Abstract
Despite recently uncovered connections between autophagy and the endocytic pathway, the role of autophagy in regulating endosomal function remains incompletely understood. Here, we find that the ablation of autophagy‐essential players disrupts EGF‐induced endocytic trafficking of EGFR. Cells lacking ATG7 or ATG16L1 exhibit increased levels of phosphatidylinositol‐3‐phosphate (PI(3)P), a key determinant of early endosome maturation. Increased PI(3)P levels are associated with an accumulation of EEA1‐positive endosomes where EGFR trafficking is stalled. Aberrant early endosomes are recognised by the autophagy machinery in a TBK1‐ and Gal8‐dependent manner and are delivered to LAMP2‐positive lysosomes. Preventing this homeostatic regulation of early endosomes by autophagy reduces EGFR recycling to the plasma membrane and compromises downstream signalling and cell survival. Our findings uncover a novel role for the autophagy machinery in maintaining early endosome function and growth factor sensing.
Keywords: autophagy, early endosomes, EGFR, galectin, signalling
Subject Categories: Autophagy & Cell Death, Membrane & Intracellular Transport, Signal Transduction
Introduction
Vesicular trafficking processes are important in controlling various aspects of cell fate decisions and homeostasis. Of these processes, both endocytosis and autophagy are known to deliver cellular cargoes to lysosomes for degradation and are involved in relaying cues from the extracellular milieu. Recent studies have begun to uncover mechanisms through which the endocytic and autophagic compartments can act in concert to facilitate their optimal activities.
Endocytosis begins with the internalisation of plasma membrane cargoes into early, or “sorting”, endosomes marked by specific adaptor proteins and small Rab GTPases 1, 2, 3. These vesicles can then mature into endosomes destined for various cellular compartments including the plasma membrane (recycling endosomes) or lysosomes (late endosomes), thereby regulating the activities of endocytic cargoes such as receptor tyrosine kinases (RTKs) 4. Efficient signalling from a subset of endocytosed RTKs has been shown to utilise autophagy‐related membranes as signalling platforms 5, 6, 7, suggesting that autophagy proteins can regulate pro‐growth signalling independent of their degradative function. This is of particular interest given that the perturbed activities of many RTKs, including the epidermal growth factor receptor (EGFR), have been associated with various cancers and developmental defects 8, 9, 10, 11.
Autophagy‐mediated degradation of various cellular components is critical to maintain cellular homeostasis 12. Recently, autophagy has been shown to target damaged lysosomal membranes and bacteria‐containing vesicles, suggesting that it can mediate the degradation of these vesicles 13, 14, 15. Such endomembrane damage is recognised by the Galectin family of proteins that bind glycans on the luminal face of the vesicles 15, 16. Tank‐binding kinase 1 (TBK1) is then required to recruit autophagy players, including WIPI2 and ATG16L1, to the damage site where they facilitate the ubiquitin‐like conjugation of ATG8 paralogues, such as LC3, to phosphatidylethanolamine and subsequently autophagosome biogenesis 16, 17. While the role of LC3 in cargo recognition by binding to receptor proteins has been well studied, increasing evidence suggests that upstream autophagy players can also play an important role in recruiting autophagosome substrates 18, 19.
Autophagy and the endocytic pathway share multiple commonalities 20. For example, phosphoinositide‐3‐phosphate (PI(3)P) is essential for the proper function of both early endosomes and autophagosomes 3, 21, 22, 23. Its biogenesis from phosphoinositol is mediated by vacuolar protein sorting‐associated protein 34 (VPS34), also known as phosphoinositide‐3‐kinase class III (PIK3C3) 24. VPS34 can generate PI(3)P on either endocytic or autophagic membranes by existing in two distinct complexes: the “autophagic” complex I (comprised of ATG14, Beclin‐1 and VPS15) and the “endocytic” complex II (comprised of UVRAG, Beclin‐1 and VPS15) 25, 26, 27, 28. These complexes can be regulated by the availability of growth factors and nutrients 29, 30, 31. The endocytic pathway can also contribute to autophagosome biogenesis as a number of autophagy players (such as ATG16L1, ATG9, WIPI2 and ULK1) can localise to Rab11‐positive (Rab11+) recycling endosomes 32, 33, 34. Furthermore, endocytic regulators (e.g. VAMP3, Rab7, CHMP2A and STX17) have been found to be required for autophagosome maturation, closure and their eventual fusion with lysosomes 35, 36, 37, 38. While extensive studies highlight the contribution of the endocytic pathway to autophagosome formation 20, less is known on how autophagy is required for the optimal function of endosomes.
Here, we uncover a novel role for autophagy in maintaining endosomal homeostasis by recognising and targeting perturbed early endosomes. A consequence of inhibiting this process is the disruption of EGFR endocytic sorting which impacts overall cellular response to growth factor signalling.
Results
Loss of autophagy perturbs EGFR endocytic trafficking
Given the close interplay between endocytic and autophagic vesicular trafficking, we were interested to understand how the loss of autophagy can impact the endocytic pathway. To test this, we used EGFR as a model endocytic cargo as its regulation has been extensively studied 39. Glial cells transformed by Ink4a/Arf deletion as well as Tp53 and Nf‐1 knockdown (shNf‐1/shTp53) were used as a model system 40, 41, and CRISPR/Cas9‐mediated gene editing of autophagy‐related genes (denoted as sgAtg) was introduced to inhibit autophagy (Fig EV1). To observe the overall endocytic trafficking of EGFR in real time, Alexa 555‐labelled EGF (555‐EGF) was monitored in cells by live spinning disc confocal microscopy. Tracking the paths of these vesicles revealed that while EGF exhibited a dynamic trafficking pattern in control cells, it accumulated in the perinuclear region of Atg7 knockout cells (Fig 1A). Immunofluorescence staining of EGFR confirmed its perinuclear accumulation in the absence of ATG7 after 15 min of EGF treatment (Fig 1B and C). To further investigate this disrupted endocytic trafficking, we assessed changes in ligand–receptor colocalisation 42 and observed an increased percentage of EGFR vesicles that remained positive for 555‐EGF in autophagy‐deficient cells (Fig 1D and E). This elevated ligand–receptor binding is suggestive of a perturbed trafficking of EGFR at early endosomes. To test this, we assessed the colocalisation between EGFR and the early endosome marker Rab5. Figure 1F and G shows that at early time points (5 min), EGFR occupancy in Rab5+ endosomes was comparable between control and sgAtg7 cells, indicating that early endocytic uptake of EGFR from the plasma membrane is not affected by ATG7 loss. However, at a later time point (15 min), EGFR residency in early endosomes was strikingly increased in sgAtg7 cells compared to control cells. Overall, these data suggest that autophagy inhibition alters EGFR trafficking resulting in its accumulation at early endosomes.
Figure EV1. Confirmation of CRISPR/Cas9‐mediated gene editing and autophagy inhibition in cells.
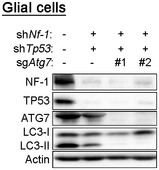
Western blot analyses of glial cells infected with shRNA against Nf‐1 and Tp53, and then with Cas9 and sgRNAs against Atg7.
Figure 1. Loss of autophagy perturbs EGFR endocytic trafficking.
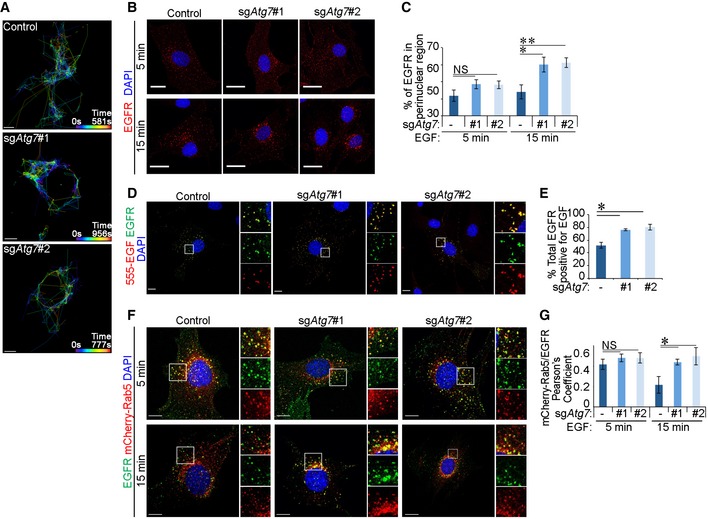
- Spinning disc confocal live cell imaging of Alexa 555‐EGF (555‐EGF) shown as vesicle tracking with time represented as a colour spectrum. Tracking started 5 min after addition of 20 ng/ml 555‐EGF for the indicated durations. Scale bar: 10 μm.
- Immunofluorescence staining of EGFR following 5‐ or 15‐min stimulation with 20 ng/ml EGF. Scale bar: 20 μm.
- Quantification of EGFR vesicles in a perinuclear region (within 30 μm diameter of the centre of the nucleus) (in B).
- Cells were stimulated with 20 ng/ml 555‐EGF for 15 or 30 min before immunofluorescence staining against EGFR. Scale bar: 10 μm.
- Quantification of percentage of total EGFR vesicles that colocalise with 555‐EGF (in D).
- Cells stably expressing mCherry‐Rab5 were stimulated with 20 ng/ml EGF for indicated times before immunofluorescence staining against EGFR. Scale bar: 10 μm.
- Pearson's colocalisation coefficient between mCherry‐Rab5 and EGFR (in F).
Autophagy inhibition increases PI(3)P‐positive early endosomes
To further investigate a potential defect in early endosomes upon autophagy deficiency, we examined the levels of PI(3)P, a key phosphoinositide determinant of autophagosome biogenesis and early endosome function 2, 43. To do this, we employed a post‐fixation technique using an Alexa 488‐conjugated recombinant FYVE‐domain probe in order to avoid interference with endosomal trafficking as a result of PI(3)P‐binding domain overexpression in cells 44. Interestingly, inhibition of autophagy in glial cells by ATG7 or ATG16L1 deletion (Fig EV2A) resulted in increased overall cellular levels of PI(3)P (Figs 2A and B, and EV2B). We further tested whether this increase in PI(3)P levels was associated with early endosomes marked by the PI(3)P effector EEA1 2 and observed a significant increase in PI(3)P+ EEA1 vesicles in sgAtg7 and sgAtg16l1 glial cells (Fig 2A and C). Similar results were obtained in sgAtg7 mouse embryonic fibroblasts (MEFs), suggesting that changes in early endosomal PI(3)P as a result of autophagy inhibition are consistent in other cell types (Fig EV2C and D). As observed with Rab5 (Fig 1F and G), EGFR exhibited a higher residency in EEA1+ endosomes upon autophagy inhibition in glial cells (Fig 2D and E).
Figure EV2. Total PI(3)P levels increase in autophagy‐inhibited cells.
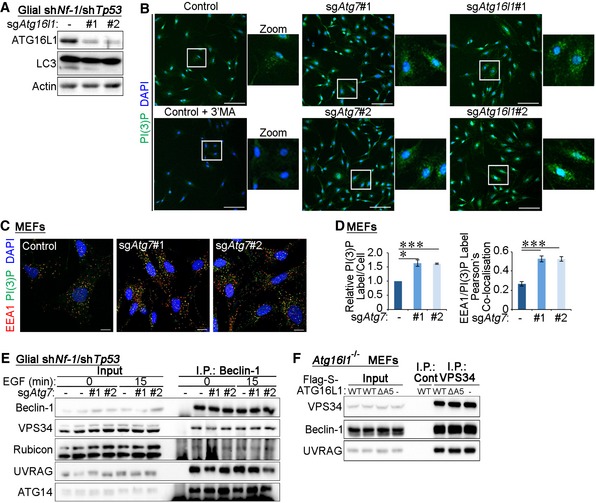
- Western blot analyses of shNf‐1/shTp53 glial cells expressing gRNA sequences targeting Atg16l1.
- 20× magnification of images in Fig 2A. Control, sgAtg7, or sgAtg16l1 cells were treated with 2 ng/ml EGF for 15 min. Cells were then processed for staining using a PI(3)P probe (Alexa 488‐labelled 2XFYVE domain). To ensure the specificity of the probe, control cells were pre‐treated with 5 mM 3'MA for 30 min. Scale bar: 100 μm.
- Control or sgAtg7 MEF cells were treated with 2 ng/ml EGF for 15 min before fixation and staining using EEA1 antibodies and a PI(3)P probe (Alexa 488‐labelled 2XFYVE domains). Scale bar: 10 μm.
- Quantification of PI(3)P+ vesicles per cell and the Pearson's colocalisation coefficient between PI(3)P and EEA1 (in C).
- Endogenous Beclin‐1 was immunoprecipitated from control and sgAtg7 cells that were stimulated with 2 ng/ml EGF for 15 min. Cells were lysed in CHAPS buffer followed by immunoprecipitation of Beclin‐1 and analyses by Western blotting.
- Atg16l1 −/− MEFs were reconstituted with wild type (WT) ATG16L1 or a mutant of ATG16L1 containing a deletion in the ATG5 binding domain (residues 1–39, ΔA5). Endogenous VPS34 was immunoprecipitated from these cells following 15 min of EGF (2 ng/ml). Binding partners were assessed by Western blotting.
Figure 2. Autophagy inhibition increases PI(3)P‐Positive early endosomes.
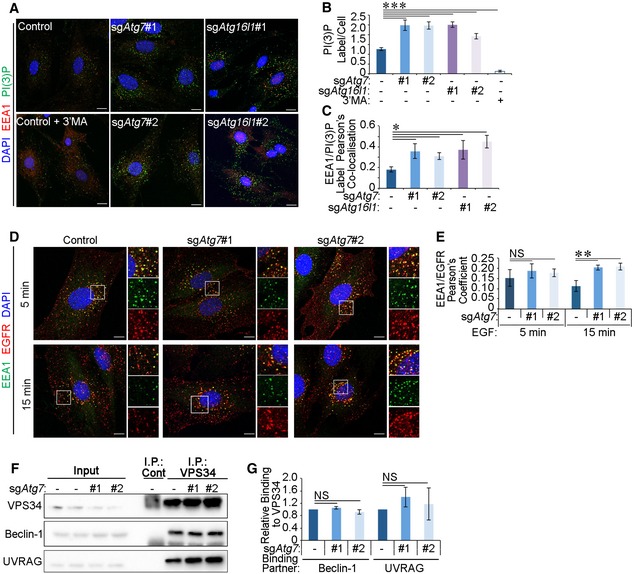
- Control, sgAtg7 or sgAtg16l1 cells were treated with 2 ng/ml EGF for 15 min. Cells were then processed for staining using anti‐EEA1 antibodies and PI(3)P probe (Alexa 488‐labelled 2XFYVE domains). To ensure the specificity of the probe, control cells were pre‐treated with 5 mM 3'MA for 30 min. Scale bar: 10 μm.
- Quantification of PI(3)P+ label intensity per cell (in A).
- Pearson's colocalisation coefficient between PI(3)P and EEA1 (in A).
- Control and sgAtg7 cells were stimulated 20 ng/ml EGF for 5 or 15 min before immunofluorescence staining against EEA1 and EGFR. Scale bar: 10 μm.
- Pearson's colocalisation coefficient between EEA1 and EGFR (in D).
- Endogenous VPS34 was immunoprecipitated from control and sgAtg7 cells that were treated with 2 ng/ml EGF for 15 min and then lysed in CHAPS‐containing detergent buffer and binding partners detected by Western blotting.
- Densitometry analyses of proteins coimmunoprecipitated with endogenous VPS34 (in F).
The production of PI(3)P catalysed by VPS34 is regulated by adaptor proteins that form part of the endocytic or autophagic VPS34 complexes 25, 26, 27, 28. To test whether changes in PI(3)P levels upon autophagy inhibition were due to differences in the binding of VPS34 to its adaptor proteins, immunoprecipitations of endogenous VPS34, or its binding partner Beclin‐1, were performed from control or sgAtg7 cells. The interactions between VPS34 and its complex components, including Beclin‐1, Rubicon, UVRAG and ATG14, were unaltered by autophagy status (Figs 2F and G, and EV2E). Similar results were obtained in MEF cells (Fig EV2F). Together, these findings suggest that the compositions of VPS34 complexes are not altered upon the deletion of autophagy genes.
Early endosomes are disrupted in the absence of autophagy machinery and stain positively for Gal8
To further examine the cause of EGFR accumulation at Rab5+ and EEA1+ endosomes, we tested the possibility that damaged early endosomes accumulated in cells that lack autophagy. Recognition of damaged endomembranes can be mediated by a family of glycan‐binding proteins known as Galectins that act as “eat‐me” signals 45. Of these, Gal3, Gal8 and Gal9 have been involved in the recognition of damaged lysosomes and salmonella‐containing vesicles 13, 14, 15. To assess whether autophagy‐deficient cells accumulate damaged early endosomes that may be recognised by these Galectins, we expressed YFP‐tagged Gal3, Gal8 or Gal9 in glial cells and their colocalisation with EEA1 was compared between control and sgAtg7 cells. Figure 3A and B shows that while the colocalisation between EEA1 and Gal3 or Gal9 was not significantly affected by ATG7 depletion, Gal8 localisation to early endosomes was strongly increased upon ATG7 loss. This suggests that damaged early endosomes may accumulate when autophagosome maturation is inhibited by ATG7 deletion. Moreover, Gal8 labelled EGF+ vesicles in ATG7‐deficient cells, indicating that EGFR can localise to this subset of damaged early endosomes (Fig 3C and D). Disruption of early endosomal function can also be induced chemically by monensin treatment in control glial cells resulting in enhanced localisation of Gal8 at early endosomes (Fig 3E and F) 46. Together, these data suggest that damaged early endosomes can accumulate in the absence of autophagy genes or during chemical disruption of endomembranes.
Figure 3. Early endosomes stain positively for Gal8 upon autophagy inhibition or monensin treatment.
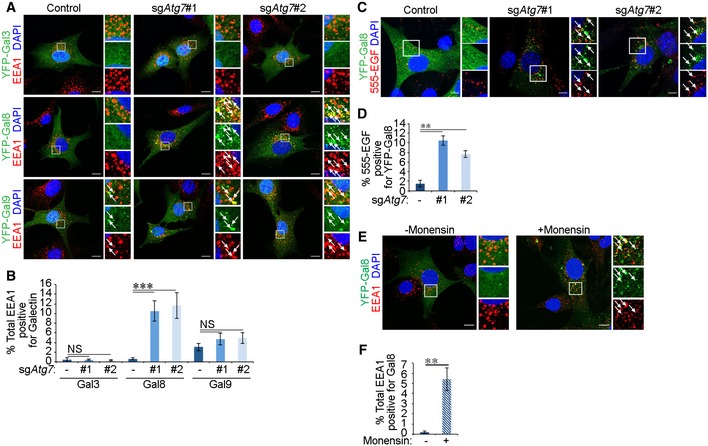
- Cells transiently expressing YFP‐Galectins (YFP‐Gal3, YFP‐Gal8, or YFP‐Gal9) were stimulated with 2 ng/ml EGF for 15 min before fixation and immunofluorescence staining against EEA1. Scale bar: 10 μm.
- Quantification of percentage of total EEA1 vesicles that colocalise with YFP‐Galectins (in A).
- Cells transiently expressing YFP‐Gal8 were stimulated with 20 ng/ml Alexa 555‐EGF (555‐EGF) for 15 min. Scale bar: 10 μm.
- Quantification of the percentage of YFP‐Gal8‐labelled vesicles that colocalise with 555‐EGF in control or ATG7‐deficient cells (in C).
- Cells transiently expressing YFP‐Gal8 were treated 100 μM monensin for 1 h and stimulated with 2 ng/ml EGF for 15 min before fixation and immunofluorescence staining against EEA1. Scale bar: 10 μm.
- Quantification of percentage of total EEA1 vesicles that colocalise with YFP‐Gal8 upon monensin treatment (in E).
Autophagy machinery targets early endosomes
We hypothesised that damaged early endosomes may be recognised by the autophagy machinery facilitating their targeting to the lysosome system. We tested this during monensin‐induced endosomal damage in autophagy‐competent cells. Indeed, monensin treatment resulted in the labelling of EEA1+ endosomes with GFP‐LC3 stably expressed in control glial or MEF cells but not in those lacking ATG7 (Figs 4A and B, and EV3A and B). The localisation of LC3 to EEA1+ vesicles suggests that the autophagy machinery may be required to recruit lysosomes to early endosomes. Indeed, EEA1+ vesicles stained positive for the lysosomal marker LAMP2 following monensin‐induced endomembrane stress in control but not sgAtg7 cells (Fig 4C and D). Interestingly, treatment of cells with bafilomycin A1 to inhibit lysosomal acidification led to the enhanced colocalisation between LC3 and EEA1, suggesting that the lysosomal targeting of early endosomes by the autophagy machinery occurs under basal levels (Fig 4A and B). A potential increase in total early endosome number was not significant between autophagy‐competent and autophagy‐incompetent cells (Fig EV3C) likely due to high variations in endosome numbers between cells or the existence of compensatory mechanisms that affect endosome biogenesis or maturation. Altogether, these findings suggest that targeting of early endosomes to lysosomes requires components of the autophagy machinery.
Figure 4. Autophagy machinery targets early endosomes.
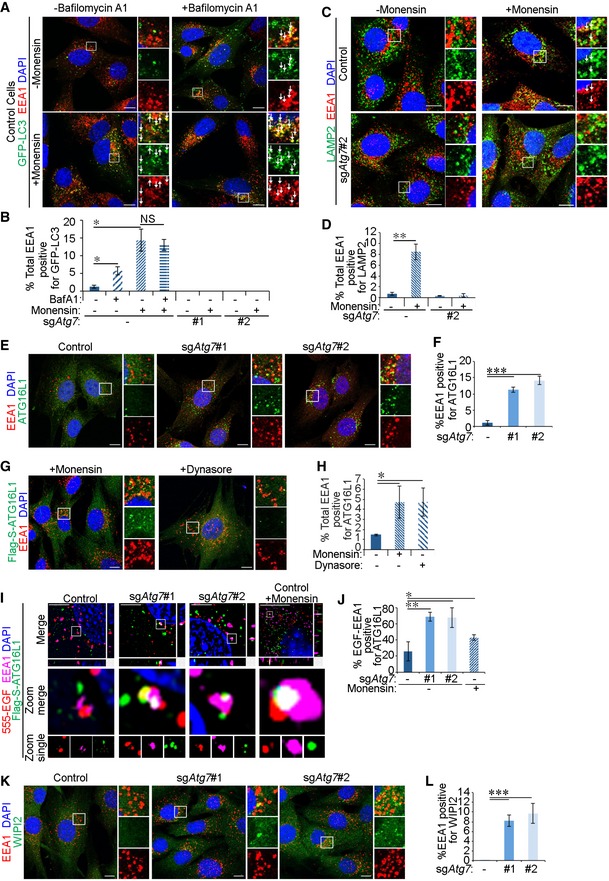
- Cells stably expressing GFP‐LC3 were treated with 100 μM monensin in the presence or absence of bafilomycin A1 (20 nM) for 1 h and stimulated with 2 ng/ml EGF for 15 min before fixation and immunofluorescence staining against EEA1. White arrows indicate colocalisation. Scale bar: 10 μm.
- Quantification of percentage of total EEA1 vesicles that colocalise with GFP‐LC3 (in A).
- Cells were treated for 1 h with 100 μM monensin and then stimulated with 2 ng/ml EGF for 15 min. LAMP2 and EEA1 were then detected by immunofluorescence staining. White arrows indicate colocalisation. Scale bar: 10 μm.
- Quantification of the percentage of total EEA1 vesicles that colocalise with LAMP2 (in C).
- Cells were treated with 2 ng/ml EGF for 15 min before immunofluorescence against endogenous ATG16L1 and EEA1. Scale bar: 10 μm.
- Quantification of percentage of total EEA1 puncta that colocalise with ATG16L1 (in E).
- Cells stably expressing Flag‐S‐ATG16L1 were treated for 1 h with either 100 μM monensin or 30 μM Dynasore, and then stimulated with 2 ng/ml EGF for 15 min. Cells were then stained by immunofluorescence against EEA1 and Flag tag. Scale bar: 10 μm.
- Quantification of percentage of total EEA1 vesicles that colocalise with Flag‐S‐ATG16L1 (in G).
- Untreated control or sgAtg7 cells, or control cells pre‐treated with 100 μM monensin 1 h, stably expressing Flag‐S‐ATG16L1 were stimulated 20 ng/ml Alexa 555‐EGF (555‐EGF) for 15 min before fixation and immunofluorescence staining against Flag tag and EEA1. Cells were then imaged by structured illumination microscopy (SIM), and images were reconstructed in Nikon Elements software. Scale bar: 10 μm.
- Quantification of the percentage of EGF‐EEA1 colocalised vesicles that stained triple‐positive with ATG16L1 by SIM (in I). Due to the low‐throughput nature of this assay, the following cell numbers were counted: control untreated (9 cells), sgAtg7#1 (10 cells), sgAtg7#2 (9 cells) and control + monensin (11 cells).
- Cells were treated with 2 ng/ml EGF for 15 min before immunofluorescence against endogenous WIPI2 and EEA1. Scale bar: 10 μm.
- Quantification of percentage of total EEA1 puncta that colocalise with WIPI2 (in K).
Figure EV3. The canonical autophagy machinery is required for early endosome targeting.
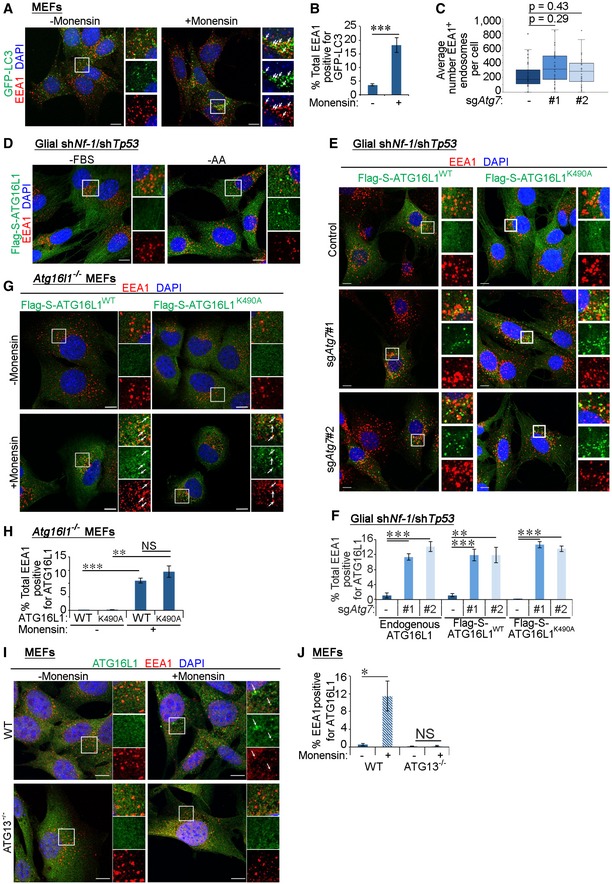
- MEF cells stably expressing GFP‐LC3 were serum starved for 4 h, then treated with 100 μM monensin for 1 h and stimulated with 2 ng/ml EGF for 15 min before immunofluorescence staining against EEA1. White arrows indicate colocalisation. Scale bar: 10 μm.
- Quantification of percentage of total EEA1 vesicles that colocalise with GFP‐LC3 (in A).
- Box‐and‐whisker representation of the quantification of the number of EEA1‐positive vesicles per cell in control and sgAtg7 cell lines. No significant differences are observed in early endosome numbers between control and autophagy‐inhibited cells (control versus sgAtg7#2: P = 0.29, control versus sgAtg7#3: P = 0.43).
- Glial shNf‐1/shTp53 control cells stably expressing Flag‐S‐ATG16L1 were either serum starved (‐FBS) or treated with amino acid‐free DMEM (‐AA) for 4 h before immunofluorescence staining against Flag tag and EEA1. Scale bar: 10 μm.
- Glial shNf‐1/shTp53 control and sgAtg7 cells stably expressing wild‐type ATG16L1 (Flag‐S‐ATG16L1WT) or LAP‐deficient K490A mutant of ATG16L1 (Flag‐S‐ATG16L1K490A) were serum starved for 4 h followed by stimulation with 2 ng/ml EGF for 15 min and immunofluorescence staining to detect Flag tag and EEA1. Scale bar: 10 μm.
- Quantification of percentage of total EEA1 vesicles positive for Flag‐S‐ATG16L1 (in E) with quantification of endogenous ATG16L1 included (see Fig 4E).
- Atg16l1 −/− MEF cells stably expressing wild‐type (Flag‐S‐ATG16L1WT) or LAP‐deficient mutant (Flag‐S‐ATG16L1K490A) of ATG16L1 were serum starved for 4 h followed by stimulation with 2 ng/ml EGF for 15 min and immunofluorescence staining to detect Flag tag and EEA1. White arrows indicate colocalisation. Scale bar: 10 μm.
- Quantification of the percentage of total EEA1 vesicles that stain positive for wild‐type or the K490A point mutant of ATG16L1 expressed in Atg16l1 −/− MEFs (in G).
- Atg13 −/− MEF cells were treated for 1 h with 100 μM monensin and then stimulated with 2 ng/ml EGF for 15 min. ATG16L1 and EEA1 were then detected by immunofluorescence staining. White arrows indicate colocalisation. Scale bar: 10 μm.
- Quantification of percentage of total EEA1 vesicles positive for Flag‐S‐ATG16L1 (in I).
We further interrogated the recruitment of upstream autophagy players to early endosomes. Staining of EEA1 showed no significant colocalisation with endogenous ATG16L1 in autophagy‐competent glial cells (Fig 4E and F) consistent with previously published findings 47. Similarly, induction of autophagy by amino acid starvation did not enhance the colocalisation of EEA1 and ATG16L1 in control cells (Fig EV3D). Intriguingly, a marked increase in the colocalisation between endogenous ATG16L1 and a subset of EEA1+ early endosomes was observed in ATG7‐deficient glial cells (Fig 4E and F). This was also observed with Flag‐S‐tagged ATG16L1 (Fig EV3E and F), demonstrating that both endogenous and stably expressed ATG16L1 show comparable localisation 48. Enhanced colocalisation between ATG16L1 and EEA1 was also observed upon chemically induced endomembrane damage triggered by monensin or Dynasore treatment 46, 49, 50 (Fig 4G and H). This recruitment is less than that observed in ATG7‐deficient cells potentially due to the transient localisation of early autophagy machinery during autophagosome maturation, which is arrested by ATG7 deletion 51. Importantly, structured illumination microscopy revealed that ATG16L1 localised to a subpopulation of EEA1+ early endosomes that were also positive for EGF in either sgAtg7‐ or monensin‐treated cells (Fig 4I and J), suggesting that upstream autophagy players can recognise damaged early endosomes where EGFR is arrested.
We explored the possibility that the autophagy machinery may be recruited to early endosomes through an LC3‐associated phagocytosis (LAP)‐like process which occurs on single membranes requiring the core autophagy machinery (such as ATG7 and ATG16L1) but not PI(3)P, WIPI2 or components of the ULK1 complex 52. Assessment of endogenous WIPI2 localisation revealed its positive staining on EEA1+ endosomes in sgAtg7 cells (Fig 4K and L). In addition, a mutant of ATG16L1 that cannot support non‐canonical LC3 lipidation on single membranes (ATG16L1K490A) 52 was also recruited to EEA1+ endosomes in glial cells deficient for ATG7 (Fig EV3E and F) or MEFs lacking endogenous ATG16L1 (Fig EV3G and H) further excluding a LAP‐like event. Moreover, the association between ATG16L1 and EEA1 was not observed in MEF cells lacking ATG13 (a component of the ULK1 complex required for canonical autophagy but not LAP‐like LC3 lipidation) (Fig EV3I and J) 53. Finally, the addition of bafilomycin A1, which inhibits the membrane recruitment of LC3 during LAP‐like processes 54, did not influence the localisation of GFP‐LC3 to early endosomes during monensin treatment (Fig 4A and B). Altogether, these findings uncover a novel process of early endosomal targeting by the canonical autophagy pathway.
Targeting of early endosomes by the autophagy machinery requires TBK1 activity and Gal8
Previous studies have shown that clearance of damaged endomembranes, as well as selective forms of autophagy, requires the activity of the TBK1 kinase, which phosphorylates autophagic receptors 15, 55. To test whether TBK1 also plays a role during the recognition of damaged early endosomes by autophagy, costaining of active phosphorylated TBK1 (p‐TBK1) and EEA1 was performed. EEA1 vesicles stained positively for p‐TBK1 in ATG7‐deficient cells (Fig 5A and B). Furthermore, treatment with the TBK1 inhibitors MRT68601 56 and momelotinib 57 ablated EEA1‐ATG16L1 colocalisation in sgAtg7‐ and monensin‐treated control cells (Fig 5C and D). These findings therefore suggest that TBK1 activity lies upstream of the recruitment of autophagy machinery to damaged early endosomes.
Figure 5. TBK1 and Gal8 are required for the recruitment of autophagy machinery to early endosomes.
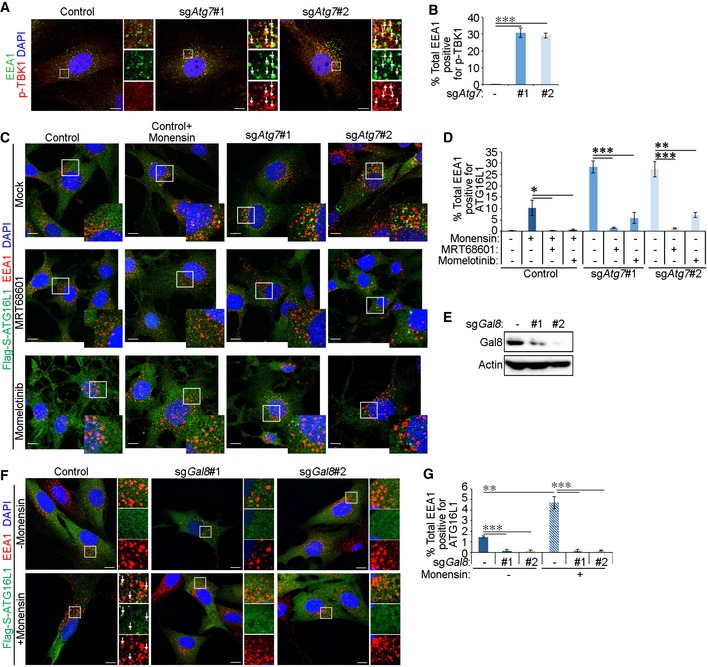
- Cells were treated for 15 min with 2 ng/ml EGF before fixation and immunofluorescence staining against EEA1 and p‐TBK1. White arrows indicate colocalisation. Scale bar: 10 μm.
- Quantification of percentage of total EEA1 vesicles that colocalise with p‐TBK1 (in A).
- Cells stably expressing Flag‐S‐ATG16L1 were pre‐treated for 1 h with TBK1 inhibitors (100 μM MRT68601 or 5 μM momelotinib). Control cells were also treated 100 μM monensin for 1 h as indicated. All cells were stimulated for 15 min with 2 ng/ml EGF followed by fixation and immunofluorescence staining against Flag tag and EEA1. Scale bar: 10 μm.
- Quantification of total EEA1 vesicles that colocalise with Flag‐S‐ATG16L1 (in C).
- Western blotting of shNf‐1/shTp53 glial cells expressing gRNA sequences targeting Gal8.
- Control and sgGal8 cells stably expressing Flag‐S‐ATG16L1 were treated 100 μM monensin for 1 h and stimulated with 2 ng/ml EGF for 15 min before fixation and immunofluorescence staining against EEA1 and Flag tag. White arrows indicate colocalisation. Scale bar: 10 μm.
- Quantification of percentage of total EEA1 vesicles that colocalise with Flag‐S‐ATG16L1 in sgGal8 cells (in F).
Having observed that damaged early endosomes are recognised by Gal8, we further examined whether the recruitment of the autophagy machinery to early endosomes required Gal8 expression. To do so, we ablated Gal8 expression by CRISPR/Cas9 (Fig 5E) and treated cells with monensin to induce endosome damage. As can be seen in Fig 5F and G, knockout of Gal8 disrupted the colocalisation between ATG16L1 and EEA1 during monensin treatment, suggesting that Gal8 is required for the recognition of damaged early endosomes by the autophagy machinery.
Autophagy loss disrupts Rab11‐mediated recycling of EGFR
We then investigated the consequences of EGFR accumulation in early endosomes during autophagy inhibition. Labelling of cells with fluorescent EGF showed that sgAtg7 cells exhibited an overall decrease in EGF signal (Figs 6A and B, and EV4A and B), despite the observed increase in EGF and EGFR colocalisation in autophagy‐deficient cells compared to their control counterparts (Fig 1D and E). Reduced EGF staining was not due to perturbed lysosomal function 58 (Fig EV4C) or altered EGFR degradation rate (Fig EV4D and E) consistent with the lack of change in EGFR‐late endosome colocalisation in the absence of autophagy (Fig EV4F and G). These data indicate that late endosome‐to‐lysosome trafficking axis is not affected by autophagy loss. Similarly, cell surface protein biotinylation experiments (outlined in Fig EV4H) showed no change in EGFR endocytosis rate in sgAtg7 or sgAtg16l1 cells (Fig 6C) in agreement with comparable EGFR‐Rab5/EEA1 colocalisation at early time points of EGF stimulation (Figs 1F and 2D). On the other hand, EGF chase experiments revealed that the recycling of EGFR to the plasma membrane was reduced upon the deletion of autophagy genes (Fig 6D). This defective recycling of EGFR reduces receptor availability at the plasma membrane, which, coupled with intact receptor degradation, can lead to diminished intracellular levels of EGF at later time points in autophagy‐deficient cells (Fig 6A). Interestingly, EGFR recycling was also reduced in the presence of the endosome damaging agent, monensin, when calculated as a percentage from endocytosed population (Fig 6D). Monensin treatment also perturbed EGFR endocytosis (Fig 6C) likely due to additional effects of this inhibitor. To mimic the recycling defect caused by autophagy loss, EGFRvIII was expressed in glial cells. This oncogenic mutant of EGFR lacks the EGF‐binding domain but can heterodimerise with the full‐length receptor, thereby altering its endocytic trafficking and increasing downstream signalling 8. EGFRvIII expression reduced the endocytic trafficking of full‐length EGFR (Fig 6C), as previously described 8, and prevented its recycling in control cells to a similar extent as loss of ATG7 or ATG16L1 (Fig 6D).
Figure 6. Autophagy loss disrupts Rab11‐mediated recycling of EGFR.
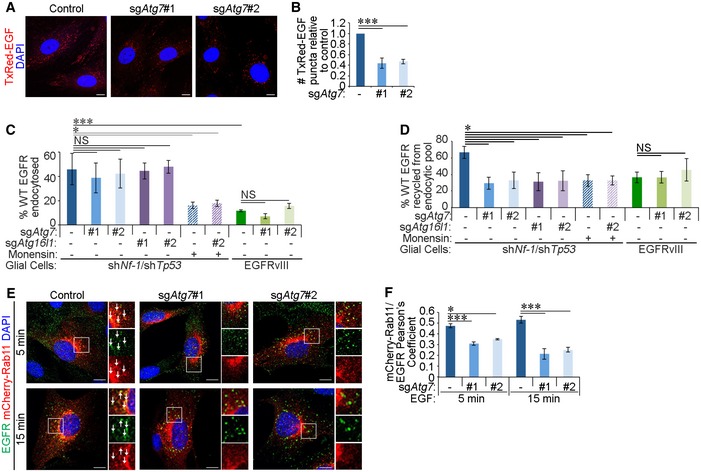
- Glial shNf‐1/shTp53 control and sgAtg7 cells were serum starved for 4 h and then stimulated with 20 ng/ml Texas Red‐EGF (TxRed‐EGF) for 30 min. Scale bar: 10 μm.
- Quantification of TxRed‐EGF puncta per cell, relative to the number taken up by control cells (in A).
- Endocytosis rate of wild‐type (WT) EGFR was assayed in control, sgAtg7 or sgAtg16l1 glial shNf‐1/shTp53 or EGFRvIII‐expressing cells by cell surface biotinylation and one application of 2 ng/ml EGF for 15 min. Monensin treatment was added as indicated.
- Plasma membrane recycling rate of WT EGFR in control, sgAtg7 or sgAtg16l1 glial shNf‐1/shTp53 or EGFRvIII‐expressing cells was assayed by cell surface biotinylation and successive applications of 2 ng/ml EGF treatments for 15 min (detailed in Fig EV4H). Monensin treatment was added as indicated.
- Glial shNf‐1/shTp53 control and sgAtg7 cells stably overexpressing mCherry‐Rab11 were serum starved for 4 h, then stimulated with 20 ng/ml EGF for 15 min, and then fixed and stained by immunofluorescence against EGFR. White arrows indicate colocalisation. Scale bar: 10 μm.
- Quantification of Pearson's colocalisation coefficient between mCherry‐Rab11 and EGFR (in E).
Figure EV4. Targeting of EGFR to late endosomes and Rab4+ recycling endosomes remain intact in the absence of autophagy.
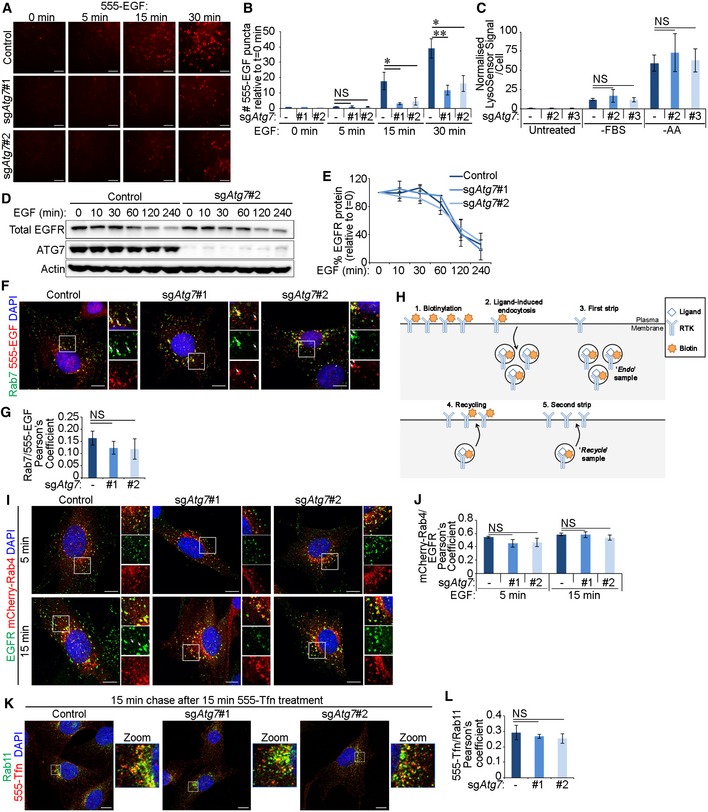
- Cells were treated with 20 ng/ml Alexa 555‐EGF (555‐EGF) for the indicated times before fixation. 555‐EGF was detected using the ImageXpress high‐content imaging platform, and representative images of 555‐EGF are shown. Scale bar: 100 μm.
- Following high‐content imaging of 555‐EGF and DAPI, 555‐EGF uptake relative to cell number was quantified and made relative in each cell line to background readings from unstimulated cells (t = 0 min) (in A).
- Control or sgAtg7 glial shNf‐1/shTp53 cells were either untreated, serum starved (‐FBS) for 4 h, or amino acid starved (‐AA) for 2 h and LysoSensor probe added for 1 h. Shown are quantifications of LysoSensor fluorescence intensity per cell normalised to untreated control cells.
- Cells were stimulated with 20 ng/ml EGF for the indicated times before EGFR levels were analysed by Western blotting.
- Densitometry analyses of Western blotting showing EGFR degradation with measurements made relative to EGFR levels in unstimulated cells (t = 0 min) (in D).
- Cells were stimulated with 20 ng/ml 555‐EGF for 15 min before immunofluorescence staining against endogenous Rab7. White arrows indicate colocalisation. Scale bar: 10 μm.
- Quantification of Pearson's colocalisation coefficient between 555‐EGF and Rab7 (in F).
- Schematic representation of cell surface protein biotinylation assay to assess endocytosis and recycling rates (see Fig 6C and D).
- Cells stably expressing mCherry‐Rab4 were stimulated with 20 ng/ml EGF for 5 or 15 min and then fixed and stained by immunofluorescence against EGFR. White arrows indicate colocalisation. Scale bar: 10 μm.
- Quantification of Pearson's colocalisation coefficient between mCherry‐Rab4 and EGFR (in I).
- Cells were stimulated with Alexa 555‐transferrin (555‐Tfn, 20 ng/ml) for 15 min and chased with media without transferrin for 15 min. Cells were then stained by immunofluorescence against Rab11. Scale bar: 10 μm.
- Quantification of Pearson's colocalisation coefficient between 555‐Tfn and Rab11 (in K).
To characterise the disruption of EGFR recycling in the absence of autophagy, we assessed EGFR colocalisation with the recycling endosome markers Rab4 and Rab11. Although the colocalisation of EGFR with Rab4 was not affected by autophagy inhibition (Fig EV4I and J), its colocalisation with Rab11 was markedly reduced (Fig 6E and F). This reduced recycling of EGFR in the absence of autophagy was not associated with a general defect in recycling endosomes as assessed by the recycling rate of the transferrin receptor, which was not affected by ATG7 loss (Fig EV4K and L). Together, these data indicate that autophagy is required for the efficient endocytic recycling of EGFR to the plasma membrane via Rab11 recycling endosomes.
Autophagy is required for the maintenance of EGF‐mediated signalling and survival
The recycling of RTKs to the plasma membrane is important for the maintenance of their signalling activities 4. To test the impact of reduced EGFR recycling upon autophagy inhibition, we assessed receptor activation and downstream signalling pathways during EGF stimulation in glial cells. Cells lacking the core canonical autophagy players, including ATG7, ATG16L1, ATG3, ATG13 or the cargo recognition protein Gal8, exhibited reduced EGF‐induced phosphorylation of EGFR, AKT and ERK (Figs 7A–C and EV5A and B). Importantly, no significant differences in signalling were observed at early time points of EGF stimulation in ATG7‐deficient cells, supporting our findings that the initial activation and endocytosis of EGFR were not affected by autophagy loss (Fig 7D and E). Expression of the endocytosis‐defective EGFRvIII mutant that enhances downstream signalling 59 restored EGF‐mediated AKT phosphorylation in sgAtg7 cells, thereby suggesting that altering the intracellular trafficking of EGFR can overcome the signalling defect caused by ATG7 loss (Fig EV5C). In addition, EGF‐induced nuclear translocation of EGFR, which is required for its role as a co‐transcriptional activator for pro‐growth and survival genes 60, was also reduced by autophagy loss (Fig 7F). Together, these data support the conclusion that the targeting of early endosomes by the autophagy machinery is required for proper cellular responses to EGF stimulation.
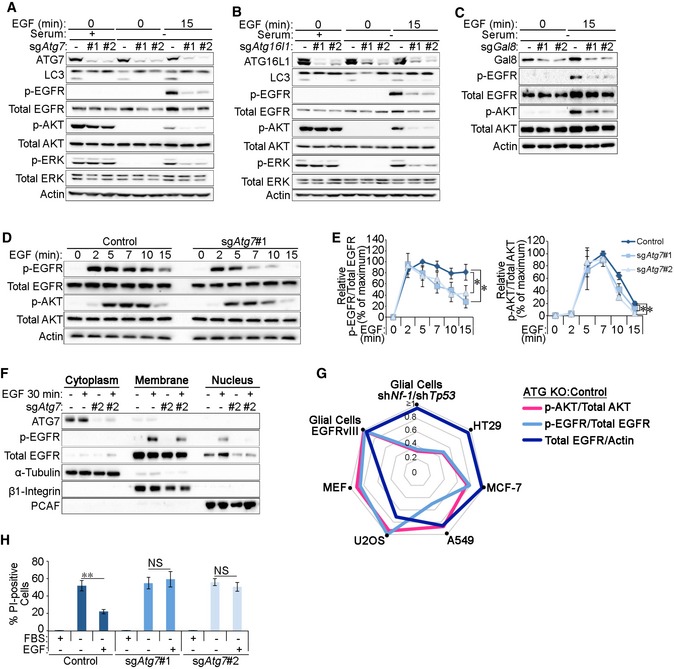
Figure 7. Autophagy is required for the maintenance of EGF‐mediated signalling and survival.
-
A–CGlial shNf‐1/shTp53 sgAtg7 (A), sgAtg16l1 (B) or sgGal8 (C) cells were either left untreated (+serum) or serum starved (−serum) for 4 h and then stimulated 2 ng/ml EGF for 15 min before analyses by Western blotting.
-
DGlial shNf‐1/shTp53 control and sgAtg7 cells were serum starved for 4 h and then stimulated with 2 ng/ml EGF for the indicated times before analysis of EGFR signalling pathway activity by Western blotting.
-
EPhosphorylated:total protein ratios for EGFR and AKT calculated from densitometry analyses of Western blots were made relative to their respective points of maximal stimulation (100%) (for EGFR: t = 5 min in control cells and for AKT: t = 7 min in control cells) (in D).
-
FGlial shNf‐1/shTp53 control and sgAtg7 cells were serum starved for 4 h with or without 20 ng/ml EGF 30‐min stimulation before subcellular fractionation and analysis by Western blotting.
-
GRadar chart indicating the relative levels of pAKT (pink line), pEGFR (light blue line) or total EGFR levels (dark blue line), in autophagy‐deficient cell lines relative to their control counterparts. The following knockouts were introduced: glial cells (sgAtg7), HT29 (crATG7), MCF‐7 (sgATG5), A549 (sgATG5), U2OS (sgATG5) and MEFs (Atg16l1 −/−).
-
HControl or sgAtg7 glial shNf‐1/shTp53 cells were serum starved for 24 h in the presence or absence of 20 ng/ml EGF. Quantification of the percentage of propidium iodide (PI)‐positive cells is shown.
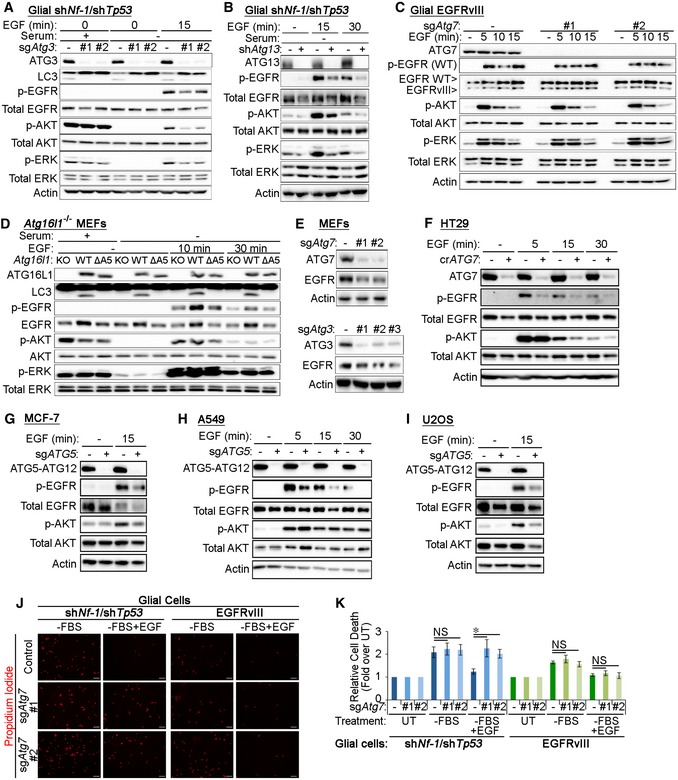
Figure EV5. Analyses of EGFR signalling and levels in various cell lines upon autophagy inhibition.
-
AGlial shNf‐1/shTp53 sgAtg3 cells were either left untreated (+serum) or serum starved for 4 h and then stimulated with 2 ng/ml EGF for 15 min before analysis by Western blotting.
-
BGlial shNf‐1/shTp53 shAtg13 cells were serum starved for 4 h and then stimulated with 2 ng/ml EGF for 15 or 30 min before analysis by Western blotting.
-
CGlial EGFRvIII‐expressing control and sgAtg7 cells were serum starved for 4 h and then stimulated with 2 ng/ml EGF before analysis by Western blotting.
-
DAtg16l1 −/− MEFs were reconstituted with wild‐type (WT) ATG16L1 or with a mutant of ATG16L1 with deletion of the ATG5‐binding domain (residues 1‐39, ΔA5). Cells were either untreated or serum starved for 4 h and then stimulated with 2 ng/ml EGF for 10 or 30 min before signalling pathway analyses by Western blotting.
-
ETotal EGFR levels in untreated sgAtg7 and sgAtg3 MEFs were analysed by Western blotting.
-
F–IThe following cells were serum starved for 4 h followed by stimulation with 2 ng/ml EGF before Western blot analyses: HT29 control and crATG7 (F); MCF‐7 control and sgATG5 (G); A549 control and sgATG5 (H); and U2OS control and sgATG5 (I).
-
JRepresentative images of propidium iodide staining in control or sgAtg7 glial shNf‐1/shTp53 or EGFRvIII‐expressing cells that were serum starved for 24 h in the presence or absence of 20 ng/ml EGF. Scale bar: 100 μm.
-
KQuantification of the experiment in J with propidium iodide relative fluorescence units (RFU) made relative to cell number as assessed by DAPI RFU measured by a fluorescence plate reader. These values were then made relative to the values in the negative control setting (untreated cells, UT).
Having observed similar early endosome defects in MEFs upon autophagy inhibition, we tested EGF‐mediated signalling in these cells. Intriguingly, AKT and ERK phosphorylation during EGF stimulation were comparable in autophagy‐deficient Atg16l1 −/− MEFs and those reconstituted with wild‐type ATG16L1 (ATG16L1WT, Fig EV5D). However, EGFR protein levels appeared to rely on functional autophagy in MEFs as total EGFR levels were elevated by the expression of ATG16L1WT in Atg16l1 −/− cells but not by an autophagy‐deficient mutant that cannot bind ATG5 (ATG16L1ΔA5) 61, 62. Similar reduction in EGFR levels was also observed in autophagy‐deficient MEFs lacking ATG7 or ATG3 (Fig EV5E) and are likely due to reduced EGFR plasma membrane localisation that may decrease its stability in the absence of acute EGF stimulation. These observations indicate that the disruption of endosomal homeostasis as a result of autophagy inhibition may impact the EGFR pathway in a cell‐type‐specific manner. To further investigate this, we tested cellular response to EGF stimulation in a panel of cell lines genetically engineered to inhibit autophagy (Fig EV5F–I). As can be seen in Fig 7G, autophagy inhibition led to a striking perturbation in EGFR phosphorylation or total levels in the majority of lines tested. This suggests that targeting of endosomes by autophagy can affect EGFR trafficking and activation in a panel of cell lines. On the other hand, the downstream Akt activation in response to EGF stimulation differed between the cell lines tested. This variation in signalling response to growth factor stimulation is likely dependent on additional oncogenic mutations that enhance pro‐growth signalling.
To test whether reduced EGFR signalling in the absence of autophagy impacted EGF‐mediated cell survival, glial cells were starved of growth factors (serum starvation) in the presence or absence of EGF and cell death was measured by propidium iodide staining. Figures 7H and EV5J and K show that while serum starvation‐induced cell death can be rescued in control cells by the addition of EGF, survival of ATG7‐deficient cells remained unaltered, mirroring their inability to maintain pro‐growth signalling. Reduced EGF‐mediated cell survival in sgAtg7 cells was rescued by the expression of EGFRvIII, thereby indicating that rescuing the signalling defect in autophagy‐deficient cells can prevent the onset of cell death (Fig EV5J and K). Taken together, these data demonstrate that quality control of early endosomes by autophagy is important for EGFR trafficking, pro‐growth signalling and cell survival.
Discussion
The results presented here outline a novel role for autophagy in regulating early endosome homeostasis. In the absence of autophagy, damaged or non‐functional early endosomes accumulate EGFR and inhibit its recycling back to the plasma membrane. This results in the perturbation of EGF‐mediated signalling and survival and underlies a novel mechanism by which autophagy can regulate RTK signalling.
Ligand binding stimulates the activation of RTKs leading to their internalisation via clathrin‐dependent or clathrin‐independent means 63. While the former is favoured by low doses of growth factors (such as those used in this study) and primarily results in receptor recycling to the plasma membrane, higher doses largely engage clathrin‐independent endocytosis machinery and direct the receptor to the lysosome 4. After their internalisation, active RTKs occupy pre‐early endosomes that are negative for PI(3)P. Production of PI(3)P following the recruitment of the endosome‐specific VPS34 complex II represses RTK signalling 3. Our study suggests that autophagy inhibition can reduce EGF‐mediated signalling by prolonging the residence of EGFR on PI(3)P+ early endosomes. Whether the overall increase in PI(3)P levels upon autophagy inhibition have additional consequences in the cell is yet to be determined.
Autophagy inhibition also suppresses EGF‐mediated signalling by reducing receptor recycling. Our data demonstrate that EGFR is arrested at early endosomes that fail to mature into Rab11+ recycling endosomes, while trafficking to Rab7+ or Rab4+ endosomes remain intact. Interestingly, autophagy knockdown does not appear to interfere with transferrin receptor recycling thereby suggesting an additional degree of cargo or endosomal population specificity. The mechanisms mediating the maturation of early to recycling endosomes are not fully understood, although it is known that phosphoinositol and Rab switching are required 64, 65. Previous studies comparing the internalisation of EGF and transferrin show that each cargo was incorporated at distinct sites with EGF being associated with vesicles enriched with EEA1 66, 67. This suggests that autophagy inhibition may affect a subset of early endosomes, where EGFR is localised, that are destined to mature into Rab11+ recycling endosomes. The precise mechanism through which this occurs remains unknown and is suggestive of the existence of unknown regulatory mechanisms governing endosome maturation. As autophagy appears to affect a subset of membrane receptors rather than general recycling, its inhibition may serve as a useful tool to decipher such mechanisms.
Previous studies suggest that ATG16L1 can traffic from the plasma membrane via clathrin‐coated pits to recycling endosomes without intermediately localising to early endosomes 33, 34, 68. In support of this, our data indicate that the colocalisation between ATG16L1 and early endosomes occurs at low levels and is only augmented upon autophagy inhibition or treatment with endosomal damaging agents. It may be that ATG16L1 traffics through early endosomes en route from the plasma membrane to recycling endosomes but is arrested in early endosomes when damage is recognised. This transient or lack of localisation between ATG16L1 and early endosomes under normal conditions could be important to prevent unwanted degradation of functional early endosomes. How early endosomes can be marked for degradation while recycling endosomes contribute to autophagosome biogenesis is yet to be elucidated.
The specific recruitment of Gal8, but not Gal3 or Gal9, to aberrant early endosomes suggests that these Galectins may differentially recognise a binding partner on the damaged endomembrane. Interestingly, out of these three Galectins, only Gal8 has been shown to bind the mTOR apparatus during lysosome injury 14. Furthermore, studies have shown that although the carbohydrate recognition domain of Galectins is structurally similar, they differ considerably in their amino acid compositions potentially leading to the recognition of specific carbohydrate moieties on damaged endosomes by Gal8 but not by other Galectins 69, 70. The precise signal that facilitates the recruitment of Gal8 to damaged early endosomes and the subsequent events that lead to the activation of the autophagy machinery remain to be elucidated.
The recruitment of LAMP2+ lysosomes to perturbed early endosomes in an autophagy‐dependent manner is suggestive of an “endosome‐phagy” process. Our findings thereby contribute to an expanding picture of organelle homeostasis mediated by autophagy. The selective degradation of ER, mitochondria and ribosomes has been shown to be critical for a variety of cellular processes 71, 72. Targeting of early endosomes by autophagy relies on the recognition of damaged membranes by Gal8‐ and TBK1‐mediated signalling, in a similar mechanism to the clearance of damaged lysosomes or bacteria‐containing vesicles 14, 15, 16. We find that the accumulation of damaged early endosomal membranes perturbs EGFR trafficking. Localisation of autophagy proteins to endosomes has also been observed during amphisome formation as well as during direct lipidation of LC3 on single membranes 73, 74. Whether these phenomena also contribute to the requirement of autophagy proteins in the proper trafficking of EGFR remains to be determined. Interestingly, EGFR knockout animals have neurological phenotypes that resemble autophagy loss 75, 76. Additionally, pro‐growth signalling pathways downstream of EGFR, which are known to be key drivers of oncogenesis, have been shown to be reduced in autophagy‐deficient tumour models 10, 41, 77. Altered RTK trafficking upon autophagy ablation may contribute to these defects. Therefore, it would be interesting to test whether additional plasma membrane endocytic cargoes are also affected by the lack of autophagy. Such mechanisms may provide novel means by which the homeostatic role of autophagy impacts important physiological and pathological processes such as in development, immunity and cancer.
Materials and Methods
Cell culture, generation and treatment
The following cell lines were maintained at 37°C and 5% CO2 in Dulbecco's modified Eagle's media (DMEM) supplemented with 10 units/ml penicillin, 100 μg/ml streptomycin, 2 mM l‐glutamine and 10% foetal bovine serum (FBS) (all reagents were manufactured by Thermo Fisher Scientific): XFM/tv‐a glial cells expressing the avian virus receptor, TV‐A, and harbouring a deletion in the Ink4a/Arf locus 40, 41, DF‐1 chicken fibroblasts (ATCC, CRL‐12203), mouse embryonic fibroblasts (MEFs), human embryonic kidney cells (293T), A549 adenocarcinoma cells (WT and ΔATG5, kind gift from Dr Simon Wilkinson, University of Edinburgh) 78, U2OS osteosarcoma cells, MCF‐7 breast cancer cells, Atg16l1 −/− MEFs (kind gift from Dr Shizuo Akira, Osaka University) 79 and Atg13 −/− MEFs.
HT29 colorectal cancer cells were maintained at 37°C and 5% CO2 in McCoy's 5A medium supplemented with 10 units/ml penicillin, 100 μg/ml streptomycin, 2 mM l‐glutamine and 10% FBS (all Thermo Fisher Scientific).
293T cells were used to generate retroviral particles by transfection with polyethylenimine‐based lipopolyplexes. For generation of RCAS viruses, DF‐1 cells were transfected with the respective RCAS vectors using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer's instructions. Following several passages, DF‐1 supernatants containing RCAS viruses were collected and used for 3 rounds of infection in XFM/tv‐a glial cells. All viruses were filtered and used to infect cells in the presence of 1 μg/ml polybrene (Millipore). Gene expression was tested by Western blotting to confirm enhanced or reduced protein levels. Importantly, pooled glial cells were assayed within 10 passages and were not freeze‐thawed.
To genetically inhibit autophagy in the array of cell lines tested in Figs 7 and EV5, several methods were required. Atg3 and Atg7 knockout MEFs were generated by CRISPR/Cas9‐mediated gene editing using Lipofectamine 2000‐mediated transient transfection of Cas9 and gRNA constructs, and pooled cells were confirmed by Western blotting. crRNA/tracRNA complexes targeting ATG7 sequences (Dharmacon, CM‐020112‐01‐0005) were introduced using DharmaFECT 2 Transfection Reagent (Dharmacon) in Cas9‐expressing HT29 cells according to the manufacturer's instructions. For glial cells, MEFs and HT29 cells, CRISPR/Cas9 knockout lines were generated by multiple rounds of transfection/infection (usually up to 3 rounds) in the absence of any antibiotics treatment, FACS sorting or single‐cell clone selection, and successful inhibition of autophagy was confirmed by Western blotting. Conversely, ATG5 knockout MCF‐7 and U2OS cell lines stably expressing Cas9 were generated by infection with lentiviral vectors expressing sgRNA targeting ATG5 sequences followed by puromycin selection (Millipore).
All EGF and transferrin stimulations were preceded by 4‐h incubation in serum‐free DMEM. EGF was used at 2 ng/ml or 20 ng/ml (as indicated in the figure legends), and fluorescently labelled EGF was used at 20 ng/ml. While lower concentrations of EGF favour receptor recycling and higher concentrations trigger lysosomal degradation 4, the observed effects of autophagy inhibition on the endocytic system were found to be independent of EGF concentrations.
For amino acid starvation, cells were incubated in amino acid‐free DMEM for 4 h.
The following inhibitors were used: Dynasore (30 μM, Sigma), monensin (100 μM, Sigma), bafilomycin A1 (20 nM, Cell Signaling Technologies), momelotinib (5 μM, Selleck Chemicals) and MRT68601 (100 μM, Tocris).
Plasmids
For expression in XFM/tv‐a glial cells, the following plasmids were used: lentiCas9‐Blast (gift from Feng Zhang, Addgene plasmid #52962), RCAS‐Y vector (gift from William Pavan, Addgene #11478), MSCV‐EGFRvIII (gift from Alonzo Ross, Addgene #20737), YFP‐Galectin‐3, YFP‐Galectin‐8 or YFP‐Galectin‐9 (kind gifts from Felix Randow: Thurston et al, 2012), mCherry‐Rab4 (gift from Michael Davidson, Addgene #55125), mCherry‐Rab5 and mCherry‐Rab11 (gift from Michael Davidson, Addgene #55124).
For the generation of the RCAS‐shRNA vectors, shRNA sequences were initially cloned into pSUPER.retro vectors (OligoEngine, VEC‐PRT‐0002) 41. The shRNA and H1 promoter sequences were then amplified by PCR and cloned into RCAS‐Y vectors using NotI and PacI restriction sites. The shRNA target sequences are as follows: RCAS‐shNf‐1 (5′ CAAGGAGTGTCTGATCAAC), RCAS‐shTp53 (5′ GTACATGTGTAATAGCTCC) and RCAS‐shAtg13 (5′ GAGAA GAATGTCCGAGAAT).
For CRISPR/Cas9‐mediated gene editing using RCAS‐Y vectors, sgRNA sequences were cloned into sgRNA‐expressing vectors (gift from George Church, Addgene #41824) as previously described 80. The sgRNA and U6 promoter sequences were then amplified by PCR and cloned into RCAS‐Y vectors using NotI restriction site. The following sgRNA targeting sequences were used: sgAtg3 #1 (5′ ATGTGATCAACACGGTGAA, sgAtg3 #2 (5′ GTTTACACCGCTTGTAGCA), sgAtg7 #1 (5′ TCACAGGTCCCCGGATTAG), sgAtg7 #2 (5′ GAAACTTGTTGAGGAGCAT), sgAtg16l1 #1 (5′ CGAACTGCACAAGAAGCGT), sgAtg16l1 #2 (5′ AAAGCATGACATGCCAAAT), sgGal8 #1 (5′ AGGCGTTGTCCTGTTGTTT) and sgGal8 #2 (5′ GGCTGCCTGCTTCACATAC). RCAS vectors expressing sgRNA sequences were infected into XFM/tv‐a glial cells expressing lentiCas9. Control cells were generated expressing lentiCas9 alone and mock infected with DF‐1 media.
For CRISPR/Cas9‐mediated gene editing in MEFs, the sgRNA vectors described above (Addgene #41824) were transfected along a Cas9 vector (hCas9, gift from George Church, Addgene #41815) using Lipofectamine 2000. Control cells were generated expressing Cas9 alone and empty gRNA vector.
For CRISPR/Cas9‐mediated gene editing in MCF‐7 and U2OS cells, lentiGuide‐puro lentivirus was used to target ATG5 (5′ GAGATATGGTTTGAATATGA).
Plasmids expressing retroviral pBabe‐Flag‐S‐ATG16L1WT, pBabe‐Flag‐S‐ATG16L1ΔA5 (deletion of Atg5‐binding domain, residues 1‐39) and pBabe‐GFP‐LC3 were described previously 61. pBabe‐Flag‐S‐ATG16L1K490A mutant was a kind gift from Oliver Florey (University of Cambridge) 52.
Cell lysis, Western blotting and immunoprecipitation
For cell lysate analyses, cells were washed twice in ice‐cold PBS before lysis in RIPA buffer (10 mM Tris pH 7.5, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.1% SDS, 1% Triton X‐100, 1 mM β‐ME, 0.5% sodium deoxycholate and 10% glycerol) supplemented with fresh protease inhibitor cocktail V and phosphatase inhibitors (Fisher Scientific, UK). Lysates were cleared by spinning at 21,000 g for 10 min at 4°C. SDS loading buffer (200 mM Tris pH 6.8, 8% SDS, 40% glycerol, 4% β‐mercaptoethanol) was diluted 1:4 into lysates. Following 5‐min incubation at 95°C, lysates were separated by SDS–PAGE and then transferred onto nitrocellulose membranes (Bio‐Rad). Membranes were blocked with 5% non‐fat milk TBST before probing with primary antibodies at room temperature for 2–4 h or at 4°C overnight. Following washes in TBST, membranes were then incubated with HRP‐conjugated secondary antibodies for 1 h at RT in milk/TBST and developed under UV light using Clarity™ Western ECL substrate (Bio‐Rad, 1705061). Western blotting densitometry analysis was performed in ImageLab (Bio‐Rad). The radar chart of pAKT, pEGFR and total EGFR ratios comparing ATG knockout to control cells (Fig 7G) was generated from densitometry quantifications of 3 independent experiments for each cell line (representative blots in Fig EV5).
For immunoprecipitations, cells were lysed in buffer containing either 0.8% CHAPS or 0.5% NP‐40 and 150 mM NaCl, 25 mM HEPES pH 7.5, 1 mM β‐mercaptoethanol, 0.2 mM CaCl2 and 0.5 mM MgCl2 supplemented with fresh phosphatase and protease inhibitors and the proteasome inhibitor MG132 (50 μM, Sigma).
Cell fractionation
To obtain cytosolic, membrane and nuclear fractions, cells were serum starved for 4 h and then stimulated with 20 ng/ml EGF for 30 min. Cells were then scrapped in an isotonic buffer (150 mM NaCl, 25 mM HEPES pH 7.5, 1 mM β‐mercaptoethanol, 0.2 mM CaCl2 and 0.5 mM MgCl2) supplemented with fresh phosphatase and protease inhibitors. For cytosolic fraction isolation, 1 μg/ml digitonin (Sigma) was added and lysates incubated for 30 min at 4°C before spinning for 1 min at 15,000 g. The resulting pellet was washed three times with the isotonic buffer and then resuspended in isotonic buffer containing 0.5% NP‐40 (Source BioScience). Lysates were then vortexed and spun at 15,000 g for 1 min. The resulting pellet containing the nuclear fraction was similarly washed and resuspended in SDS loading buffer diluted 1:4 in RIPA buffer followed by mechanical sheering using a syringe. The various cell fractions were then analysed by SDS–PAGE and Western blotting.
Antibodies
Antibodies against the following targets were used for either Western blotting (WB) or immunofluorescence (IF) as indicated: β‐actin (WB: Sigma, A5316), AKT (WB: CST, 9272), pSer473‐AKT (WB: CST, 4060), ATG3 (WB: MBL, M133‐3), ATG7 (WB: Sigma, A2856), ATG13 (WB: Sigma, SAR4200100), ATG5 (WB: Sigma, A0731), ATG14 (WB: MBL, PD026), ATG16L1 (WB and IF: MBL, PD040), ATG16L1 (WB: MBL, M150‐3), Beclin‐1 (WB: MBL, PD017), EEA1 (IF: CST, 3288), EEA1 (IF: BD Biosciences, 410456), EGFR (WB and IF: Santa Cruz, sc‐03), EGFR (WB: Millipore, 04‐290), pTyr1068‐EGFR (WB: CST, 2234), ERK (WB: CST, 9102), pThr202/pTyr204‐ERK (WB: CST, 4370), FIP200 (IF: Abcam, ab176816), Galectin‐3 (WB: Santa Cruz, sc‐32790), Galectin‐8 (WB: Abcam, ab109519), β1‐Integrin (WB: CST, 4706), LAMP2 (IF: Abcam, ab25631), LC3B (WB: Sigma, L7543), NF‐1 (WB: Bethyl Labs, A300‐140A), PCAF (WB: Santa Cruz, E‐08), VPS34/PIK3C3 (WB: CST, 4263), Rab5 (WB and IF: CST, 3547), Rab7 (IF: CST, 9367), Rubicon (WB: MBL, PD027), pSer172‐TBK1 (IF: CST, 5483), α‐Tubulin (WB: CST, 2144), UVRAG (WB: MBL, M160‐3B), WIPI2 (IF: Biorad, 2A2), Flag tag (WB and IF: Sigma, F1804), S tag (WB and IF: Bethyl Labs, A190‐135A), anti‐rabbit‐HRP secondary (WB: CST, 7074), anti‐mouse‐HRP secondary (WB: CST, 7076), anti‐rabbit‐Alexa 488 (IF: Invitrogen, A11008), anti‐rabbit‐Alexa 594 (IF: Invitrogen, A11012), anti‐mouse‐Alexa 488 (IF: Invitrogen, A1101), anti‐mouse‐Alexa 594 (IF: Invitrogen, A11032) and anti‐mouse‐Alexa 647 (IF: Invitrogen, A28181).
Fluorescence imaging
For immunofluorescence analyses, cells were grown on glass coverslips in a 6‐well plate. Twenty‐four hours later, cells were treated as indicated. Coverslips were then fixed with 3.7% paraformaldehyde (PFA) in 20 mM HEPES pH 7.5 for 10 min on ice and then 20 min at RT. Cells were permeabilised in 0.1% Triton X‐100 in PBS for 5 min at RT. Slides were then incubated in primary antibodies in blocking buffer (1% BSA in PBS) at 37°C for 2–3 h or overnight at 4°C, followed by incubation with Alexa‐conjugated secondary antibodies (Invitrogen) for 1 h at room temperature. Finally, nuclei were stained with 1 μg/ml DAPI (Sigma) for 5 min at RT; then, coverslips were mounted on microscope slides with Prolong Gold Anti‐fade (Invitrogen). Images were acquired using either the Leica SP5 confocal microscope or, for super‐resolution structured illumination microscopy (SIM), the Nikon N‐SIM. SIM images were reconstructed using the NIS Elements software.
Perinuclear EGFR localisation was assessed by staining endogenous EGFR following stimulation with 2 ng/ml EGF. Analyses were performed using ImageJ software by drawing circles around the nucleus with a diameter of 30 μm, which was classed as “perinuclear”. The number of EGFR puncta inside or outside this region was measured using the “Analyze particles” feature and a constant threshold for each experiment.
For live cell imaging, cells were plated on glass‐bottom plates (World Precision Instruments, FluoroDish FD35‐100) and starved of serum for 4 h before the addition of Alexa 555‐EGF (555‐EGF, Invitrogen E35350). Live cell imaging was performed using the Andor Dragonfly spinning disc confocal microscope. Vesicle tracking was performed by Imaris Tracking software.
To assay EGF uptake, cells plated on either coverslips or in a 12‐well plastic‐bottomed plate were stimulated with 20 ng/ml of TxRed‐EGF and 555‐EGF, respectively. Cells were then fixed with 3.7% PFA and stained for DAPI. 555‐EGF images were acquired using the ImageXpress high‐content plate reader, and TxRed‐EGF images were taken by confocal microscopy. To quantify these images, a constant threshold was applied and the ImageJ “Analyze particles” feature was used.
To assess the impact of autophagy loss on lysosomal acidification, LysoSensor (Invitrogen, L7535) was added to cells at 1 μM for 1 h before imaging unfixed cells using confocal microscopy and a 20× objective. Total fluorescence levels were then analysed in ImageJ and normalised to cell numbers followed by applying “Analyze particles” feature.
Transferrin recycling was assayed by stimulating cells with 20 ng/ml Alexa 555‐Transferrin (555‐Tfn, Invitrogen, T35352) and then chasing with Tfn‐free, serum‐free media to allow recycling of the Tfn before fixing with 3.7% PFA and staining for Rab11.
For EGF/EGFR colocalisation, serum‐starved cells were treated for 4 h before stimulating with 20 ng/ml 555‐EGF. Cells were then stained for EGFR, and numbers of colocalised puncta were quantified manually in ImageJ. Total puncta numbers were determined using a constant threshold on the “Analyze particles” feature.
The ImageJ “Coloc2” plugin was used to quantify Pearson's colocalisation coefficients between: EGFR and EEA1, Rab4, Rab5, Rab7 or Rab11; EEA1 and PI(3)P; and 555‐Tfn and Rab11. Alternatively, the Imaris Coloc software was used to calculate the Pearson's coefficient between 555‐EGF and EEA1, Rab5 or Rab7.
For colocalisation between EEA1 and ATG16L1, WIPI2, FIP200, Galectins, p‐TBK1, GFP‐LC3 or LAMP2 quantifications of colocalised and total puncta numbers were conducted manually and using the ImageJ “Analyze particles” feature with constant threshold, respectively.
Post‐fixation PI(3)P labelling
PI(3)P staining in cells was performed using a FYVE‐Alexa 488 probe (kind gift from Ian Ganley, University of Dundee) as previously described 44. Briefly, cells plated on coverslips were stimulated with 2 ng/ml EGF stimulation for 15 min. Treatment with 10 μg/ml 3‐methyladenine (3'MA, Sigma) for 1 h was used as a negative control. Cells were washed with PBS and placed in glutamate buffer (25 mM HEPES pH 7.4, 25 mM KCl, 2.5 mM MgAc, 5 mM EGTA, 150 mM potassium glutamate). Coverslips were then briefly submerged in liquid nitrogen before thawing at RT and then placed in glutamate buffer. Following washing with glutamate buffer, cells were fixed with 3.7% PFA for 30 min followed by quenching and incubation twice in DMEM with 10 mM HEPES pH 7.4 for 10 min. After blocking in 1% BSA in PBS, cells were processed for immunostaining with the inclusion of FYVE‐Alexa 488 (1:300) with the secondary antibody incubation.
Biotinylation endocytosis/recycling assay
Measurement of EGFR endocytosis and recycling was adapted from previously described protocols (outlined in Fig EV4H 81, 82). Briefly, serum‐starved cells (4 h) were washed twice with ice‐cold PBS and labelled with 0.2 mg/ml biotin (EZ‐Link sulfo‐NHS‐SS‐biotin, Thermo Fisher) for 30 min at 4°C. Excess sulfo‐NHS‐SS‐biotin was washed off with PBS and then quenched with 20 mM glycine for 15 min at 4°C. EGFR endocytosis was induced by treatment with 2 ng/ml EGF for 15 min at 37°C and then stopped with ice‐cold PBS washes. To strip biotin remaining on the cell surface, a reduction reaction was performed using a buffer containing 37.5 mM NaOH, 37.5 mM NaCl, 25 mM L‐glutathione, 25 mM MesNA and 0.5% BSA for 30 min at 4°C, which was quenched with 20 mM iodoacetamide (Sigma) in PBS 20 min at 4°C. Receptor recycling was then performed by incubating cells at 37°C in the presence of EGF for a further 15 min. The stripping procedure was then repeated. To isolate biotinylated proteins, cells were lysed in RIPA buffer before or after the recycling step, and then incubated overnight at 4°C with Streptavidin‐sepharose high‐performance beads (GE Healthcare 17‐5113‐01). Beads were washed three times with RIPA and then once with RIPA supplemented with 300 mM NaCl final concentration, followed by two washes with PBS. Beads were then analysed by SDS–PAGE and Western blotting.
EGF rescue of cell death assay
To investigate growth factor starvation‐induced cell death, cells were plated in a 12‐well dish and serum starved in the presence or absence of 20 ng/ml EGF. Twenty‐four hours later, propidium iodide (1 μg/ml, #10008351, Cayman) was added to cells and analysed using a Tecan Spark20M plate reader. Cells were then fixed with 3.7% PFA and total cell number was obtained by staining with DAPI and measurement using the Tecan plate reader. The ratio of propidium iodide/DAPI relative fluorescence was then calculated to obtain relative values of cell death. Representative cell images were taken on a Leica DM IL LED microscope using Qimaging Retiga EXi Fast1394.
Graphs and statistical analyses
All chart‐ and graph‐making and statistical analyses were performed using Microsoft Excel. A minimum of three independent experiments were used in each analysis, and statistical significance was measured using an unpaired 2‐sided Student's t‐test with significance set at: NS P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001.
Author contributions
JF, JS, RF & CK‐I performed the experiments; NTK provided critical input; JF and NG designed the study, analysed the data and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Review Process File
Acknowledgements
We would like to thank Ian Ganley (University of Dundee), Oliver Florey (University of Cambridge) and Felix Randow (University of Cambridge) for providing reagents. We thank Simon Wilkinson for critical reading of the manuscript. We are grateful to members of the N.G. laboratory for discussions and critical reading of the manuscript. N.G. is supported by a Cancer Research UK fellowship (C52370/A21586).
EMBO Reports (2019) 20: e47734
References
- 1. Grant BD, Donaldson JG (2009) Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 10: 597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simonsen A, Lippe R, Christoforidis S, Gaullier J‐M, Brech A, Callaghan J, Toh B‐H, Murphy C, Zerial M, Stenmark H (1998) EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394: 494–498 [DOI] [PubMed] [Google Scholar]
- 3. Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De Camilli P (2009) A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell 136: 1110–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP (2008) Clathrin‐mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell 15: 209–219 [DOI] [PubMed] [Google Scholar]
- 5. Barrow‐McGee R, Kishi N, Joffre C, Ménard L, Hervieu A, Bakhouche BA, Noval AJ, Mai A, Guzmán C, Robert‐Masson L et al (2016) Beta 1‐integrin‐c‐Met cooperation reveals an inside‐in survival signalling on autophagy‐related endomembranes. Nat Commun 7: 11942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lampada A, O'Prey J, Szabadkai G, Ryan KM, Hochhauser D, Salomoni P (2017) mTORC1‐independent autophagy regulates receptor tyrosine kinase phosphorylation in colorectal cancer cells via an mTORC2‐mediated mechanism. Cell Death Differ 24: 1045–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinez‐Lopez N, Athonvarangkul D, Mishall P, Sahu S, Singh R (2013) Autophagy proteins regulate ERK phosphorylation. Nat Commun 4: 2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gan HK, Cvrljevic AN, Johns TG (2013) The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J 280: 5350–5370 [DOI] [PubMed] [Google Scholar]
- 9. Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R (1995) Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 376: 337–341 [DOI] [PubMed] [Google Scholar]
- 10. Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS (2006) Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366: 2–16 [DOI] [PubMed] [Google Scholar]
- 11. Olayioye MA, Neve RM, Lane HA, Hynes NE (2000) The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19: 3159–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anding AL, Baehrecke EH (2017) Cleaning house: selective autophagy of organelles. Dev Cell 41: 10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chauhan S, Kumar S, Jain A, Ponpuak M, Mudd MH, Kimura T, Choi SW, Peters R, Mandell M, Bruun J‐A et al (2016) TRIMs and galectins globally cooperate and TRIM16 and Galectin‐3 Co‐direct autophagy in endomembrane damage homeostasis. Dev Cell 39: 13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jia J, Abudu YP, Claude‐Taupin A, Gu Y, Kumar S, Choi SW, Peters R, Mudd MH, Allers L, Salemi M et al (2018) Galectins control mTOR in response to endomembrane damage. Mol Cell 70: 120–135.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thurston TLM, Wandel MP, von Muhlinen N, Foeglein Á, Randow F (2012) Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482: 414–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thurston TL, Boyle KB, Allen M, Ravenhill BJ, Karpiyevich M, Bloor S, Kaul A, Noad J, Foeglein A, Matthews SA et al (2016) Recruitment of TBK1 to cytosol‐invading Salmonella induces WIPI2‐dependent antibacterial autophagy. EMBO J 35: 1779–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamb CA, Yoshimori T, Tooze SA (2013) The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol 14: 759–774 [DOI] [PubMed] [Google Scholar]
- 18. Fracchiolla D, Sawa‐Makarska J, Zens B, de Ruiter A, Zaffagnini G, Brezovich A, Romanov J, Runggatscher K, Kraft C, Zagrovic B et al (2016) Mechanism of cargo‐directed Atg8 conjugation during selective autophagy. ELife 5: e18544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith MD, Harley ME, Kemp AJ, Wills J, Lee M, Arends M, von Kriegsheim A, Behrends C, Wilkinson S (2017) CCPG1 Is a non‐canonical autophagy cargo receptor essential for ER‐Phagy and pancreatic ER proteostasis. Dev Cell 44: 217–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fraser J, Cabodevilla AG, Simpson J, Gammoh N (2017) Interplay of autophagy, receptor tyrosine kinase signalling and endocytic trafficking. Essays Biochem 61: 597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT (2008) Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3‐phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182: 685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kutateladze TG (2010) Translation of the phosphoinositide code by PI effectors. Nat Chem Biol 6: 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marat AL, Haucke V (2016) Phosphatidylinositol 3‐phosphates‐at the interface between cell signalling and membrane traffic. EMBO J 35: 561–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Volinia S, Dhand R, Vanhaesebroeck B, MacDougall LK, Stein R, Zvelebil MJ, Domin J, Panaretou C, Waterfield MD (1995) A human phosphatidylinositol 3‐kinase complex related to the yeast Vps34p‐Vps15p protein sorting system. EMBO J 14: 3339–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Itakura E, Kishi C, Inoue K, Mizushima N (2008) Beclin 1 forms two distinct phosphatidylinositol 3‐kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 19: 5360–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kihara A, Noda T, Ishihara N, Ohsumi Y (2001) Two distinct Vps34 phosphatidylinositol 3–kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae . J Cell Biol 152: 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, Maejima I, Shirahama‐Noda K, Ichimura T, Isobe T et al (2009) Two Beclin 1‐binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol 11: 385–396 [DOI] [PubMed] [Google Scholar]
- 28. Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, Heintz N, Yue Z (2009) Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1–phosphatidylinositol‐3‐kinase complex. Nat Cell Biol 11: 468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wei Y, Zou Z, Becker N, Anderson M, Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW et al (2013) EGFR‐mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell 154: 1269–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuan H‐X, Russell RC, Guan K‐L (2013) Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient stress‐induced autophagy. Autophagy 9: 1983–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang D, Wang W, Sun X, Xu D, Wang C, Zhang Q, Wang H, Luo W, Chen Y, Chen H et al (2016) AMPK regulates autophagy by phosphorylating BECN1 at threonine 388. Autophagy 12: 1447–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Longatti A, Lamb CA, Razi M, Yoshimura S, Barr FA, Tooze SA (2012) TBC1D14 regulates autophagosome formation via Rab11‐ and ULK1‐positive recycling endosomes. J Cell Biol 197: 659–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Puri C, Vicinanza M, Ashkenazi A, Gratian MJ, Zhang Q, Bento CF, Renna M, Menzies FM, Rubinsztein DC (2018) The RAB11A‐positive compartment is a primary platform for autophagosome assembly mediated by WIPI2 recognition of PI3P‐RAB11A. Dev Cell 45: 114–131.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Søreng K, Munson MJ, Lamb CA, Bjørndal GT, Pankiv S, Carlsson SR, Tooze SA, Simonsen A (2018) SNX18 regulates ATG9A trafficking from recycling endosomes by recruiting Dynamin‐2. EMBO Rep 19: e44837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fader CM, Sánchez DG, Mestre MB, Colombo MI (2009) TI‐VAMP/VAMP7 and VAMP3/cellubrevin: two v‐SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta BBA ‐ Mol Cell Res 1793: 1901–1916 [DOI] [PubMed] [Google Scholar]
- 36. Itakura E, Kishi‐Itakura C, Mizushima N (2012) The hairpin‐type tail‐anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151: 1256–1269 [DOI] [PubMed] [Google Scholar]
- 37. Jager S (2004) Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 117: 4837–4848 [DOI] [PubMed] [Google Scholar]
- 38. Takahashi Y, He H, Tang Z, Hattori T, Liu Y, Young MM, Serfass JM, Chen L, Gebru M, Chen C et al (2018) An autophagy assay reveals the ESCRT‐III component CHMP2A as a regulator of phagophore closure. Nat Commun 9: 2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wee P, Wang Z (2017) Epidermal growth factor receptor cell proliferation signaling pathways. Cancers 9: 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC (2001) PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo . Genes Dev 15: 1913–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gammoh N, Fraser J, Puente C, Syred HM, Kang H, Ozawa T, Lam D, Acosta JC, Finch AJ, Holland E et al (2016) Suppression of autophagy impedes glioblastoma development and induces senescence. Autophagy 12: 1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sorkin A, Goh LK (2009) Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res 315: 683–696 [DOI] [PubMed] [Google Scholar]
- 43. Nascimbeni AC, Codogno P, Morel E (2017) Phosphatidylinositol‐3‐phosphate in the regulation of autophagy membrane dynamics. FEBS J 284: 1267–1278 [DOI] [PubMed] [Google Scholar]
- 44. Munson M, Ganley I (2016) Determination of cellular phosphatidylinositol‐3‐phosphate (PI3P) levels using a fluorescently labelled selective PI3P binding domain (PX). BIO‐Protoc 6: e1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boyle KB, Randow F (2013) The role of “eat‐me” signals and autophagy cargo receptors in innate immunity. Curr Opin Microbiol 16: 339–348 [DOI] [PubMed] [Google Scholar]
- 46. Basu SK, Goldstein JL, Anderson RGW, Brown MS (1981) Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell 24: 493–502 [DOI] [PubMed] [Google Scholar]
- 47. Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC (2013) Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell 154: 1285–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li J, Chen Z, Stang MT, Gao W (2017) Transiently expressed ATG16L1 inhibits autophagosome biogenesis and aberrantly targets RAB11‐positive recycling endosomes. Autophagy 13: 345–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A (2009) The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell 17: 712–723 [DOI] [PubMed] [Google Scholar]
- 50. Mesaki K, Tanabe K, Obayashi M, Oe N, Takei K (2011) Fission of tubular endosomes triggers endosomal acidification and movement. PLoS ONE 6: e19764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koyama‐Honda I, Itakura E, Fujiwara TK, Mizushima N (2013) Temporal analysis of recruitment of mammalian ATG proteins to the autophagosome formation site. Autophagy 9: 1491–1499 [DOI] [PubMed] [Google Scholar]
- 52. Fletcher K, Ulferts R, Jacquin E, Veith T, Gammoh N, Arasteh JM, Mayer U, Carding SR, Wileman T, Beale R et al (2018) The WD40 domain of ATG16L1 is required for its non‐canonical role in lipidation of LC3 at single membranes. EMBO J 37: e97840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jacquin E, Leclerc‐Mercier S, Judon C, Blanchard E, Fraitag S, Florey O (2017) Pharmacological modulators of autophagy activate a parallel noncanonical pathway driving unconventional LC3 lipidation. Autophagy 13: 854–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Florey O, Gammoh N, Kim SE, Jiang X, Overholtzer M (2015) V‐ATPase and osmotic imbalances activate endolysosomal LC3 lipidation. Autophagy 11: 88–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Heo J‐M, Ordureau A, Paulo JA, Rinehart J, Harper JW (2015) The PINK1‐PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy. Mol Cell 60: 7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Newman AC, Scholefield CL, Kemp AJ, Newman M, McIver EG, Kamal A, Wilkinson S (2012) TBK1 kinase addiction in lung cancer cells is mediated via autophagy of Tax1bp1/Ndp52 and non‐canonical NF‐κB signalling. PLoS ONE 7: e50672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhu Z, Aref AR, Cohoon TJ, Barbie TU, Imamura Y, Yang S, Moody SE, Shen RR, Schinzel AC, Thai TC et al (2014) Inhibition of KRAS‐driven tumorigenicity by interruption of an autocrine cytokine circuit. Cancer Discov 4: 452–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou J, Tan S‐H, Nicolas V, Bauvy C, Yang N‐D, Zhang J, Xue Y, Codogno P, Shen H‐M (2013) Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome‐lysosome fusion. Cell Res 23: 508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fan Q‐W, Cheng CK, Gustafson WC, Charron E, Zipper P, Wong RA, Chen J, Lau J, Knobbe‐Thomsen C, Weller M et al (2013) EGFR phosphorylates tumor‐derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell 24: 438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brand TM, Iida M, Luthar N, Starr MM, Huppert EJ, Wheeler DL (2013) Nuclear EGFR as a molecular target in cancer. Radiother Oncol 108: 370–377 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 61. Gammoh N, Florey O, Overholtzer M, Jiang X (2012) Interaction between FIP200 and ATG16L1 distinguishes ULK1 complex–dependent and –independent autophagy. Nat Struct Mol Biol 20: 144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dudley LJ, Cabodevilla AG, Makar AN, Sztacho M, Michelberger T, Marsh JA, Houston DR, Martens S, Jiang X, Gammoh N (2019) Intrinsic lipid binding activity of ATG16L1 supports efficient membrane anchoring and autophagy. EMBO J 38: e100554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ketel K, Krauss M, Nicot A‐S, Puchkov D, Wieffer M, Müller R, Subramanian D, Schultz C, Laporte J, Haucke V (2016) A phosphoinositide conversion mechanism for exit from endosomes. Nature 529: 408–412 [DOI] [PubMed] [Google Scholar]
- 65. Ullrich O (1996) Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol 135: 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lakadamyali M, Rust MJ, Zhuang X (2006) Ligands for clathrin‐mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell 124: 997–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leonard D, Hayakawa A, Lawe D, Lambright D, Bellve KD, Standley C, Lifshitz LM, Fogarty KE, Corvera S (2008) Sorting of EGF and transferrin at the plasma membrane and by cargo‐specific signaling to EEA1‐enriched endosomes. J Cell Sci 121: 3445–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC (2010) Plasma membrane contributes to the formation of pre‐autophagosomal structures. Nat Cell Biol 12: 747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Guardia CMA, Gauto DF, Di Lella S, Rabinovich GA, Martí MA, Estrin DA (2011) An integrated computational analysis of the structure, dynamics, and ligand binding interactions of the human galectin network. J Chem Inf Model 51: 1918–1930 [DOI] [PubMed] [Google Scholar]
- 70. Kumar S, Frank M, Schwartz‐Albiez R (2013) Understanding the specificity of human galectin‐8C domain interactions with its glycan ligands based on molecular dynamics simulations. PLoS ONE 8: e59761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gatica D, Lahiri V, Klionsky DJ (2018) Cargo recognition and degradation by selective autophagy. Nat Cell Biol 20: 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rogov V, Dötsch V, Johansen T, Kirkin V (2014) Interactions between autophagy receptors and ubiquitin‐like proteins form the molecular basis for selective autophagy. Mol Cell 53: 167–178 [DOI] [PubMed] [Google Scholar]
- 73. Florey V, Kim SE, Sandoval CP, Haynes CM, Overholtzer M (2011) Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol 13: 1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hoyvik H, Gordon PB, Berg TO, Strømhaug PE, Seglen PO (1991) Inhibition of autophagic‐lysosomal delivery and autophagic lactolysis by asparagine. J Cell Biol 113: 1305–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki‐Migishima R, Yokoyama M, Mishima K, Saito I, Okano H et al (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441: 885–889 [DOI] [PubMed] [Google Scholar]
- 76. Sibilia M, Steinbach JP, Stingl L, Aguzzi A, Wagner EF (1998) A strain‐independent postnatal neurodegeneration in mice lacking the EGF receptor. EMBO J 17: 719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Karsli‐Uzunbas G, Guo JY, Price S, Teng X, Laddha SV, Khor S, Kalaany NY, Jacks T, Chan CS, Rabinowitz JD et al (2014) Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov 4: 914–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Newman AC, Kemp AJ, Drabsch Y, Behrends C, Wilkinson S (2017) Autophagy acts through TRAF3 and RELB to regulate gene expression via antagonism of SMAD proteins. Nat Commun 8: 1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Saitoh T, Fujita N, Jang MH, Uematsu S, Yang B‐G, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M et al (2008) Loss of the autophagy protein Atg16L1 enhances endotoxin‐induced IL‐1β production. Nature 456: 264–268 [DOI] [PubMed] [Google Scholar]
- 80. Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM (2013) RNA‐guided human genome engineering via Cas9. Science 339: 823–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nishimura N, Sasaki T (2008) Cell‐surface biotinylation to study endocytosis and recycling of occludin In Exocytosis and endocytosis, Ivanov AI. (ed.), pp 89–96. Totowa, NJ: Humana Press; [DOI] [PubMed] [Google Scholar]
- 82. Wu X, Fleming A, Ricketts T, Pavel M, Virgin H, Menzies FM, Rubinsztein DC (2016) Autophagy regulates Notch degradation and modulates stem cell development and neurogenesis. Nat Commun 7: 10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Review Process File


