Abstract
Centromeres are defined epigenetically by nucleosomes containing the histone H3 variant CENP‐A, upon which the constitutive centromere‐associated network of proteins (CCAN) is built. CENP‐C is considered to be a central organizer of the CCAN. We provide new molecular insights into the structure of human CENP‐A nucleosomes, in isolation and in complex with the CENP‐C central region (CENP‐CCR), the main CENP‐A binding module of human CENP‐C. We establish that the short αN helix of CENP‐A promotes DNA flexibility at the nucleosome ends, independently of the sequence it wraps. Furthermore, we show that, in vitro, two regions of human CENP‐C (CENP‐CCR and CENP‐Cmotif) both bind exclusively to the CENP‐A nucleosome. We find CENP‐CCR to bind with high affinity due to an extended hydrophobic area made up of CENP‐AV 532 and CENP‐AV 533. Importantly, we identify two key conformational changes within the CENP‐A nucleosome upon CENP‐C binding. First, the loose DNA wrapping of CENP‐A nucleosomes is further exacerbated, through destabilization of the H2A C‐terminal tail. Second, CENP‐CCR rigidifies the N‐terminal tail of H4 in the conformation favoring H4K20 monomethylation, essential for a functional centromere.
Keywords: CENP‐A, CENP‐C, centromere, cryo‐EM, nucleosome
Subject Categories: Chromatin, Epigenetics, Genomics & Functional Genomics; Structural Biology
Introduction
The centromere is a chromosomal locus that directs accurate segregation of chromosomes during cell division 1. Defects in chromosome segregation lead to aneuploidy, a hallmark of cancer 2.
DNA sequences underlining human centromeres are composed of AT‐rich repeats (termed α‐satellites), but these are neither necessary nor sufficient for centromere function. Instead, centromeres are specified epigenetically by the presence of the histone H3 variant, CENP‐A [reviewed in 3]. Chromatin containing CENP‐A nucleosomes must have unique structural properties to organize the constitutive centromere‐associated network (CCAN). It is therefore critical to gain a full structural understanding of the CENP‐A nucleosome alone and in complex with the two key components of the CCAN with which it interacts directly and specifically—CENP‐C and CENP‐N 4. Initial clues on CENP‐A nucleosome‐specific features came from crystallographic studies of the (CENP‐A/H4)2 tetramer 5 and of the CENP‐A nucleosome 6. These studies implied that the CENP‐A nucleosome has an octameric histone core, similar to canonical nucleosomes in composition and structure. DNA ends in the crystal structure of CENP‐A nucleosome 6 are disordered, indicating increased DNA flexibility. Recently, cryo‐EM structures of the human CENP‐A nucleosome in complex with human CENP‐N have been reported by several groups 7, 8, 9, revealing high‐resolution molecular determinants for the CENP‐A/CENP‐N interaction.
CENP‐C is a central component of the CCAN, responsible for interactions both with the CENP‐A nucleosome on the chromatin‐side and with subunits of the Mis12 complex on the kinetochore side 10. Human CENP‐C is a 934 amino acid long disordered protein, depletion of which leads to cell division defects and chromosome mis‐segregation 11, 12. Two regions in human CENP‐C have been identified as nucleosome binding regions: (i) the central region (aa 426–537), CENP‐CCR, that is necessary and sufficient to promote CENP‐A nucleosome binding in vitro and kinetochore targeting in vivo and (ii) the CENP‐C motif (aa 736–758), CENP‐Cmotif, that is conserved across species, but is not sufficient for centromere targeting in the absence of endogenous CENP‐C as it requires the CENP‐C dimerization domain. The CENP‐Cmotif is dispensable for epigenetic stability of the CENP‐A nucleosomes 10, 11, 13.
The current molecular understanding of the CENP‐A nucleosome/CENP‐C interactions is based on the crystal structure of the canonical D. melanogaster nucleosome in which the C‐terminal tail of histone H3 is replaced by the C‐terminal tail of rat CENP‐A, in complex with the rat CENP‐C motif 14.
Here, we report a 3.8 Å cryo‐EM structure of the CENP‐A nucleosome that confirms flexibility of DNA ends as an intrinsic property of CENP‐A nucleosomes. We find that terminal DNA flexibility is independent of the nature of the underlining DNA sequence and is instead dictated primarily by the N‐terminal tail of CENP‐A. Furthermore, we find both nucleosome binding domains of CENP‐C, CENP‐CCR and CENP‐Cmotif, to be specific for CENP‐A nucleosomes, where CENP‐CCR shows stronger binding. We also determined the cryo‐EM structure of the human CENP‐A nucleosome in complex with human CENP‐CCR at 3.1 Å resolution and identified CENP‐AV532 and CENP‐AV533 as the key determinants for strong affinity of the CENP‐A/CENP‐C interaction. We notice conformational changes within the CENP‐A nucleosome upon binding of CENP‐CCR. The enhanced DNA unwrapping is facilitated by destabilization of the H2A C‐terminal tail while the H4 N‐terminal tail is stabilized in the conformation that favors centromere‐specific H4K20 monomethylation.
In summary, our work provides a high‐resolution, integrated view of the human CENP‐A nucleosome with its key CCAN partner, human CENP‐C. We establish CENP‐A nucleosomes as the sole CENP‐C binder, and we provide a molecular understanding for the higher specificity of the CENP‐CCR compared to the CENP‐Cmotif. Finally, our study identifies conformational changes in the nucleosome, taking place upon binding.
Results
CENP‐A nucleosome has flexible DNA ends, irrespective of DNA sequence
Ever since CENP‐A has been identified as the key epigenetic mark of the centromere, a central question has been how it is distinguished from canonical nucleosomes 15. Initial in vitro studies 5, 6 together with recent research in cells strongly support an octameric nucleosome, similar to the canonical one 16, 17. In the last 10 years, several studies both in vivo 16, 18 and in vitro 6, 18, 19, 20 have identified flexible DNA ends as a unique feature of CENP‐A nucleosomes but to which degree DNA sequence and/or crystal packing contributed to unwrapping remained unclear. To determine this directly, we MNase digested CENP‐A nucleosomes assembled both on synthetic super‐positioning DNA “601” 21 and on two natural α‐satellite DNA constructs 16, with and without the CENP‐B box, a 17 bp sequence recognized by CENP‐B 22, respectively. Since the exact nucleosome positioning on the sequence that contains the CENP‐B box is not precisely mapped, we used a full‐length α‐satellite repeat (171 bp) with a CENP‐B box at one of the ends. For all three DNA sequences, we observed faster DNA digestion when assembled on CENP‐A nucleosomes in comparison with H3 nucleosomes (Figs 1A and EV1A). The DNA unwrapping of the CENP‐A nucleosome has been linked to properties of the N‐terminal sequence of CENP‐A 18, 23. Indeed, when we substitute residues 1–49 of CENP‐A with 1–50 of H3, we completely loose the DNA flexibility (Fig EV1B). The converse is also true where H3 nucleosomes bearing the N‐terminal tail of CENP‐A undergo DNA unwrapping to a similar extent as CENP‐A nucleosomes (Fig EV1B). We therefore conclude that flexibility of the DNA ends is an intrinsic property of the CENP‐A nucleosome that is regulated by its N‐terminal tail and is independent of DNA sequence.
Figure 1. CENP‐A nucleosome has flexible DNA ends independently of the wrapped DNA sequence.
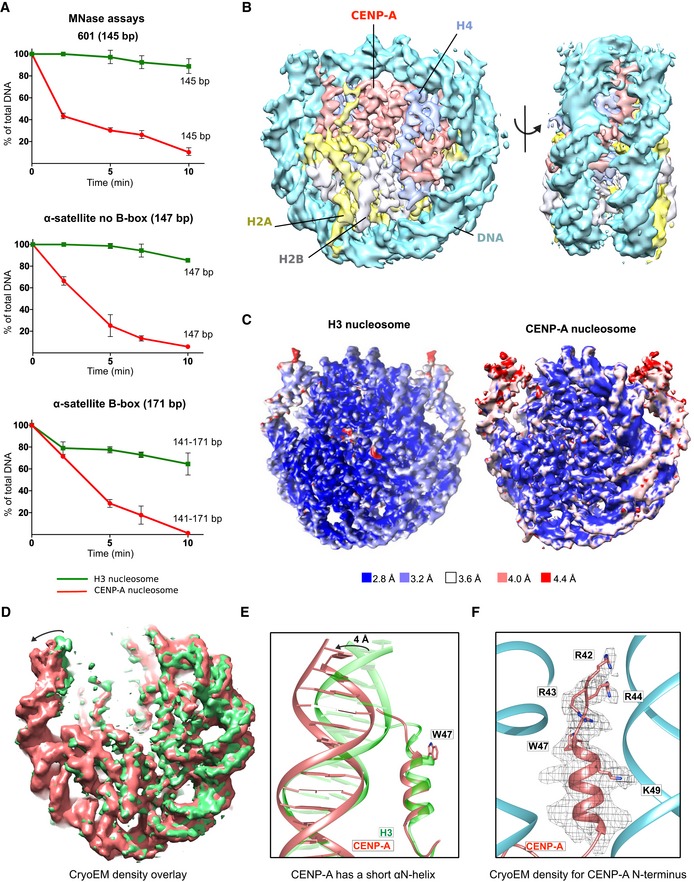
- Graphs showing the relative abundance of undigested DNA as a function of time during digestion with micrococcal nuclease (MNase) for three types of DNA wrapped around the CENP‐A nucleosome (red) and the H3 nucleosome (green). For α‐satellite DNA with initial size of 171 bp, size ranges (141–171) corresponding to DNA lengths above NCP (nucleosome core particle) is presented. Data are presented as mean (SD) for each time point based on three independent experiments. Corresponding virtual gels from Bioanalyzer are in Fig EV1A.
- Cryo‐EM density map of the human CENP‐A nucleosome, color‐coded for histones and DNA.
- Cryo‐EM maps of the H3 nucleosome (PDB 6ESF) and the CENP‐A nucleosome, colored based on local resolution.
- Overlay of cryo‐EM maps of the H3 nucleosome (green; PDB 6ESF) and the CENP‐A nucleosome (red). Note shorter and moved density for DNA on the CENP‐A nucleosome indicated by the arrow.
- Overlay of the N‐terminal tail of CENP‐A (red) and H3 (green; PDB 6ESF), illustrating shorter αN helix of CENP‐A (obstructed by the presence of bulky CENP‐AW47) and terminal nucleosomal DNA moved by 4 Å.
- The N‐terminal tail of CENP‐A (red) makes contacts with the DNA (cyan) at the entry/exit sites.
Figure EV1. CENP‐A nucleosome structural features.
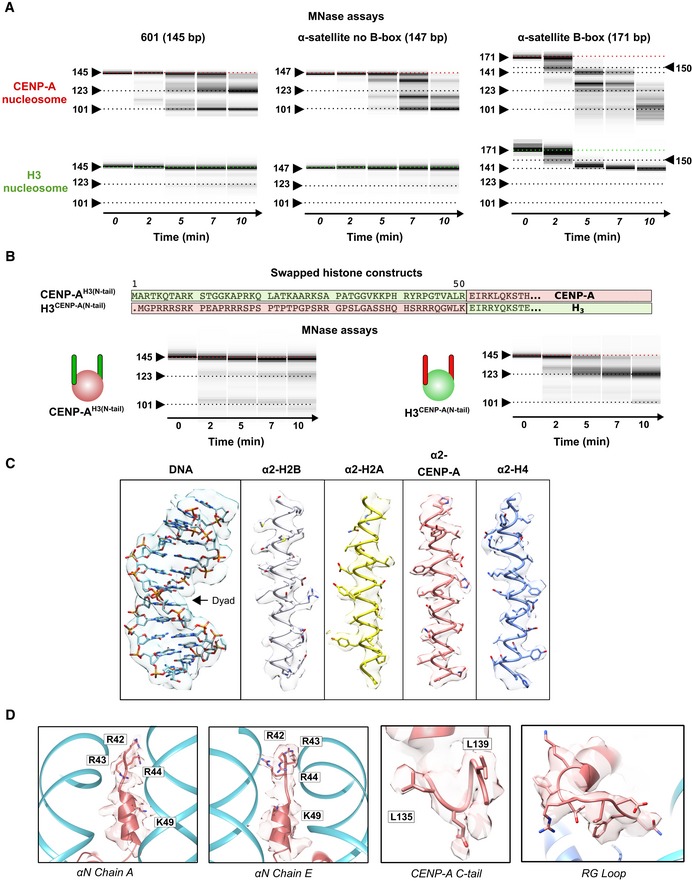
- Virtual gels from Bioanalyzer showing DNA digestion for CENP‐A and H3 nucleosomes on three different DNA templates.
- (Top) Sequence overlay of the N‐terminus of H3 and CENP‐A, indicating swapped sequences used in CENP‐AH3(N‐tail) and H3CENP‐A(N‐tail) constructs. (Bottom) Virtual gels of MNase digestion for CENP‐AH3(N‐tail) and H3CENP‐A(N‐tail) nucleosomes assembled on 601 DNA.
- Representative cryo‐EM map of the CENP‐A nucleosome, illustrating quality of map and model fitting.
- EM density of CENP‐A‐specific features on the nucleosome: αN helix (2 different sides), C‐terminal tail and RG‐loop.
Next, we used cryo‐EM to obtain a high‐resolution structure of CENP‐A nucleosomes on 601 DNA at 3.8 Å (Figs 1B and EV1C and EV2, Appendix Table S1). In contrast to the crystal structure 6 where electron density for the terminal 13 DNA bp is missing, probably due to its flexible nature, our cryo‐EM structure of CENP‐A nucleosomes reveals density for the entire 145 bp of DNA used in nucleosome reconstitutions. However, the map is less defined and has a lower local resolution for the terminal DNA (Figs 1C and EV2E), indicating local flexibility. The modeled DNA is shifted by 4 Å in comparison with the one in the H3 nucleosome 24 (Fig 1D and E). These results are in agreement with our MNase experiments and with a recent antibody‐stabilized structure of the CENP‐A nucleosome 20. Interestingly, despite the low resolution for the DNA, we can clearly model the N‐terminus of CENP‐A all the way to CENP‐AR42, including the αN helix (Fig 1F). The αN helix of CENP‐A is shorter, disrupted by CENP‐AG46 and the bulky CENP‐AW47, while H3 continues with one extra turn (Fig 1E). CENP‐AR42, CENP‐AR43, and CENP‐AR44 are all involved in DNA binding, although the interactions are slightly different on the two sides of the nucleosome (Figs 1F and EV1D). In our structure, we can also clearly see other CENP‐A‐specific features of the nucleosome (Fig EV1D): the C‐terminal tail (‐LEEGLG) that specifically binds CENP‐C, and L1 loop containing CENP‐AR80 (“RG‐loop”) that specifically binds CENP‐N.
Figure EV2. Cryo‐EM analysis of CENP‐A nucleosome.
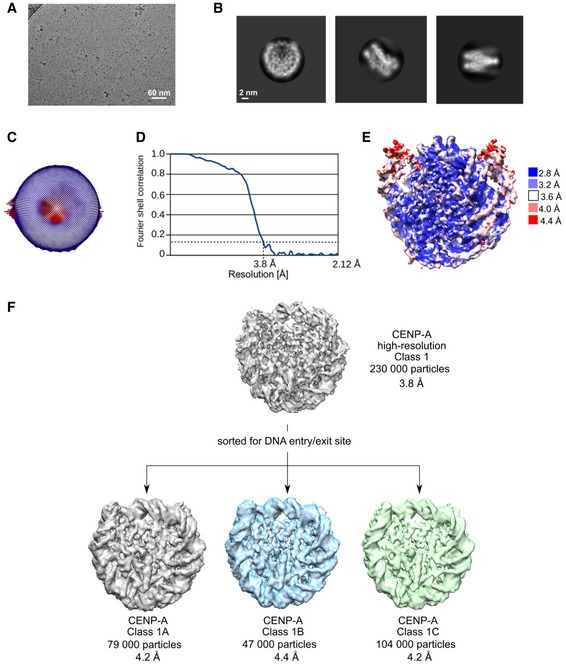
- Representative cryo‐EM raw micrograph.
- Subset of selected 2D class averages.
- Euler angle distribution of particles used in the final 3D reconstruction.
- Fourier shell correlation (FSC) curves of the final density map (CENP‐A high resolution).
- Local resolution of the final 3D density map.
- Particles used for the high‐resolution CENP‐A map were further classified for DNA entry/exit site in order to highlight differences at this part of the nucleosome. Gray map (Class 1A) has loosest DNA wrap, and green map (Class 1C) has tightest DNA wrap. The blue map represents particles that were in‐between two extreme conformations.
CENP‐CCR competes out the CENP‐Cmotif on CENP‐A nucleosomes
Initial efforts to identify human CCAN proteins that directly and specifically bind CENP‐A nucleosomes uncovered the CENP‐C and CENP‐N proteins 4, 25. In those studies, only the central region of human CENP‐C (CENP‐CCR, aa 426–537) was characterized for CENP‐A binding (Fig 2A). In 2013, Kato et al 14 reported a crystal structure of the canonical fruit fly H3 nucleosome in which the C‐terminal tail of H3 was replaced by a mutated rat CENP‐A C‐terminus in complex with the rat CENP‐C residues 710–734, a region known as the CENP‐C motif (CENP‐Cmotif). Analysis of the structure identified the hydrophobic interactions between the CENP‐A C‐terminal tail and the CENP‐Cmotif, and the authors proposed that both CENP‐CCR and CENP‐Cmotif bind nucleosomes, with 5–10 times higher affinity for CENP‐A then for H3 nucleosomes. To determine whether CENP‐CCR binds the nucleosome with a different affinity than the CENP‐Cmotif, we prepared complexes of both and analyzed their mobility on native PAGE. We found that both the CENP‐CCR and the CENP‐Cmotif make complexes with CENP‐A nucleosomes in a ∼2:1 ratio. The complexes travel as sharp bands on the native gel, indicating their uniform nature (Fig 2B and C). Surprisingly, when mixed with H3 nucleosomes, both CENP‐C constructs result in a smear on the native gel, indicating non‐specific binding (Fig 2B and C). We conclude that in vitro CENP‐CCR and CENP‐Cmotif bind only CENP‐A nucleosomes specifically.
Figure 2. Both CENP‐CCR and CENP‐Cmotif bind specifically to the CENP‐A nucleosome, and CENP‐CCR easily competes out CENP‐Cmotif bound to CENP‐A.
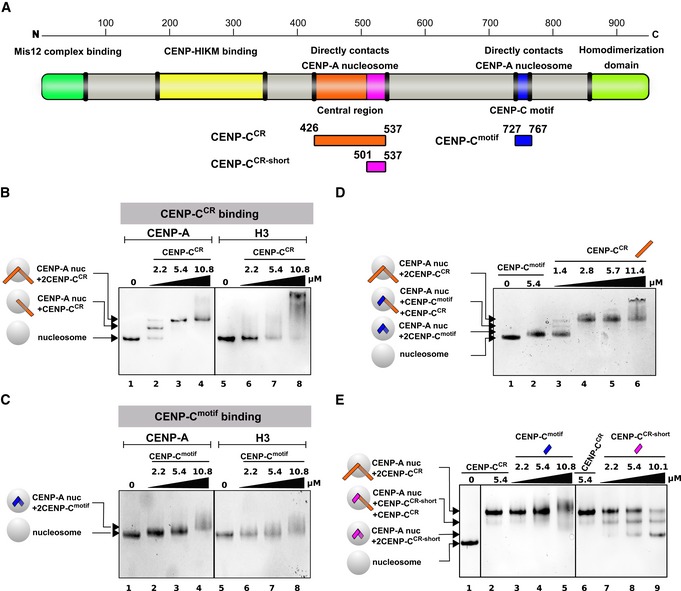
- Schematic diagram of the full‐length CENP‐C protein, indicating parts involved in interactions with other proteins or homo‐dimerization. Constructs used in this study are depicted below the diagram.
- Native PAGE gel stained with Coomassie blue showing complexes formed between CENP‐A or H3 nucleosome and CENP‐CCR. Lane 1: CENP‐A nucleosome, Lanes 2–4: Increasing amounts of CENP‐CCR are added to CENP‐A nucleosome. Generation of a sharp band with slower mobility indicates formation of a specific CENP‐A/CENP‐CCR complex. Lane 5: H3 nucleosome. Lanes 6–8: Increasing amounts of CENP‐CCR are added to H3 nucleosome. Smear on the gel indicates formation of non‐specific H3/CENP‐CCR complexes.
- Same experiment as in (B) using CENP‐Cmotif. Lane 1: CENP‐A nucleosome. Lanes 2–4: Increasing amounts of CENP‐Cmotif are added to CENP‐A nucleosome. Upon binding CENP‐Cmotif, CENP‐A nucleosome migrates slower through the gel. Note only modest change in mobility due to small size of CENP‐Cmotif, comparing to CENP‐CCR in (B). Lane 5: H3 nucleosome. Lanes 6–8: Increasing amounts of CENP‐Cmotif are added to H3 nucleosome. Smear on the gel indicates formation of non‐specific H3/CENP‐Cmotif complexes.
- Native gel showing CENP‐CCR competing out CENP‐Cmotif bound to CENP‐A nucleosome. Lane 1: CENP‐A nucleosome. Lane 2: CENP‐A/CENP‐Cmotif complex. Lane 3–6: Increasing amounts of CENP‐CCR are added to the pre‐formed CENP‐A/CENP‐Cmotif complex. Formation of slower migrating bands indicates that longer CENP‐CCR is replacing shorter CENP‐Cmotif bound to the CENP‐A nucleosome.
- Native gel showing the inability of CENP‐Cmotif to compete out CENP‐CCR bound to CENP‐A nucleosome. Lane 1: CENP‐A nucleosome. Lanes 2 and 6: CENP‐CCR/CENP‐A nucleosome complex. Lanes 3–5: Increasing amounts of CENP‐Cmotif are added to the pre‐formed CENP‐A/CENP‐CCR complex. Formation of smear at high amounts of CENP‐Cmotif added indicates that CENP‐Cmotif, at high concentrations, non‐specifically binds CENP‐A/CENP‐CCR complex rather than replacing bound CENP‐CCR. Lanes 7–9: Increasing amounts of CENP‐CCR‐short are added to the pre‐formed CENP‐A/CENP‐CCR complex. Formation of bands with higher mobility indicates that smaller CENP‐CCR‐short is effectively replacing bigger CENP‐CCR bound to CENP‐A nucleosome.
Furthermore, we performed a competition experiment to determine which CENP‐C region has higher affinity for CENP‐A nucleosomes. When we preassemble the human CENP‐A/CENP‐Cmotif complex and titrate in CENP‐CCR, we observe formation of the CENP‐A/CENP‐CCR complex with almost full saturation at 2 × molar access of CENP‐CCR (Fig 2D). In contrast, titrating in the CENP‐Cmotif to a pre‐formed CENP‐A/CENP‐CCR complex results in a smear on the native PAGE, indicative of non‐specific binding of CENP‐Cmotif to CENP‐A/CENP‐CCR complexes (Fig 2E). The CENP‐Cmotif is 71 residues shorter than CENP‐CCR (40 versus 111 residues), and it is possible that extra residues beyond those directly interacting with the CENP‐A C‐terminus are providing additional affinity. To test this, we made a truncated CENP‐CCR that has only residues predicted to interact with the CENP‐A C‐terminal tail (501–537; CENP‐CCR‐short) and is comparable in size to the CENP‐Amotif. First, we confirmed specificity of CENP‐CCR‐short for CENP‐A nucleosomes (Appendix Fig S1). Interestingly, we also observed that CENP‐CCR‐short completely loses its ability to bind H3 nucleosomes. This suggests that the additional 71 residues present in the CENP‐CCR are responsible for the non‐specific H3 nucleosome binding, likely through interactions with DNA. Next, we tested if CENP‐CCR‐short can compete out CENP‐CCR from pre‐assembled CENP‐A/CENP‐CCR complexes. We find that, in contrast to the CENP‐Cmotif, CENP‐CCR‐short efficiently replaces CENP‐CCR bound to CENP‐A nucleosomes (Fig 2E), demonstrating that residues within the nucleosome binding region of CENP‐CCR contribute to high affinity binding.
In summary, we conclude that CENP‐C binds only CENP‐A nucleosomes specifically and that CENP‐CCR provides the major interactions between CENP‐A and CENP‐C. Our findings are consistent with studies in cells that found CENP‐CCR to be essential for epigenetic propagation of the centromere 13.
Strong hydrophobic interactions are contributing specificity and high affinity between the CENP‐A nucleosome and CENP‐CCR
Next, we aimed to define how the high affinity CENP‐CCR interaction is achieved. The crystal structure of the H3‐GIEGGL/rat CENP‐Cmotif has identified two types of interactions involved in complex formation: (i) electrostatic interactions of rat CENP‐CR717/R719 with the acidic patch on H2A/H2B, and (ii) hydrophobic interactions between rat CENP‐CY725, W726 and the hydrophobic C‐terminal tail of CENP‐A 14. A sequence comparison of the rat CENP‐Cmotif with the human CENP‐Cmotif and human CENP‐CCR reveals the existence of analogous residues in the human protein but does not explain the higher affinity of the CENP‐CCR (Fig 3A). Also, since crystallographic studies 14 used a canonical nucleosome with a grafted C‐terminus of CENP‐A, it failed to capture possible conformational changes that could occur within the CENP‐A nucleosome upon CENP‐C binding. To resolve this, we solved the 3.1 Å cryo‐EM structure of the human CENP‐A nucleosome in complex with human CENP‐CCR (Fig 3B, Appendix Table S2, Figs EV3 and EV4). Our maps show CENP‐C bound to both sides of the nucleosome via the CENP‐AR521, R522 anchoring residues, while the remaining residues in CENP‐CCR show fuzzy interactions. In order to visualize a larger CENP‐C fragment, we have further sorted particles and increased CENP‐C occupancy on one of the sides, so that we can trace residues 519‐536. We observe strong electrostatic interactions between human CENP‐CR521, R522 and the acidic patch formed by H2A/H2B (Figs 3C and EV3A). Neutralization of a negative surface contributed by H2A/H2B on the nucleosome is a feature common to a handful of other nucleosome binding molecules (reviewed in 26) that was also seen in the chimeric H3 nucleosome/CENP‐Cmotif structure 14. We also observe human CENP‐CW530, W531 tightly fitting the hydrophobic cleft formed by the C‐terminal tail of CENP‐A (Figs 3C and EV3B). Furthermore, the conformation of the C‐terminal tail of CENP‐A changes slightly upon binding (Fig 3D), and we observe more extended hydrophobic interactions in comparison with those reported in the crystal structure (Fig 3E) 14. Two bulky tryptophans (CENP‐CW530, W531) are clamped between CENP‐AR131 and CENP‐AL135, L139 while CENP‐CV532 and CENP‐CV533 stabilize the hydrophobic patch on H4 formed by H4V58 and H4V61. These interactions explain the perturbations observed by NMR in H4 residues within the nucleosome upon CENP‐CCR binding 14. To test whether extended hydrophobicity contributed by CENP‐CV532, V533 plays a role in stronger CENP‐A nucleosome/CENP‐CCR interactions, we generated a CENP‐C mutant devoid of residues 530–537, CENP‐CCR‐ΔC (Fig 3F). This mutant fails to form a complex with the CENP‐A nucleosome (Fig 3G), indicating that the residues following CENP‐CW530, W531 are important for binding. Point mutations further revealed that replacement of CENP‐CV533 with aspartic acid is sufficient to eliminate binding to the CENP‐A nucleosome (Fig 3F and G).
Figure 3. Strong hydrophobic interactions are contributing specificity and high affinity between the CENP‐A nucleosome and CENP‐CCR .
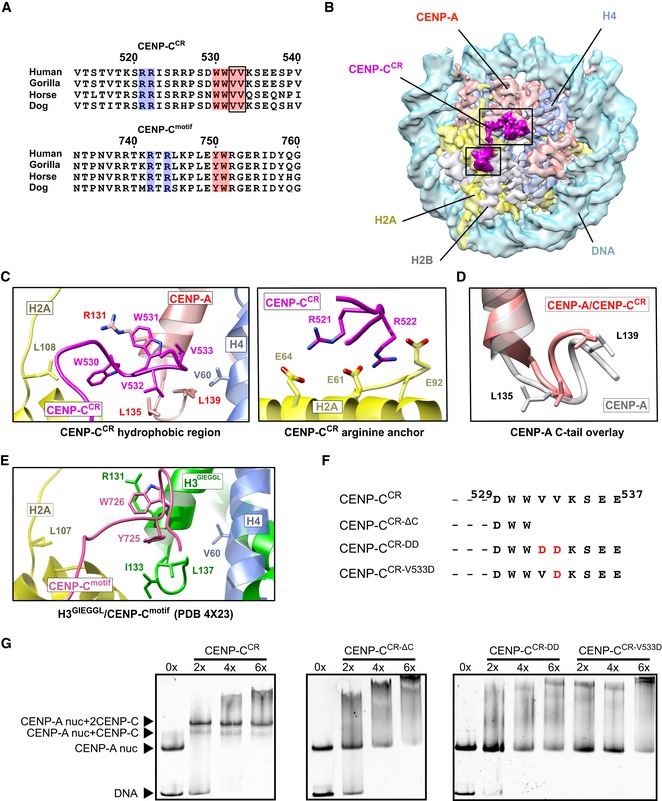
- Sequence alignment of CENP‐CCR and CENP‐Cmotif regions from different mammals. Conserved residues involved in CENP‐A binding are highlighted in blue (electrostatic interactions) and pink (hydrophobic interactions). Residues identified in this study to contribute higher affinity of CENP‐CCR comparing to CENP‐Cmotif are boxed.
- Cryo‐EM density map of the human CENP‐A nucleosome, color‐coded for histones, in complex with CENP‐CCR (magenta). CENP‐CCR binds CENP‐A nucleosome through hydrophobic region (big box) and arginine anchor (small box). Interacting residues in each of the regions are shown in (C).
- Ribbon diagram showing interactions of the CENP‐CCR hydrophobic region (left) and CENP‐CCR arginine anchor (right) with the CENP‐A nucleosome.
- Overlay of the CENP‐A C‐terminal tail before (gray) and after (red) binding of CENP‐CCR.
- Interactions between H3‐GIEGGL and rat CENP‐Cmotif as observed in PDB 4X23 14.
- Schematic diagram of mutated CENP‐CCR sequences used to test importance of CENP‐CV532 and CENP‐CV533 for generation of CENP‐A/CENP‐CCR complexes.
- Native gels showing the impaired ability of mutated CENP‐CCR to form complexes with the CENP‐A nucleosome. The molar ratio of CENP‐CCR/CENP‐A nucleosomes is shown above each lane.
Figure EV3. CENP‐A nucleosome/CENP‐CCR complex structure.
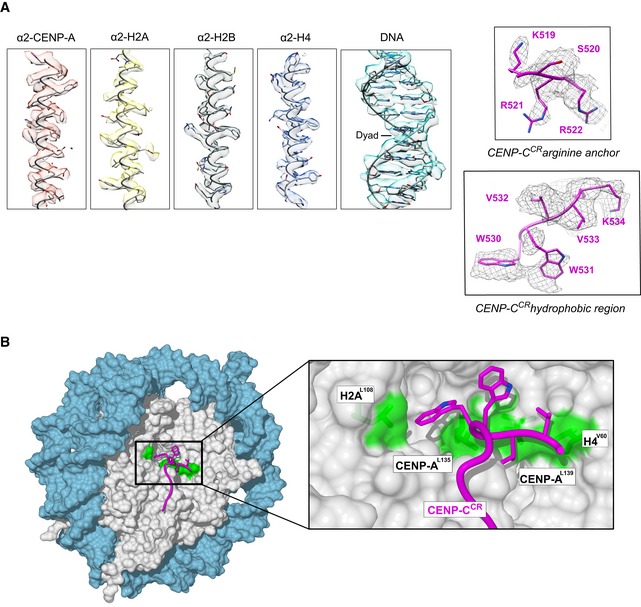
- Representative cryo‐EM densities showing fitted model for DNA and each of the histones (left), arginine anchor, and hydrophobic regions of CENP‐CCR (right).
- Surface representation of nucleosome (histone core—gray, DNA—blue), showing a hydrophobic groove (green) on the nucleosome formed by H2AL108, CENP‐AL135, CENP‐AL139, and H4V60. CENP‐CCR is shown as a purple coil with hydrophobic sidechains in stick representation.
Figure EV4. Cryo‐EM analysis of CENP‐A/CENP‐CCR complex.
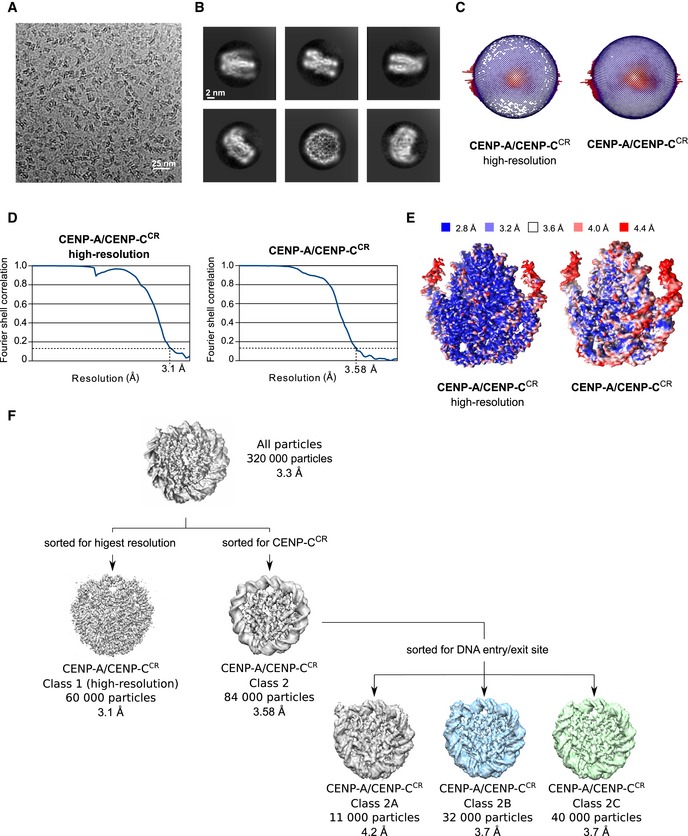
- Representative cryo‐EM raw micrograph.
- Subset of selected 2D class averages.
- Euler angle distribution of particles used in the final 3D reconstruction for CENP‐A/CENP‐CCR high resolution and CENP‐A/CENP‐CCR complex enriched for CENP‐CCR.
- Fourier shell correlation (FSC) curves of the final density map for CENP‐A/CENP‐CCR high resolution and CENP‐A/CENP‐CCR enriched for CENP‐CCR.
- Local resolution of the final 3D density maps.
- First, particles were sorted for high resolution, and this map was used for initial model building. A map enriched in CENP‐CCR was generated to increase map quality around CENP‐CCR. Particles used for the later map were further classified for DNA entry/exit site in order to highlight extend of DNA unwrapping. The gray map (Class 2A) has the loosest DNA wrap, and the green map (Class 2C) has tightest DNA wrap. The blue map presents particles that were in‐between two extreme conformations.
We conclude that human CENP‐CCR binds the human CENP‐A nucleosome through electrostatic interactions, involving CENP‐CR521, R522 (neutralizing the acidic patch on the nucleosome) as well as via an extensive hydrophobic network formed between CENP‐CW530, W531, V532, V533 and the C‐terminal tail of CENP‐A, aided by the hydrophobic patch of H4. Our data show that CENP‐CCR makes additional hydrophobic contacts with the CENP‐A nucleosome that have not been observed for CENP‐Cmotif. These interactions are required for the higher affinity of CENP‐CCR versus the CENP‐Cmotif.
Conformational changes within the human CENP‐A nucleosome upon CENP‐C binding
The nucleosome in our experiments bears the full‐length human CENP‐A, which enables us to assess conformational changes upon CENP‐C binding. It has previously been proposed that CENP‐C binding rigidifies the histone core, rendering it more histone H3‐like, while at the same time further enhancing DNA unwrapping 27. The MNase experiments on CENP‐A nucleosomes in complex with CENP‐CCR or CENP‐Cmotif show increased DNA digestion in both cases (Figs 4A and EV5A). Comparison of the local resolution maps between CENP‐A and CENP‐A/CENP‐CCR complex (Figs EV2E and EV4E) indicates more extensive DNA unwrapping in samples with bound CENP‐CCR. To further confirm this, we performed careful cryo‐EM classification of particles based on the conformations at DNA entry/exit sites for each of the two samples (Fig EV4F). We find that, although flexible, the DNA at the entry/exit site in CENP‐A nucleosome samples only a limited space (distance between most extreme DNA conformation is 2 Å) (Fig 4B). In contrast, in the CENP‐A/CENP‐CCR complex the most extreme distance between different subpopulations is 9 Å (Fig 4C). Concomitantly, we observe no density for the C‐terminal tail of H2A (Fig 4C), indicating disorder in this part of the nucleosome upon CENP‐C binding. This is in striking contrast to the cryo‐EM structure of the canonical nucleosome with tightly wrapped DNA, where the density for the C‐terminal tail of H2A is one of the most well‐resolved parts of the structure 24. It has been reported that removal of the C‐terminal tail of H2A leads to decreased stability of the nucleosome and alters nucleosome positioning and interactions with H1 28, 29. Molecular dynamic studies have identified residues H2AK118 and H2AK119 to interact with DNA, most likely securing the DNA wrap 30. Indeed, if we remove residues 110–130 in H2A from the CENP‐A nucleosome (CENP‐AΔC‐tail H2A), we observe increased DNA digestion (Figs 4D and EV5B, top), and addition of CENP‐CCR to these nucleosomes does not change the nuclease digestion profile (Figs 4D and EV5B, bottom). In contrast, DNA digestion of H3 nucleosomes was only mildly affected by removal of the C‐terminus of H2A (Fig EV5C, top). We next hypothesized that the different MNase pattern of CENP‐A versus H3 nucleosomes (in the background of ΔC‐tail H2A) must be contributed by the residues in the N‐terminal tail of CENP‐A/H3, since this region of the nucleosome is in close contact with the C‐terminal tail of H2A. Indeed, in the context of ΔC‐tail H2A, MNase digestion of nucleosomes with an H3 core and CENP‐A tail is highly similar to CENP‐A nucleosomes while digestion of nucleosomes with a CENP‐A core and H3 tail is similar to that of the H3 nucleosome (Fig EV5C, middle). These results demonstrate a contribution of the C‐terminal tail of H2A to nucleosome DNA wrapping that synergizes with the N‐terminal tail of CENP‐A. In the context of the CENP‐A nucleosome that has a shorter αN helix, DNA wrapping is already compromised and removal of C‐terminal H2A results in further unwrapping (Fig 4E). Consistently, we find that in CENP‐A nucleosomes with an H3 N‐tail, binding of CENP‐CCR alone cannot induce DNA unwrapping (Fig EV5C, bottom). From this, we conclude that the N‐terminal tail of CENP‐A favors DNA unwrapping which is counteracted by the C‐terminus of H2A. CENP‐C binding destabilizes the C‐terminus of H2A, leading to increased DNA unwinding. We find that this alternative, destabilized, conformation of the H2A C‐terminal tail is most likely caused by an interaction between the bulky hydrophobic CENP‐CW530, W531 and H2AL108. In addition, CENP‐CW530, W531 is orienting CENP‐AR131 to establish a salt bridge with H2AQ112 (Fig EV5D).
Figure 4. The C‐terminus of H2A is destabilized in the CENP‐A/CENP‐CCR complex.
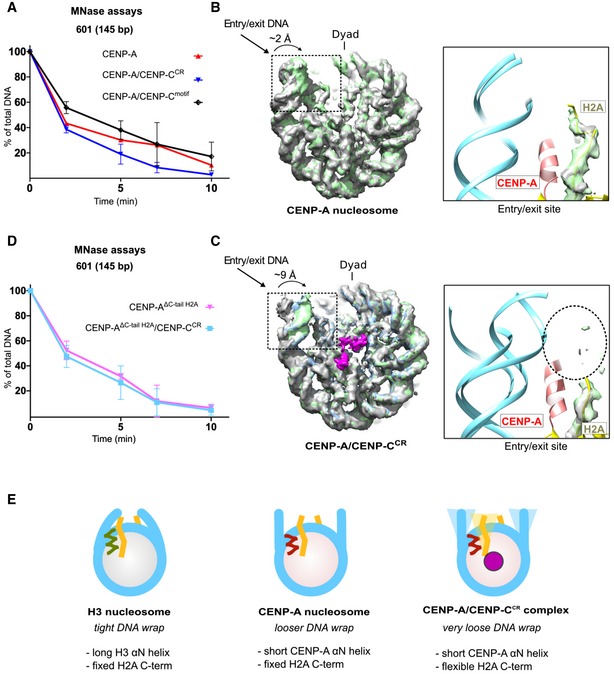
- Graph showing the relative abundance of undigested DNA (145 bp) as a function of time during digestion with micrococcal nuclease (MNase) for the CENP‐A nucleosome (red), the CENP‐A/CENP‐CCR complex (blue), and the CENP‐A/CENP‐Cmotif complex (black).
- Overlay of three cryo‐EM maps (gray, blue, and green) of the CENP‐A nucleosome obtained by sorting on the DNA entry/exit site (Fig EV2F). Distance between most open (gray) and most closed (green) map is 2 Å. The entry/exit site is boxed, and the corresponding model is shown on the right. Note that the density of the H2A C‐terminus is well defined.
- Overlay of three cryo‐EM maps (gray, blue, and green) of the CENP‐A/CENP‐CCR complex obtained by sorting on the DNA entry/exit site (Fig EV4F). The distance between the most open (gray) and most closed (green) maps is 9 Å. Map assigned to CENP‐CCR is shown in magenta. The entry/exit site is boxed, and the corresponding model is shown on the right. Note the absence of a clearly defined density of the H2A C‐tail (indicated by the dotted circle).
- Same type of data as in (A) for the CENP‐A nucleosome assembled with tailless H2A (H2A, 1–109) alone (pink) and in complex with CENP‐CCR (light blue).
- Schematic representation of the interplay between the N‐terminus of H3 or CENP‐A and the C‐terminus of H2A in regulating flexibility of nucleosomal DNA wrap. DNA (cyan); longer H3 αN (green); shorter CENP‐A αN (red); H2A C‐terminus (yellow); CENP‐C (magenta).
Figure EV5. Conformational changes on the CENP‐A nucleosome upon CENP‐CCR binding.
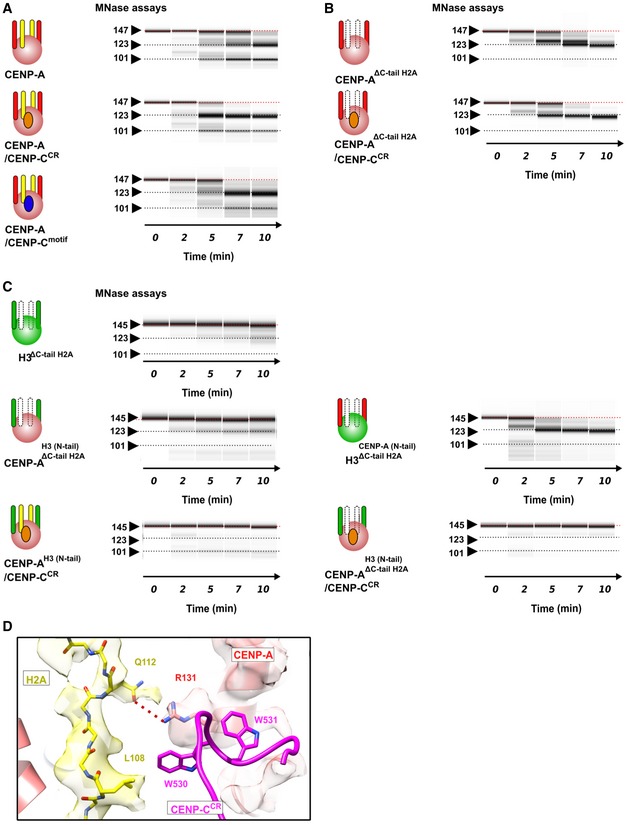
-
A–CVirtual gels for MNase digestion. CENP‐A nucleosomes are pink, and H3 nucleosomes are green balls. CENP‐A N‐terminal tail (aa 1–49) is red stick, H3 N‐terminal tail (aa 1–50) is green stick, H2A C‐terminal tail (aa 110–130) is yellow stick, CENP‐CCR is orange circle, and CENP‐Cmotif is blue circle. Deleted H2A C‐terminal tail is indicated as dotted white stick. (A) MNase digestion of the CENP‐A nucleosome alone and in complex with CENP‐CCR or CENP‐Cmotif (also in Fig 4A). (B) MNase digestion of the CENP‐A nucleosome assembled with H2A lacking 110–130 residues, alone (top) or in complex with CENP‐CCR (bottom), showing similar magnitude of digestion. (C) (Top) MNase digestion of the H3ΔC‐tail H2A, indicating that removal of H2A110–130 does not have an effect on the DNA digestion speed in the context of H3 nucleosome. (Middle) MNase digestion of CENP‐AH3(N‐tail), ΔC‐tail H2A and H3CENP‐A(N‐tail), ΔC‐tail H2A, indicating that removal of H2A110–130 does not have an effect on the DNA digestion speed in the context of CENP‐AH3(N‐tail) nucleosome, but digestion is slightly increased in the context of H3CENP‐A(N‐tail). (Bottom) MNase digestion of the CENP‐AH3(N‐tail) is unaffected with CENP‐CCR binding independently of the presence of H2A110–130.
-
DInteractions between H2A C‐terminal tail and CENP‐CCR.
Furthermore, we noticed that the N‐terminal tail of H4 clearly adopts an upwards conformation on both sides of the CENP‐A nucleosome relative to canonical nucleosomes (Fig 5A, left), but in the presence of CENP‐CCR, this conformation is more rigidified (Fig 5A, right). Monomethylation of H4 is enriched on CENP‐A nucleosomes, and it is necessary for epigenetic establishment of the kinetochore 31. A recent structural study 32 proposed an upward conformation of the H4 tail to be required for establishment and/or maintenance of H4K20 monomethylation. In our cryo‐EM maps, we observe the N‐terminus of H4 in a slightly different but still upwards conformation in comparison with the crystal structure of the H3CATD nucleosome (Fig 5B) 32. A very recent study 33 indicates that the conformation of the N‐terminus of H4 is further modified upon CENP‐N binding.
Figure 5. The N‐terminal tail of H4 is stabilized in upwards conformation in the CENP‐A/CENP‐CCR complex.
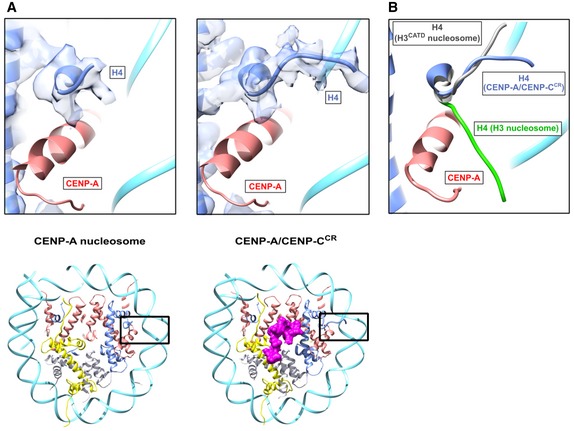
- Structure of the H4 N‐terminal tail with corresponding cryo‐EM density in the CENP‐A nucleosome (left) and CENP‐A/CENP‐CCR complex (right). Position of the H4 N‐terminal tail within the nucleosome is boxed on ribbon diagram below. Histones and DNA are color‐coded as in Fig 1, and cryo‐EM density assigned to CENP‐C is shown in magenta.
- Overlay of the H4 N‐tail from the CENP‐A/CENP‐CCR complex (blue) with the H4 N‐terminus from the H3 nucleosome (green; PDB 6ESF) and H3CATD nucleosome (gray; PDB 5Z23).
In summary, we find that binding of CENP‐CCR to the CENP‐A nucleosome enhances DNA unwrapping by destabilizing the C‐terminal tail of H2A while, at the same time, stabilizing the H4 N‐terminal tail in the upward conformation that may be important for centromere‐specific monomethylation of H4K20.
Discussion
CENP‐A is a key epigenetic mark to maintain centromere identity, a chromosome locus essential for genome integrity. CENP‐A is a histone H3 variant with a histone core bearing 64% identity to H3 while featuring completely divergent tails. As CENP‐A is a central epigenetic determinant of the centromere, an important question is how CENP‐A containing nucleosomes are distinguished from bulk chromatin. Over the years, a number of models have been proposed, including different histone stoichiometries, presence of non‐histone proteins, and alternative DNA wrapping [summarized in 15]. Finally, an octameric nucleosome with a right‐handed DNA wrap, much like canonical nucleosomes, emerged as the favorite model for the human CENP‐A nucleosome, based on both in vitro and in vivo studies 5, 6, 16, 17, leaving open the question of what is making CENP‐A nucleosomes so unique. A crystal structure of the CENP‐A nucleosome 6, in vivo MNase experiments 16, and initial cryo‐EM studies 18 all pointed toward enhanced DNA unwrapping as a CENP‐A‐specific feature where unwrapped DNA results in a different chromatin architecture, potentially important for accommodating CCAN components. Indeed, the experiments presented here, together with the structure of human CENP‐A, confirm high flexibility of the DNA ends as an intrinsic feature of CENP‐A nucleosomes encoded in the N‐terminal tail of CENP‐A, independent on the DNA sequence.
A key question is how the CENP‐A nucleosome directly binds two CCAN components, CENP‐N and CENP‐C, and how their binding is changing the nucleosome. Recent work 7, 8, 9 has provided a view of the CENP‐A/CENP‐N interaction at atomic resolution, which involves recognition of the solvent‐exposed, positively charged L1 loop on CENP‐A and an interaction with DNA, with minimal changes to the rest of the nucleosome. For the CENP‐C interactions with the CENP‐A nucleosome, 2 different parts of CENP‐C are proposed to bind: CENP‐CCR and CENP‐Cmotif. CENP‐CCR is necessary and sufficient for CENP‐A nucleosome binding in vitro and centromere targeting and stability in vivo while the CENP‐Cmotif can be recruited to kinetochores only in the presence of a homo‐dimerization domain 10, 11, 13. Having two independent CENP‐C modules able to bind nucleosome at centromeres with different affinities, led to the proposal 10 of CENP‐C, as a direct mediator of CENP‐A loading during the cell cycle. The model assumes that the CENP‐C module with weaker nucleosome binding, CENP‐Cmotif, alternates between binding of an H3.3 nucleosome and a CENP‐A nucleosome while the stronger module, CENP‐CCR, remains stably associated with the CENP‐A nucleosome in all phases of the cell cycle. We show that, indeed, CENP‐CCR binds the CENP‐A nucleosome with higher affinity than CENP‐Cmotif, but neither of the CENP‐C modules can make uniform complexes with H3 nucleosome.
Furthermore, structural insights of the CENP‐A/CENP‐C interactions are based on the crystal structure of a chimeric fruit fly H3 nucleosome with the C‐terminal tail of rat CENP‐A and interaction of this tail with the rat CENP‐Cmotif 14. We here report a 3.1 Å structure of a complete human CENP‐A nucleosome in complex with human CENP‐CCR which is essential for epigenetic stability of centromeres 13. We find a longer hydrophobic stretch on CENP‐CCR, formed by CENP‐CV532, V533, to be essential for robustness of the CENP‐A nucleosome/CENP‐CCR interactions, and we re‐define the minimal fragment of CENP‐CCR necessary for productive CENP‐A binding (residues 501–537).
Previous experiments 27 and those reported here confirm enhanced DNA unwrapping of the CENP‐A nucleosome, induced by CENP‐CCR binding. In our structure of the CENP‐A/CENP‐CCR complex, we observe a disordered H2A C‐terminal tail and using a combination of MNase experiments and mutagenesis we establish a role for the H2A histone tail in regulating the extent of DNA wrapping on nucleosomes. We find that the C‐terminal tail of H2A secures nucleosomal DNA wrapping that counteracts the unwrapping of CENP‐A nucleosomes promoted by the short CENP‐A αN helix. Upon CENP‐C binding, the hydrophobic interaction between H2A and CENP‐C is displacing the H2A C‐terminal tail, resulting in a very loose DNA wrapping in the CENP‐A/CENP‐CCR complex. Regulation of the DNA wrap by the C‐terminal tail of H2A is likely exploited in general chromatin. For example, H2A variants with different C‐terminal tails are known to regulate various biological processes, conferring special properties to the chromatin 34.
Furthermore, in both our structures of the CENP‐A nucleosome alone and the CENP‐A/CENP‐CCR complex, we see an upwards conformation of the N‐terminal tail of H4. The conformation is additionally stabilized by CENP‐CCR binding, most likely through hydrophobic interactions between CENP‐C and H4. H4K20 monomethylation is essential for kinetochore assembly 31, 32, and binding of CENP‐CCR could be enforcing this centromere‐specific epigenetic chromatin modification.
Combined, our structures provide an essential and long anticipated high‐resolution view of the fundamental building block of the centromere, the human CENP‐A nucleosome alone and in complex with CENP‐C, a protein that forms the backbone of the constitute centromere complex. Our biochemical and structural analysis establishes CENP‐C as an exclusive and multivalent binder of CENP‐A nucleosomes that employs two independent modules and homo‐dimerization to crosslink sparse CENP‐A domains in centromeric chromatin 35, 36 and provides framework for functional CCAN (Fig 6). Binding of CENP‐C to CENP‐A nucleosomes not only spatially organizes CENP‐A nucleosomes and recruits other CCAN components, it also induces conformational changes (DNA unwrapping, neutralization of the acidic patch on H2A and facilitation of H4K20 monomethylation) that might be required for establishment and maintenance of functional centromeres (Fig 6).
Figure 6. Model illustrating putative role of CENP‐C in centromeric chromatin.
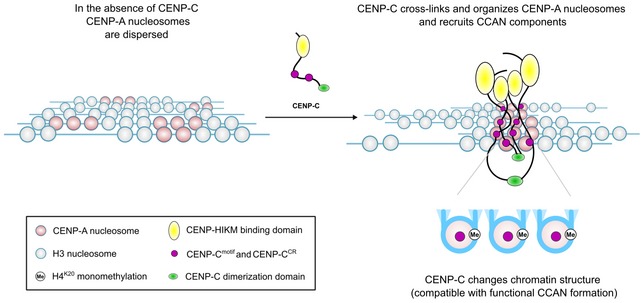
CENP‐C crosslinks CENP‐A nucleosomes, recruits other CCAN components, and pre‐conditions chromatin for formation of a functional centromere.
Materials and Methods
Protein purification
Human histones, CENP‐A and the CENP‐C central domain, were expressed and purified as previously described in 37. Briefly, the CENP‐A/H4 hetero‐tetramer was expressed from a bicistronic plasmid in E. coli pLysS under soluble conditions and purified on a hydroxyapatite column followed by cation exchange chromatography. H3, H4, H2A, and H2B were expressed in inclusion bodies and purified under denaturing conditions using a Sephacryl size exclusion column followed by cation exchange chromatography. H2A/H2B and H3/H4 histones were subsequently co‐refolded to form hetero‐dimers and hetero‐tetramers, respectively, and purified with size exclusion chromatography.
GST‐tagged recombinant human CENP‐C central region (CENP‐CCR, aa 426–537), CENP‐C motif (CENP‐Cmotif, aa 727–767), and short CENP‐C central region (CENP‐CCR‐short, aa 501–537) were expressed and affinity‐purified on a glutathione column. GST was subsequently cleaved overnight by PreScission protease and separated from CENP‐C, using cation exchange and size exclusion chromatography.
PCR site‐directed mutagenesis was performed to generate CENP‐CV532D/V533D, CENP‐CV533D, CENP‐C426–531, and CENP‐C426–533. All mutants were expressed and purified as described for the central CENP‐C region.
The H3 histone with a CENP‐A N‐tail1–49 (H3CENP‐A (N‐tail)), CENP‐A histone with a H3 N‐tail1–50 (CENP‐AH3(N‐tail)) (Appendix Fig S1B), and a H2A histone lacking the C‐terminal residues 110–130 were cloned using In‐Fusion® HD cloning strategy (Takara Bio).
145 bp 601 super‐positioning DNA and 147 bp α‐satellite DNA were purified as described in 38. Briefly, XL10 cells transformed with pUC57 plasmids containing 6 × 147 bp α‐satellite DNA and 8 × 145 bp 601 super‐positioning DNA (gift from Ben Black, UPenn) were cultivated, and DNA was extracted with phenol/chloroform, digested using EcoRV restriction enzyme and further purified using anion‐exchange chromatography. 171 bp α‐satellite DNA with a CENP‐B box was amplified from plasmid using PCR and further purified using anion‐exchange chromatography.
601 (145 bp): ATCAGAATCCCGGTGCCGAGGCCGCTCAATTGGTCGTAGACAGCTCTAGCACCGCTTAAACGCACGTACGCGCTGTCCCCCGCGTTTTAACCGCCAAGGGGATTACTCCCTAGTCTCCAGGCACGTGTCAGATATATACATCGAT
α‐satellite no B‐box (147 bp): ATCAAATATCCACCTGCAGATTCTACCAAAAGTGTATTTGGAAACTGCTCCATCAAAAGGCATGTTCAGCTCTGTGAGTGAAACTCCATCATCACAAAGAATATTCTGAGAATGCTTCCGTTTGCCTTTTATATGAACTTCCTCGAT
α‐satellite B‐box (171 bp): GGAGGATTTCGTTGGAAACGGGATCAACTTCCCATAACTGAACGGAAGCAAACTCAGAACATTCTTTGTGATGTTTGTATTCAACTCACAGAGTTGAACCTTCCTTTGATAGTTCAGGTTTGCAACACCCTTGTAGTAGAATCTGCAAGTGTATATTTTGACCACTTTGGA
Assembly of nucleosomes and nucleosome complexes
CENP‐A and H3 nucleosomes were assembled using 601 (145 bp), α‐satellite no B‐box (147 bp), or α‐satellite with B‐box (171 bp) DNA. H2A/H2B hetero‐dimers, (CENP‐A/H4)2 hetero‐tetramers, and DNA were mixed with a molar ratio of 2:1:1 at high salt concentration (2 M NaCl). A gradient dialysis to low salt was performed overnight with a flow rate of 1.5 ml/min using a two‐channel peristaltic pump as described in 39. Assembled nucleosomes were then uniquely positioned on the DNA by a thermal shift for 2 h at 55°C. CENP‐A nucleosome and CENP‐CCR were complexed by adding 2.2 moles of CENP‐CCR to each mole of CENP‐A nucleosome. The complex quality was controlled on a 5% native PAGE gel.
Binding experiments
2.4 μM of CENP‐A and H3 nucleosomes assembled on 601 (145 bp) DNA were mixed with different amounts of CENP‐CCR, CENP‐Cmotif, or CENP‐CCR‐short and incubated for 1 h at 4°C. Complex formation was verified on a 5% native PAGE gel.
Competition experiments
2.4 μM of the CENP‐A/CENP‐Cmotif complex was mixed with different amounts of CENP‐CCR, and the competition between the two domains was tested using 5% native PAGE gel. 2.4 μM of CENP‐A/CENP‐CCR complex was mixed with different amounts of CENP‐Cmotif and CENP‐CCR‐short. The competition was then followed using 5% native PAGE gel.
Micrococcal nuclease digestion
2 μg of nucleosomes was incubated with 2 Kunitz units of micrococcal nuclease (NEB) in buffer containing 10 mM Tris HCl pH 7.5, 3 mM CaCl2, and 1 mM DTT at room temperature. Reactions were quenched at different time points (2, 5, 7, 10, and 20 min) by the addition of 250 μl of PB buffer (Qiagen QIAquick PCR Purification Kit) supplemented with 10 mM of EGTA. DNA from each sample was purified with the QIAquick PCR purification kit, and the extent of DNA digestion was quantified by 2100 Bioanalyzer (Agilent). All experiments were done in triplicates.
Cryo‐EM grid preparation and data collection and processing
CENP‐A nucleosome and CENP‐A/CENP‐CCR complex were prepared as described above. 3 μl of the sample (1–1.2 mg/ml) was applied to freshly glow‐discharged Quantifoil R2/1 holey carbon grid. After 3 s blotting time, grids were plunge‐frozen in liquid ethane using a FEI Vitrobot automatic plunge freezer. Humidity in the chamber was kept at 95%.
Electron micrographs were recorded on FEI Titan Krios at 300 kV with a Gatan Summit K2 electron detector (~4,700 micrographs) (Cryo‐EM facility at MPI for Biochemistry Martinsried, Germany). The image pixel size was 0.65 Å per pixel on the object scale. Data were collected in a defocus range of 7,000–30,000 Å with a total exposure of 100 e/Å2. Fifty frames were collected and aligned with the Unblur software package using a dose filter 40.
Several thousand particles were manually picked and carefully cleaned in Relion to remove inconsistent particles. The resulting useful particles were then used for semi‐automatic and automatic particle picking in Relion. The contrast transfer function parameters were determined using CTFFIND4 41. The 2D class averages were generated with the Relion software package 42. Inconsistent class averages were removed from further data analysis. The 3D refinements and classifications were subsequently done in Relion. All final refinements were done using the auto refine option (Relion). The initial reference was filtered to 60 Å, and C1 symmetry was applied during refinements for all classes. Particles were split into two datasets and refined independently, and the resolution was determined using the 0.143 cut‐off (Relion auto refine option). Local resolution was determined with Resmap. All maps were filtered to local resolution using Relion with a B‐factor determined by Relion.
Model building
The model was built in Coot 43 and refined using Phenix real_space_refine 44. Figures are prepared with Chimera 45.
Author contributions
NS and MH conceived and supervised the project. AA‐A carried out mutagenesis, protein expression, purification and assembled nucleosomes and CENP‐C complexes and did MNase experiments and analysis. SB prepared grids for cryo‐EM. SB, IBS, and MH collected cryoEM data. SB and MH processed cryo‐EM data. AA, NS, SB, and MH analyzed data and built the structure, and AA refined the final models. AA and NS wrote the manuscript, and all authors commented on it.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Acknowledgements
N.S. and A.A. are supported by Centre for Molecular Medicine Norway (NCMM), Department of Chemistry at University of Oslo and Norwegian Research Council, grant # 263195. S.B. and M.H. are supported by St. Jude Children's Research Hospital, the American Lebanese Syrian Associated Charities and ERC‐smallRNAhet‐309584. We would like to thank Elena Conti and the cryo‐EM facility at Max Planck Institute for Biochemistry in Martinsried for access to cryo‐EM microscopes. Without their support this work would not be possible. We also thank Dario Segura‐Peña (UiO, Norway) and Lars Jansen (Oxford, UK) for critical reading of the manuscript, and our families for daily support.
EMBO Reports (2019) 20: e48913
Contributor Information
Mario Halić, Email: mario.halic@stjude.org.
Nikolina Sekulić, Email: nikolina.sekulic@ncmm.uio.no.
Data availability
The datasets produced in this study are available in the following databases:
CENP‐A nucleosome and CENP‐A/CENP‐CCR complex cryo‐EM maps: Electron Microscopy Data Bank (EMDB, www.ebi.ac.uk/pdbe/emdb), accession codes:
EMD‐10155 (CENP‐A, Class 1), www.emdb-empiar.org/emd-10155
EMD‐10152 (CENP‐A, Class 2), www.emdb-empiar.org/emd-10152
EMD‐10153 (CENP‐A, Class 2A), www.emdb-empiar.org/emd-10153
EMD‐10156 (CENP‐A, Class 2B), www.emdb-empiar.org/emd-10156
EMD‐10154 (CENP‐A, Class 2C), www.emdb-empiar.org/emd-10154
EMD‐10151 (CENP‐A/CENP‐CCR, Class 1), www.emdb-empiar.org/emd-10151
EMD‐10157 (CENP‐A/CENP‐CCR, Class 1A), www.emdb-empiar.org/emd-10157
EMD‐10158 (CENP‐A/CENP‐CCR, Class 1B), www.emdb-empiar.org/emd-10158
EMD‐10159 (CENP‐A/CENP‐CCR, Class 1C), www.emdb-empiar.org/emd-10159
CENP‐A nucleosome and CENP‐A/CENP‐CCR complex molecular models: Protein Data Bank (PDB, www.rcsb.org), accession codes:
6SE0 (CENP‐A, Class 1), www.pdbe.org/6se0
6SEG (CENP‐A/CENP‐CCR, Class 1), www.pdbe.org/6seg
6SE6 (CENP‐A/CENP‐CCR, Class 2), www.pdbe.org/6se6
6SEE (CENP‐A/CENP‐CCR, Class 2A), www.pdbe.org/6see
6SEF (CENP‐A/CENP‐CCR, Class 2C), www.pdbe.org/6sef
References
- 1. McKinley KL, Cheeseman IM (2016) The molecular basis for centromere identity and function. Nat Rev Mol Cell Biol 17: 16–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Potapova T, Gorbsky GJ (2017) The consequences of chromosome segregation errors in mitosis and meiosis. Biology 6: 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sekulic N, Black BE (2012) Molecular underpinnings of centromere identity and maintenance. Trends Biochem Sci 37: 220–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carroll CW, Milks KJ, Straight AF (2010) Dual recognition of CENP‐A nucleosomes is required for centromere assembly. J Cell Biol 189: 1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sekulic N, Bassett EA, Rogers DJ, Black BE (2010) The structure of (CENP‐A‐H4)2 reveals physical features that mark centromeres. Nature 467: 347–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tachiwana H, Kagawa W, Shiga T, Osakabe A, Miya Y, Saito K, Hayashi‐Takanaka Y, Oda T, Sato M, Park S‐Y et al (2011) Crystal structure of the human centromeric nucleosome containing CENP‐A. Nature 476: 232–235 [DOI] [PubMed] [Google Scholar]
- 7. Pentakota S, Zhou K, Smith C, Maffini S, Petrovic A, Morgan GP, Weir JR, Vetter IR, Musacchio A, Luger K (2017) Decoding the centromeric nucleosome through CENP‐N. Elife 6: e33442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chittori S, Hong J, Saunders H, Feng H, Ghirlando R, Kelly AE, Bai Y, Subramaniam S (2018) Structural mechanisms of centromeric nucleosome recognition by the kinetochore protein CENP‐N. Science 359: 339–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tian T, Li X, Liu Y, Wang C, Liu X, Bi G, Zhang X, Yao X, Zhou ZH, Zang J (2018) Molecular basis for CENP‐N recognition of CENP‐A nucleosome on the human kinetochore. Cell Res 28: 374–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Musacchio A, Desai A (2017) A molecular view of kinetochore assembly and function. Biology 6: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Milks KJ, Moree B, Straight AF (2009) Dissection of CENP‐C–directed centromere and kinetochore assembly. Mol Biol Cell 20: 4246–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song K, Gronemeyer B, Lu W, Eugster E, Tomkiel JE (2002) Mutational analysis of the central centromere targeting domain of human centromere protein C, (CENP‐C). Exp Cell Res 275: 81–91 [DOI] [PubMed] [Google Scholar]
- 13. Guo LY, Allu PK, Zandarashvili L, McKinley KL, Sekulic N, Dawicki‐McKenna JM, Fachinetti D, Logsdon GA, Jamiolkowski RM, Cleveland DW et al (2017) Centromeres are maintained by fastening CENP‐A to DNA and directing an arginine anchor‐dependent nucleosome transition. Nat Commun 8: 15775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kato H, Jiang J, Zhou B‐R, Rozendaal M, Feng H, Ghirlando R, Xiao TS, Straight AF, Bai Y (2013) A conserved mechanism for centromeric nucleosome recognition by centromere protein CENP‐C. Science 340: 1110–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Black BE, Cleveland DW (2011) Epigenetic centromere propagation and the nature of CENP‐A nucleosomes. Cell 144: 471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hasson D, Panchenko T, Salimian KJ, Salman MU, Sekulic N, Alonso A, Warburton PE, Black BE (2013) The octamer is the major form of CENP‐A nucleosomes at human centromeres. Nat Struct Mol Biol 20: 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nechemia‐Arbely Y, Fachinetti D, Miga KH, Sekulic N, Soni GV, Kim DH, Wong AK, Lee AY, Nguyen K, Dekker C et al (2017) Human centromeric CENP‐A chromatin is a homotypic, octameric nucleosome at all cell cycle points. J Cell Biol 216: 607–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roulland Y, Ouararhni K, Naidenov M, Ramos L, Shuaib M, Syed SH, Lone IN, Boopathi R, Fontaine E, Papai G et al (2016) The flexible ends of CENP‐A nucleosome are required for mitotic fidelity. Mol Cell 63: 674–685 [DOI] [PubMed] [Google Scholar]
- 19. Conde e Silva N, Black BE, Sivolob A, Filipski J, Cleveland DW, Prunell A (2007) CENP‐A‐containing nucleosomes: easier disassembly versus exclusive centromeric localization. J Mol Biol 370: 555–573 [DOI] [PubMed] [Google Scholar]
- 20. Zhou B‐R, Yadav KNS, Borgnia M, Hong J, Cao B, Olins AL, Olins DE, Bai Y, Zhang P (2019) Atomic resolution cryo‐EM structure of a native‐like CENP‐A nucleosome aided by an antibody fragment. Nat Commun 10: 2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lowary PT, Widom J (1998) New DNA sequence rules for high affinity binding to histone octamer and sequence‐directed nucleosome positioning 1. J Mol Biol 276: 19–42 [DOI] [PubMed] [Google Scholar]
- 22. Masumoto H, Masukata H, Muro Y, Nozaki N, Okazaki T (1989) A human centromere antigen (CENP‐B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J Cell Biol 109: 1963–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panchenko T, Sorensen TC, Woodcock CL, Kan Z, Wood S, Resch MG, Luger K, Englander SW, Hansen JC, Black BE (2011) Replacement of histone H3 with CENP‐A directs global nucleosome array condensation and loosening of nucleosome superhelical termini. Proc Natl Acad Sci USA 108: 16588–16593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bilokapic S, Strauss M, Halic M (2018) Histone octamer rearranges to adapt to DNA unwrapping. Nat Struct Mol Biol 25: 101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carroll CW, Silva MCC, Godek KM, Jansen LET, Straight AF (2009) Centromere assembly requires the direct recognition of CENP‐A nucleosomes by CENP‐N. Nat Cell Biol 11: 896–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGinty RK, Tan S (2016) Recognition of the nucleosome by chromatin factors and enzymes. Curr Opin Struct Biol 37: 54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falk SJ, Guo LY, Sekulic N, Smoak EM, Mani T, Logsdon GA, Gupta K, Jansen LET, Duyne GDV, Vinogradov SA et al (2015) CENP‐C reshapes and stabilizes CENP‐A nucleosomes at the centromere. Science 348: 699–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eickbush TH, Godfrey JE, Elia MC, Moudrianakis EN (1988) H2a‐specific proteolysis as a unique probe in the analysis of the histone octamer. J Biol Chem 263: 18972–18978 [PubMed] [Google Scholar]
- 29. Vogler C, Huber C, Waldmann T, Ettig R, Braun L, Izzo A, Daujat S, Chassignet I, Lopez‐Contreras AJ, Fernandez‐Capetillo O, et al (2010) Histone H2A C‐terminus regulates chromatin dynamics, remodeling, and histone H1 binding. PLoS Genet 6: e1001234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Biswas M, Voltz K, Smith JC, Langowski J (2011) Role of histone tails in structural stability of the nucleosome. PLoS Comput Biol 7: e1002279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hori T, Shang W‐H, Toyoda A, Misu S, Monma N, Ikeo K, Molina O, Vargiu G, Fujiyama A, Kimura H et al (2014) Histone H4 Lys 20 monomethylation of the CENP‐A nucleosome is essential for kinetochore assembly. Dev Cell 29: 740–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arimura Y, Tachiwana H, Takagi H, Hori T, Kimura H, Fukagawa T, Kurumizaka H (2019) The CENP‐A centromere targeting domain facilitates H4K20 monomethylation in the nucleosome by structural polymorphism. Nat Commun 10: 576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Allu PK, Dawicki‐McKenna JM, Van Eeuwen T, Slavin M, Braitbard M, Xu C, Kalisman N, Murakami K, Black BE (2019) Structure of the human core centromeric nucleosome complex. Curr Biol 29: 2625–2639.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bönisch C, Hake SB (2012) Histone H2A variants in nucleosomes and chromatin: more or less stable? Nucleic Acids Res 40: 10719–10741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bodor DL, Mata JF, Sergeev M, David AF, Salimian KJ, Panchenko T, Cleveland DW, Black BE, Shah JV, Jansen LE (2014) The quantitative architecture of centromeric chromatin. Elife 3: e02137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ross JE, Woodlief KS, Sullivan BA (2016) Inheritance of the CENP‐A chromatin domain is spatially and temporally constrained at human centromeres. Epigenetics Chromatin 9: 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sekulic N, Black BE (2016) Preparation of recombinant centromeric nucleosomes and formation of complexes with nonhistone centromere proteins. Methods Enzymol 573: 67–96 [DOI] [PubMed] [Google Scholar]
- 38. Dyer PN, Edayathumangalam RS, White CL, Bao Y, Chakravarthy S, Muthurajan UM, Luger K (2003) Reconstitution of nucleosome core particles from recombinant histones and DNA In Methods in Enzymology, (ed.), pp 23–44. Academic Press; 10.1016/s0076-6879(03)75002-2 [DOI] [PubMed] [Google Scholar]
- 39. Luger K, Rechsteiner TJ, Richmond TJ (1999) Preparation of nucleosome core particle from recombinant histones In Methods in Enzymology, (ed.), pp 3–19. Academic Press; 10.1016/s0076-6879(99)04003-3 [DOI] [PubMed] [Google Scholar]
- 40. Grant T, Grigorieff N (2015) Measuring the optimal exposure for single particle cryo‐EM using a 2.6 Å reconstruction of rotavirus VP6. Elife 4: e06980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rohou A, Grigorieff N (2015) CTFFIND4: fast and accurate defocus estimation from electron micrographs. J Struct Biol 192: 216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scheres SHW (2012) RELION: implementation of a Bayesian approach to cryo‐EM structure determination. J Struct Biol 180: 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of coot. Acta Crystallogr D Biol Crystallogr 66: 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L‐W, Kapral GJ, Grosse‐Kunstleve RW et al (2010) PHENIX: a comprehensive Python‐based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File
Data Availability Statement
The datasets produced in this study are available in the following databases:
CENP‐A nucleosome and CENP‐A/CENP‐CCR complex cryo‐EM maps: Electron Microscopy Data Bank (EMDB, www.ebi.ac.uk/pdbe/emdb), accession codes:
EMD‐10155 (CENP‐A, Class 1), www.emdb-empiar.org/emd-10155
EMD‐10152 (CENP‐A, Class 2), www.emdb-empiar.org/emd-10152
EMD‐10153 (CENP‐A, Class 2A), www.emdb-empiar.org/emd-10153
EMD‐10156 (CENP‐A, Class 2B), www.emdb-empiar.org/emd-10156
EMD‐10154 (CENP‐A, Class 2C), www.emdb-empiar.org/emd-10154
EMD‐10151 (CENP‐A/CENP‐CCR, Class 1), www.emdb-empiar.org/emd-10151
EMD‐10157 (CENP‐A/CENP‐CCR, Class 1A), www.emdb-empiar.org/emd-10157
EMD‐10158 (CENP‐A/CENP‐CCR, Class 1B), www.emdb-empiar.org/emd-10158
EMD‐10159 (CENP‐A/CENP‐CCR, Class 1C), www.emdb-empiar.org/emd-10159
CENP‐A nucleosome and CENP‐A/CENP‐CCR complex molecular models: Protein Data Bank (PDB, www.rcsb.org), accession codes:
6SE0 (CENP‐A, Class 1), www.pdbe.org/6se0
6SEG (CENP‐A/CENP‐CCR, Class 1), www.pdbe.org/6seg
6SE6 (CENP‐A/CENP‐CCR, Class 2), www.pdbe.org/6se6
6SEE (CENP‐A/CENP‐CCR, Class 2A), www.pdbe.org/6see
6SEF (CENP‐A/CENP‐CCR, Class 2C), www.pdbe.org/6sef


