe H, Hirano M, et al. Risk Factors for Predicting Severe Neutropenia Induced by Pemetrexed Plus Carboplatin Therapy in Patients with Advanced Non-small Cell Lung Cancer. Biol Pharm Bull 2015;38:1192-8. 10.1248/bpb.b15-00162 [DOI] [PubMed] [Google Scholar]
- 19.López-Pousa A, Rifà J, Casas de Tejerina A, et al. Risk assessment model for first-cycle chemotherapy-induced neutropenia in patients with solid tumours. Eur J Cancer Care (Engl) 2010;19:648-55. 10.1111/j.1365-2354.2009.01121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamanaka T, Matsumoto S, Teramukai S, et al. Analysis of risk factors for severe adverse effects of oral 5-fluorouracil S-1 in patients with advanced gastric cancer. Gastric Cancer 2007;10:129-34. 10.1007/s10120-007-0422-y [DOI] [PubMed] [Google Scholar]
- 21.Tsuji D, Kamezato M, Daimon T, et al. Retrospective analysis of severe neutropenia in patients receiving concomitant administration of docetaxel and clarithromycin. Chemotherapy 2013;59:407-13. 10.1159/000362437 [DOI] [PubMed] [Google Scholar]
- 22.Kawachi S, Shinoda Y, Kimura M, et al. Risk factors for severe neutropenia induced by combination therapy of S-1 and cisplatin in patients with advanced/recurrent gastric cancer. Pharmazie 2018;73:174-7. [DOI] [PubMed] [Google Scholar]
- 23.Saito T, Sugiyama K, Hama S, et al. Prognostic importance of temozolomide-induced neutropenia in glioblastoma, IDH-wildtype patients. Neurosurg Rev 2018;41:621-8. 10.1007/s10143-017-0903-3 [DOI] [PubMed] [Google Scholar]