Abstract
The PDH bypass and the GABA shunt serve to maintain mainline metabolic fluxes during episodes of organellar thiamin diphosphate deficiency.
Plants have pathways that bypass two α-keto-acid dehydrogenase complexes: the pyruvate dehydrogenase (PDH) bypass around the PDH complex (PDHc) and the γ-aminobutyrate (GABA) shunt around the α-ketoglutarate dehydrogenase complex (KGDHc; Fig. 1A). The PDH bypass is suggested to enhance pyruvate flux to acetyl-CoA but is otherwise enigmatic (Tadege and Kuhlemeier, 1997; Wei et al., 2009); the GABA shunt is proposed to have various metabolic and signaling functions (Fait et al., 2008). The first enzymes of these bypasses, pyruvate decarboxylase (PDC) and Glu decarboxylase (GAD), are expressed constitutively in leaves and further induced by stresses (Fait et al., 2008; Mithran et al., 2014). It is therefore generally thought that the bypasses, although energetically less efficient than the PDHc and KGDHc, have adaptive roles in both unstressed and stressed leaves. What has not been considered for plants, although it has been for mammals, is that the PDHc and KGDHc are thiamin diphosphate (ThDP)-dependent, and hence vulnerable to ThDP deficiency; that ThDP is labile, particularly in organelles and under stress conditions; and that episodic organellar ThDP deficiency is consequently probable. Here, we marshal data from the literature on these points, show that the properties of leaf PDCs equip them to mediate the PDH bypass, and provide evidence that this bypass operates in vivo. We then argue that a major function of the PDH bypass and the GABA shunt is to maintain mainline metabolic fluxes when organellar ThDP levels run low.
Figure 1.
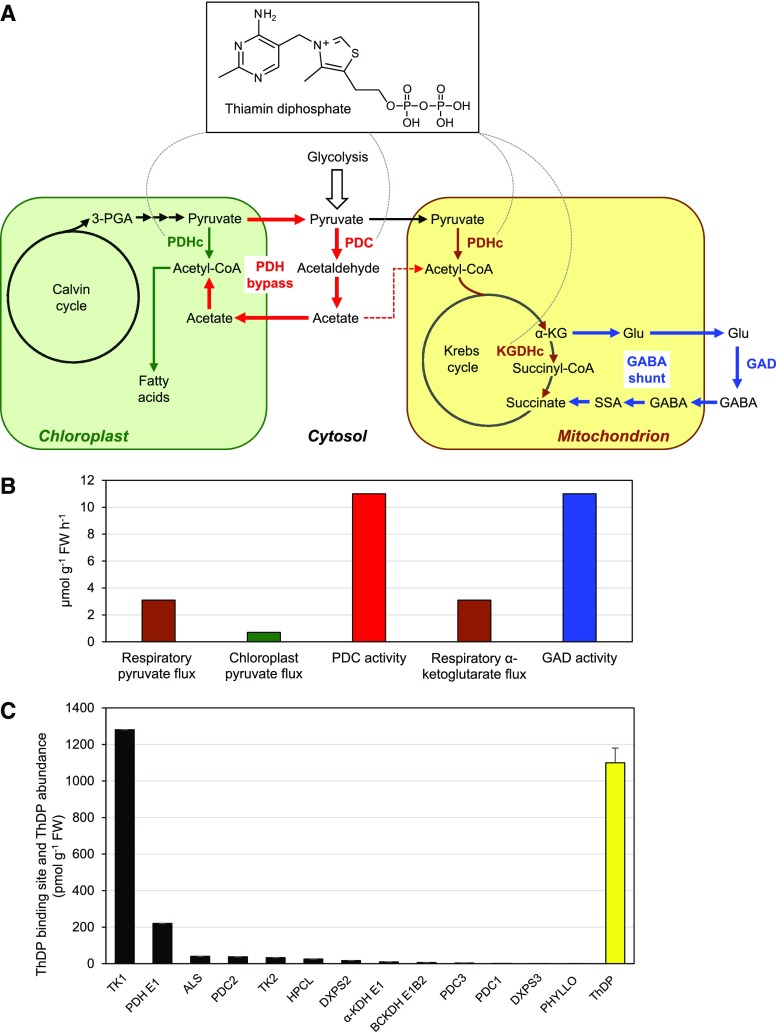
The PDH bypass, the GABA shunt, and their relationship to ThDP. A, Scheme showing the PDH bypass (red arrows), the GABA shunt (blue arrows), and the α-keto-acid dehydrogenase complexes that they circumvent. The PDHc occurs in mitochondria and chloroplasts; the KGDHc is solely mitochondrial. The dashed red arrow leading from cytosolic acetate to mitochondrial acetyl-CoA denotes an undefined pathway by which acetate is respired (Eastmond et al., 2000). The dashed arcs radiating from the ThDP structure indicate the enzymes in the scheme that are ThDP-dependent. B, Estimated in vivo fluxes through the mitochondrial PDHc, the chloroplast PDHc, and the KGDHc in Arabidopsis leaves compared with the extractable activities of Arabidopsis leaf PDC and GAD. Supporting literature and calculations are given in Supplemental Data. C, Abundance of ThDP binding sites (black bars) and ThDP cofactor (yellow bar) in Arabidopsis leaves, estimated from PaxDb abundance data for ThDP-dependent enzymes, and from ThDP contents taken from three independent articles. The ThDP values are means ± sd. Calculations are detailed in Supplemental Data. FW, fresh weight. Enzyme abbreviations: TK, transketolase; PDH E1, pyruvate dehydrogenase E1 subunit; ALS, acetolactate synthase; HPCL, 2-hydroxyphytanoyl-CoA lyase; DXPS, 1-deoxy-d-xylulose-5-P synthase; α-KDH E1, α-ketoglutarate dehydrogenase E1 subunit; BCKDH E1, branched-chain ketoacid dehydrogenase E1 subunit; PHYLLO, MenF/MenD/MenC/MenH fusion enzyme.
GAD and the GABA shunt are well documented in leaves and other tissues, and have proposed roles that include maintenance of carbon/nitrogen balance, pH regulation during hypoxia, and modulation of growth and development (Fait et al., 2008; Ramesh et al., 2017). Notwithstanding all the data available, however, the physiological significance of the GABA shunt remains quite speculative (Fait et al., 2008; Ramesh et al., 2017), as indeed are the roles in leaves of the Krebs cycle itself, especially in C4 plants (Zhang and Fernie, 2018). PDC has been much studied in the context of ethanol glycolysis (Mithran et al., 2014) but not in the context of the PDH bypass in leaves. PDC is clearly present in leaves and could provide an alternative path to acetyl-CoA as well as to acetaldehyde and ethanol during hypoxia, but what it does in normoxia remains conjectural (Nguyen et al., 2009; Wei et al., 2009; Mithran et al., 2014). The presence of the enzymes of the GABA shunt and the PDH bypass in leaves is particularly enigmatic because, unlike roots or developing seeds, leaves rarely experience hypoxia or use ethanol glycolysis.
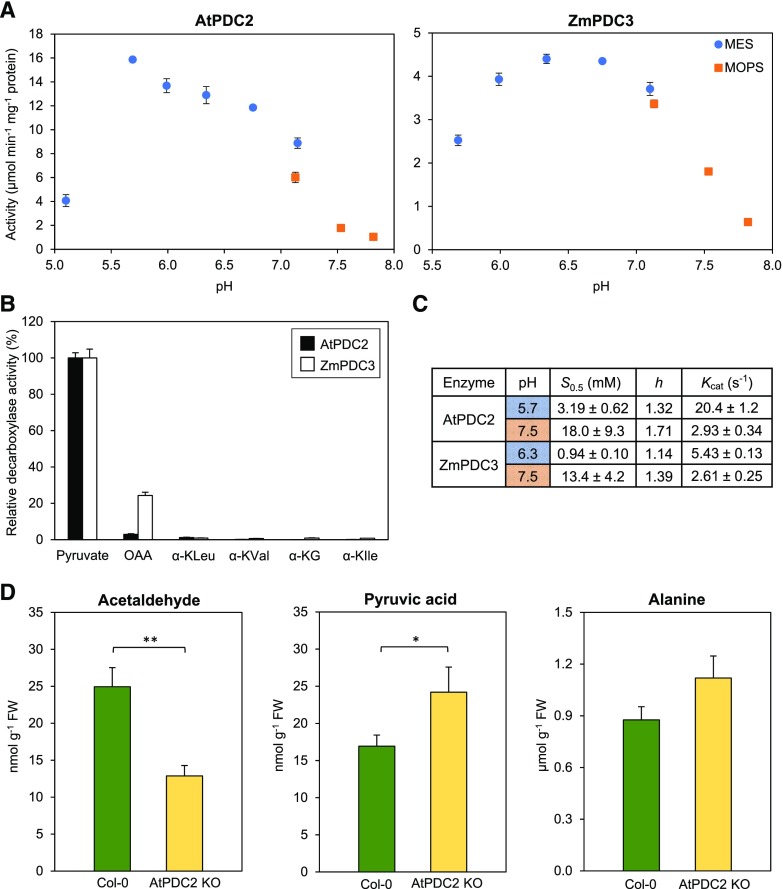
A factor adding to the enigma is that the properties of leaf PDCs from herbaceous plants have not been defined. Specifically, it is not known whether PDC leaf isoforms have acidic pH optima, this being a key characteristic that enables activation by a fall in cytosolic pH (Ismond et al., 2003). Nor have KM and Kcat values or substrate preferences been reported for leaf PDCs. We therefore expressed the major leaf PDC isoforms of Arabidopsis (Arabidopsis thaliana; AtPDC2; Mithran et al., 2014) and maize (Zea mays; ZmPDC3; Walley et al., 2016) in Escherichia coli and characterized the purified enzymes. Both have acidic pH optima (pH 5.7 for AtPDC2, 6.3 for ZmPDC3; Fig. 2A), strongly prefer pyruvate to other physiological α-keto acids (Fig. 2B), and show moderately sigmoidal kinetics consistent with allosteric activation by pyruvate (Lee and Langston-Unkefer, 1985; Supplemental Fig. S1). Their S0.5, Vmax (Kcat), and Hill coefficient (h) values are pH-dependent in ways that favor activation at low pH (Fig. 2C; Supplemental Fig. S1). First, the S0.5 values of AtPDC2 and ZmPDC3 at optimal pH are in the low millimolar range (∼1 and 3 mm, respectively), whereas at pH 7.5 (a typical cytosolic pH) the S0.5 values of both enzymes are 6- to 13-fold higher and their Kcat values are 2- to 6-fold lower (Fig. 2C; Supplemental Fig. S1). Second, as for PDCs purified from maize kernels and roots (Lee and Langston-Unkefer, 1985), the cooperativity of both enzymes is greater at higher pH. Activation by low pH might be further enhanced if affinity for ThDP increases as pH falls, as for yeast PDC (Gounaris et al., 1971). The properties of leaf PDCs are thus consistent with their functioning in the bypass pathway provided that there is a fall in cytosolic pH.
Figure 2.
Characteristics of the predominant leaf PDCs from Arabidopsis and maize, and metabolic effects of knocking out the Arabidopsis enzyme. A, pH-activity profiles for recombinant AtPDC2 and ZmPDC3. Activities were assayed in 50 mm MES-KOH or MOPS-KOH buffer using a pyruvate concentration of 15 mm. Data are means ± se for three replicate experiments with the same enzyme preparations. B, Substrate preferences of AtPDC2 and ZmPDC3, assayed at pH 6.4 using substrate concentrations of 5 mm for AtPDC2 and 0.94 mm for ZmPDC3, to approximate their respective S0.5 values. Data are means ± se for three replicates and are expressed as percentages of activity with pyruvate, which was 10.5 μmol min−1 mg−1 protein for AtPDC2 and 0.76 μmol min−1 mg−1 protein for ZmPDC3. Substrate abbreviations: OAA, oxaloacetate; α-KLeu, α-keto-Leu; α-KVal, α-keto-Val; α-KG, α-ketoglutarate; α-KIle, α-keto-Ile. C, S0.5, Hill coefficient (h), and Kcat values of AtPDC2 and ZmPDC3 at their respective pH optima and at pH 7.5. Data are means ± se for three independent experiments. Values for AtPDC2 at pH 7.5 could not be measured as precisely as other values because the S0.5 value approached the maximum acceptable pyruvate concentration (30 mM). Initial velocity versus substrate concentration plots for AtPDC2 and ZmPDC3 are shown in Supplemental Figure S1. D, Acetaldehyde, pyruvate, and Ala contents of wild-type Arabidopsis (Col-0) and an AtPDC2 knockout line (AtPDC2 KO). Data are means ± se for five or six replicates. The knockout and wild-type mean values for acetaldehyde and pyruvate differ significantly by Student’s t test. *P < 0.05; **P < 0.01. FW, fresh weight.
If there is flux through the PDH bypass, ablating PDC is predicted to shrink the pool of acetaldehyde and expand the pools of pyruvate and/or its transamination product Ala. We therefore compared the levels of these metabolites in leaves of an AtPDC2 knockout line (Supplemental Fig. S2) with those of wild-type plants. Knocking out AtPDC2 significantly decreased acetaldehyde level (by 48%) and significantly increased pyruvate level (by 43%; Fig. 2D). As the plants were grown in near-optimal conditions, these data imply that the PDH bypass carries substantial flux even in the absence of stresses.
The PDH bypass and the GABA shunt each provides a route around a mitochondrial α-keto-acid dehydrogenase complex and, for the PDH bypass, around a chloroplastic α-keto-acid dehydrogenase complex as well (Fig. 1A). These complexes require ThDP, the cofactor for their E1 subunits (Fig. 1A). ThDP is inactivated by catalytic misfires (Bunik et al., 2011) and has a short half-life (∼10 h in Arabidopsis leaves) that probably shortens further as fluxes through ThDP-dependent enzymes increase, and as a result of stress (Hanson et al., 2016). Evidence from plants and mammals suggests that mitochondrial and chloroplastic ThDP are especially susceptible to depletion (Bunik et al., 2011; Hanson et al., 2016), and thiamin deficiency has been known for >70 years to reduce the activities of mammalian PDHc and KGDHc (Bender, 1999). These points make it reasonable to think that the PDH bypass and GABA shunt are adaptations to ThDP deficiency. For the GABA shunt in mammals, this idea is 30 years old and has experimental support (Page et al., 1989). It is thus striking that this simple idea has apparently not been invoked to explain why leaves can run the GABA shunt and PDH bypass. For the PDH bypass, an objection to the idea is that PDC is itself ThDP-dependent—but a counterargument is that PDC is cytosolic whereas the proposed ThDP deficiency is organellar (as discussed below). The GABA shunt is not subject to such an objection as GAD is not ThDP-dependent.
Three lines of reasoning support the idea that the PDH bypass and the GABA shunt are workarounds for organellar ThDP deficiency in leaves.
(1) Calculations from published data show that the PDC and GAD activities in Arabidopsis leaves, assayed in optimal conditions, are at least 3-fold greater than the total in vivo fluxes through the PDHc and KGDHc (Fig. 1B). These enzymes thus have the capacity to handle all the flux that normally goes through the reaction they bypass. Isotope-labeling data for the GABA shunt support this inference (Fait et al., 2008).
(2) For the PDH bypass, estimates based on a respiratory flux to pyruvate of 3.1 μmol g−1 fresh weight h−1 (Fig. 1B) and standard values for cytosolic volume and the buffer capacity of leaf cytoplasm show that, in the absence of PDH complex activity, it would take only 5–10 min for pyruvate accumulation to decrease the cytosolic pH by 1 unit, and hence to strongly activate PDC (Fig. 2A; Supplemental Data). Although complete loss of PDH complex activity is physiologically unlikely, these estimates suggest that even a modest loss could quickly cause sufficient cytosolic acidification to activate PDC.
(3) Many published results point to organellar ThDP deficiency as an existential threat in leaves. Thus, estimates of the abundance of ThDP-binding sites (i.e. the active sites of ThDP-dependent enzymes) in Arabidopsis leaves give a total of ∼1,700 pmol g−1 FW, with ∼1,300 (76%) coming from a single chloroplast enzyme, transketolase1 (Fig. 1C). As total leaf ThDP content is only ∼1,100 pmol g−1 FW (Fig. 1C, yellow bar), transketolase1 could theoretically sequester all available ThDP, leaving none for other enzymes, particularly extrachloroplastic ones. Other studies have reached similar conclusions (Piques et al., 2009; Khozaei et al., 2015). These numbers imply that chloroplast transketolase, and by extension the chloroplast PDH complex, usually operate close to ThDP deficiency. That there is probably flux through the PDH bypass even in mild conditions (Fig. 2D) supports the possibility that incipient ThDP deficiency is normal. Further supporting this possibility, doubling chloroplast transketolase expression in tobacco (Nicotiana benthamiana) leaves led to systemic ThDP deficiency (Khozaei et al., 2015) and raising ThDP level in Arabidopsis leaves by just ∼20% increased extractable transketolase activity without changing transketolase protein level (Bocobza et al., 2013). Increasing ThDP level also increased mitochondrial PDH complex activity, but not protein, indicating that leaf mitochondria are likewise normally borderline ThDP-deficient (Bocobza et al., 2013). The classical finding that isolated leaf mitochondria exhaust their ThDP pool within ∼1 h points to the same conclusion (Douce et al., 1977). More generally, a corollary of the tightly regulated, just-in-time character of ThDP synthesis in leaves (Bocobza et al., 2013) is the possibility of transient ThDP deficiency at any time and prolonged deficiency if stress disrupts the system. As ThDP deficiency is a priori less likely in the cytosol (where ThDP is made) than in organelles (Hanson et al., 2018), PDC may well be able to keep its ThDP cofactor when α-keto-acid dehydrogenase complexes are losing theirs. Relative immunity of PDC to ThDP deficiency would allow the PDH bypass to take over when the PDH complex fails.
If the risk of organellar ThDP deficiency is a baked-in feature of the plant thiamin economy, then constitutive bypasses around ThDP-dependent organellar enzymes would neatly solve the problem of keeping central metabolism running. Was, then, the primordial function of the PDH bypass and the GABA shunt to provide workarounds for ThDP deficiency? And are the diverse metabolic and signaling roles of these two pathways secondary functions that evolved later? Whatever the case, the solid evidence that leaves usually operate with borderline-deficient ThDP levels raises the question for future research of why this is so. Two nonexclusive possibilities are (1) that cells use a limiting ThDP supply to actively orchestrate core central metabolism, as proposed by Bocobza et al. (2013), or (2) that cells minimize the level of free ThDP because it is highly reactive and potentially toxic (Lerma-Ortiz et al., 2016).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Initial velocity versus substrate concentration plots for AtPDC2 and ZmPDC3 at their respective pH optima and at pH 7.5.
Supplemental Figure S2. Validation of AtPDC2 knockout mutant.
Supplemental Data. Supplementary materials, methods, and calculations.
Acknowledgments
We thank Michael J. Ziemak for general technical assistance and Maria A. Ralat for assistance with acetaldehyde analysis.
Footnotes
This work was supported by the National Science Foundation (awards IOS-1444202 to A.D.H. and MCB-1611846 to O.F.), and by the C.V. Griffin Sr. Foundation (endowment).
Articles can be viewed without a subscription.
References
- Bender DA. (1999) Optimum nutrition: Thiamin, biotin and pantothenate. Proc Nutr Soc 58: 427–433 [DOI] [PubMed] [Google Scholar]
- Bocobza SE, Malitsky S, Araújo WL, Nunes-Nesi A, Meir S, Shapira M, Fernie AR, Aharoni A (2013) Orchestration of thiamin biosynthesis and central metabolism by combined action of the thiamin pyrophosphate riboswitch and the circadian clock in Arabidopsis. Plant Cell 25: 288–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunik VI, Schloss JV, Pinto JT, Dudareva N, Cooper AJ (2011) A survey of oxidative paracatalytic reactions catalyzed by enzymes that generate carbanionic intermediates: Implications for ROS production, cancer etiology, and neurodegenerative diseases. Adv Enzymol Relat Areas Mol Biol 77: 307–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R, Moore AL, Neuburger M (1977) Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol 60: 625–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ, Germain V, Lange PR, Bryce JH, Smith SM, Graham IA (2000) Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc Natl Acad Sci USA 97: 5669–5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fait A, Fromm H, Walter D, Galili G, Fernie AR (2008) Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci 13: 14–19 [DOI] [PubMed] [Google Scholar]
- Gounaris AD, Turkenkopf I, Buckwald S, Young A (1971) Pyruvate decarboxylase. I. Protein dissociation into subunits under conditions in which thiamine pyrophosphate is released. J Biol Chem 246: 1302–1309 [PubMed] [Google Scholar]
- Hanson AD, Beaudoin GA, McCarty DR, Gregory JF III (2016) Does abiotic stress cause functional B vitamin deficiency in plants? Plant Physiol 172: 2082–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson AD, Amthor JS, Sun J, Niehaus TD, Gregory JF III, Bruner SD, Ding Y (2018) Redesigning thiamin synthesis: Prospects and potential payoffs. Plant Sci 273: 92–99 [DOI] [PubMed] [Google Scholar]
- Ismond KP, Dolferus R, de Pauw M, Dennis ES, Good AG (2003) Enhanced low oxygen survival in Arabidopsis through increased metabolic flux in the fermentative pathway. Plant Physiol 132: 1292–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khozaei M, Fisk S, Lawson T, Gibon Y, Sulpice R, Stitt M, Lefebvre SC, Raines CA (2015) Overexpression of plastid transketolase in tobacco results in a thiamine auxotrophic phenotype. Plant Cell 27: 432-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TC, Langston-Unkefer PJ (1985) Pyruvate decarboxylase from Zea mays L.: I. Purification and partial characterization from mature kernels and anaerobically treated roots. Plant Physiol 79: 242–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma-Ortiz C, Jeffryes JG, Cooper AJ, Niehaus TD, Thamm AM, Frelin O, Aunins T, Fiehn O, de Crécy-Lagard V, Henry CS, et al. (2016) ‘Nothing of chemistry disappears in biology’: The top 30 damage-prone endogenous metabolites. Biochem Soc Trans 44: 961–971 [DOI] [PubMed] [Google Scholar]
- Mithran M, Paparelli E, Novi G, Perata P, Loreti E (2014) Analysis of the role of the pyruvate decarboxylase gene family in Arabidopsis thaliana under low-oxygen conditions. Plant Biol (Stuttg) 16: 28–34 [DOI] [PubMed] [Google Scholar]
- Nguyen T, Drotar AM, Monson RK, Fall R (2009) A high affinity pyruvate decarboxylase is present in cottonwood leaf veins and petioles: A second source of leaf acetaldehyde emission? Phytochemistry 70: 1217–1221 [DOI] [PubMed] [Google Scholar]
- Page MG, Ankoma-Sey V, Coulson WF, Bender DA (1989) Brain glutamate and gamma-aminobutyrate (GABA) metabolism in thiamin-deficient rats. Br J Nutr 62: 245–253 [DOI] [PubMed] [Google Scholar]
- Piques M, Schulze WX, Höhne M, Usadel B, Gibon Y, Rohwer J, Stitt M (2009) Ribosome and transcript copy numbers, polysome occupancy and enzyme dynamics in Arabidopsis. Mol Syst Biol 5: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh SA, Tyerman SD, Gilliham M, Xu B (2017) γ-Aminobutyric acid (GABA) signalling in plants. Cell Mol Life Sci 74: 1577–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadege M, Kuhlemeier C (1997) Aerobic fermentation during tobacco pollen development. Plant Mol Biol 35: 343–354 [DOI] [PubMed] [Google Scholar]
- Walley JW, Sartor RC, Shen Z, Schmitz RJ, Wu KJ, Urich MA, Nery JR, Smith LG, Schnable JC, Ecker JR, et al. (2016) Integration of omic networks in a developmental atlas of maize. Science 353: 814–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Lin M, Oliver DJ, Schnable PS (2009) The roles of aldehyde dehydrogenases (ALDHs) in the PDH bypass of Arabidopsis. BMC Biochem 10: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fernie AR (2018) On the role of the tricarboxylic acid cycle in plant productivity. J Integr Plant Biol 60: 1199–1216 [DOI] [PubMed] [Google Scholar]