Abstract
Molecular mechanisms controlling the thermal response in Arabidopsis.
A severe consequence of climate change is global warming with heat waves occurring across the world, including in many important agricultural production areas (Lobell et al., 2011; Sun et al., 2014; Christidis et al., 2015; Lesk et al., 2016). This phenomenon leads to a significant drop in crop yield and quality and to a shift in the distribution of native and invasive plant species (Challinor et al., 2014; Merow et al., 2017).
As sessile organisms, plants possess complex mechanisms that help them cope with even the slightest increase in temperature. A large number of studies explored the response and acclimation to heat shock that occurs at, for plants, potentially lethal high temperatures (Driedonks et al., 2015; Bäurle, 2016; Ohama et al., 2017). For Arabidopsis (Arabidopsis thaliana), a sudden heat shock for a short time without previous heat acclimation at temperatures higher than 40°C may lead to plant death (Yeh et al., 2012). However, acquired thermotolerance from a previous exposure to moderate heat (less than 37°C) can increase the chance to survive (Larkindale and Vierling, 2008; Finka et al., 2012; Yeh et al., 2012; Sedaghatmehr et al., 2016; Ling et al., 2018). In contrast, a mildly increased ambient temperature, typically up to 27°C to 32°C (referred to as high [ambient] temperature in the context of this review) for Arabidopsis, causes numerous significant changes in plant architecture and the transition between developmental stages without resulting in plant death (Fig. 1; Quint et al., 2016; Casal and Balasubramanian, 2019; Jin and Zhu, 2019). The response to high ambient temperature is controlled by distinct mechanisms with limited overlap in response to extreme heat (Quint et al., 2016).
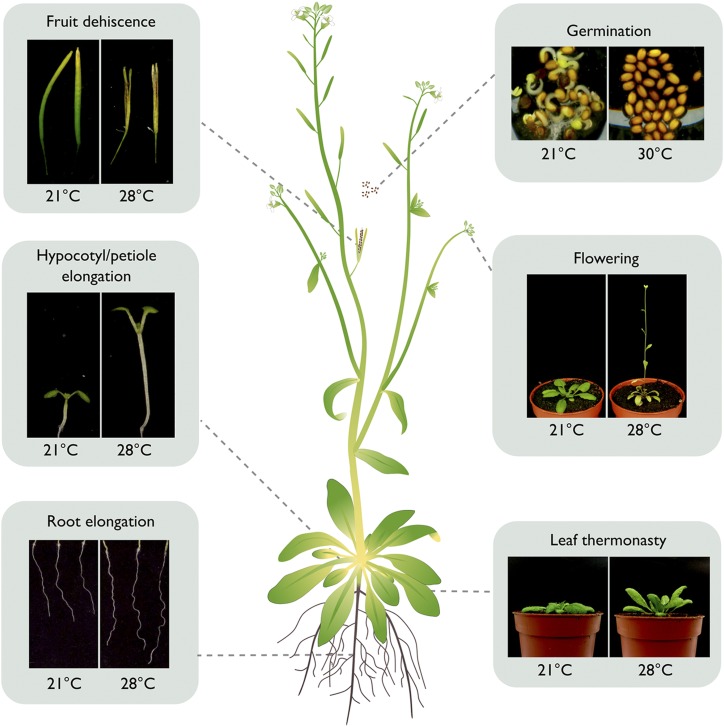
Figure 1.
High ambient temperature has effects on plant architecture and development. Representative images are shown for the indicated phenotypes of organs and processes at optimal (21°C) and high (28°C and 30°C) temperatures.
In this review, we assess thermal responses during plant growth at different developmental stages, including seed germination, root and shoot thermomorphogenesis (the effect of high ambient temperature on the organization and shape of the plant body; Quint et al., 2016; Casal and Balasubramanian, 2019), floral transition, and fruit dehiscence, and the underlying molecular mechanisms (e.g. transcriptional, posttranscriptional, and protein levels), mainly focusing on knowledge gained from the model plant Arabidopsis. High-temperature effects on other processes, such as metabolic pathways and redox homeostasis, have been reviewed elsewhere (Salvucci and Crafts-Brandner, 2004; de Pinto et al., 2015; Suzuki and Katano, 2018; Wang et al., 2018).
HIGH TEMPERATURE INHIBITS SEED GERMINATION
Germinating under favorable seasonal conditions can increase the survival chance and the fitness of young seedlings (Finch-Savage and Leubner-Metzger, 2006). Temperature is an important factor, and high temperature has an inhibitory effect on seed germination, which is termed thermoinhibition (Hills et al., 2003; Fig. 1). For Arabidopsis, seed germination is reduced by ∼40% at 32°C compared with 21°C (Chiu et al., 2016). Abscisic acid (ABA) and gibberellic acid (GA) act antagonistically to regulate seed germination, in which GAs are promoting factors (Debeaujon and Koornneef, 2000). High temperature promotes ABA biosynthesis (Toh et al., 2008). Increased ABA levels dampen protein ubiquitination and proteasome activity, which is required to degrade seed dormancy-promoting proteins, such as DELLA proteins (negative regulators of GA signaling), ABA INSENSITIVE3 (ABI3), and ABI5 (Chiu et al., 2016). ABI3, ABI5, and DELLAs induce the expression of high temperature-responsive genes in the seeds, such as SOMNUS (SOM; Lim et al., 2013). Subsequently, SOM promotes the expression of ABA biosynthesis genes and decreases the expression GA biosynthesis genes (Toh et al., 2008; Lim et al., 2013; Chiu et al., 2016). However, the epigenetic factor POWERDRESS (PWR) interacts with ABI3 and negatively controls SOM expression to control seed germination at high temperature (Yang et al., 2019). Another thermoinhibitory pathway is regulated by DELAY OF GERMINATION1 (DOG1). DOG1 inhibits GA metabolism in seeds in a temperature-dependent manner (Graeber et al., 2014), and inhibition of DOG1 expression allows seeds to germinate at 32°C (Huo et al., 2016).
After a period of exposure to high temperature, the accumulation of PHYTOCHROME INTERACTING FACTOR3-LIKE5 (PIL5)/PHYTOCHROME-INTERACTING FACTOR1 (PIF1), a negative regulator of seed germination, leads to a secondary dormancy at conditions that are normally permissive for germination (Martel et al., 2018). Interestingly, when seeds are transferred back to light and 22°C after a preincubation at 32°C in the dark, PIL5 accumulation is reduced by an increased expression of PHYTOCHROME D (PHYD). phyD, together with other phytochromes, marks PIL5 for degradation via phosphorylation and relieves the repression of seed germination.
In summary, high ambient temperature inhibits seed germination mainly by up-regulating ABA signaling and reducing GA signaling. Furthermore, high temperature-induced reactive oxygen species generation by mitochondrial small heat shock proteins also plays a role (Ma et al., 2019).
PHYTOHORMONES REGULATE THERMOMORPHOGENIC GROWTH OF SEEDLINGS AT HIGH TEMPERATURE
At high temperature, the elongation of the hypocotyl and the upward bending of the leaves in seedlings (or thermonastic movement) gives plants an advantage to maximize cooling capacity by moving away from heat-absorbing soil and having better access to cooling air streams (Fig. 1; Gray et al., 1998; Crawford et al., 2012; Bridge et al., 2013; Quint et al., 2016; Park et al., 2019). The phytohormone auxin contributes to this response by inducing the expression of AUXIN RESPONSE FACTORs, which activate cell expansion-promoting genes in the hypocotyl epidermis (Reed et al., 2018). In addition, auxin signaling is enhanced by stabilization of the major auxin receptor TRANSPORT INHIBITOR RESPONSE1 (TIR1) at increased temperature (Wang et al., 2016) and by alterations in the auxin transport system (Hanzawa et al., 2013).
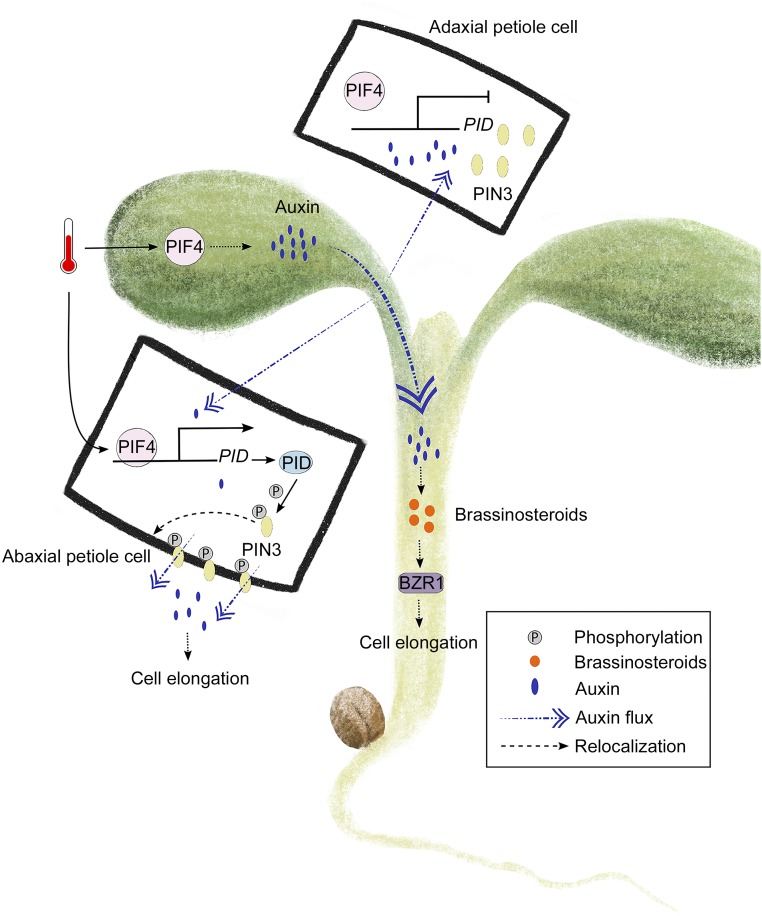
The transcription factor PIF4, a central regulator of many high temperature-related responses in Arabidopsis, promotes auxin biosynthesis in cotyledons and leaf blades at high temperature (Franklin et al., 2011; Sun et al., 2012; Bellstaedt et al., 2019). Auxin then travels from cotyledons to the hypocotyl, where it induces growth-promoting brassinosteroid (BR) biosynthesis and signaling (Fig. 2; Ibañez et al., 2018; Bellstaedt et al., 2019). Furthermore, auxin biosynthesis and redistribution play similar roles in the regulation of thermonastic leaf movement (Park et al., 2019). Excision of cotyledon or leaf blades abolishes thermoresponsive cell elongation in the abaxial petiole zone. Indeed, inhibiting PIN-FORMED (PIN)-mediated auxin redistribution leads to a significant reduction in thermonastic petiole movement. In addition, the expression of PINOID (PID), encoding a protein kinase that controls phosphorylation-mediated PIN polarization to regulate polar auxin transport, is promoted by PIF4 at high temperature (Fig. 2; Park et al., 2019).
Figure 2.
Organ-specific perception of high temperature and subsequent auxin distribution and downstream signaling. At high temperature, auxin moves from the leaf blade through the petiole toward the hypocotyl. In the latter organ, auxin promotes BR-mediated growth. In the petiole, PIF4 controls PID expression, which impacts phosphorylation-mediated PIN3 localization to control auxin efflux and cell elongation.
The cross talk of auxin and BR signaling plays an important role in the regulation of plant growth and development (Tian et al., 2018). The transcription factor BZR1 (BRASSINAZOLE RESISTANT1), a positive regulator of BR signaling, AUXIN RESPONSE FACTOR6, and PIF4 interact with each other and compose the "BAP" module that promotes hypocotyl elongation in response to diverse environmental stimuli (Oh et al., 2014). Especially, PIF4 and BZR1 form a complex and regulate the expression of common target genes interdependently, including those required for thermomorphogenesis (Oh et al., 2012). High temperature promotes this interaction by translocating BZR1 to the nucleus in a BR-dependent manner (Ibañez et al., 2018). Besides, at high temperature, BR also promotes PIF4 expression via BZR1 activation (Ibañez et al., 2018). Remarkably, in plants lacking auxin biosynthesis and signaling components, a defect in thermomorphogenic elongation growth can be rescued by exogenous BR treatment (Ibañez et al., 2018). However, this defect cannot be rescued by auxin treatment if the plants lack BR biosynthesis and signaling components. This indicates that BR acts downstream of auxin and promotes PIF4 expression via BZR1 to generate a signaling amplification loop to promote hypocotyl growth under extended exposure to high temperature (Fig. 2). In contrast, FLOWERING CONTROL LOCUS A attenuates PIF4 activity by triggering the dissociation with, for example, the YUCCA8 promoter, especially under long-term exposure to high temperature (Lee et al., 2014).
PIF4 also binds to the BR-responsive transcription factor BRI1-EMS-SUPPRESSOR1 (BES1) and modifies the DNA-binding specificity and transcriptional activity of BES1 (Martínez et al., 2018). The BES1 homodimer represses the expression of BR biosynthesis genes, while the PIF4-BES1 complex coactivates BR biosynthesis, which is essential for thermomorphogenic growth. The formation of this complex depends on the availability of PIF4 and BES1 (Martínez et al., 2018). Furthermore, PIF4 has been shown to be phosphorylated by the BR signaling repressor BRASSINOSTEROID-INSENSITIVE2 to control its stability and repress diurnal hypocotyl growth (Bernardo-García et al., 2014). It would be interesting to investigate whether thermomorphogenesis is also regulated by phytohormone-controlled posttranslational modifications of PIF4.
In Arabidopsis, expression of the GA oxidases GA20ox1 and GA3ox1, major GA biosynthetic genes, is highly up-regulated in plants grown at 29°C compared with plants grown at 20°C, whereas the GA catabolism gene GA2ox1 is down-regulated (Stavang et al., 2009). The resulting increased level of GA induces the degradation of DELLA proteins, such as REPRESSOR OF GA, which inhibits the transcriptional activity of "BAP" module components and mediates proteasomal degradation of PIF4 to suppress elongation growth (de Lucas et al., 2008; Feng et al., 2008; Li et al., 2012, 2016; Oh et al., 2014). Furthermore, the reduction of temperature-induced hypocotyl elongation in the GA biosynthesis mutant gai-1D can be rescued by treatment with the bioactive BR epibrassinolide, indicating that GA also regulates thermomorphogenesis via BR signaling pathways (Ibañez et al., 2018).
THERMOMORPHOGENIC GROWTH INTERSECTS WITH LIGHT SIGNALING
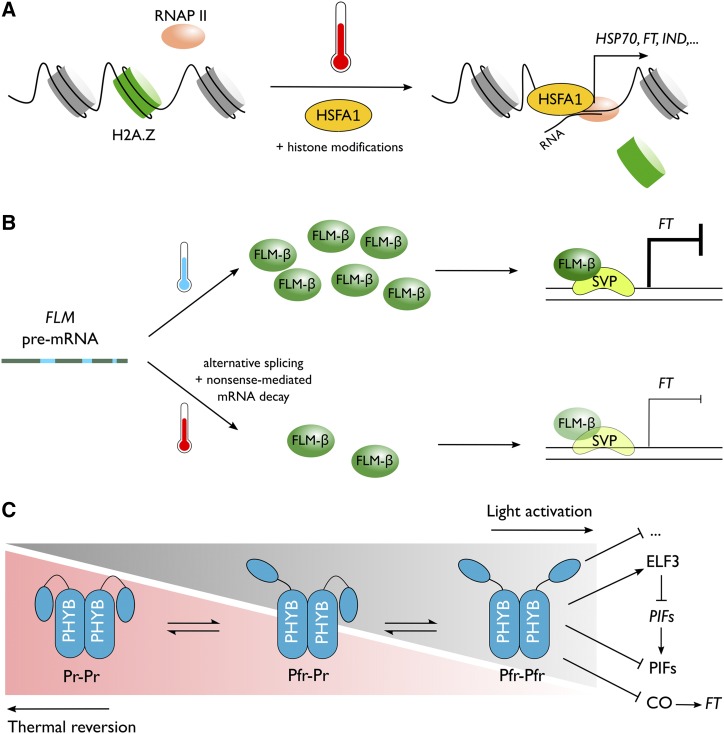
Light and temperature share a large set of common regulators, including PIF proteins, and likely act interdependently to control plant growth (Legris et al., 2017). Induction of hypocotyl elongation by PIFs is antagonized by red light-sensing phytochromes, which promote photomorphogenesis (Zhu et al., 2000; Shen et al., 2008). High temperature spontaneously reverts the light-activated Pfr form of phytochrome B (phyB) to the inactive Pr form in a light-independent process called thermal reversion or dark reversion (Fig. 3C; Sweere, 2001; Klose et al., 2015; Legris et al., 2016). Alteration in the activity of phytochromes affects thermoresponsive elongation growth in Arabidopsis (Jung et al., 2016). Therefore, phyB, and conceivably other phytochromes, have been put forward as major sensors in Arabidopsis that integrate both light and temperature cues (Jung et al., 2016; Legris et al., 2016; Vu et al., 2019).
Figure 3.
Molecular regulation of thermomorphogenesis. A, Transcriptional regulation by chromatin remodeling. B, Posttranscriptional regulation by alternative splicing. C, Posttranslational regulation by thermal reversion of photoreceptors.
Similar to phyB, in Marchantia polymorpha, high temperature shortens the lifetime of photoactivated PHOTOTROPIN (PHOT), a homolog of the Arabidopsis blue light receptors phot1 and phot2 (Fujii et al., 2017). This leads to inactivation of the PHOT kinase domain and repositioning of the chloroplasts, establishing its role as a thermosensor in M. polymorpha (Fujii et al., 2017; Vu et al., 2019). However, as of yet, there is no evidence showing whether phototropins also play a role in thermosensing and thermomorphogenesis in Arabidopsis. In addition, blue light represses thermomorphogenesis via its receptor CRYPTOCHROME1 (CRY1; Ma et al., 2016). CRY1 interacts physically with PIF4 and binds to the promoter of PIF4 targets to repress PIF4 transcriptional activity without affecting PIF4 affinity to DNA. The repressive activity of CRY1 is enhanced by both blue light and high temperature. Similarly, temperature-dependent PIF4 activity is also repressed by UVB-RESISTANCE8 (UVR8) under UV light (Hayes et al., 2017). However, unlike CRY1, UVR8 does not interact directly with PIF4. It was proposed that UVB-dependent inhibition of thermomorphogenesis might be mediated by the UVR8-promoted stabilization of LONG HYPOCOTYL IN FAR-RED1, which represses PIF4 activity via heterodimer formation (Hornitschek et al., 2009; Hayes et al., 2017). Between 20°C and 30°C, the inversion of the active UVR8 monomer to the inactive homodimer is more rapid than at lower temperature (5°C–10°C; Findlay and Jenkins, 2016). It remains to be investigated how thermosensing information is transduced to the dimerization of cryptochromes and UVR8 or whether temperature modulates their activity via an alternative pathway.
In addition to photoreceptors, other key components of light signaling pathways, such as DE-ETIOLATED1 (DET1) and the E3 ubiquitin ligase CONSTITUTIVE PHOTOMORPHOGENESIS1 (COP1), also play important roles in thermomorphogenesis, possibly by promoting PIF4 expression and stabilizing the PIF4 protein via yet unknown mechanisms (Delker et al., 2014; Gangappa and Kumar, 2017). Upon light perception, phyB and CRY1 suppress COP1 activity to promote photomorphogenesis (Sheerin et al., 2015; Holtkotte et al., 2017). The transcriptional repressor LONG HYPOCOTYL5, which is a downstream target of DET1 and COP1, modulates PIF4 transcriptional activity, not by repressing PIF4 expression but rather by competitive binding to the promoter of common downstream targets (Fig. 4; Gangappa and Kumar, 2017; Park et al., 2017).
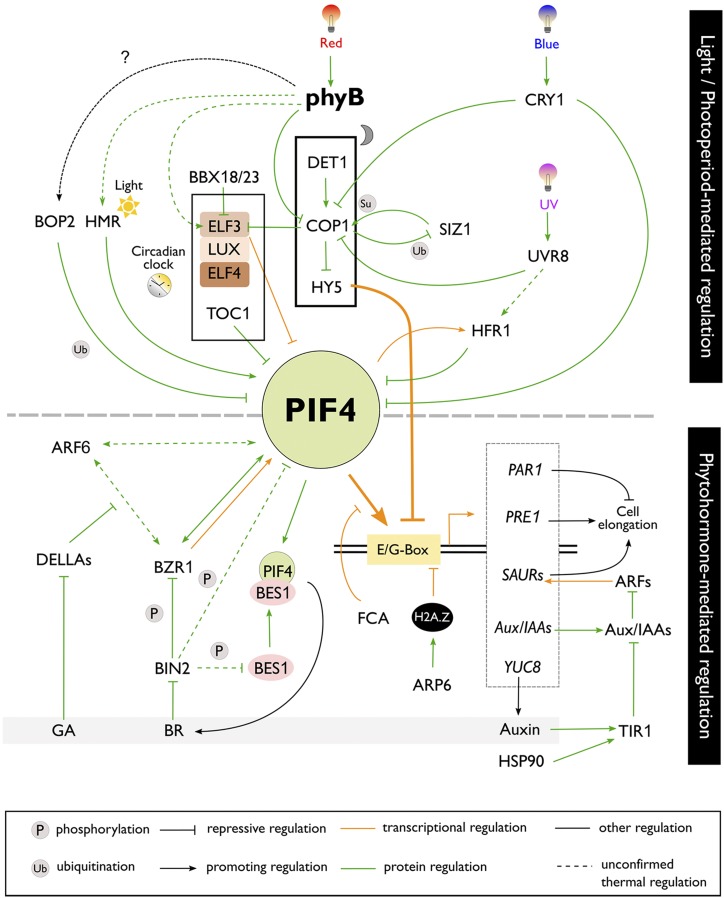
Figure 4.
Light/photoperiod- and phytohormone-dependent thermoresponsive pathways converge at PIF4. Here, light and circadian clock components are integrated with temperature information to mediate thermoresponsive growth, which is controlled by phytohormone signaling. The thermosensory machinery is mainly controlled by phyB, while the roles of other light receptors are still largely elusive. PIF4 mainly mediates thermomorphogenesis by promoting auxin biosynthesis and signaling, while BR signaling plays an important downstream function. For further details on the components of these pathways, see the main text.
The CULLIN3-BLADE-ON-PETIOLE1 (BOP1)/BOP2 E3 ligase complex acts in the phyB signaling pathway to suppress elongation growth under red light by decreasing PIF4 levels following polyubiquitination (Zhang et al., 2017). Loss-of-function mutants of BOP2 and its homolog BOP1 show no difference in elongation growth at 22°C but display a significantly longer hypocotyl at 28°C, indicating their role in modulating thermomorphogenesis. Astoundingly, while light enhances BOP2 activity, BOP2 can still ubiquitinate PIF4 in the dark (Zhang et al., 2017). This suggests that phyB-mediated phosphorylation of PIF4 is not required for its ubiquitination by BOP2. Whether BOP2 modulates PIF4 during thermomorphogenesis by a phyB-independent mechanism or phyB controls BOP2 activity in an alternative pathway remains to be investigated.
LIGHT SIGNALING AND CIRCADIAN COMPONENTS COORDINATE TO CONTROL THERMOMORPHOGENIC GROWTH
Physiological and morphological responses to external stimuli are often the result of the interaction between the circadian clock components and the environmental input (Hotta et al., 2007). Overexpression of the circadian clock gene TIMING OF CAB EXPRESSION1 suppresses thermomorphogenic growth (Zhu et al., 2016). Conversely, the loss-of-function toc1-2 mutant shows hypersensitivity to high temperature, which can be suppressed in the presence of the pif4 mutation. Indeed, TIMING OF CAB EXPRESSION1 functions upstream of PIF4 and transmits circadian information into PIF4-mediated thermal responses by binding to PIF4 and inhibiting PIF4 transcriptional activity.
Another clock component, EARLY FLOWERING3 (ELF3), a subunit of the clock-regulating Evening Complex (EC; Fig. 4), also regulates thermomorphogenesis via PIF4 (Box et al., 2015; Raschke et al., 2015). At 22°C, ELF3 directly binds to the PIF4 promoter and represses PIF4 transcription. This repression can be reversed when the temperature increases to 27°C. Furthermore, ELF3 can bind directly to the PIF4 protein independently from the EC to suppress PIF4 activity (Nieto et al., 2015). During thermomorphogenesis, ELF3 accumulation is partially negatively regulated by COP1 and by its interaction with two B-box domain (BBX) proteins, BBX18 and BBX23 (Ding et al., 2018). Overexpression of BBX18 increases thermomorphogenic response in the wild type but not in pif4. Transcriptional targets of BBX18, BBX23, and PIF4 show a large overlap, further demonstrating the role of BBX18 and BBX23 in thermomorphogenesis. Altogether, this indicates that BBX18 and BBX23 mediate thermomorphogenesis via the ELF3-PIF4 pathway.
Transcriptomic and chromatin immunoprecipitation data indicate that the EC can directly bind to promoters and regulate the expression of a number of target genes at night in a thermoresponsive manner independently from PIF4 and phyB (Ezer et al., 2017). Conversely, phyB and the EC also share a significant overlap of thermoresponsive binding loci. Furthermore, hypocotyl elongation caused by loss of functional ELF3 is completely abolished by overexpression of PHYB. These observations indicate that a large portion of light and temperature information may be transmitted to the circadian EC via phyB. Indeed, ELF3 interacts with phyB-Pfr directly, and this leads to the accumulation of ELF3 in the light (Liu et al., 2001; Nieto et al., 2015). Under short-day conditions, a large portion of misregulated thermoresponsive transcripts in phytochrome mutants occurs at night, leading to the previous belief that phyB mainly regulates thermal response during the night (Jung et al., 2016). However, phyB also senses temperature and controls thermomorphogenesis during the daytime via a distinct pathway composed of HEMERA (HMR) and PIF4 (Fig. 4; Qiu et al., 2019). In the light, HMR interacts with photoactivated phyB and mediates the formation of phyB nuclear bodies to initiate photomorphogenesis (Chen et al., 2010). Under long-day or continuous red light conditions, HMR interacts with PIF4 to activate thermoresponsive genes by increasing PIF4 protein accumulation in warm temperatures (Qiu et al., 2019). In contrast, HMR function only plays a minor role under short-day conditions. Interestingly, during photomorphogenesis, HMR accumulates by interacting with the Pfr form of phyB (Galvão et al., 2012). Since high temperature decreases Pfr abundance, phyB should regulate the HMR-PIF4 interaction via an unknown mechanism during thermomorphogenesis in the light.
CHROMATIN REMODELING REGULATES THERMOMORPHOGENIC GROWTH
Dynamic nucleosome positioning is an important regulatory mechanism of gene transcription (Bai and Morozov, 2010). At low temperature, the H2A histone variant H2A.Z is found to be enriched at many heat-inducible genes (Kumar and Wigge, 2010; Cortijo et al., 2017; Kumar, 2018). At temperatures higher than 22°C, H2A.Z occupation is reduced and chromatin accessibility for RNA polymerase II is increased, which allows higher transcription of thermoresponsive genes (Fig. 3A; Kumar and Wigge, 2010). Expectedly, loss-of-function mutants in ACTIN-RELATED PROTEIN6 (ARP6), which is required for proper H2A.Z deposition, display a constitutive high-temperature phenotype, including a long hypocotyl (Choi et al., 2005; Deal et al., 2005, 2007; Kumar and Wigge, 2010). However, the depletion of H2A.Z is not directly affected by high temperature but is rather mediated by the recruitment of transcription factors, such as those of the HEAT SHOCK FACTOR A1 family (Cortijo et al., 2017). Furthermore, H3 deacetylation, which is controlled by PWR and HISTONE DEACETYLASE9, regulates genes that are also regulated by H2A.Z dynamics (Tasset et al., 2018). Loss-of-function pwr mutants are suppressed in hypocotyl growth, largely by attenuating the expression of PIF4 and auxin biosynthesis.
HIGH TEMPERATURE AFFECTS FLORAL TRANSITION
Seasonal cues such as daylength, light quality, and temperature control the transition from vegetative to reproductive growth (Amasino, 2010; Capovilla et al., 2015; Song et al., 2015). A mild increase in temperature can trigger floral transition in Arabidopsis under noninductive short-day conditions (Fig. 1; Balasubramanian et al., 2006). This is mainly attributed to an increase in expression of the major flowering-promoting genes FLOWERING LOCUS T (FT), TWIN SISTER OF FT, and SUPPRESSOR OF OVEREXPRESSION OF CO1 (Blázquez et al., 2003; Balasubramanian et al., 2006; Seo et al., 2009; Lee et al., 2013).
Similar to thermomorphogenic elongation growth, light receptors also play an important role in thermoresponsive flowering. In Arabidopsis, phyB and CRY2 negatively and positively regulate thermal flower transition, respectively (Blázquez et al., 2003; Halliday et al., 2003). Under long-day conditions, phyB destabilizes CONSTANS (CO), which induces FT expression to promote photoperiodic flowering, while cryptochromes and phyA enhance CO stability (Valverde et al., 2004). It will be interesting to investigate whether light receptors couple photoperiodic flowering with thermoresponsive flowering via known photoperiodic pathways (Kaiserli et al., 2015; Zhang et al., 2018).
Chromatin remodeling at H2A.Z also plays a role in the regulation of temperature-dependent flowering (Kumar et al., 2012; Tasset et al., 2018). It was previously suggested that an increase in temperature would reduce the occupancy of H2A.Z at the FT locus, leading to a higher enrichment of PIF4 at the FT locus and promoting FT expression (Kumar et al., 2012). However, in more recent studies, the difference in the flowering phenotype of pif4 plants and the wild type at high temperature appeared negligible in both short- and long-day conditions (Galvão et al., 2015; Fernández et al., 2016). Conversely, arp6 and pwr mutants show early- and late-flowering phenotypes, respectively, constitutively at both low and high temperature (Kumar and Wigge, 2010; Tasset et al., 2018). Therefore, in contrast to hypocotyl growth, thermal induction of flowering mediated by H2A.Z depletion requires an unknown transcriptional regulator independent from PIF4. For example, GA-regulated temperature-mediated flowering independently of FT and PIFs might play a crucial role (Galvão et al., 2015).
Another important regulator of gene expression in response to environmental stimuli is alternative splicing that results in different protein isoforms (Syed et al., 2012). For example, thermally primed splicing memory can lead to a higher survival chance in Arabidopsis during heat stress (Ling et al., 2018). It was previously suggested that the flowering regulator FLOWERING LOCUS M (FLM) is targeted by temperature-dependent alternative splicing, which results in two main spliced forms, FLM-β and FLM-δ, that control flowering (Posé et al., 2013). However, in many Arabidopsis accessions, temperature-sensitive flowering can be largely explained by altered accumulation of the flowering-repressive FLM-β isoform, whereas FLM-δ does not play a significant role (Fig. 3B; Lutz et al., 2015, 2017). The level of nonsense FLM transcripts is increased at high temperature and leads to nonsense-mediated mRNA decay of FLM, which also compromises the level of the functional FLM-β transcripts (Sureshkumar et al., 2016). Furthermore, SHORT VEGETATIVE PHASE, which promotes FT expression and is inhibited by interaction with FLM-β, is itself destabilized at high temperature (Lee et al., 2013). The expression of other related flowering genes in the FLM clade is also affected by temperature-controlled splicing (Airoldi et al., 2015; Verhage et al., 2017). Surprisingly, the splicing machineries themselves are targeted by temperature-induced alternative splicing. Hence, it is suggested that this may serve as the earlier step of a temperature-dependent mechanism that mediates altered splicing of other downstream thermoresponsive genes (Verhage et al., 2017). Furthermore, similar to light-responsive alternative splicing (Shikata et al., 2014) and alternative promoter selection (Ushijima et al., 2017), it is likely that phytochromes also affect high temperature-dependent generation of protein isoforms.
MicroRNAs serve as another regulatory checkpoint of transcriptional machineries controlling FT expression at high temperature (Lee et al., 2010). High temperature induces the expression of miR172, which targets the mRNA of a subset of APETALA2 flowering repressors (Lee et al., 2010; Zhu and Helliwell, 2011). Conversely, an increased temperature from 15°C to 21°C to 27°C reduces the level of miR169, which targets the mRNAs of JASMONATE-ZIM-DOMAIN PROTEIN4 and the gene encoding NF-YA2, a subunit of the transcriptional complex Nuclear Factor Y (NF-Y), which both positively affect the expression of FT (Gyula et al., 2018). In addition to microRNAs, thermoregulation of floral transition by long noncoding RNAs also occurs. For example, FLOWERING LONG INTERGENIC NON CODING RNA (FLINC), a long intergenic noncoding RNA, is down-regulated at 25°C compared with 16°C (Severing et al., 2018). Knockout mutants of FLINC have higher FT expression and flower earlier than wild-type plants, while overexpression of FLINC delays flowering. However, the molecular mechanisms underlying FLINC activity remain unexplored.
It is clear that Arabidopsis flowering is regulated by temperature at multiple checkpoints. In agreement with this, a recent mathematical model has predicted multiple thermosensing inputs for FLC expression during vernalization, and they highly depend on the cold duration as well as spikes of warm temperature (Antoniou-Kourounioti et al., 2018). Similarly, in silico simulations also show different thermosensitivities for FT expression (Kinmonth-Schultz et al., 2019). This suggests that thermosensory information controlling a specific thermal response is highly variable and depends largely on growth conditions and length of thermal exposure, instead of being generated by only a few core thermosensors (Antoniou-Kourounioti et al., 2018; Vu et al., 2019).
FRUIT DEHISCENCE IS PROMOTED BY HIGH TEMPERATURE
In several plant species, including Brassicaceae species, hydrolytic enzymes decompose the cell wall during ripening, promoting cell separation precisely at an abscission cell layer between the fruit valve and the replum (called fruit dehiscence) and leading to the release and dispersal of the fruit content (Spence et al., 1996). In agriculture, however, early seed dispersal by fruit dehiscence is an undesirable trait that significantly reduces crop yield (Price et al., 1996).
In Arabidopsis, fruit dehiscence is accelerated by high ambient temperature (Fig. 1; Li et al., 2018). INDEHISCENT (IND), a transcription factor specifically expressed at the fruit valve-replum junction, positively regulates fruit opening (Liljegren et al., 2004; Ogawa et al., 2009). Expression of IND is significantly increased at 27°C compared with 17°C, seemingly independently from its upstream transcriptional regulators (Li et al., 2018). Intriguingly, the thermal induction of IND expression is promoted by the eviction of histone H2A.Z from its promoter (Li et al., 2018). As expected, early dehiscence could also be observed in the loss-of-function arp6 mutants (Li et al., 2018). This also suggests that transcriptional regulation by chromatin dynamics is conserved across different processes and developmental stages, from general thermal responsive factors (such as HEAT SHOCK PROTEIN70; Kumar and Wigge, 2010) to flowering genes (such as FT; Kumar et al., 2012) and fruit dehiscence regulators (such as IND; Li et al., 2018).
FEELING THE HEAT UNDERGROUND: ROOT THERMOMORPHOGENESIS
Increased temperature can affect root growth, either directly or indirectly via signaling of the shoot for water and nutrient demand or via carbon supply from the shoot to the root (Heckathorn et al., 2013). However, it was recently shown that the primary root can autonomously sense and respond to high temperature (Bellstaedt et al., 2019). While the associated molecular mechanism is largely unknown, the stability of the auxin receptor TIR1, for example, is positively regulated by the heat shock protein HEAT SHOCK PROTEIN 90 at high temperature (29°C; Fig. 4). This boosts auxin signaling at high temperature and enhances primary root growth and lateral root formation (Wang et al., 2016). Furthermore, temperature-dependent root growth is regulated by intracellular distribution of auxin (Feraru et al., 2019). At high temperature (29°C), the intracellular auxin carrier PIN-LIKES6 is less stable in the root meristem (Feraru et al., 2019). This increases nuclear distribution of auxin and auxin signaling in the root meristem, which contributes to promotion of root growth at high temperature.
BRs also play a role in root responses to high temperature, but independently of auxin or players that regulate the shoot thermomorphogenesis response. Elevated ambient temperature reduces the level of the BR receptor BRI1, likely by controlling BRI1 ubiquitination status, to negatively regulate BR signaling and root growth (Martins et al., 2017).
Of note, while root growth is unquestionably affected by increased temperature (Fig. 1), root thermomorphogenesis is not consistently observed in different species or experimental setups (Gladish and Rost, 1993; Seiler, 1998; Martins et al., 2017). For example, in contrast to the observations mentioned above in Arabidopsis, both lateral and primary root growth of garden pea (Pisum sativum) are inhibited by 32°C (Gladish and Rost, 1993).
TEMPERATURE COORDINATES THE THERMOMORPHOGENIC GROWTH AND PLANT IMMUNITY TRADEOFF
Plants have developed two different lines of defense against pathogen infection (Bigeard et al., 2015). First, plants can perceive pathogens by recognition of microbe-associated molecular patterns by pattern-recognition receptors, inducing pattern-triggered immunity. Second, effector molecules synthesized by pathogens can be injected into the plant cells to weaken the cell for further infection and induce effector-triggered immunity (ETI). Elevated temperature increases susceptibility toward infection in Arabidopsis and other plant species, mainly via inhibition of ETI (Cheng et al., 2013). The reduced ETI response is also observed for the constitutively thermoresponsive arp6 mutants. Furthermore, the biosynthesis and accumulation of salicylic acid, which is pivotal for defense against pathogens, are suppressed at high temperature, enhancing pathogenesis (Huot et al., 2017).
As a result of infection, at optimal growth temperature, plant growth is repressed in favor of defense against pathogens (Alcázar and Parker, 2011; Wang and Wang, 2014). However, high temperature is inhibitory to immunity and promotes growth (Alcázar and Parker, 2011). For example, growth defects and autoimmunity in snc1-1, a gain-of-function mutant in SUPPRESSOR OF npr1-1, CONSTITUTIVE1 (SNC1), are abolished at 28°C (Zhu et al., 2010). Temperature induces the expression of HOPZ‐ETI‐DEFICIENT1 (ZED1)‐RELATED KINASES and TEOSINTE BRANCHED1, CYCLOIDEA, AND PROLIFERATING CELL FACTOR transcription factors (Wang et al., 2017, 2019). ZED1 and ZED1‐RELATED KINASES interact with TEOSINTE BRANCHED1, CYCLOIDEA, AND PROLIFERATING CELL FACTORs and repress SNC1 expression to inhibit immunity at high temperature (Wang et al., 2017, 2019).
Interestingly, the DET1-COP1-PIF4 module integrates seasonal cues, such as light and temperature, to coordinate immunity and growth (Gangappa et al., 2017; Gangappa and Kumar, 2018). The loss-of-function pif4 mutant shows an increase in transcription of pathogenesis-related genes. Expectedly, enhancing PIF4 signaling via DET1 and COP1 activity increased susceptibility to infection even at low temperature (Gangappa et al., 2017; Gangappa and Kumar, 2018). In addition, by physically connecting COP1 and small ubiquitin-related modifier (SUMO) conjugation activity in nuclear bodies, the SUMO E3 ligase SAP and Miz1 (SIZ1) acts as a positive regulator of thermomorphogenesis (Hammoudi et al., 2018; Mazur et al., 2019). At the same time, SIZ1 negatively regulates the immune response at high temperature. Hereby, SIZ1 acts upstream of PIF4 and BZR1 via enhancement of COP1 activity by sumoylation. Therefore, SIZ1 is another hub for the temperature-dependent tradeoff between growth and immunity.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Molecular mechanisms that control a multitude of high temperature-mediated effects on plant growth and development are complex, including extensive cross talk with other pathways, such as hormone regulation and light signaling. PIF4 is considered as the central regulator of thermal response, with the majority of described thermoresponsive pathways converging at PIF4 (Fig. 4). Hereby, the effects of auxin, BRs, and GAs have been extensively explored (Stavang et al., 2009; Galvão et al., 2015; Martins et al., 2017; Ibañez et al., 2018; Martínez et al., 2018), and links between the phyB-PIF4 module with ABA, ethylene, and jasmonate have emerged in other biological contexts (Sakuraba et al., 2014; Song et al., 2014; Campos et al., 2016; Jin et al., 2018). It is furthermore important to note that depending on the developmental process, plant organ, or tissue, high temperature can lead, for example, to increased auxin levels, such as during hypocotyl elongation (Franklin et al., 2011; Sun et al., 2012), or decreased auxin levels, such as in Arabidopsis anthers resulting in male sterility or during apical hook opening in the dark (Sakata et al., 2010; Jin et al., 2018). This suggests that high-temperature responses are highly regulated, even on the level of the same set of temperature signaling molecules. There are thus many open questions that need to be tackled in the future (see “Outstanding Questions”).
Since a change in temperature can be detected at any location in the cell, we suspect that there are still other thermoresponsive pathways, which can act independently of phyB-PIF4, to be discovered. Nearly all analyses described above are performed in laboratory conditions, but diurnal and seasonal temperature fluctuations display specific magnitudes, rhythms, and durations, making it important to study plant developmental plasticity and the underlying molecular mechanisms in more natural conditions or even in the field (Song et al., 2018).
Here, we have mainly discussed the molecular regulation of developmental plasticity at high temperature in the dicot model plant Arabidopsis. Thus, a big remaining question is: is all of this relevant in other species, including crops? For example, high temperature-induced hypocotyl elongation seems conserved among dicot crop species, such as cabbage (Brassica oleracea) and tomato (Solanum lycopersicum; Quint et al., 2016). However, in contrast to Arabidopsis (Capovilla et al., 2017), species such as Chrysanthemum morifolium, Populus ssp., or Boechera stricta (the perennial relative of Arabidopsis) are delayed in flowering when the ambient temperature increases (Anderson et al., 2011; Hsu et al., 2011; Nakano et al., 2013). Therefore, it is important for future molecular studies to focus on specific and relevant high temperature-associated phenotypes in the crop of interest. In this context, some components associated with high temperature were already studied in other species, including monocot crops, such as the effect of high temperature on yield and the role of H2A.Z in Brachypodium distachyon (Boden et al., 2013), the role of phyB in rice (Oryza sativa) anther development and pollen viability (Sun et al., 2017), or the role of auxin in male sterility in wheat (Triticum aestivum) caused by high temperature (Sakata et al., 2010). Nevertheless, investigating whether central high-temperature sensing and response regulators are functionally conserved in crop plants is very much needed.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Footnotes
This work was supported by the VIB International Ph.D. Scholarship in Life Sciences to L.D.V. and by a grant from the Chinese Scholarship Council to X.X.
Articles can be viewed without a subscription.
References
- Airoldi CA, McKay M, Davies B (2015) MAF2 is regulated by temperature-dependent splicing and represses flowering at low temperatures in parallel with FLM. PLoS ONE 10: e0126516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcázar R, Parker JE (2011) The impact of temperature on balancing immune responsiveness and growth in Arabidopsis. Trends Plant Sci 16: 666–675 [DOI] [PubMed] [Google Scholar]
- Amasino R. (2010) Seasonal and developmental timing of flowering. Plant J 61: 1001–1013 [DOI] [PubMed] [Google Scholar]
- Anderson JT, Lee CR, Mitchell-Olds T (2011) Life-history QTLS and natural selection on flowering time in Boechera stricta, a perennial relative of Arabidopsis. Evolution 65: 771–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou-Kourounioti RL, Hepworth J, Heckmann A, Duncan S, Qüesta J, Rosa S, Säll T, Holm S, Dean C, Howard M (2018) Temperature sensing is distributed throughout the regulatory network that controls FLC epigenetic silencing in vernalization. Cell Syst 7: 643–655.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Morozov AV (2010) Gene regulation by nucleosome positioning. Trends Genet 26: 476–483 [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Sureshkumar S, Lempe J, Weigel D (2006) Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet 2: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäurle I. (2016) Plant heat adaptation: Priming in response to heat stress. F1000 Res 5: 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellstaedt J, Trenner J, Lippmann R, Poeschl Y, Zhang X, Friml J, Quint M, Delker C (2019) A mobile auxin signal connects temperature sensing in cotyledons with growth responses in hypocotyls. Plant Physiol 180: 757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo-García S, de Lucas M, Martínez C, Espinosa-Ruiz A, Davière JM, Prat S (2014) BR-dependent phosphorylation modulates PIF4 transcriptional activity and shapes diurnal hypocotyl growth. Genes Dev 28: 1681–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigeard J, Colcombet J, Hirt H (2015) Signaling mechanisms in pattern-triggered immunity (PTI). Mol Plant 8: 521–539 [DOI] [PubMed] [Google Scholar]
- Blázquez MA, Ahn JH, Weigel D (2003) A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat Genet 33: 168–171 [DOI] [PubMed] [Google Scholar]
- Boden SA, Kavanová M, Finnegan EJ, Wigge PA (2013) Thermal stress effects on grain yield in Brachypodium distachyon occur via H2A.Z-nucleosomes. Genome Biol 14: R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box MS, Huang BE, Domijan M, Jaeger KE, Khattak AK, Yoo SJ, Sedivy EL, Jones DM, Hearn TJ, Webb AAR, et al. (2015) ELF3 controls thermoresponsive growth in Arabidopsis. Curr Biol 25: 194–199 [DOI] [PubMed] [Google Scholar]
- Bridge LJ, Franklin KA, Homer ME (2013) Impact of plant shoot architecture on leaf cooling: A coupled heat and mass transfer model. J R Soc Interface 10: 20130326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos ML, Yoshida Y, Major IT, de Oliveira Ferreira D, Weraduwage SM, Froehlich JE, Johnson BF, Kramer DM, Jander G, Sharkey TD, et al. (2016) Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat Commun 7: 12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla G, Schmid M, Posé D (2015) Control of flowering by ambient temperature. J Exp Bot 66: 59–69 [DOI] [PubMed] [Google Scholar]
- Capovilla G, Symeonidi E, Wu R, Schmid M (2017) Contribution of major FLM isoforms to temperature-dependent flowering in Arabidopsis thaliana. J Exp Bot 68: 5117–5127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Balasubramanian S (2019) Thermomorphogenesis. Annu Rev Plant Biol 70: 321–346 [DOI] [PubMed] [Google Scholar]
- Challinor AJ, Watson J, Lobell DB, Howden SM, Smith DR, Chhetri N (2014) A meta-analysis of crop yield under climate change and adaptation. Nat Clim Chang 4: 287–291 [Google Scholar]
- Chen M, Galvão RM, Li M, Burger B, Bugea J, Bolado J, Chory J (2010) Arabidopsis HEMERA/pTAC12 initiates photomorphogenesis by phytochromes. Cell 141: 1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Gao X, Feng B, Sheen J, Shan L, He P (2013) Plant immune response to pathogens differs with changing temperatures. Nat Commun 4: 2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu RS, Pan S, Zhao R, Gazzarrini S (2016) ABA-dependent inhibition of the ubiquitin proteasome system during germination at high temperature in Arabidopsis. Plant J 88: 749–761 [DOI] [PubMed] [Google Scholar]
- Choi K, Kim S, Kim SY, Kim M, Hyun Y, Lee H, Choe S, Kim SG, Michaels S, Lee I (2005) SUPPRESSOR OF FRIGIDA3 encodes a nuclear ACTIN-RELATED PROTEIN6 required for floral repression in Arabidopsis. Plant Cell 17: 2647–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christidis N, Jones GS, Stott PA (2015) Dramatically increasing chance of extremely hot summers since the 2003 European heatwave. Nat Clim Chang 5: 46–50 [Google Scholar]
- Cortijo S, Charoensawan V, Brestovitsky A, Buning R, Ravarani C, Rhodes D, van Noort J, Jaeger KE, Wigge PA (2017) Transcriptional regulation of the ambient temperature response by H2A.Z nucleosomes and HSF1 transcription factors in Arabidopsis. Mol Plant 10: 1258–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AJ, McLachlan DH, Hetherington AM, Franklin KA (2012) High temperature exposure increases plant cooling capacity. Curr Biol 22: R396–R397 [DOI] [PubMed] [Google Scholar]
- Deal RB, Kandasamy MK, McKinney EC, Meagher RB (2005) The nuclear actin-related protein ARP6 is a pleiotropic developmental regulator required for the maintenance of FLOWERING LOCUS C expression and repression of flowering in Arabidopsis. Plant Cell 17: 2633–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal RB, Topp CN, McKinney EC, Meagher RB (2007) Repression of flowering in Arabidopsis requires activation of FLOWERING LOCUS C expression by the histone variant H2A.Z. Plant Cell 19: 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Koornneef M (2000) Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol 122: 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delker C, Sonntag L, James GV, Janitza P, Ibañez C, Ziermann H, Peterson T, Denk K, Mull S, Ziegler J, et al. (2014) The DET1-COP1-HY5 pathway constitutes a multipurpose signaling module regulating plant photomorphogenesis and thermomorphogenesis. Cell Rep 9: 1983–1989 [DOI] [PubMed] [Google Scholar]
- de Lucas M, Davière JM, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blázquez MA, Titarenko E, Prat S (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451: 480–484 [DOI] [PubMed] [Google Scholar]
- de Pinto MC, Locato V, Paradiso A, De Gara L (2015) Role of redox homeostasis in thermo-tolerance under a climate change scenario. Ann Bot 116: 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Wang S, Song ZT, Jiang Y, Han JJ, Lu SJ, Li L, Liu JX (2018) Two B-box domain proteins, BBX18 and BBX23, interact with ELF3 and regulate thermomorphogenesis in Arabidopsis. Cell Rep 25: 1718–1728.e4 [DOI] [PubMed] [Google Scholar]
- Driedonks N, Xu J, Peters JL, Park S, Rieu I (2015) Multi-level interactions between heat shock factors, heat shock proteins, and the redox system regulate acclimation to heat. Front Plant Sci 6: 999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezer D, Jung JH, Lan H, Biswas S, Gregoire L, Box MS, Charoensawan V, Cortijo S, Lai X, Stöckle D, et al. (2017) The evening complex coordinates environmental and endogenous signals in Arabidopsis. Nat Plants 3: 17087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L, Yu L, Iglesias-Pedraz JM, Kircher S, et al. (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451: 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraru E, Feraru MI, Barbez E, Waidmann S, Sun L, Gaidora A, Kleine-Vehn J (2019) PILS6 is a temperature-sensitive regulator of nuclear auxin input and organ growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 116: 3893–3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández V, Takahashi Y, Le Gourrierec J, Coupland G (2016) Photoperiodic and thermosensory pathways interact through CONSTANS to promote flowering at high temperature under short days. Plant J 86: 426–440 [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytol 171: 501–523 [DOI] [PubMed] [Google Scholar]
- Findlay KMW, Jenkins GI (2016) Regulation of UVR8 photoreceptor dimer/monomer photo-equilibrium in Arabidopsis plants grown under photoperiodic conditions. Plant Cell Environ 39: 1706–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finka A, Cuendet AFH, Maathuis FJM, Saidi Y, Goloubinoff P (2012) Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24: 3333–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, Gu C, Ye S, Yu P, Breen G, Cohen JD, et al. (2011) Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci USA 108: 20231–20235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y, Tanaka H, Konno N, Ogasawara Y, Hamashima N, Tamura S, Hasegawa S, Hayasaki Y, Okajima K, Kodama Y (2017) Phototropin perceives temperature based on the lifetime of its photoactivated state. Proc Natl Acad Sci USA 114: 9206–9211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão RM, Li M, Kothadia SM, Haskel JD, Decker PV, Van Buskirk EK, Chen M (2012) Photoactivated phytochromes interact with HEMERA and promote its accumulation to establish photomorphogenesis in Arabidopsis. Genes Dev 26: 1851–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão VC, Collani S, Horrer D, Schmid M (2015) Gibberellic acid signaling is required for ambient temperature-mediated induction of flowering in Arabidopsis thaliana. Plant J 84: 949–962 [DOI] [PubMed] [Google Scholar]
- Gangappa SN, Kumar SV (2017) DET1 and HY5 control PIF4-mediated thermosensory elongation growth through distinct mechanisms. Cell Rep 18: 344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa SN, Kumar SV (2018) DET1 and COP1 modulate the coordination of growth and immunity in response to key seasonal signals in Arabidopsis. Cell Rep 25: 29–37.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa SN, Berriri S, Kumar SV (2017) PIF4 coordinates thermosensory growth and immunity in Arabidopsis. Curr Biol 27: 243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladish DK, Rost TL (1993) The effects of temperature on primary root growth dynamics and lateral root distribution in garden pea (Pisum sativum L., cv. “Alaska”). Environ Exp Bot 33: 243–258 [Google Scholar]
- Graeber K, Linkies A, Steinbrecher T, Mummenhoff K, Tarkowská D, Turečková V, Ignatz M, Sperber K, Voegele A, de Jong H, et al. (2014) DELAY OF GERMINATION 1 mediates a conserved coat-dormancy mechanism for the temperature- and gibberellin-dependent control of seed germination. Proc Natl Acad Sci USA 111: E3571–E3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M (1998) High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA 95: 7197–7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyula P, Baksa I, Tóth T, Mohorianu I, Dalmay T, Szittya G (2018) Ambient temperature regulates the expression of a small set of sRNAs influencing plant development through NF-YA2 and YUC2. Plant Cell Environ 41: 2404–2417 [DOI] [PubMed] [Google Scholar]
- Halliday KJ, Salter MG, Thingnaes E, Whitelam GC (2003) Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. Plant J 33: 875–885 [DOI] [PubMed] [Google Scholar]
- Hammoudi V, Fokkens L, Beerens B, Vlachakis G, Chatterjee S, Arroyo-Mateos M, Wackers PFK, Jonker MJ, van den Burg HA (2018) The Arabidopsis SUMO E3 ligase SIZ1 mediates the temperature dependent trade-off between plant immunity and growth. PLoS Genet 14: e1007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzawa T, Shibasaki K, Numata T, Kawamura Y, Gaude T, Rahman A (2013) Cellular auxin homeostasis under high temperature is regulated through a sorting NEXIN1-dependent endosomal trafficking pathway. Plant Cell 25: 3424–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Sharma A, Fraser DP, Trevisan M, Cragg-Barber CK, Tavridou E, Fankhauser C, Jenkins GI, Franklin KA (2017) UV-B perceived by the UVR8 photoreceptor inhibits plant thermomorphogenesis. Curr Biol 27: 120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn SA, Giri A, Mishra S, Bista D (2013) Heat stress and roots. In Climate Change and Plant Abiotic Stress Tolerance. Tuteja N, Gill SS, eds, Wiley-VCH Verlag, Weinheim, Germany, pp 109–136 [Google Scholar]
- Hills PN, van Staden J, Thomas TH (2003) Thermoinhibition of seed germination. S Afr J Bot 69: 455–461 [Google Scholar]
- Holtkotte X, Ponnu J, Ahmad M, Hoecker U (2017) The blue light-induced interaction of cryptochrome 1 with COP1 requires SPA proteins during Arabidopsis light signaling. PLoS Genet 13: e1007044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornitschek P, Lorrain S, Zoete V, Michielin O, Fankhauser C (2009) Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J 28: 3893–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta CT, Gardner MJ, Hubbard KE, Baek SJ, Dalchau N, Suhita D, Dodd AN, Webb AAR (2007) Modulation of environmental responses of plants by circadian clocks. Plant Cell Environ 30: 333–349 [DOI] [PubMed] [Google Scholar]
- Hsu CY, Adams JP, Kim H, No K, Ma C, Strauss SH, Drnevich J, Vandervelde L, Ellis JD, Rice BM, et al. (2011) FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc Natl Acad Sci USA 108: 10756–10761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo H, Wei S, Bradford KJ (2016) DELAY OF GERMINATION1 (DOG1) regulates both seed dormancy and flowering time through microRNA pathways. Proc Natl Acad Sci USA 113: E2199–E2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot B, Castroverde CDM, Velásquez AC, Hubbard E, Pulman JA, Yao J, Childs KL, Tsuda K, Montgomery BL, He SY (2017) Dual impact of elevated temperature on plant defence and bacterial virulence in Arabidopsis. Nat Commun 8: 1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez C, Delker C, Martinez C, Bürstenbinder K, Janitza P, Lippmann R, Ludwig W, Sun H, James GV, Klecker M, et al. (2018) Brassinosteroids dominate hormonal regulation of plant thermomorphogenesis via BZR1. Curr Biol 28: 303–310.e3 [DOI] [PubMed] [Google Scholar]
- Jin H, Zhu Z (2019) Dark, light and temperature: Key players in plant morphogenesis. Plant Physiol 180: 1793–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Pang L, Fang S, Chu J, Li R, Zhu Z (2018) High ambient temperature antagonizes ethylene-induced exaggerated apical hook formation in etiolated Arabidopsis seedlings. Plant Cell Environ 41: 2858–2868 [DOI] [PubMed] [Google Scholar]
- Jung JH, Domijan M, Klose C, Biswas S, Ezer D, Gao M, Khattak AK, Box MS, Charoensawan V, Cortijo S, et al. (2016) Phytochromes function as thermosensors in Arabidopsis. Science 354: 886–889 [DOI] [PubMed] [Google Scholar]
- Kaiserli E, Páldi K, O’Donnell L, Batalov O, Pedmale UV, Nusinow DA, Kay SA, Chory J (2015) Integration of light and photoperiodic signaling in transcriptional nuclear foci. Dev Cell 35: 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinmonth-Schultz HA, MacEwen MJS, Seaton DD, Millar AJ, Imaizumi T, Kim SH (2019) An explanatory model of temperature influence on flowering through whole-plant accumulation of FLOWERING LOCUS T in Arabidopsis thaliana. In Silico Plants 1: diz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose C, Venezia F, Hussong A, Kircher S, Schäfer E, Fleck C (2015) Systematic analysis of how phytochrome B dimerization determines its specificity. Nat Plants 1: 15090. [DOI] [PubMed] [Google Scholar]
- Kumar SV. (2018) H2A.Z at the core of transcriptional regulation in plants. Mol Plant 11: 1112–1114 [DOI] [PubMed] [Google Scholar]
- Kumar SV, Wigge PA (2010) H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140: 136–147 [DOI] [PubMed] [Google Scholar]
- Kumar SV, Lucyshyn D, Jaeger KE, Alós E, Alvey E, Harberd NP, Wigge PA (2012) Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484: 242–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Vierling E (2008) Core genome responses involved in acclimation to high temperature. Plant Physiol 146: 748–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Yoo SJ, Lee JH, Kim W, Yoo SK, Fitzgerald H, Carrington JC, Ahn JH (2010) Genetic framework for flowering-time regulation by ambient temperature-responsive miRNAs in Arabidopsis. Nucleic Acids Res 38: 3081–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Jung JH, Cortés Llorca L, Kim SG, Lee S, Baldwin IT, Park CM (2014) FCA mediates thermal adaptation of stem growth by attenuating auxin action in Arabidopsis. Nat Commun 5: 5473. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ryu HS, Chung KS, Pose D, Kim S, Schmid M, Ahn JH (2013) Regulation of temperature-responsive flowering by MADS-box transcription factor repressors. Science 342: 628–632 [DOI] [PubMed] [Google Scholar]
- Legris M, Klose C, Burgie ES, Rojas CC, Neme M, Hiltbrunner A, Wigge PA, Schäfer E, Vierstra RD, Casal JJ (2016) Phytochrome B integrates light and temperature signals in Arabidopsis. Science 354: 897–900 [DOI] [PubMed] [Google Scholar]
- Legris M, Nieto C, Sellaro R, Prat S, Casal JJ (2017) Perception and signalling of light and temperature cues in plants. Plant J 90: 683–697 [DOI] [PubMed] [Google Scholar]
- Lesk C, Rowhani P, Ramankutty N (2016) Influence of extreme weather disasters on global crop production. Nature 529: 84–87 [DOI] [PubMed] [Google Scholar]
- Li K, Yu R, Fan LM, Wei N, Chen H, Deng XW (2016) DELLA-mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis. Nat Commun 7: 11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QF, Wang C, Jiang L, Li S, Sun SSM, He JX (2012) An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci Signal 5: ra72. [DOI] [PubMed] [Google Scholar]
- Li XR, Deb J, Kumar SV, Østergaard L (2018) Temperature modulates tissue-specification program to control fruit dehiscence in Brassicaceae. Mol Plant 11: 598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljegren SJ, Roeder AH, Kempin SA, Gremski K, Østergaard L, Guimil S, Reyes DK, Yanofsky MF (2004) Control of fruit patterning in Arabidopsis by INDEHISCENT. Cell 116: 843–853 [DOI] [PubMed] [Google Scholar]
- Lim S, Park J, Lee N, Jeong J, Toh S, Watanabe A, Kim J, Kang H, Kim DH, Kawakami N, et al. (2013) ABA-insensitive3, ABA-insensitive5, and DELLAs interact to activate the expression of SOMNUS and other high-temperature-inducible genes in imbibed seeds in Arabidopsis. Plant Cell 25: 4863–4878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, Serrano N, Gao G, Atia M, Mokhtar M, Woo YH, Bazin J, Veluchamy A, Benhamed M, Crespi M, et al. (2018) Thermopriming triggers splicing memory in Arabidopsis. J Exp Bot 69: 2659–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Covington MF, Fankhauser C, Chory J, Wagner DR (2001) ELF3 encodes a circadian clock-regulated nuclear protein that functions in an Arabidopsis PHYB signal transduction pathway. Plant Cell 13: 1293–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell DB, Schlenker W, Costa-Roberts J (2011) Climate trends and global crop production since 1980. Science 333: 1186–1189 [DOI] [PubMed] [Google Scholar]
- Lutz U, Posé D, Pfeifer M, Gundlach H, Hagmann J, Wang C, Weigel D, Mayer KFX, Schmid M, Schwechheimer C (2015) Modulation of ambient temperature-dependent flowering in Arabidopsis thaliana by natural variation of FLOWERING LOCUS M. PLoS Genet 11: e1005588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz U, Nussbaumer T, Spannagl M, Diener J, Mayer KF, Schwechheimer C (2017) Natural haplotypes of FLM non-coding sequences fine-tune flowering time in ambient spring temperatures in Arabidopsis. eLife 6: 156–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Li X, Guo Y, Chu J, Fang S, Yan C, Noel JP, Liu H (2016) Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc Natl Acad Sci USA 113: 224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Guan X, Li J, Pan R, Wang L, Liu F, Ma H, Zhu S, Hu J, Ruan YL, et al. (2019) Mitochondrial small heat shock protein mediates seed germination via thermal sensing. Proc Natl Acad Sci USA 116: 4716–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel C, Blair LK, Donohue K (2018) PHYD prevents secondary dormancy establishment of seeds exposed to high temperature and is associated with lower PIL5 accumulation. J Exp Bot 69: 3157–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez C, Espinosa-Ruíz A, de Lucas M, Bernardo-García S, Franco-Zorrilla JM, Prat S (2018) PIF4-induced BR synthesis is critical to diurnal and thermomorphogenic growth. EMBO J 37: 99552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins S, Montiel-Jorda A, Cayrel A, Huguet S, Roux CPL, Ljung K, Vert G (2017) Brassinosteroid signaling-dependent root responses to prolonged elevated ambient temperature. Nat Commun 8: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur MJ, Kwaaitaal M, Mateos MA, Maio F, Kini RK, Prins M, van den Burg HA (2019) The SUMO conjugation complex self-assembles into nuclear bodies independent of SIZ1 and COP1. Plant Physiol 179: 168–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merow C, Bois ST, Allen JM, Xie Y, Silander JA Jr (2017) Climate change both facilitates and inhibits invasive plant ranges in New England. Proc Natl Acad Sci USA 114: E3276–E3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Higuchi Y, Sumitomo K, Hisamatsu T (2013) Flowering retardation by high temperature in chrysanthemums: Involvement of FLOWERING LOCUS T-like 3 gene repression. J Exp Bot 64: 909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto C, López-Salmerón V, Davière JM, Prat S (2015) ELF3-PIF4 interaction regulates plant growth independently of the Evening Complex. Curr Biol 25: 187–193 [DOI] [PubMed] [Google Scholar]
- Ogawa M, Kay P, Wilson S, Swain SM (2009) ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21: 216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Zhu JY, Wang ZY (2012) Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat Cell Biol 14: 802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Zhu JY, Bai MY, Arenhart RA, Sun Y, Wang ZY (2014) Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife 3: e0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K (2017) Transcriptional regulatory network of plant heat stress response. Trends Plant Sci 22: 53–65 [DOI] [PubMed] [Google Scholar]
- Park YJ, Lee HJ, Ha JH, Kim JY, Park CM (2017) COP1 conveys warm temperature information to hypocotyl thermomorphogenesis. New Phytol 215: 269–280 [DOI] [PubMed] [Google Scholar]
- Park YJ, Lee HJ, Gil KE, Kim JY, Lee JH, Lee H, Cho HT, Vu LD, De Smet I, Park CM (2019) Developmental programming of thermonastic leaf movement. Plant Physiol 180: 1185–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posé D, Verhage L, Ott F, Yant L, Mathieu J, Angenent GC, Immink RGH, Schmid M (2013) Temperature-dependent regulation of flowering by antagonistic FLM variants. Nature 503: 414–417 [DOI] [PubMed] [Google Scholar]
- Price JS, Hobson RN, Neale MA, Bruce DM (1996) Seed losses in commercial harvesting of oilseed rape. J Agric Eng Res 65: 183–191 [Google Scholar]
- Qiu Y, Li M, Kim RJA, Moore CM, Chen M (2019) Daytime temperature is sensed by phytochrome B in Arabidopsis through a transcriptional activator HEMERA. Nat Commun 10: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint M, Delker C, Franklin KA, Wigge PA, Halliday KJ, van Zanten M (2016) Molecular and genetic control of plant thermomorphogenesis. Nat Plants 2: 15190. [DOI] [PubMed] [Google Scholar]
- Raschke A, Ibañez C, Ullrich KK, Anwer MU, Becker S, Glöckner A, Trenner J, Denk K, Saal B, Sun X, et al. (2015) Natural variants of ELF3 affect thermomorphogenesis by transcriptionally modulating PIF4-dependent auxin response genes. BMC Plant Biol 15: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Wu MF, Reeves PH, Hodgens C, Yadav V, Hayes S, Pierik R (2018) Three auxin response factors promote hypocotyl elongation. Plant Physiol 178: 864–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N, Miyazawa Y, Takahashi H, Watanabe M, Higashitani A (2010) Auxins reverse plant male sterility caused by high temperatures. Proc Natl Acad Sci USA 107: 8569–8574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba Y, Jeong J, Kang MY, Kim J, Paek NC, Choi G (2014) Phytochrome-interacting transcription factors PIF4 and PIF5 induce leaf senescence in Arabidopsis. Nat Commun 5: 4636. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004) Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120: 179–186 [DOI] [PubMed] [Google Scholar]
- Sedaghatmehr M, Mueller-Roeber B, Balazadeh S (2016) The plastid metalloprotease FtsH6 and small heat shock protein HSP21 jointly regulate thermomemory in Arabidopsis. Nat Commun 7: 12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler GJ. (1998) Influence of temperature on primary and lateral root growth of sunflower seedlings. Environ Exp Bot 40: 135–146 [Google Scholar]
- Seo E, Lee H, Jeon J, Park H, Kim J, Noh YS, Lee I (2009) Crosstalk between cold response and flowering in Arabidopsis is mediated through the flowering-time gene SOC1 and its upstream negative regulator FLC. Plant Cell 21: 3185–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severing E, Faino L, Jamge S, Busscher M, Kuijer-Zhang Y, Bellinazzo F, Busscher-Lange J, Fernández V, Angenent GC, Immink RGH, et al. (2018) Arabidopsis thaliana ambient temperature responsive lncRNAs. BMC Plant Biol 18: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheerin DJ, Menon C, zur Oven-Krockhaus S, Enderle B, Zhu L, Johnen P, Schleifenbaum F, Stierhof Y-D, Huq E, Hiltbrunner A (2015) Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell 27: 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Zhu L, Castillon A, Majee M, Downie B, Huq E (2008) Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell 20: 1586–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata H, Hanada K, Ushijima T, Nakashima M, Suzuki Y, Matsushita T (2014) Phytochrome controls alternative splicing to mediate light responses in Arabidopsis. Proc Natl Acad Sci USA 111: 18781–18786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Yang C, Gao S, Zhang W, Li L, Kuai B (2014) Age-triggered and dark-induced leaf senescence require the bHLH transcription factors PIF3, 4, and 5. Mol Plant 7: 1776–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T (2015) Photoperiodic flowering: Time measurement mechanisms in leaves. Annu Rev Plant Biol 66: 441–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Kubota A, Kwon MS, Covington MF, Lee N, Taagen ER, Laboy Cintrón D, Hwang DY, Akiyama R, Hodge SK, et al. (2018) Molecular basis of flowering under natural long-day conditions in Arabidopsis. Nat Plants 4: 824–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J, Vercher Y, Gates P, Harris N (1996) ‘Pod shatter’ in Arabidopsis thaliana, Brassica napus and B. juncea. J Microsc 181: 195–203 [Google Scholar]
- Stavang JA, Gallego-Bartolomé J, Gómez MD, Yoshida S, Asami T, Olsen JE, García-Martínez JL, Alabadí D, Blázquez MA (2009) Hormonal regulation of temperature-induced growth in Arabidopsis. Plant J 60: 589–601 [DOI] [PubMed] [Google Scholar]
- Sun J, Qi L, Li Y, Chu J, Li C (2012) PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating Arabidopsis hypocotyl growth. PLoS Genet 8: e1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Hui Xu X, Lu X, Xie L, Bai B, Zheng C, Sun H, He Y, Xie XZ (2017) The rice phytochrome genes, PHYA and PHYB, have synergistic effects on anther development and pollen viability. Sci Rep 7: 6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang X, Zwiers FW, Song L, Wan H, Hu T, Yin H, Ren G (2014) Rapid increase in the risk of extreme summer heat in eastern China. Nat Clim Chang 4: 1082–1085 [Google Scholar]
- Sureshkumar S, Dent C, Seleznev A, Tasset C, Balasubramanian S (2016) Nonsense-mediated mRNA decay modulates FLM-dependent thermosensory flowering response in Arabidopsis. Nat Plants 2: 16055. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Katano K (2018) Coordination between ROS regulatory systems and other pathways under heat stress and pathogen attack. Front Plant Sci 9: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweere U. (2001) Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science 294: 1108–1111 [DOI] [PubMed] [Google Scholar]
- Syed NH, Kalyna M, Marquez Y, Barta A, Brown JWS (2012) Alternative splicing in plants: Coming of age. Trends Plant Sci 17: 616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasset C, Singh Yadav A, Sureshkumar S, Singh R, van der Woude L, Nekrasov M, Tremethick D, van Zanten M, Balasubramanian S (2018) POWERDRESS-mediated histone deacetylation is essential for thermomorphogenesis in Arabidopsis thaliana. PLoS Genet 14: e1007280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Lv B, Ding T, Bai M, Ding Z (2018) Auxin-BR interaction regulates plant growth and development. Front Plant Sci 8: 2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, Hanada A, Aso Y, Ishiyama K, Tamura N, et al. (2008) High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol 146: 1368–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima T, Hanada K, Gotoh E, Yamori W, Kodama Y, Tanaka H, Kusano M, Fukushima A, Tokizawa M, Yamamoto YY, et al. (2017) Light controls protein localization through phytochrome-mediated alternative promoter selection. Cell 171: 1316–1325.e12 [DOI] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303: 1003–1006 [DOI] [PubMed] [Google Scholar]
- Verhage L, Severing EI, Bucher J, Lammers M, Busscher-Lange J, Bonnema G, Rodenburg N, Proveniers MCG, Angenent GC, Immink RGH (2017) Splicing-related genes are alternatively spliced upon changes in ambient temperatures in plants. PLoS ONE 12: e0172950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu LD, Gevaert K, De Smet I (2019) Feeling the heat: Searching for plant thermosensors. Trends Plant Sci 24: 210–219 [DOI] [PubMed] [Google Scholar]
- Wang W, Wang ZY (2014) At the intersection of plant growth and immunity. Cell Host Microbe 15: 400–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QL, Chen JH, He NY, Guo FQ (2018) Metabolic reprogramming in chloroplasts under heat stress in plants. Int J Mol Sci 19: 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhang Y, Kieffer M, Yu H, Kepinski S, Estelle M (2016) HSP90 regulates temperature-dependent seedling growth in Arabidopsis by stabilizing the auxin co-receptor F-box protein TIR1. Nat Commun 7: 10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Cui D, Liu J, Zhao J, Liu C, Xin W, Li Y, Liu N, Ren D, Tang D, et al. (2017) Arabidopsis ZED1-related kinases mediate the temperature-sensitive intersection of immune response and growth homeostasis. New Phytol 215: 711–724 [DOI] [PubMed] [Google Scholar]
- Wang Z, Cui D, Liu C, Zhao J, Liu J, Liu N, Tang D, Hu Y (2019) TCP transcription factors interact with ZED1-related kinases as components of the temperature-regulated immunity. Plant Cell Environ 42: 2045–2056 [DOI] [PubMed] [Google Scholar]
- Yang W, Chen Z, Huang Y, Chang G, Li P, Wei J, Yuan X, Huang J, Hu X (2019) Powerdress as the novel regulator enhances Arabidopsis seeds germination tolerance to high temperature stress by histone modification of SOM locus. Plant Sci 284: 91–98 [DOI] [PubMed] [Google Scholar]
- Yeh CH, Kaplinsky NJ, Hu C, Charng YY (2012) Some like it hot, some like it warm: Phenotyping to explore thermotolerance diversity. Plant Sci 195: 10–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Holmlund M, Lorrain S, Norberg M, Bakó L, Fankhauser C, Nilsson O (2017) BLADE-ON-PETIOLE proteins act in an E3 ubiquitin ligase complex to regulate PHYTOCHROME INTERACTING FACTOR 4 abundance. eLife 6: e26759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li C, Zhou Y, Wang X, Li H, Feng Z, Chen H, Qin G, Jin D, Terzaghi W, et al. (2018) TANDEM ZINC-FINGER/PLUS3 is a key component of phytochrome A signaling. Plant Cell 30: 835–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QH, Helliwell CA (2011) Regulation of flowering time and floral patterning by miR172. J Exp Bot 62: 487–495 [DOI] [PubMed] [Google Scholar]
- Zhu JY, Oh E, Wang T, Wang ZY (2016) TOC1-PIF4 interaction mediates the circadian gating of thermoresponsive growth in Arabidopsis. Nat Commun 7: 13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tepperman JM, Fairchild CD, Quail PH (2000) Phytochrome B binds with greater apparent affinity than phytochrome A to the basic helix-loop-helix factor PIF3 in a reaction requiring the PAS domain of PIF3. Proc Natl Acad Sci USA 97: 13419–13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Qian W, Hua J (2010) Temperature modulates plant defense responses through NB-LRR proteins. PLoS Pathog 6: e1000844. [DOI] [PMC free article] [PubMed] [Google Scholar]