Short moderate heat stress during the tetrad stage of pollen development targets vital metabolic pathways, ultimately leading to sterility and yield losses in maize.
Abstract
Shifts in the duration and intensity of ambient temperature impair plant development and reproduction, particularly male gametogenesis. Stress exposure causes meiotic defects or premature spore abortion in male reproductive organs, leading to male sterility. However, little is known about the mechanisms underlying stress and male sterility. To elucidate these mechanisms, we imposed a moderate transient heat stress on maize (Zea mays) plants at the tetrad stage of pollen development. After completion of pollen development at optimal conditions, stress responses were assessed in mature pollen. Transient heat stress resulted in reduced starch content, decreased enzymatic activity, and reduced pollen germination, resulting in sterility. A transcriptomic comparison pointed toward misregulation of starch, lipid, and energy biosynthesis-related genes. Metabolomic studies showed an increase of Suc and its monosaccharide components, as well as a reduction in pyruvate. Lipidomic analysis showed increased levels of unsaturated fatty acids and decreased levels of saturated fatty acids. In contrast, the majority of genes involved in developmental processes such as those required for auxin and unfolded protein responses, signaling, and cell wall biosynthesis remained unaltered. It is noteworthy that changes in the regulation of transcriptional and metabolic pathway genes, as well as heat stress proteins, remained altered even though pollen could recover during further development at optimal conditions. In conclusion, our findings demonstrate that a short moderate heat stress during the highly susceptible tetrad stage strongly affects basic metabolic pathways and thus generates germination-defective pollen, ultimately leading to severe yield losses in maize.
High temperatures caused by climate change are predicted to decrease cereal productivity in many parts of the world. Yield losses due to rising temperatures have been reported for major cereal crops including maize (Zea mays; Lobell et al., 2013), wheat (Triticum, sp.; Lobell et al., 2012; Begcy et al., 2018b), and rice (Oryza sativa; Folsom et al., 2014; Chen et al., 2016; Begcy et al., 2018a). These cereals together are the main sources of caloric intake of the people in most countries. Therefore, it is necessary to elucidate the most critical stages of plant development and reproduction, to understand the underlying molecular stress responses, and to use the knowledge generated with the long-term goal to develop heat stress-tolerant crops.
In particular, high temperature spikes have become more extreme and are occurring with increasing frequency, and in many cases with greater intensity. A detrimental effect has been observed on plant development, and even more drastically during male reproductive development, generating sterile pollen (De Storme and Geelen, 2014). This directly decreases crop productivity. Male gametophyte (pollen) development appears to be most sensitive to environmental stresses. In flowering plants, pollen grains are generated during microsporogenesis and microgametogenesis. During microsporogenesis, meioses I and II take place leading to the formation of four haploid microspores from spore mother cells (meiocytes). Several reports have pointed out that stress exposure during microgametogenesis leads to microspore abortion and associated male sterility (De Storme and Geelen, 2014; Rieu et al., 2017; Begcy and Dresselhaus, 2018). In most investigated species, the onset of meiosis and transition to pollen mitosis seems to be the most sensitive to environmental stresses (Boyer and McLaughlin, 2007; De Storme and Geelen, 2014; Müller and Rieu, 2016; Rieu et al., 2017). In rice and wheat, for instance, the meiotic tetrad stage and its subsequent transition to microgametogenesis, which generates the vegetative pollen tube cells and two sperm cells during pollen mitoses I and II inside the pollen grain, were most sensitive to low temperatures (Oliver et al., 2005; Barton et al., 2014; Sharma and Nayyar, 2016). Increased temperatures during the microspore stage reduced spikelet fertility in rice and wheat by affecting endogenous levels of indole-3-acetic acid, gibberellic acid (GA), and abscisic acid (Tang et al., 2008; Endo et al., 2009; Alghabari et al., 2014). Thus, although the microsporogenesis-to-microgametogenesis transition appears to be the most critical stage and the most sensitive to abiotic stresses, the molecular and biochemical bases that contribute to male sterility and the effects of transient heat stress are not well understood.
During pollen formation, several metabolic processes are fundamental to ensure developmental progression and viability. These include glycolysis as a primary metabolic pathway whose main function is to allow energy to be harnessed in the form of ATP. Additionally, Glc is converted into pyruvate, which is the major substrate entering the citrate cycle (Muñoz-Bertomeu et al., 2010; Selinski and Scheibe, 2014). Lipid biosynthesis is also critical, as lipids are fundamental for pollen grain formation, germination, and growth, but also for penetration into the maternal tissues of the stigma (Evans et al., 1992; Piffanelli et al., 1997; Wolters-Arts et al., 1998; Zhang et al., 2016). Lipid biosynthesis was reported to be highly active, especially during early microgametogenesis (Piffanelli et al., 1997).
In this study, we used maize as a cereal model, since its pollen is highly susceptible to heat stress and it is amenable to study transient stress at various pollen developmental stages, which can be easily recognized using the nondestructive so-called Leaf Collar Method (Begcy and Dresselhaus, 2017). Pre-experiments confirmed that meiosis, as well as the transition from microsporogenesis to microgametogenesis, is very sensitive to heat stress. Therefore, we investigated the effect of a transient moderate temperature increase applied during the tetrad stage of maize pollen development using a number of approaches. These include morphological and physiological measurements as well as RNA sequencing (RNA-Seq) and metabolomic analyses. Mature pollen was investigated, and pollen was allowed to recover and continue development after transient heat stress was applied during the tetrad stage.
RESULTS AND DISCUSSION
Heat Stress Affects Maize Pollen Development
To study the effect of increased temperatures on developing pollen, we imposed a transient moderate heat stress (35°C/25°C light/dark period) for 48 h on maize plants, specifically at the tetrad stage. A parallel set of maize plants was maintained under optimal growth conditions (25°C/21°C light/dark period) in a parallel chamber and was used as a control for all experiments (see Fig. 1A for the experimental setup). First, we monitored whether a short spike of increased temperature was able to alter the physiological status of maize plants. We observed that moderate heat stress strongly affects gas exchange parameters. Under control (nonstressed) conditions, maize plants did not show variations in net photosynthesis and transpiration rate (Supplemental Fig. S1A). After 48 h of increased temperature, heat-stressed plants showed a steady decline in net photosynthesis and transpiration rate (Supplemental Fig. S1B). These results indicate that a transient spike in temperature during reproductive development negatively affects the physiological status of maize plants. Similar responses to increased temperatures during reproductive development have been observed in rice (Chaturvedi et al., 2017), wheat (Feng et al., 2014; Begcy et al., 2018b), sorghum (Sorghum bicolor; Sunoj et al., 2017), and tomato (Solanum lycopersicum; Zhou et al., 2017). A short heat stress imposition negatively affected photosynthetic characteristics and gas exchange parameters in general.
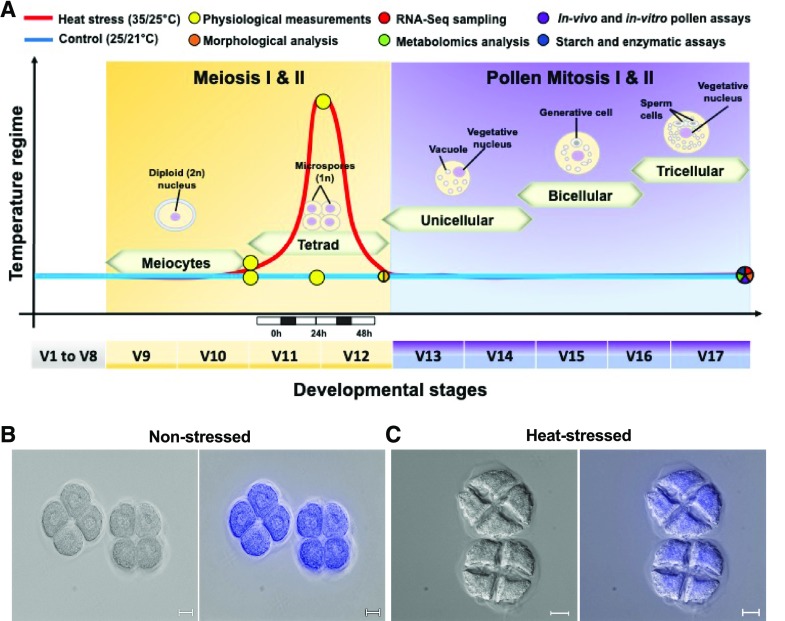
Figure 1.
A short heat stress period applied at the tetrad stage affects pollen development in maize. A, Experimental setup and sampling scheme. Maize plants were grown in control conditions (25°C/21°C light/dark period) until they reached the tetrad stage of pollen development. Stages were identified according to Begcy and Dresselhaus (2017). At stage V11, plants were exposed to moderate heat stress (35°C/25°C light/dark period) for 48 h and afterward transferred back to control conditions until maturity. Red and blue lines indicate heat stress and control temperature regimes, respectively. Pie-chart colors and position indicate assay types and time points, respectively. B and C, Bright-field images (left) and merged bright-field and UV images (right) of DAPI-stained maize pollen at the tetrad stage of V12 nonstressed and heat-stressed plants, as indicated. Scale bars = 10 μm.
To explore in detail the effect of heat stress on pollen morphology during the microsporogenesis-to-microgametogenesis transition, we dissected maize plants at the tetrad stage as described previously (Begcy and Dresselhaus, 2017). We observed a dramatic effect of heat stress on pollen development. Heat-stressed plants displayed irregular tetrads immediately after 48 h of heat exposure, while nonstressed plants presented a normal and regular shape of pollen tetrads (Fig. 1, B and C). This result indicates that the tetrad stage is a developmental stage in which pollen is highly susceptible to increased temperatures. Even though the effect of heat stress on pollen morphology has been observed in mature stages in several plant species (Jäger et al., 2008; Giorno et al., 2013; Prasad and Djanaguiraman, 2014), in pea (Pisum sativum), for example, morphological differences in pollen grains and their surface were not observed between control and heat-stressed plants for 7 d (Jiang et al., 2015). Altogether, these observations indicate that pollen development in cereals is especially susceptible during heat stress. Another intriguing observation was the less intense coloration observed in nuclei of stressed pollen grains at the tetrad stage after staining with 4',6-diamidino-2-phenylindole (DAPI) when compared with those from nonstressed plants (Fig. 1, B and C). These observations indicate that heat stress at the tetrad stage may cause irreversible damage to DNA during pollen development in maize or lead to changes in the tetrad wall, thus preventing efficient DAPI uptake. In summary, this study shows that pollen morphology in maize is severely altered after application of transient heat stress at the highly susceptible tetrad stage of pollen development.
To further explore the effect of transient heat stress on the progression of pollen development and degeneration of surrounding maternal tissues, we performed methacrylate-mix cross-sections of developing anthers at the tetrad, vacuolated microspore, vacuolated bicellular pollen, early tricellular pollen, and mature tricellular pollen stages (Fig. 2). Anthers at the same developmental stages kept under nonstressed conditions were used as controls. In general, pollen development appeared not to be severely affected after heat stress. However, we observed a slightly delayed degeneration of anther cell layers including the endothecium, middle layer, and tapetum after transient heat stress exposure. Moreover, mature pollen of plants heat stressed at the tetrad stage displayed less intense toluidine blue staining, indicating lower starch content. Delay in the degeneration of the tapetum and middle cell layer by programmed cell death has also been observed in male sterile cotton (Gossypium hirsutum) varieties (Min et al., 2014) and kiwifruit (Actinidia deliciosa; Falasca et al., 2013). These observations suggest a negative effect of transient heat stress on pollen development, which prompted us to investigate in greater detail whether heat stress applied transiently during the tetrad stage has an impact on pollen function at maturity.
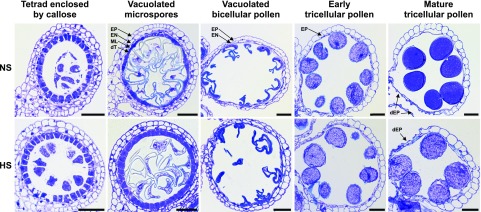
Figure 2.
Disintegration of anther cell layers was slightly delayed after heat stress was applied at the tetrad stage. Anther sections were stained with toluidine blue. Note that large and highly vacuolated cells collapsed during the staining procedure. Top row, Representative images showing anther development in nonstressed (NS) plants at the stages indicated. Bottom row, Representative images showing anther developmental stages after a 48 h heat stress was applied at the tetrad stage (HS). Degeneration of the tapetum (dT) and middle layer (ML) of anthers were delayed after heat stress. At the bicellular stage, these cell layers were fully disintegrated in both samples. Degeneration of the endothecium (EN) was delayed in HS samples at the bicellular/tricellular stage. At maturity, only the epidermis cell layer (EP) is left and partly disintegrated (dEP) for pollen release in control plants. Pollen of heat-stressed plants shows less intense staining and the endothecium is less disintegrated. Scale bars = 50 µm.
Transient Heat Stress at the Tetrad Stage Inhibits Germination of Mature Pollen Grains and Alters Starch Content and Enzymatic Activity
To test the long-term effects of transient moderate heat stress on pollen development and function at the tetrad stage at maturity, we allowed nonstressed and heat-stressed plants to continue their development under normal conditions until pollen maturation (Fig. 1A). After pollen reached maturity, we observed that heat stress substantially reduced the number of pollen grains adhering to the anther locules, indicating accelerated development (Supplemental Fig. S1, C–F). We did not notice any obvious difference in the size of mature florets after application of moderate heat stress (Supplemental Fig. S1G). However, the size of mature anthers significantly (P < 0.05) increased (Supplemental Fig. S1, H and I).
Next, we compared mature pollen from both sets of plants. A few pollen grains were defective after heat stress was applied at the tetrad stage (Supplemental Fig. S1, J and K). We germinated released pollen on media and observed that a large portion of pollen originating from heat-stressed plants showed a low germination rate (∼20%) and often burst, while nonstressed plants produced pollen that germinated and grew normally (∼80%; Fig. 3, A–C). Since pollen germination was carried out in petri dishes containing pollen germination media, we could not exclude the possibility that this nonnatural condition might have restricted pollen tube growth. Therefore, we also analyzed pollen germination in vivo by pollinating nonstressed maize silks with pollen of nonstressed and heat-stressed plants. Aniline blue staining was used to monitor pollen germination and growth. Nonstressed pollen was able to germinate and penetrate maize silks at high frequency (Fig. 3D). However, pollen from heat-stressed plants failed to germinate and consequently did not develop a functional pollen tube (Fig. 3E).
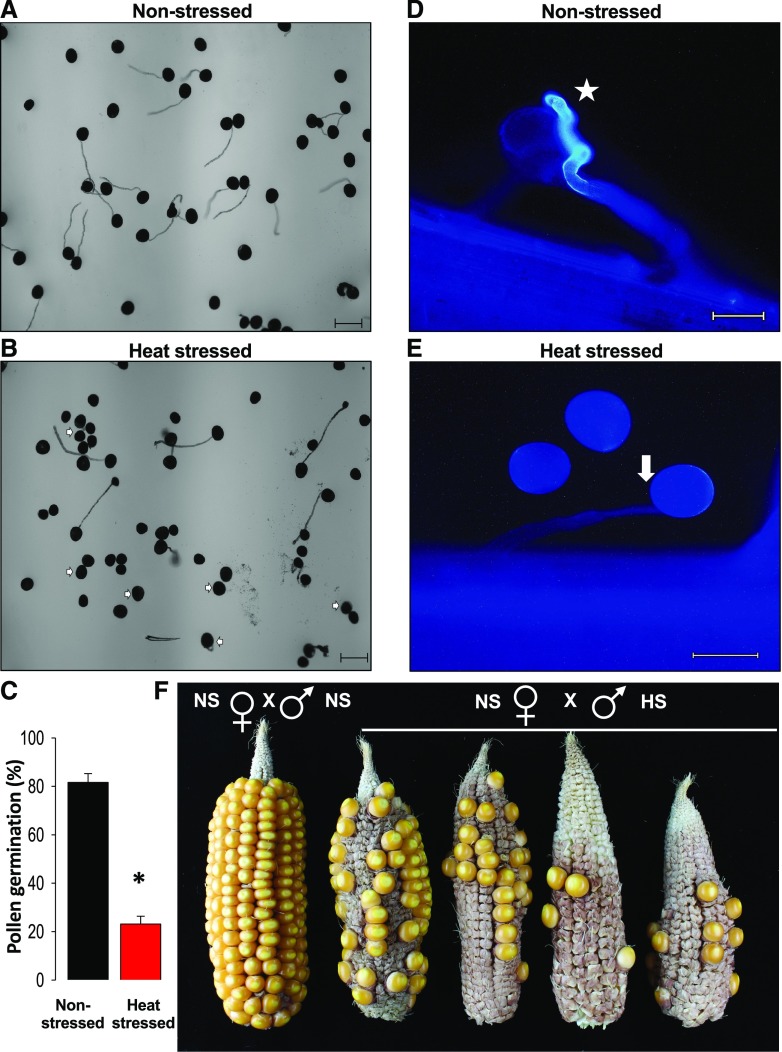
Figure 3.
Heat stress applied during the tetrad stage of pollen development inhibits pollen germination in maize. A and B, In vitro germination assay of pollen isolated from nonstressed (A) and heat-stressed plants (B) show a reduced germination rate and burst of stressed pollen. Arrows indicate nongerminated pollen tubes. Scale bars = 100 μm. C, Percentage of in vitro germination rate of pollen harvested from nonstressed and heat-stressed plants. The asterisk indicates a significant difference at P < 0.001; one-tailed t test comparing heat-stressed samples to nonstressed samples. D and E, Aniline blue staining of nonstressed (D) and heat-stressed (E) pollen germinating on papilla hair cells show lack of pollen tubes and penetration after heat stress application. The star indicates a normal germinating pollen tube. Scale bars = 100 μm. F, Nonstressed cobs were pollinated with both nonstressed pollen (NS × NS) and heat-stressed pollen (NS × HS). Seed set is strongly reduced after using heat-stressed pollen. Data are presented as the mean ± sd. n = 400–500.
Since maize plants are able to produce a large amount of pollen, we tested whether the 20% in vitro germinated pollen from heat-stressed plants was sufficient to maintain full seed set. When we crossed cobs of control plants with heat-stressed pollen, a strong reduction in the number of seeds per cob was observed (Fig. 3F). In contrast, control cobs showed full seed set. These results demonstrate that a short transient spike of heat stress applied during the tetrad stage of pollen development negatively impacts pollen function by drastically reducing its germination, both in vitro and in vivo, and thus strongly affects maize yield. Similarly, in sorghum (Nguyen et al., 2013; Singh et al., 2015) and pea (Jiang et al., 2018), a reduction in pollen germination across different genotypes and an increase in temperature regimes correlated with a decrease in seed setting.
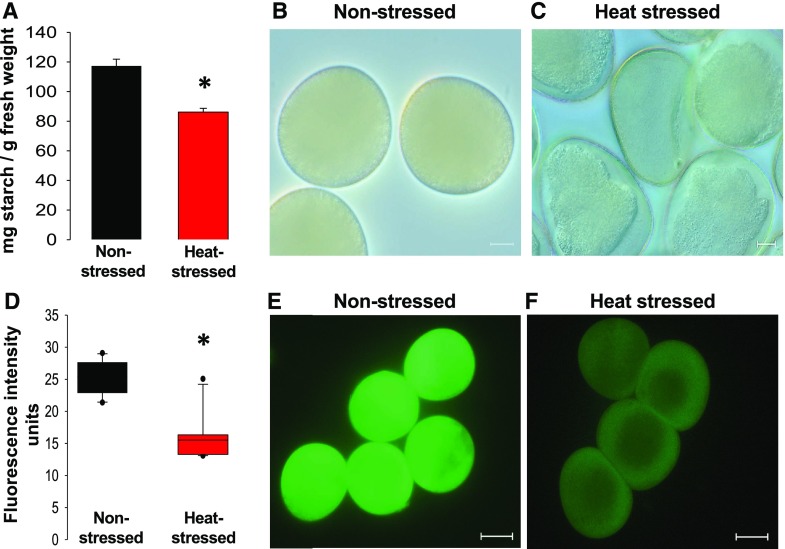
To further examine the causes of the strong reduction in pollen tube germination, we quantified the total starch content of mature pollen grains. Total starch was reduced by ∼30% (Figs. 2 and 4A) in heat-stressed pollen when compared to nonstressed pollen. A closer examination of pollen from both sets of plants revealed that heat-stressed pollen contained fewer starch granules (Fig. 4, B and C; Supplemental Fig. S1, J and K), explaining the reduction in starch content. We next asked whether this reduction might have been caused by reduced enzymatic activity. Therefore, we carried out fluorescein diacetate (FDA) staining and detected a strong reduction of general enzymatic activity in heat-stressed pollen; a drop in fluorescence intensity of ∼60% was observed (Fig. 4, D–F). These results suggest that the reduction in pollen germination and enzymatic activity might be directly linked to reduced starch and/or sugar biosynthesis, but might also be associated with reduced energy metabolism as well as lipid composition, as these are all essential prerequisites for proper pollen tube growth.
Figure 4.
Transient moderate heat stress at the tetrad stage leads to reduced starch content and enzymatic activity in mature pollen. A, Total starch quantification of mature pollen subjected to heat stress at the tetrad stage and the nonstressed control pollen. B and C, Light microscopic images of nonstressed (B) and heat-stressed pollen (C). D, Quantification of enzymatic activity (D) in mature pollen stained with FDA in nonstressed (E) and heat-stressed pollen (F). Data are presented as the mean ± sd. Scale bars = 10 μm (B and C) and 20 μm (E and F).
In developing microspores and anthers (Sato et al., 2006; Jain et al., 2007; Rieu et al., 2017) and developing seeds (Yang et al., 2018), heat stress was previously reported to affect sugar-to-starch metabolism, which inevitably led to decreased Suc content in those tissues. Heat stress strongly suppressed the expression of genes related to Suc and starch biosynthesis (Phan et al., 2013). Pollen viability and tube growth highly depend on the ability to accumulate enough reserve metabolites. Starch is a major form of reserve resource in mature pollen grains. During reproductive development, Suc has to be transferred from photosynthetically active tissues to reproductive organs via phloem and transported into developing pollen, where it is incorporated and stored as starch granules (De Storme and Geelen, 2014). The reduction observed in the net photosynthetic rate (Supplemental Fig. S1B) of heat-stressed maize plants indicates that the decline in production of photoassimilates influences starch accumulation and enzymatic activity (Fig. 4) and, consequently, reduced pollen germination capability (Fig. 3).
Genes Involved in Stress Responses, Signaling, Transcriptional Regulation and Energy Metabolism Are Misregulated in Heat-Stressed Pollen
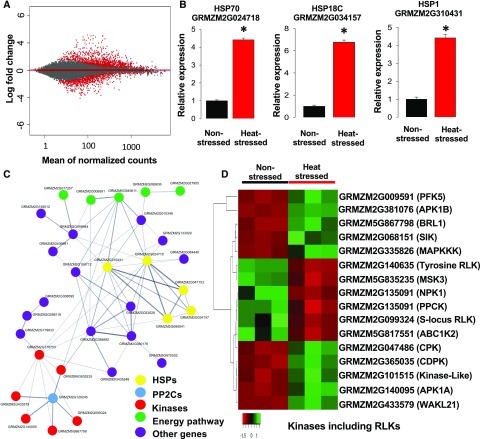
In order to elucidate the molecular basis of the reduction in pollen starch content, enzymatic activity, and germination capability, we further explored the transcriptional changes imposed by increased temperatures using an RNA-Seq approach. We found that ∼300 genes are differentially expressed between nonstressed and heat-stressed pollen samples collected at flower maturity (Fig. 5A; Supplemental Table S1). Gene ontology analysis showed enrichment of this gene set for genes involved in metabolic and cellular processes (Supplemental Fig. S2, A–C). Although samples were taken ∼14–16 d after heat application at the tetrad stage, RNA-Seq data analysis still indicated a strong increased expression of several heat shock protein genes (HSPs), including HSP70 (GRMZM2G024718), HSP18C (GRMZM2G034157), and HSP1 (GRMZM2G310431). We used these genes to validate our RNA-Seq results by reverse-transcription quantitative PCR (RT-qPCR; Fig. 5B).
Figure 5.
Differential gene expression analysis and data validation of mature pollen transiently heat stressed at the tetrad stage. A, Differential gene expression (the base 2 logarithm fold change) in mature maize pollen samples from plants exposed to heat stress relative to control (nonstressed) is plotted versus average gene expression level (i.e. logarithm of mean counts normalized for difference in library sizes). Red color indicates differentially expressed genes (DEG) exhibiting significant expression. B, RT-qPCR analysis shows that expression of HSP genes is still increased in mature pollen 2 weeks after application of heat stress at the tetrad stage. The asterisk indicates significant difference at P < 0.001; one-tailed t test comparing heat-stressed samples to nonstressed samples. n = 3 biological replicates, each with 3 technical replicates. C, Analysis of interactions of differentially expressed genes in response to heat stress. Well-known HSP markers for heat-stress responses are highlighted in yellow. A threshold of 0.7 of edge confidence was used. Thicker and thinner lines represent edge confidence of 0.9 and 0.7, respectively. A detailed list of the genes included in the gene interaction analysis can be found in Supplemental Table S4A. D, A number of genes encoding kinases, including RLKs, are differentially expressed in mature pollen after application of heat stress at the tetrad stage.
Many uncharacterized genes were overrepresented among the top 20 up- and downregulated genes (Supplemental Tables S2 and S3). To further explore their putative functions, we searched for orthologous genes in rice and Arabidopsis (Arabidopsis thaliana). In addition to HSPs, we found a heat-induced upregulated gene encoding a Bcl-2-associated athanogene protein (fold change [FC] 3.5). This protein represents a plant homolog of mammalian regulators of apoptosis. Its expression in Arabidopsis leaves was also shown to be strongly induced by heat stress (Doukhanina et al., 2006). Notably, a putative uncharacterized protein in maize (AC209062.3_FG002) was also strongly upregulated (Supplemental Table S2). Its orthologous gene in Arabidopsis (AT1G74950) encodes a member of the JAZ/TIFY family known to be involved in defense responses. Genome-scale transcriptional analysis among 10 different Arabidopsis ecotypes during heat stress showed a strong heat-induced expression of AT1G74950, which was one of the key genes in their predicted regulatory network analysis (Barah et al., 2013). Further genes encoding proteins associated with stress responses were also upregulated: while the characterization of GRMZM2G066213 orthologs in Arabidopsis suggested involvement in a variety of abiotic and biotic stress responses (Visscher et al., 2015), overexpression of the orthologous gene of GRMZM2G129246, glycolate oxidase, enhanced photosynthesis under high temperatures in rice (Cui et al., 2016). The connection between these genes in stress responses suggests that even though several vital processes important for pollen development were strongly affected by heat stress, pollen grains still tried to mitigate the damage suffered.
Downregulated genes encode a number of proteins associated, for instance, with growth and translation initiation (Supplemental Table S3). Interestingly, growth-regulating factor 4 (GRF4, GRMZM2G018414, FC −2.68), a plant-specific transcription factor (TF) involved in fundamental developmental processes such as stem, leaf, flower, and root development, as well as seed formation, has also been shown to be involved in the coordination of growth processes under adverse environmental conditions (Omidbakhshfard et al., 2015). Downregulation of this gene in heat-stressed maize pollen suggests an internal mechanism employed by the pollen to cope with stress, since downregulation of several members of the growth-regulating factor family resulted in increased stress tolerance in Arabidopsis (Kim et al., 2012; Omidbakhshfard et al., 2015). Under nonstressed conditions, GRFs repress the expression of stress-responsive genes such as dehydration-responsive element-binding factors. However, when the plant senses stresses, GRF levels are reduced and dehydration-responsive element-binding factors are increased, which enhances tolerance (Kim et al., 2012). Similarly, reduced transcription of GRMZM2G386430 (FC −2.8), a eukaryotic translation initiation factor 4G, shows the effort of stressed pollen grains to recover from the stress. A strategy to prevent damage caused by heat stress is to inhibit protein biosynthesis in order to limit the accumulation of unfolded proteins that might damage cells. In humans, for example, heat stress induces the expression of HSP27, which inhibits mRNA translation by binding to eukaryotic translation initiation factor 4G and thus preventing the cap-binding initiation complex (Cuesta et al., 2000).
To further understand pollen development-associated genetic responses to heat stress using the list of DEGs, we conducted a cluster correlation analysis using the “cor” function implemented in the R package WGCNA to identify groups of genes with shared expression profiles. Considering that a large portion of the maize genome is still poorly annotated and functions of many genes are unknown, it was likely that the correlation analysis would contain uncharacterized genes. Therefore, we combined cluster correlation analysis with the web-tool STRING (Szklarczyk et al., 2015), which provides protein interaction data in order to extract pathways that are affected by heat stress. After removal of unrooted genes, the remaining genes were queried in STRING, which identified 33 interacting genes associated with the heat stress response (Supplemental Table S4A). We selected candidate genes with an edge confidence of at least 0.7. This approach resulted in a cluster that grouped genes into five main hubs (Fig. 5C). These hubs contained heat shock proteins (yellow), protein phosphatases (blue), protein kinases (red), genes involved in the energy biosynthesis pathway (green), and others (purple). Since our analysis includes several protein kinases, we explored all DEGs in greater detail. We found that a number of protein kinases and receptor-like kinase (RLK) genes were differentially regulated after heat stress application (Fig. 5D). Similarly, a transcriptome comparison of heat-stressed mature pollen in Arabidopsis revealed an extensive misregulation of kinases. Several Ca2+-dependent protein kinases (CPKs) and calcineurin B-like-interacting protein kinase were downregulated as a result of heat stress imposition (Rahmati Ishka et al., 2018). CPKs and calcineurin B-like-interacting protein kinases play an essential role during pollen tube growth (Weinl and Kudla, 2009; Muschietti and Wengier, 2018; Zhu et al., 2018). A maize pollen-specific CPK (ZmCPK32) has recently been shown to regulate pollen tube growth (Li et al., 2018). In conclusion, misregulation of protein kinases and their interactor genes is very likely directly associated with a reduction in pollen germination and growth capability.
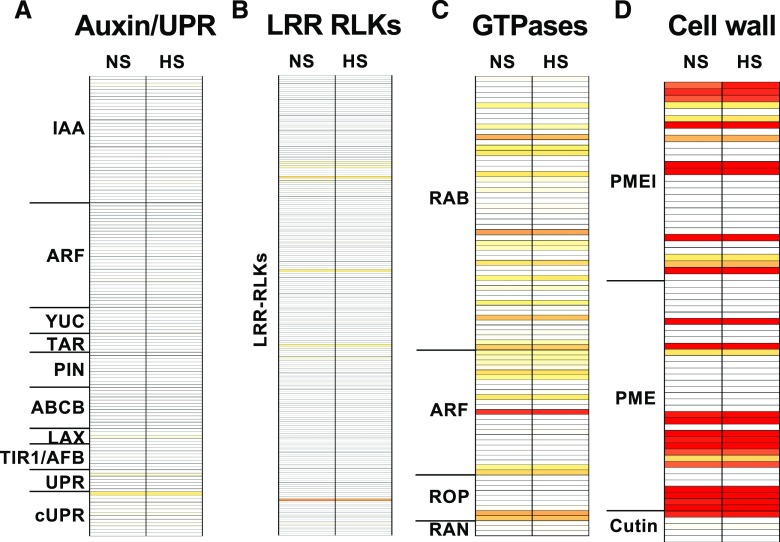
In Arabidopsis, the unfolded protein response (UPR) is an important mechanism to maintain fertility upon heat stress (Zhang et al., 2017). In order to investigate whether a similar response was triggered in maize pollen during heat stress, we searched for maize genes homologous to the ones described in Arabidopsis as being involved in the UPR (Zhang et al., 2017). This identified seven homologous genes (Supplemental Table S5). An alteration in gene expression was not found in UPR genes in mature maize pollen as a consequence of transient heat stress applied during the tetrad stage of pollen development. We also explored other canonical UPR genes in maize (Supplemental Table S5) as described by Srivastava et al. (2018). None of the canonical UPR genes, including Zm basic leucine zipper60 (bZIP60; GRMZM2G025812) and ZmbZIP17 (GRMZM2G060109), showed altered transcriptional expression (Fig. 6A). In conclusion, transient heat stress applied during the tetrad stage of pollen development did not lead to altered expression patterns of UPR genes in pollen at maturity. However, this does not exclude the possibility that UPR gene expression changed directly after the heat stress was applied.
Figure 6.
Expression of genes involved in auxin and UPRs, signaling as well as cell wall biosynthesis, is largely unaltered in mature pollen grains after application of moderate heat stress at the tetrad stage. A, Genes involved in auxin biosynthesis, transport, and response as well as UPRs. cUPR, canonical UPR genes, B and C, Genes encoding LRR RLKs and small GTPases as examples of genes involved in signaling. D, Examples of genes encoding cell wall modification enzymes (PMEs) and their inhibitors (PMEIs), as well as enzymes for cutin/suberin biosynthesis. White boxes indicate lack of gene expression, yellow boxes TPM values of 50, and dark red boxes TPM values >1,000; mixed colors indicate intermediate values. See the summary in Supplemental Table S5 for exact TPM values.
To further explore the impact of heat stress on pollen germination, we searched for gene families previously described as being involved in pollen development, germination, and growth. We particularly focused on genes encoding proteins involved in signaling, such as small GTPases, nucleotide-binding site Leu-rich repeat proteins (LRRs), and LRR-RLKs; cell wall biosynthesis genes encoding pectin methylesterases (PMEs) and PME inhibitors (PMEIs); wax biosynthesis; cutin and suberin formation genes; genes involved in auxin biosynthesis, transport, and signaling; and flavone, flavonol, flavonoid, isoflavonoid, and phenylpropanoid biosynthesis genes (Fig. 6; Supplemental Table S5). Auxin pathway genes were almost completely switched off (Fig. 6A), likely because pollen and anther development were completed. We further observed that transient heat stress did not affect flavone, flavonol, flavonoid, isoflavonoid, and phenylpropanoid biosynthesis genes. Only GRMZM2G167613, a gene involved in phenylpropanoid biosynthesis, showed a moderate transcriptional induction after heat stress. GRMZM2G167613 is thought to encode a protein that may function in the pollen cell wall during pollen tube stigma coupling (Yue et al., 2014) and that is induced in response to Ustilago maydis infection (Tanaka et al., 2014). The altered expression of this gene in response to heat stress might be linked to a putative function in response to biotic and abiotic stresses during maize reproduction. Other fundamental gene families required for pollen germination and growth in the transmitting tissue are RLKs and small GTPases. With the exception of the RLKs shown in Figure 5D, LRR RLKs are not altered in gene expression pattern (Fig. 6B). Moreover, at pollen maturity, only seven LRR RLKs showed a substantial expression, with GRMZM2G028643 being strongly expressed, but their expression levels were not significantly altered. Similarly, a number of small GTPases of various subfamilies were substantially expressed in mature pollen, but their expression levels were unaltered (Fig. 6C). Genes shown to play important roles in pollen germination and growth are PMEs and PMEIs. Both act to stabilize/destabilize certain domains of the pollen tube wall during tube elongation (Röckel et al., 2008; Guan et al., 2013). In general, both gene families contain members that are highly expressed in pollen grains, but whose expression was not affected by heat stress. Only GRMZM2G431856 and GRMZM2G321870 encoding a PME and GRMZM2G079661 and GRMZM2G122230 encoding a PMEI showed a small reduction at the transcriptional level (Fig. 6D). These genes were also found to be downregulated in a comparative analysis of the male inflorescence transcriptome of an ms22 maize mutant, a well-known male sterile mutant used for heterosis in maize (Gao et al., 2018).
In other plants, it has been shown that high temperatures induce tissue-specific auxin responses (Gray et al., 1998; Zhang et al., 2017). It was further shown that external auxin application reverses plant male sterility (Sakata et al., 2010). In developing anthers of barley (Hordeum vulgare) and Arabidopsis, endogenous auxin levels decreased under high temperature conditions (Sakata et al., 2010). To explore whether transient heat stress during pollen development triggers a similar response in our experimental setup, we surveyed all genes described to date as being involved in auxin signaling in maize (Galli et al., 2015; Matthes et al., 2019). As described above, we did not find major changes at the transcriptional level in either nonstressed or heat-stressed pollen grains. In general, gene expression levels of auxin response and auxin biosynthesis, and transport genes including members of the indole-3-acetic acid, auxin response factor (ARF), flavin monooxygenase (YUC), tryptophan aminotransferase-related (TAR), PIN-FORMED (PIN), like-auxin1 (LAX), ATP-binding cassette sub-family B (ABCB), and transport inhibitor resistant1/auxin signaling F-box (TIR1/AFB) families were very low at pollen maturity and not altered in response to heat-stress imposition (Fig. 6A; Supplemental Table S5). Only ZmLAX3 (GRMZM2G127949) showed transcript per million (TPM) values >10. In conclusion, our results suggest that a transient moderate heat stress applied during the tetrad stage does not lead to altered auxin signaling pathways in pollen at maturity. This observation is not unexpected given that pollen development is completed and auxin plays a major role during developmental processes. Moreover, we conclude that the above described auxin responses after high temperatures are likely attributable to developmental changes in the maternal anther tissues rather than to auxin responses within pollen grains.
Transient Heat Stress Altered Gene Expression and Metabolite Pattern Correlated with Energy and Lipid Biosynthesis
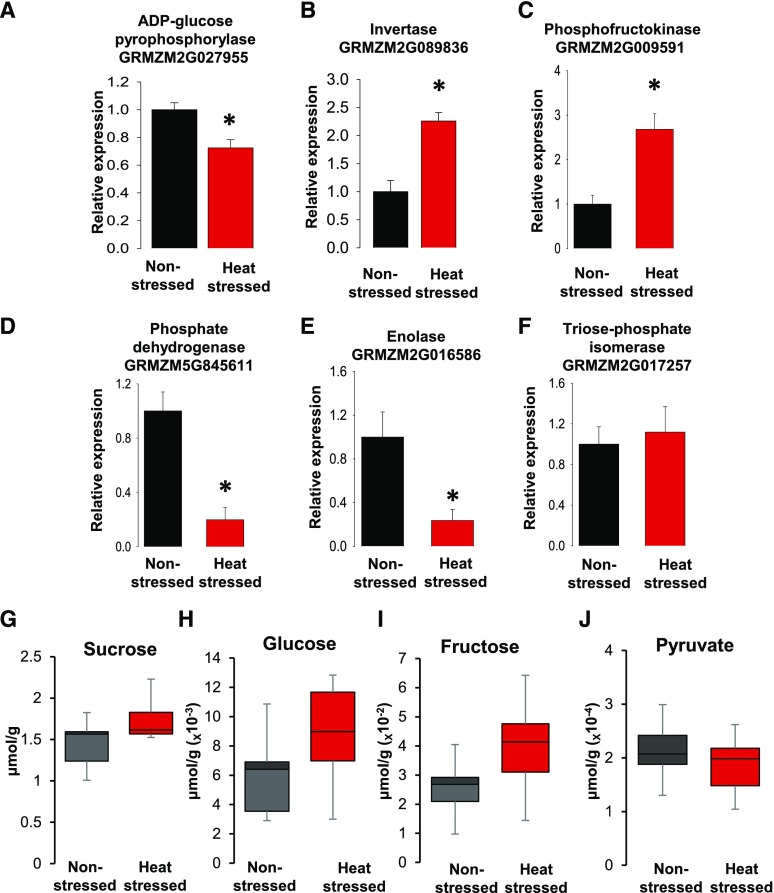
Since the clustering correlation analysis identified genes involved in energy biosynthesis, we next explored in more detail how heat stress affected the expression of genes involved in starch biosynthesis, as well as in glycolysis, a major energy biosynthesis pathway in plants and other organisms. We found that heat stress induced, for example, downregulation of the gene encoding ADP-Glc pyrophosphorylase (GRMZM2G027955) encoding an enzyme that catalyzes a key step in starch biosynthesis (Fig. 7A). Notably, an invertase gene (GRMZM2G089836) involved in the conversion of Suc into Fru and Glc was upregulated (Fig. 7B), explaining the observed reduction of starch levels and pointing toward increased levels of hexoses in heat-stressed pollen. Another gene encoding an enzyme involved in Glc catabolism, phosphofructokinase (GRMZM2G009591), was also upregulated (Fig. 7C). The corresponding enzyme participates in the conversion of Fru 6-phosphate and ATP to Fru 1,6-bisphosphate and ADP, respectively. In contrast, the phosphate dehydrogenase gene (GRMZM5G845611), involved in the breakdown of Glc for energy and carbon molecules, and enolase (GRMZM2G016586), responsible for the catalysis of 2-phosphoglycerate to phosphoenolpyruvate, were significantly downregulated (Fig. 7, D and E). These downregulations presumably created a bottleneck in the conversion of hexose pools to pyruvate and ATP (see Supplemental Fig. S3 for an overview). In order to test whether other genes in the pathway were also affected, we measured the transcriptional expression of triose-phosphate isomerase (GRMZM2G017257) as an example. This gene encodes an enzyme that catalyzes the reversible interconversion of triose phosphate isomers to dihydroxyacetone phosphate and d-glyceraldehyde 3-phosphate (Fig. 7F). We did not find any differences in gene expression levels between nonstressed and heat stressed pollen.
Figure 7.
In contrast to Suc and pyruvate, hexose phosphate is increased after transient heat stress. A to E, Transcriptional expression levels of misregulated enzymes involved in energy production. The asterisk indicates significant difference at P < 0.001. F, Triose-phosphate isomerase served as a control. The one-tailed t test was used in comparing heat-stressed samples to nonstressed samples. n = 3 biological replicates, each with 3 technical replicates. G to J, Metabolite levels of Suc (G), Glc (H), Fru (I), and pyruvate (J). Data are normalized to peak areas; n = 8 control and n = 7 heat stressed. Black and red represent nonstressed and heat-stressed pollen, respectively. Data are presented as the mean ± sd.
In conclusion, gene expression data indicated that hexose and hexose-phosphate pools might be upregulated, while pyruvate and ATP levels might be decreased in heat-stressed pollen grains. Therefore, we used gas chromatography (GC) coupled to mass spectrometry (MS) to assess the changes in sugar metabolite composition and concentration. Metabolite analysis showed that heat stress triggered the accumulation of Suc, Glc, and Fru (Fig. 7, G and I), while pyruvate levels were decreased (Fig. 7J), confirming a previous report in mammals showing that heat stress negatively affects the production of pyruvate and, thus, ATP synthesis in growing pigs (Sans-fernandez et al., 2015). Reduced pyruvate levels likely result in limited acetyl-coenzyme A (acetyl-CoA), which enters lipid biosynthesis pathways due to the increased activity of pathway entry enzymes, while less acetyl-CoA enters the citrate cycle for ATP production. Notably in yeast, a mild heat stress also affected the transcriptional expression of genes involved in the glycolytic pathway (Sakaki et al., 2003), affecting several genes related to those identified in this study. Remarkably, in human cells, increased levels of HSP70 inhibited the effects of stress and contributed to ATP balance by enhancing glycolytic activity (Wang et al., 2012). In our study, heat stress induced the transcriptional increase in HSP70; however, this enhanced expression level likely did not result in significantly increased ATP production in maize pollen. Taken together, these experiments demonstrate that transient temperature increase during the tetrad stage impaired starch and pyruvate production, while both Suc and hexose levels were substantially increased.
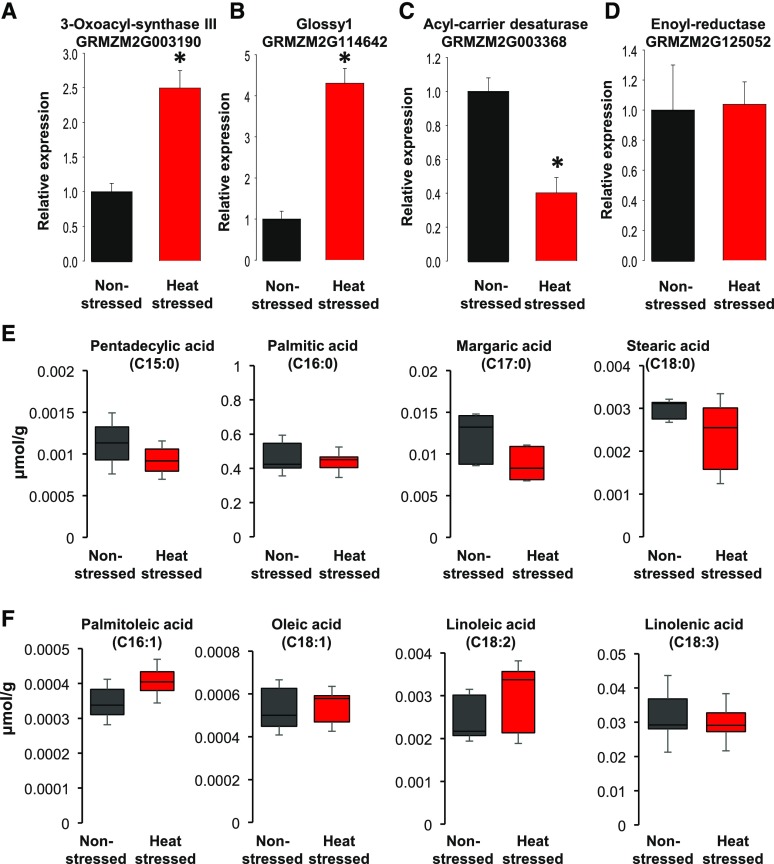
Another critical step for pollen germination and rapid tube growth involves an enormous demand for the production of lipids. Lipids not only represent one form of storage energy in plants, they also represent major constituents of cell membranes, which are required in large amounts during pollen tube growth. In this context, misregulation of lipid biosynthesis genes is critical for both pollen germination and tube growth. Lipids are synthesized from the cellular pool of acetyl-CoA generated from pyruvate delivered via glycolysis (Fig. 8). Notably, acetyl-CoA is also required to generate ATP during the citrate cycle. Thus, any upregulation of genes encoding enzymes that use acetyl-CoA from limited pyruvate pools in heat-stressed pollen will indirectly reduce ATP synthesis via the citrate cycle. Indeed, heat stress strongly increased the expression of a gene encoding 3-oxoacyl-synthase III (GRMZM2G003190), an enzyme that catalyzes the first condensation step within the fatty acid biosynthesis pathway using acetyl-CoA as a primer and malonyl-acyl-carrier protein as an acceptor (Fig. 8A). Similarly, the Glossy1 gene (GRMZM2G114642) involved in the conversion of dodecanoyl to myristoyl was 4-fold upregulated in heat-stressed pollen compared to the nonstressed control (Fig. 8B). However, the next step, which involves the generation of pentadecylic acid, a 15-carbon compound (C15:0), from myristoyl by acyl-carrier desaturase (GRMZM2G003368), was significantly down-regulated (FC −2.2, P = 2.75E−22; Fig. 8C). Similarly, as described for glycolysis, we also investigated genes that were not differentially expressed in this pathway. Figure 8D shows expression levels of a gene encoding enoyl-reductase (GRMZM2G125052), an enzyme that converts 3-oxo-dodecanoyl to dodecanoyl. Neither RNA-Seq data nor RT-qPCR analysis showed any alteration of enoyl-reductase gene expression levels between nonstressed and heat-stressed pollen.
Figure 8.
Heat stress during the tetrad stage of maize pollen development reduces levels of saturated fatty acids, while levels of unsaturated fatty acids are increased. A to C, Transcriptional expression of misregulated genes involved in lipid biosynthesis. D, Enoyl-reductase represents an unaltered gene. The asterisk indicates significant difference at P < 0.001; one-tailed t test comparing heat-stressed samples to nonstressed samples. n = 3 biological replicates, each with 3 technical replicates. E and F, Metabolite levels of saturated (E) and unsaturated (F) fatty acids. Data show normalized peak areas, n = 8 control and n = 7 heat stressed. Black and red represent nonstressed and heat-stressed pollen, respectively. Data are presented as the mean ± sd.
The alteration of lipid biosynthesis gene expression profiles observed in heat-stressed pollen hinted at a decrease in lipid production (see Supplemental Fig. S5 for an overview), which could explain the reduced pollen germination phenotype (Fig. 3). To further explore this hypothesis, we performed a metabolomics analysis and found a significant decrease in saturated fatty acid concentration in heat-stressed pollen (Fig. 6E). Pentadecylic acid, the metabolite generated by acyl-carrier desaturase, showed lower levels compared with nonstressed pollen. Similarly, all longer saturated fatty acids generated after pentadecylic acid, including palmitic acid (C16:0), margaric acid (C17:0), and stearic acid (C18:0), were significantly reduced. In contrast, longer-chain unsaturated fatty acids, including palmitoleic acid (C16:1), oleic acid (C18:1), and linoleic acid (C18:2), showed higher levels in heat-stressed pollen samples compared to nonstressed samples (Fig. 8F). Taken together, the findings indicate a negative effect of increased temperatures on lipid biosynthesis. In Arabidopsis, it was shown that heat stress resulted in a decrease in polyunsaturated fatty acids in leaf cell membranes (Higashi et al., 2015). This is expected, as a decrease in polyunsaturated fatty acids and an increase in saturated fatty acids maintains membrane dynamics at high temperatures. We found the opposite effect, which might be caused by misregulation of TF genes regulating the expression of lipid biosynthesis genes. Therefore, we next explored the effect of heat stress on the TF binding sites of all misregulated genes.
Transient Heat Stress at the Tetrad Stage Induced the Differential Expression of TFs with Highly Conserved Binding Sites
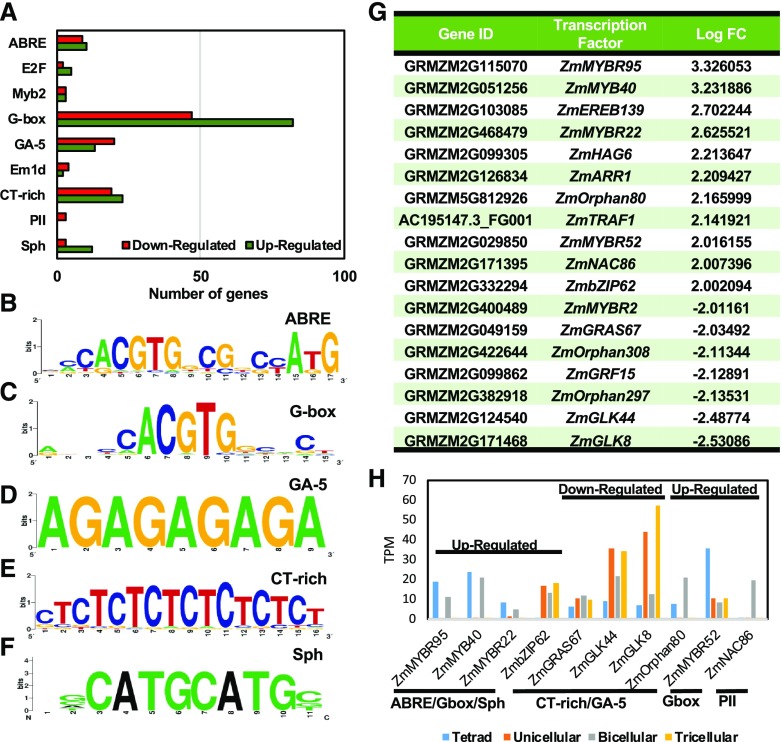
Gene expression patterns are highly dependent on TFs. Any alterations from normal conditions that result in large transcriptional changes are likely caused by changes in the activity of TFs. To identify TFs involved in gene expression alterations observed in pollen in response to heat stress, we analyzed TF binding sites in the promoter regions of all DEGs (Supplemental Table S1). We found that nine TF binding sites were significantly overrepresented in the data set (Fig. 9A). These included conserved TF binding motifs ABRE, G-box, GA-5, CT-rich, and Sph (Fig. 9, B–F), which are present in promoters of several genes responsive to abiotic stress in plants (Meier and Gruissem, 1994; Kim et al., 2011; Barah et al., 2016). About 50% of misregulated genes, both up- and downregulated, contain G-boxes. G-box cis-regulatory motifs have been shown to be overrepresented in promoters of genes upregulated in response to stresses, particularly during increased temperatures in Arabidopsis (Albihlal et al., 2018; Chow et al., 2018), indicating that the activity of interacting TFs is especially affected by heat stress. Notably, the GA-5 motif was also reported to be a cis-regulatory element involved in the heat response in animals like Caenorhabditis elegans (GuhaThakurta et al., 2002).
Figure 9.
TF binding sites of DEGs in mature pollen in response to heat stress applied at the tetrad stage. A, Main TF binding sites presented in genes differentially expressed after heat stress. B to F, Consensus TF binding site sequences identified for ABRE (B), G-box (C), GA-5 (D), CT-rich (E), and Sph (F) motifs. G, TF classes differentially expressed as the result of heat stress. H, Gene expression during pollen development of TFs containing overrepresented binding sites of DEGs.
To further identify TFs altered in response to increased transient temperatures during pollen development at the tetrad stage, a TF search was performed among the DEGs reported above. More than 16% of the DEGs were annotated as TF genes and classified into 12 TF families (Fig. 9G) according to the TF classification provided at the Grass Regulatory Information Server, Grassius (Gray et al., 2009). Notably, genes encoding MYB and MYB-related (MYBR) TFs were strongly heat stress induced, while genes encoding Golden2-Like (GLK) TFs displayed the strongest decrease after heat stress. During stress conditions, the conversion of stress signal perception to stress-responsive gene expression is modulated by TFs, which interact with cis-acting elements located in the promoter region of several target stress-responsive genes (Kim et al., 2011; Omidbakhshfard et al., 2015). These interactions activate a signaling cascade that aims to increase plant resilience. For instance, in the endosperm of rice seeds, heat stress induced profound changes in the transcriptional landscape of developing seeds (Chen et al., 2016). A large number of TFs were activated, particularly OsMADS87, which is a modulator of stress responses. We further explored the gene expression pattern of those TFs misregulated by heat stress during normal pollen development (Fig. 9H). We found that most TFs upregulated after heat stress were expressed during the early stages of pollen development. For instance, MYB and MYBR family members, which are expressed during the tetrad and bicellular stages of pollen development (Fig. 9H), were strongly upregulated in response to heat stress (Fig. 9G). Similarly, ZmGRAS44 and ZmGLK8, two downregulated TFs (Fig. 9G), are normally expressed during later stages of pollen development (Fig. 9H). These changes demonstrated that temperature-driven perturbations significantly alter the normal transcriptome-level reprogramming even 2 weeks after the stress is applied.
In conclusion, we found candidate TFs associated with translating abiotic heat stress signals into changes in gene expression (Lindemose et al., 2013; Guo et al., 2016) during pollen development. These include MYBs, MYBRs, bZIPs, GRFs, and NACs (Fig. 9G). Our results indicate that the broad transcriptional alterations in gene expression in developing pollen in response to heat stress could be largely attributed to the misregulation of TF genes. Large-scale functional studies of these candidate genes may elucidate key players whose activity could be modulated to avoid sterility. Moreover, our study suggests that transient heat stress applied at the tetrad stage of pollen development in maize leads to sterility due to altered and reduced accumulation of photoassimilates and lipids, as well as a reduction of energy production. Even though a number of studies have reported the general effect of heat stress under increased temperatures, the effects of transiently increased temperatures in a developmental context have remained unexplored. Our work further demonstrates that maize pollen at the tetrad stage is highly sensitive to heat stress.
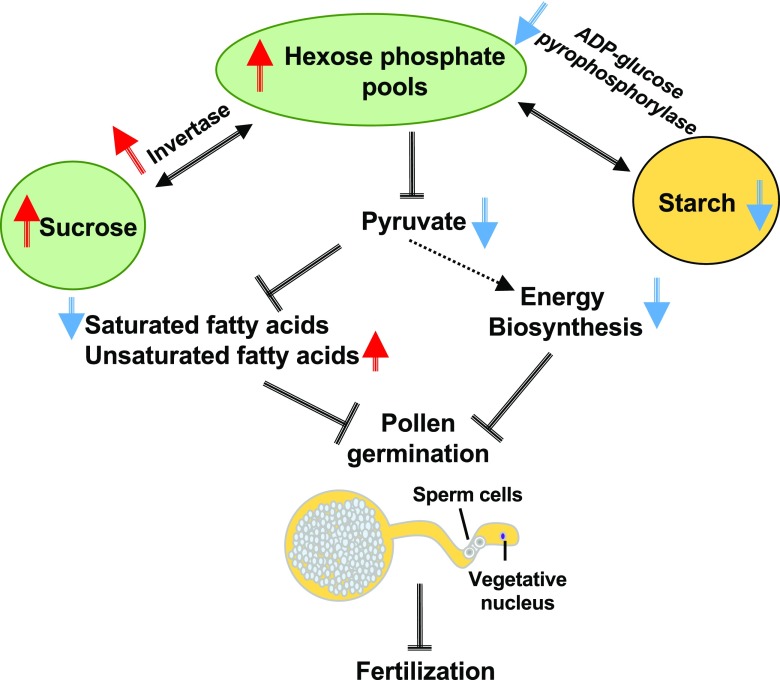
In summary, we propose a molecular mechanism that involves the control of pollen tube growth through differential regulation of genes involved in the production of energy and lipids (Fig. 10). We observed a decrease in the production of starch and an increase in the accumulation of Suc, as well as its components Glc and Fru. We also observed that the lipidome status was significantly impaired by heat stress. We also identified TF binding sites that are likely involved in regulating the expression of these DEGs. It will now be important to perform assays such as chromatin immunoprecipitation to investigate which of the TFs bind to the promoters of DEGs identified in this study. Moreover, analysis of the methylome will show how the memory effect of transient heat stress at the sensitive tetrad stage is maintained during further pollen development until maturity. This study provides a foundation for further studies to elucidate key components that can be potentially used for the long-term goal of developing crops resilient to increased temperatures.
Figure 10.
Proposed model incorporating the main pathways that are impacted when pollen is exposed to heat stress at the tetrad stage. Heat stress decreases starch concentration, but to compensate for the deficit of reserves, the activity of invertase increases, leading to high levels of hexose phosphate pools. Nevertheless, conversion of hexose pools into pyruvate is strongly affected, resulting in reduced energy production and fatty acids. Arrows and T-bars indicate induction and inhibition, respectively. Blue and red arrows represent downregulation and upregulation, respectively.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of the maize (Zea mays) inbred line B73 were germinated in an incubator and then transferred in the greenhouse to pots (10 cm diameter, 10 seedlings per pot) containing a standard substrate and soil mixture (1:1, v/v). Maize B73 seedlings were transferred after 3 weeks to 10 L pots in the greenhouse and grown under controlled conditions of 14 h of light at 25°C ± 2°C and 10 h of darkness at 21°C ± 2°C, and a constant air humidity of 60% to 65%. Supplemented light of ∼20,000 lux was provided to adjust day length duration. An automated temperature-water-based irrigation system was used to supply water according to plant consumption in a time-based preprogrammed schedule. Plants were fertilized twice a week with 2% (w/v) Hakaphos and monitored throughout their vegetative and reproductive development.
To apply heat stress treatments, plants at the tetrad stage of pollen development were identified using the Leaf Collar Method as described previously by Begcy and Dresselhaus (2017), and then transferred to walk-in growth chambers. For heat stress, growth chamber day/night temperature conditions were set at 35°/25°C and 60% humidity at 25,000 lux for 48 h. Correspondingly, nonstressed plants were maintained under a 25°/21°C day/night temperature regime and 60% humidity at 25,000 lux. After 48 h exposure to heat stress, all plants were maintained under greenhouse conditions as described above until pollen maturation. At the tetrad stage, pollen was collected for morphological and physiological analyses from control (nonstressed) and heat-stressed plants. Samples for biochemical and RNA-Seq analysis and pollen germination assays were collected upon pollen maturation (Fig. 1A).
Microscopy
Developing pollen grains at the tetrad stage were isolated as described by Begcy and Dresselhaus (2017). Pollen was mounted on glass slides containing 1 mg/mL DAPI solution in 1× phosphate-buffered saline (PBS; 0.8% [w/v] NaCl, 0.002% [w/v] KCl, 0.014% [w/v] Na2HPO4, and 0.0024% [w/v] KH2PO4), covered with a cover slip, sealed, and observed after 5 min in a Zeiss Axio Imager Z1 microscope equipped for structured illumination microscopy (Apotome) and with a Zeiss AxioCam MRM monochromatic camera. DAPI was excited with a filter set of 359 nm.
RNA Isolation and RT-qPCR
Total RNA was isolated from three biological replicates of nonstressed and heat-stressed pollen using the RNA Plant Mini kit (Ambion) following the manufacturer’s instructions. Complementary DNA (cDNA) synthesis was performed using the Invitrogen SuperScript II reverse transcription system and oligo(dT) primers. RT-qPCR reactions were performed using KAPA SYBR Fast qPCR master mix (Peqlab Biotechnologie) as described by Begcy and Walia (2015). A ubiquitin gene (GRMZM2G102471) was used as a normalization control. PCRs were performed using the Master Cycler realPlex2 in a 96-well reaction plate according to the manufacturer’s recommendations. Primers are provided in Supplemental Table S4B. Cycling parameters consisted of 5 min at 95°C followed by 40 cycles of 95°C for 15 s, 60°C for 30 s, and 70°C for 30 s. Reactions were performed in triplicate for each RNA sample using at least three biological replicates. Specificity of amplifications was verified by a melting curve analysis. Results from the Master Cycler realPlex2 detection system were further analyzed using Microsoft Excel. Relative amounts of mRNA were calculated from threshold points (Ct values) located in the log-linear range of RT-qPCR amplification plots using the 2-ΔCt method.
Pollen Germination
Freshly collected pollen from nonstressed and heat-stressed plants were immediately distributed onto petri dishes containing pollen germination medium (0.0005% [w/v] H3BO3, 10 mm CaCl2, 0.05 mm KH2PO4, 10% [w/v] Suc, and 6% [w/v] polyethylene glycol4000 in 1.2% [w/v] low-melting agarose) and kept under room temperature and dark conditions. Pollen germination was monitored after 40 min on pollen germination medium and visualized using a binocular microscope (Discovery.V8, Zeiss) with a Zeiss AxioCam MRM monochromatic camera.
Biochemical Assays
Mature pollen grains were freshly harvested and tested cytologically for metabolic viability by staining with FDA. Immediately after staining, pollen grains were visualized using a fluorescent microscope with a fluorescein isothiocyanate filter to detect fluorescein (excitation 450 nm and emission 520 nm filters, Zeiss). Fluorescein-positive cells were considered to be metabolically active. For total starch quantification, mature pollen was macerated and submitted to total hydrolysis of starch to Glc by amyloglucosidase catalysis. Then, Glc was phosphorylated by ATP in a reaction catalyzed by hexokinase. Glc-6-phosphate was oxidized to 6-phosphogluconate in the presence of NAD in a reaction catalyzed by Glc-6-phosphate dehydrogenase. During this oxidation, an equimolar amount of NAD is reduced to NADH. The increase in A340 was calculated and is directly proportional to the total starch concentration.
Toluidine Blue Staining of Anther Development
Anthers of nonstressed and heat-stressed plants were collected according to their developmental stages using the Leaf Collar Method as described previously (Begcy and Dresselhaus, 2017). Fresh samples were fixed in formaldehyde acetic acid solution (50% [v/v] ethanol, 10% [v/v] formalin, and 5% [v/v] acetic acid) overnight followed by an ethanol series (50% to 95% v/v) treatment containing 1 mm dithiothreitol (DTT) at 4°C (Begcy et al., 2018b). Afterward, samples were incubated in 100% (v/v) ethanol with 10 mm DTT for 30 min and subsequently embedded in a methacrylate mix (75% [w/v] butyl-methacrylate, 25% [w/v] methyl-methacrylate, 10 mm DTT, and 0.5% [w/v] benzoin ethyl ether). Polymerization of the methacrylate mix was done with UV light at 4°C overnight. Sectioning of anthers was done using a Leica microtome RM2255 with 2 μm thickness. Slices were fixed on polylysine-coated slides and acetone was used for removing polymerized methacrylate. Slides were incubated in 1× PBS for 5 min and images taken with an ApoTome2 microscopy system (Zeiss) after samples were stained for 1 min with 0.1% (w/v) toluidine blue.
Library Preparation and RNA-Seq
Library preparation and RNA-Seq were carried out as described in the Illumina TruSeq Stranded mRNA Sample Preparation Guide, the Illumina HiSeq 1000 System User Guide (Illumina), and the KAPA Library Quantification Kit-Illumina/ABI Prism User Guide (Kapa Biosystems). In brief, 250 ng of total RNA of each of the three biological replicates of nonstressed and heat-stressed pollen was used for purifying poly-A-containing mRNA molecules using poly-T oligo-attached magnetic beads. Following purification, mRNA was fragmented to an average insert size of 200–400 bases using divalent cations under elevated temperature (94°C for 4 min). Next, cleaved RNA fragments were reverse transcribed into first-strand cDNA using reverse transcriptase and random hexamer primers. Actinomycin D was added to improve strand specificity by preventing spurious DNA-dependent synthesis. Blunt-ended second-strand cDNA was synthesized using DNA Polymerase I, RNase H, and 2´-Deoxyuridine, 5´-Triphosphate (dUTP) nucleotides. Incorporation of dUTP in place of Deoxythymidine triphosphate (dTTP) quenched the second strand during the later PCR amplification, because the polymerase does not incorporate past this nucleotide. Resulting cDNA fragments were adenylated at their 3′ ends and ligated with indexing adapters, and specific cDNA libraries were subsequently created after PCR enrichment. Libraries were quantified using the KAPA SYBR FAST ABI Prism Library Quantification Kit (Kapa Biosystems). Equimolar amounts of each library were used for cluster generation on the cBot using the Illumina TruSeq PE Cluster Kit v3. Sequencing was performed on a HiSeq 1000 instrument using the indexed, 50 cycles paired-end read (PR) protocol and the TruSeq SBS v3 Reagents according to the Illumina HiSeq 1000 System User Guide. Image analysis and base calling resulted in bcl files, which were converted into fastq files using the bcl2fastq v2.18 software. Library preparation and RNA-Seq were performed at the Regensburg University service facility KFB (Competence Center for Fluorescent Bioanalytics).
Data Processing, Mapping, Differential Expression, and Statistical Analysis
Quality trimming and filtering of the resulting RNA-Seq PRs were done using Trimmomatic v.0.35 (Bolger et al., 2014). Data quality was assessed using FastQC. Processed PRs were aligned to the maize reference genome sequence AGPv3 assembly using annotation release-5b+ (corresponding to Gramene AGPv3.27) and a mapping program, STAR v. 2.5.2a (Dobin et al., 2013). Read pairs aligned to exonic regions were summarized per gene using featureCounts (Liao et al., 2014). Reads overlapping with more than one feature (i.e. gene region) were excluded from the analysis. To test for the presence/absence of batch effect and outliers, we performed variance stabilizing transformation of raw counts and analyzed nonmerged technical replicates using R package DESeq2 (Love et al., 2014). For pairwise comparisons, DEGs were identified using DEseq2 (Anders and Huber, 2010) by analyzing the number of reads aligned to the genes. The thresholds for differential expression were set at fold change >2 and P < 0.05 (after the false discovery rate adjustment for multiple testing) for the null hypothesis. The online tool agriGO (Du et al., 2010) was used for gene ontology analysis.
In order to identify transcriptional correlations among genes with shared expression profile analysis of nonstressed and heat-stressed pollen, the cor function was implemented in the R package WGCNA. Then, genes with shared expression profiles were considered as seed candidates and were used to obtain direct and indirect interactions using the STRING 10.5 database (Szklarczyk et al., 2015). We constructed cluster correlation analysis using as a threshold a high confidence score of 0.7. Only interactions with high levels of confidence were extracted from the database and considered as valid. Statistical analyses for morphological, biochemical, and physiological data were performed using R software/environment 3.2.2. For all measurements, data from at least four independent experiments were used. Data represented mean and median values including standard deviations. To determine statistical significance between nonstressed and heat-stressed samples, an unpaired student’s t test analysis with P = 0.05 as the significance level was used. Heat maps of expression profiles of genes of interest were generated using R packed heatmap2.
Metabolomics Analysis
Metabolite analyses were performed according to Obermeyer et al. (2013), with slight modifications. For metabolite extraction, ∼40 mg of frozen pollen grains were homogenized in a Retsch Mill (MM400) using six small stainless steel balls and a frequency of 30 Hz for 2 min, after which they were cooled in liquid N2 and the homogenizing process was repeated one more time. Then, 700 µL of methanol:chloroform:water (MCW; 2.5:1:0.5) were added to each sample and transferred to new tubes filled with ∼0.5 g of Lysing Matrix B and lysed in a Fast Prep Bead Beater (MP Biomedicals) for 6 × 20 s at 6.5 m/s, followed by incubation on ice for 10 min and centrifugation for 4 min at 21,000g. The supernatant was transferred to a new tube and the pellet reextracted with 300 µL MCW and incubated for 15 min on ice. After pellet reextraction with 300 µL of chloroform for 15 min, all supernatants generated during the different extraction steps (MCW extracts) were combined. An additional reextraction was performed on pellets with 300 µL of 80% (v/v) ethanol for 30 min at 80°C and then pelleted by centrifugation for 4 min at 21,000g. Meanwhile, 400 µL of water was added to the combined MCW extracts to induce separation into polar and apolar phases. After centrifugation, the supernatant (polar phase) was transferred to a new tube and combined with the supernatant of the 80% (v/v) ethanol extraction. The polar phase (methanol, water, and ethanol) was separated into two aliquots and dried with internal standard (5 µL of 5 mm solution of pentaerythritol) in a speed vac (Scan Vac) using a pressure gradient. The apolar (chloroform) phase was dried separately and both dried extracts were stored at −20°C until further analysis. For sample derivatization of the polar phase, one aliquot of each sample was redried in a speed vacuum for 10 min. Samples were dissolved in 20 μL solution of 40 mg/mL methoxyamine hydrochloride (CH3ONH2*HCl) in pyridine (Merck) and incubated for 90 min at 30°C in a Thermo shaker at 550 rpm. Subsequently, 80 µL of N-methyl-N-(trimethylsilyl) trifluoroacetamide (Macherey Nagel) was added to the sample solution and incubated for 30 min at 37°C. After centrifugation (2 min at 14,000g), the supernatant was transferred to glass vials with micro inserts, closed with crimp caps, and measured by GC-MS.
Analyses of fatty acids were performed according to Furuhashi et al. (2016), with slight modifications. In brief, one-third of the apolar phase was dried and used for analysis of fatty acid methylesters. Therefore, samples were dissolved in 295 µL Methyl tert-butyl ether and 5 µL of a trimethylsulfonium hydroxide solution in methanol and incubated for 30 min at room temperature for derivatization of lipids in order to analyze their fatty acid methylester content. Samples were centrifuged at 21,000g and the supernatant was aliquoted into two glass vials for GC-time-of-flight-MS analyses using two different split rates. Both extracts of a sample, polar and apolar, were derivatized as described above and analyzed on a Leco Pegasus 4D GC×GC time-of-flight MS instrument. Injection of samples was performed with a split/splitless injector at a constant temperature of 230°C and equipped with a single-tapered liner with deactivated wool. Injection volume was 1 µL of derivatized sample injection and was performed two times for each sample, namely at split rate 1:100 and 1:2 for the polar samples, as well as splitless and split 1:2 for the apolar fraction. Gas chromatographic separation was conducted on a HP-5MS column (30 m × 3 0.25 mm × 3 0.25 mm, Agilent Technologies) using helium as a carrier gas at a flow rate of 1 mL min–1. Temperature gradient started at 70°C isothermal for 1 min, followed by a heating ramp of 9°C min–1 to 330°C held for 7 min. The transfer line temperature was 250°C, and the ion source temperature was set to 200°C. Mass spectra were acquired at an acquisition rate of 20 spectra s–1 at a mass range of Thomson mass-to-charge ratio 40:600 using a detector voltage of 1625 V and electron impact ionization of 70 eV.
Data were analyzed using the instrument’s software ChromaTof (Leco). Measurements of standard mixes and of representative samples of the experiment were used as reference and were processed using the parameters peak width = 2.5 s, base line offset = 1, and S/n = 10, then used as reference samples. Peaks were annotated using spectral match factors (>850) and retention indices (alkane based) comparing to the standard mixes, to an in house database as well as the Golm Metabolome database (Kopka et al., 2005). All other samples were processed against these references, separately for the polar and apolar phases. Peak annotations as well as peak integrations were checked manually before exporting peak areas for relative quantification into Microsoft Excel. Areas of different trimethylsilyl derivatives of single metabolites were summed, and from methoxyamine products, only one peak was selected for further analyses. In Supplemental Table S6, all detected analytes of the polar and apolar phases are presented, as well as their levels of identification based on the Metabolomics Standard Initiative (Sumner et al., 2007).
Histological Analysis
Anthers of nonstressed and heat-stressed plants were collected upon maturation, fixed in 3.7% (w/v) formaldehyde, 5% (v/v) acetic acid, and 50% (v/v) ethanol, vacuum infiltrated, and stored at 4°C overnight. Samples were then embedded in 10% (w/v) low-melting agarose dissolved in distilled water. Samples kept in gelatin blocks were postfixed with 10% (v/v) formaldehyde (diluted from 37% [v/v] formaldehyde; Carl Roth) and 0.1 m PBS at 4°C overnight. All embedded blocks (1.5 × 1.5 × 1.0 cm) were stored at 4°C in PBS until sectioning. Cross-sections (50 µm thick) were prepared using a vibratome Hyrax V50 (Carl Zeiss MicroImaging), adjusting frequency, amplitude, and speed of the razor blade (Isana Men; Rossmann) for each sample. At least 20 50-µm-thick sections were prepared per sample for quality comparison. Three independent experiments were performed to collect anthers for sectioning. The images of anther sections are representative across replicates (Supplemental Fig. S1).
Physiological Measurements
Plants kept under nonstressed and heat-stressed conditions were monitored during the time course of 48 h of the heat stress experiment. Fully expanded leaves of maize plants at the V11 stage containing pollen at the tetrad stage were identified using the Leaf Collar Method as described previously (Begcy and Dresselhaus, 2017) and were used to estimate net photosynthetic rate (Supplemental Fig. S1A) and transpiration rate (Supplemental Fig. S1E). Daily measurements were taken with an Infrared Gas Analyzer (LCpro+; ADC Bioscientific) at a CO2 concentration of 360 µL L−1, a saturating light intensity of 1,000 µmol m−2 s−1, and a gas flow rate of 200 mL min−1, as described by Begcy et al. (2012, 2019)
Identification of Conserved Regulatory Elements in the Promoters of DEGs
To identify regulatory elements shared by/overrepresented in the promoters of DEGs relative to the reference set, which consists of all genes expressed in the mature maize pollen, promoter sequences of 23,000 maize protein-coding genes were obtained from the Plant Promoter Database (Shahmuradov et al., 2003). This data set includes only promoters for which canonical transcriptional start sites were identified: promoters of 14,016 out of 20,309 genes expressed in mature maize pollen (at least one library-size-normalized count in at least two libraries), including promoters of 204 of 300 DEGs in pollen after exposure to heat stress. To find conserved regulatory elements shared by at least 50% of the DEGs, promoters of the 204 DEGs were analyzed using NsiteMP v6 (Shahmuradov et al., 2003). In addition, we conducted a TF binding site (TFBS) overrepresentation test. TFBSs in the promoters of the 14,016 genes expressed in maize pollen were predicted using Nsite and RegSite Database of Plant Regulatory Elements (Softberry). TFBS overrepresented in the promoters of DEGs relative to the reference set (all 14,016 genes) were identified using an exact binomial test in R with a confidence interval of 0.99. TFBSs with P < 0.01 were considered to be overrepresented. DEGs containing overrepresented TFBSs in the promoter regions were annotated using the BLAST similarity search against the NCBI nonredundant database.
SUPPLEMENTAL DATA
The following supplemental materials are available.
Supplemental Figure S1. Heat stress negatively influences gas exchange parameters and disrupts anther and pollen development in maize inbred line B73.
Supplemental Figure S2. Gene ontology enrichment analysis of genes differentially expressed in maize pollen in response to heat stress.
Supplemental Figure S3. Schematic representation of glycolysis in mature pollen after heat stress was applied at the tetrad stage.
Supplemental Figure S4. Schematic representation of the lipid biosynthesis pathway in mature pollen after heat stress was applied at the tetrad stage.
Supplemental Table S1. DEGs between nonstressed and heat-stressed samples during the tetrad stage of pollen development.
Supplemental Table S2. Top 20 upregulated genes after developing pollen were transiently heat stressed for 48 h at the tetrad stage.
Supplemental Table S3.Top 20 downregulated genes after developing pollen at the tetrad stage were transiently heat stressed for 48 h.
Supplemental Table S4. List of genes used for gene cluster correlation analysis.
Supplemental Table S5. Transcriptional expression of genes involved in UPR, signaling, and cell wall biosynthesis, such as auxin response, biosynthesis and transport genes, nucleotide-binding site-LRRs and LRR-RLKs, PMEIs, PMEs, secondary metabolite biosynthesis genes, small GTPases, and others.
Supplemental Table S6. Chromatograms of all metabolites analyzed in nonstressed and heat-stressed pollen samples.
Acknowledgments
We thank Noureddine Djella and Armin Hildebrand for plant care and Daniel Lang for critical reading of the article.
Footnotes
This work was supported by the BayKlimaFit program of the Bavarian State Ministry of the Environment and Consumer Protection (grant no. 810100 to T.D.).
Articles can be viewed without a subscription.
References
- Albihlal WS, Obomighie I, Blein T, Persad R, Chernukhin I, Crespi M, Bechtold U, Mullineaux PM (2018) Arabidopsis HEAT SHOCK TRANSCRIPTION FACTORA1b regulates multiple developmental genes under benign and stress conditions. J Exp Bot 69: 2847–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghabari F, Lukac M, Jones HE, Gooding MJ (2014) Effect of Rht alleles on the tolerance of wheat grain set to high temperature and drought stress during booting and anthesis. J Agron Crop Sci 200: 36–45 [Google Scholar]
- Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barah P, Jayavelu ND, Mundy J, Bones AM (2013) Genome scale transcriptional response diversity among ten ecotypes of Arabidopsis thaliana during heat stress. Front Plant Sci 4: 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barah P, Mahantesha Naika BN, Jayavelu ND, Sowdhamini R, Shameer K, Bones AM (2016) Transcriptional regulatory networks in Arabidopsis thaliana during single and combined stresses. Nucleic Acids Res 44: 3147–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton DA, Cantrill LC, Law AM, Phillips CG, Sutton BG, Overall RL (2014) Chilling to zero degrees disrupts pollen formation but not meiotic microtubule arrays in Triticum aestivum L. Plant Cell Environ 37: 2781–2794 [DOI] [PubMed] [Google Scholar]
- Begcy K, Dresselhaus T (2017) Tracking maize pollen development by the Leaf Collar Method. Plant Reprod 30: 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begcy K, Dresselhaus T (2018) Epigenetic responses to abiotic stresses during reproductive development in cereals. Plant Reprod 31: 343–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begcy K, Walia H (2015) Drought stress delays endosperm development and misregulates genes associated with cytoskeleton organization and grain quality proteins in developing wheat seeds. Plant Sci 240: 109–119 [DOI] [PubMed] [Google Scholar]
- Begcy K, Mariano ED, Gentile A, Lembke CG, Zingaretti SM, Souza GM, Menossi M (2012) A novel stress-induced sugarcane gene confers tolerance to drought, salt and oxidative stress in transgenic tobacco plants. PLoS One 7: e44697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begcy K, Sandhu J, Walia H (2018a) Transient heat stress during early seed development primes germination and seedling establishment in rice. Front Plant Sci 9: 1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begcy K, Weigert A, Ogolla Egesa A, Dresselhaus T (2018b) Compared to Australian cultivars, European summer wheat (Triticum aestivum) overreacts when moderate heat stress is applied at the pollen development stage. Agronomy (Basel) 8: 99 [Google Scholar]
- Begcy K, Mariano ED, Lembke CG, Zingaretti SM, Souza GM, Araújo P, Menossi M (2019) Overexpression of an evolutionarily conserved drought-responsive sugarcane gene enhances salinity and drought resilience. Ann Bot 20: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS, McLaughlin JE (2007) Functional reversion to identify controlling genes in multigenic responses: Analysis of floral abortion. J Exp Bot 58: 267–277 [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, Bahuguna RN, Shah D, Pal M, Jagadish SVK (2017) High temperature stress during flowering and grain filling offsets beneficial impact of elevated CO2 on assimilate partitioning and sink-strength in rice. Sci Rep 7: 8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Begcy K, Liu K, Folsom JJ, Wang Z, Zhang C, Walia H (2016) Heat stress yields a unique MADS box transcription factor in determining seed size and thermal sensitivity. Plant Physiol 171: 606–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CN, Chiang-Hsieh YF, Chien CH, Zheng HQ, Lee TY, Wu NY, Tseng KC, Hou PF, Chang WC (2018) Delineation of condition specific Cis- and Trans-acting elements in plant promoters under various endo- and exogenous stimuli. BMC Genomics 19(Suppl 2): 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta R, Laroia G, Schneider RJ (2000) Chaperone hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes. Genes Dev 14: 1460–1470 [PMC free article] [PubMed] [Google Scholar]
- Cui LL, Lu YS, Li Y, Yang C, Peng XX (2016) Overexpression of glycolate oxidase confers improved photosynthesis under high light and high temperature in rice. Front Plant Sci 7: 1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Geelen D (2014) The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms. Plant Cell Environ 37: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukhanina EV, Chen S, van der Zalm E, Godzik A, Reed J, Dickman MB (2006) Identification and functional characterization of the BAG protein family in Arabidopsis thaliana. J Biol Chem 281: 18793–18801 [DOI] [PubMed] [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res 38: W64–W70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Tsuchiya T, Hamada K, Kawamura S, Yano K, Ohshima M, Higashitani A, Watanabe M, Kawagishi-Kobayashi M (2009) High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol 50: 1911–1922 [DOI] [PubMed] [Google Scholar]
- Evans DE, Taylor PE, Singh MB, Knox RB (1992) The interrelationship between the accumulation of lipids, protein and the level of acyl carrier protein during the development of Brassica napus L. pollen. Planta 186: 343–354 [DOI] [PubMed] [Google Scholar]
- Falasca G, D’Angeli S, Biasi R, Fattorini L, Matteucci M, Canini A, Altamura MM (2013) Tapetum and middle layer control male fertility in Actinidia deliciosa. Ann Bot 112: 1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Liu P, Li G, Dong ST, Wang FH, Kong LA, Zhang JW (2014) Effect of heat stress on the photosynthetic characteristics in flag leaves at the grain-filling stage of different heat-resistant winter wheat varieties. J Agron Crop Sci 200: 143–155 [Google Scholar]
- Folsom JJ, Begcy K, Hao X, Wang D, Walia H (2014) Rice Fertilization-Independent Endosperm1 regulates seed size under heat stress by controlling early endosperm development. Plant Physiol 165: 238–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi T, Nakamura T, Fragner L, Roustan V, Schön V, Weckwerth W (2016) Biodiesel and poly-unsaturated fatty acids production from algae and crop plants—A rapid and comprehensive workflow for lipid analysis. Biotechnol J 11: 1262–1267 [DOI] [PubMed] [Google Scholar]
- Galli M, Liu Q, Moss BL, Malcomber S, Li W, Gaines C, Federici S, Roshkovan J, Meeley R, Nemhauser JL, et al. (2015) Auxin signaling modules regulate maize inflorescence architecture. Proc Natl Acad Sci USA 112: 13372–13377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhang L, Zhao S, Yan Y (2018) Comparative analysis of the male inflorescence transcriptome profiles of an ms22 mutant of maize. PLoS One 13: e0199437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorno F, Wolters-Arts M, Mariani C, Rieu I (2013) Ensuring reproduction at high temperatures: The heat stress response during anther and pollen development. Plants (Basel) 2: 489–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Bevan M, Brutnell T, Buell CR, Cone K, Hake S, Jackson D, Kellogg E, Lawrence C, McCouch S, et al. (2009) A recommendation for naming transcription factor proteins in the grasses. Plant Physiol 149: 4–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M (1998) High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA 95: 7197–7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Guo J, Li H, Yang Z (2013) Signaling in pollen tube growth: Crosstalk, feedback, and missing links. Mol Plant 6: 1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GuhaThakurta D, Palomar L, Stormo GD, Tedesco P, Johnson TE, Walker DW, Lithgow G, Kim S, Link CD (2002) Identification of a novel cis-regulatory element involved in the heat shock response in Caenorhabditis elegans using microarray gene expression and computational methods. Genome Res 12: 701–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Liu JH, Ma X, Luo DX, Gong ZH, Lu MH (2016) The plant heat stress transcription factors (HSFs): Structure, regulation, and function in response to abiotic stresses. Front Plant Sci 7: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y, Okazaki Y, Myouga F, Shinozaki K, Saito K (2015) Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci Rep 5: 10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger K, Fábián A, Barnabás B (2008) Effect of water deficit and elevated temperature on pollen development of drought sensitive and tolerant winter wheat (Triticum aestivum L.) genotypes. Acta Biol Szeged 52: 67–71 [Google Scholar]
- Jain M, Prasad PVV, Boote KJ, Hartwell AL Jr., Chourey PS (2007) Effects of season-long high temperature growth conditions on sugar-to-starch metabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench). Planta 227: 67–79 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lahlali R, Karunakaran C, Kumar S, Davis AR, Bueckert RA (2015) Seed set, pollen morphology and pollen surface composition response to heat stress in field pea. Plant Cell Environ 38: 2387–2397 [DOI] [PubMed] [Google Scholar]
- Jiang YF, Bueckert RA, Warkentin TD, Davis AR (2018) High temperature effects on in vitro pollen germination and seed set in field pea. Can J Plant Sci 98: 71–80 [Google Scholar]
- Kim JS, Mizoi J, Yoshida T, Fujita Y, Nakajima J, Ohori T, Todaka D, Nakashima K, Hirayama T, Shinozaki K, et al. (2011) An ABRE promoter sequence is involved in osmotic stress-responsive expression of the DREB2A gene, which encodes a transcription factor regulating drought-inducible genes in Arabidopsis. Plant Cell Physiol 52: 2136–2146 [DOI] [PubMed] [Google Scholar]
- Kim JS, Mizoi J, Kidokoro S, Maruyama K, Nakajima J, Nakashima K, Mitsuda N, Takiguchi Y, Ohme-Takagi M, Kondou Y, et al. (2012) Arabidopsis GROWTH-REGULATING FACTOR7 functions as a transcriptional repressor of abscisic acid- and osmotic stress-responsive genes, including DREB2A. Plant Cell 24: 3393–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopka J, Schauer N, Krueger S, Birkemeyer C, Usadel B, Bergmüller E, Dörmann P, Weckwerth W, Gibon Y, Stitt M, et al. (2005) Gmd@csb.Db: The Golm Metabolome Database. Bioinformatics 21: 1635–1638 [DOI] [PubMed] [Google Scholar]
- Li J, Li Y, Deng Y, Chen P, Feng F, Chen W, Zhou X, Wang Y (2018) A calcium-dependent protein kinase, ZmCPK32, specifically expressed in maize pollen to regulate pollen tube growth. PLoS One 13: e0195787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W (2014) featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930 [DOI] [PubMed] [Google Scholar]
- Lindemose S, O’Shea C, Jensen MK, Skriver K (2013) Structure, function and networks of transcription factors involved in abiotic stress responses. Int J Mol Sci 14: 5842–5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell DB, Sibley A, Ortiz-Monasterio JI (2012) Extreme heat effects on wheat senescence in India. Nat Clim Chang 2: 186–189 [Google Scholar]
- Lobell DB, Hammer GL, McLean G, Messina C, Roberts MJ, Schlenker W (2013) The critical role of extreme heat for maize production in the United States. Nat Clim Chang 3: 497–501 [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes MS, Best NB, Robil JM, Malcomber S, Gallavotti A, McSteen P (2019) Auxin EvoDevo: Conservation and diversification of genes regulating auxin biosynthesis, transport, and signaling. Mol Plant 12: 298–320 [DOI] [PubMed] [Google Scholar]
- Meier I, Gruissem W (1994) Novel conserved sequence motifs in plant G-box binding proteins and implications for interactive domains. Nucleic Acids Res 22: 470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min L, Li Y, Hu Q, Zhu L, Gao W, Wu Y, Ding Y, Liu S, Yang X, Zhang X (2014) Sugar and auxin signaling pathways respond to high-temperature stress during anther development as revealed by transcript profiling analysis in cotton. Plant Physiol 164: 1293–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Rieu I (2016) Acclimation to high temperature during pollen development. Plant Reprod 29: 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Bertomeu J, Cascales-Miñana B, Irles-Segura A, Mateu I, Nunes-Nesi A, Fernie AR, Segura J, Ros R (2010) The plastidial glyceraldehyde-3-phosphate dehydrogenase is critical for viable pollen development in Arabidopsis. Plant Physiol 152: 1830–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschietti JP, Wengier DL (2018) How many receptor-like kinases are required to operate a pollen tube. Curr Opin Plant Biol 41: 73–82 [DOI] [PubMed] [Google Scholar]
- Nguyen CT, Singh V, van Oosterom EJ, Chapman SC, Jordan DR, Hammer GL (2013) Genetic variability in high temperature effects on seed-set in sorghum. Funct Plant Biol 40: 439–448 [DOI] [PubMed] [Google Scholar]
- Obermeyer G, Fragner L, Lang V, Weckwerth W (2013) Dynamic adaption of metabolic pathways during germination and growth of lily pollen tubes after inhibition of the electron transport chain. Plant Physiol 162: 1822–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver SN, Van Dongen JT, Alfred SC, Mamun EA, Zhao XC, Saini HS, Fernandes SF, Blanchard CL, Sutton BG, Geigenberger P, et al. (2005) Cold-induced repression of the rice anther-specific cell wall invertase gene OsINV4 is correlated with sucrose accumulation and pollen sterility. Plant Cell Environ 28: 1534–1551 [Google Scholar]
- Omidbakhshfard MA, Proost S, Fujikura U, Mueller-Roeber B (2015) Growth-regulating factors (GRFs): A small transcription factor family with important functions in plant biology. Mol Plant 8: 998–1010 [DOI] [PubMed] [Google Scholar]
- Phan TTT, Ishibashi Y, Miyazaki M, Tran HT, Okamura K, Tanaka S, Nakamura J, Yuasa T, Iwaya-Inoue M (2013) High temperature-induced repression of the rice sucrose transporter (OsSUT1) and starch synthesis-related genes in sink and source organs at milky ripening stage causes chalky grains. J Agron Crop Sci 199: 178–188 [Google Scholar]
- Piffanelli P, Ross JH, Murphy DJ (1997) Intra- and extracellular lipid composition and associated gene expression patterns during pollen development in Brassica napus. Plant J 11: 549–562 [DOI] [PubMed] [Google Scholar]
- Prasad PVV, Djanaguiraman M (2014) Response of floret fertility and individual grain weight of wheat to high temperature stress: Sensitive stages and thresholds for temperature and duration. Funct Plant Biol 41: 1261–1269 [DOI] [PubMed] [Google Scholar]
- Rahmati Ishka M, Brown E, Weigand C, Tillett RL, Schlauch KA, Miller G, Harper JF (2018) A comparison of heat-stress transcriptome changes between wild-type Arabidopsis pollen and a heat-sensitive mutant harboring a knockout of cyclic nucleotide-gated cation channel 16 (cngc16). BMC Genomics 19: 549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Twell D, Firon N (2017) Pollen development at high temperature: From acclimation to collapse. Plant Physiol 173: 1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röckel N, Wolf S, Kost B, Rausch T, Greiner S (2008) Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J 53: 133–143 [DOI] [PubMed] [Google Scholar]
- Sakaki K, Tashiro K, Kuhara S, Mihara K (2003) Response of genes associated with mitochondrial function to mild heat stress in yeast Saccharomyces cerevisiae. J Biochem 134: 373–384 [DOI] [PubMed] [Google Scholar]
- Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N, Miyazawa Y, Takahashi H, Watanabe M, Higashitani A (2010) Auxins reverse plant male sterility caused by high temperatures. Proc Natl Acad Sci USA 107: 8569–8574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans-fernandez V, Johnson JS, Abuajamieh M, Stoakes SK, Seibert JT, Cox L, Kahl S, Elsasser TH, Ross JW, Isom SC, et al. (2015) Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol Rep 3: e12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Kamiyama M, Iwata T, Makita N, Furukawa H, Ikeda H (2006) Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Ann Bot 97: 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinski J, Scheibe R (2014) Pollen tube growth: Where does the energy come from? Plant Signal Behav 9: e977200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahmuradov IA, Gammerman AJ, Hancock JM, Bramley PM, Solovyev VV (2003) PlantProm: A database of plant promoter sequences. Nucleic Acids Res 31: 114–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KD, Nayyar H (2016) Regulatory networks in pollen development under cold stress. Front Plant Sci 7: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Nguyen CT, van Oosterom EJ, Chapman SC, Jordan DR, Hammer GL (2015) Sorghum genotypes differ in high temperature responses for seed set. Field Crops Res 171: 32–40 [Google Scholar]
- Srivastava R, Li Z, Russo G, Tang J, Bi R, Muppirala U, Chudalayandi S, Severin A, He M, Vaitkevicius SI, et al. (2018) Response to persistent ER stress in plants: A multiphasic process that transitions cells from prosurvival activities to cell death. Plant Cell 30: 1220–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TW, Fiehn O, Goodacre R, Griffin JL, et al. (2007) Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3: 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunoj VSJ, Somayanda IM, Chiluwal A, Perumal R, Prasad PVV, Jagadish SVK (2017) Resilience of pollen and post-flowering response in diverse sorghum genotypes exposed to heat stress under field conditions. Crop Sci 57: 1658–1669 [Google Scholar]
- Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, et al. (2015) STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43: D447–D452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Brefort T, Neidig N, Djamei A, Kahnt J, Vermerris W, Koenig S, Feussner K, Feussner I, Kahmann R (2014) A secreted Ustilago maydis effector promotes virulence by targeting anthocyanin biosynthesis in maize. eLife 3: e01355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang RS, Zheng JC, Jin ZQ, Zhang D, Huang H, Chen LG (2008) Possible correlation between high temperature-induced floret sterility and endogenous levels of IAA, gas and ABA in rice (Oryza sativa L.). Plant Growth Regul 54: 37–43 [Google Scholar]
- Visscher AM, Belfield EJ, Vlad D, Irani N, Moore I, Harberd NP (2015) Overexpressing the multiple-stress responsive gene at1g74450 reduces plant height and male fertility in Arabidopsis thaliana. PLoS One 10: e0140368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Schumann U, Liu Y, Prokopchuk O, Steinacker JM (2012) Heat shock protein 70 (Hsp70) inhibits oxidative phosphorylation and compensates ATP balance through enhanced glycolytic activity. J Appl Physiol (1985) 113: 1669–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinl S, Kudla J (2009) The CBL-CIPK Ca2+-decoding signaling network: Function and perspectives. New Phytol 184: 517–528 [DOI] [PubMed] [Google Scholar]
- Wolters-Arts M, Lush WM, Mariani C (1998) Lipids are required for directional pollen-tube growth. Nature 392: 818–821 [DOI] [PubMed] [Google Scholar]
- Yang H, Gu X, Ding M, Lu W, Lu D (2018) Heat stress during grain filling affects activities of enzymes involved in grain protein and starch synthesis in waxy maize. Sci Rep 8: 15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Gao XQ, Wang F, Dong Y, Li X, Zhang XS (2014) Transcriptional evidence for inferred pattern of pollen tube-stigma metabolic coupling during pollination. PLoS One 9: e107046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Shi J, Yang X (2016) Role of lipid metabolism in plant pollen exine development. Subcell Biochem 86: 315–337 [DOI] [PubMed] [Google Scholar]
- Zhang SS, Yang H, Ding L, Song ZT, Ma H, Chang F, Liu JX (2017) Tissue-specific transcriptomics reveals an important role of the unfolded protein response in maintaining fertility upon heat stress in Arabidopsis. Plant Cell 29: 1007–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Kjaer KH, Rosenqvist E, Yu X, Wu Z, Ottosen CO (2017) Physiological response to heat stress during seedling and anthesis stage in tomato genotypes differing in heat tolerance. J Agron Crop Sci 203: 68–80 [Google Scholar]
- Zhu L, Chu LC, Liang Y, Zhang XQ, Chen LQ, Ye D (2018) The Arabidopsis CrRLK1L protein kinases BUPS1 and BUPS2 are required for normal growth of pollen tubes in the pistil. Plant J 95: 474–486 [DOI] [PubMed] [Google Scholar]