Abstract
Electronic waste management is one of the key challenges for the green revolution without affecting the environment. The wide use of printer devices has brought a horde of discarded waste toner, which release ∼6000 tons of processed carbon powder into the atmosphere every year that would essentially pollute the atmosphere. Here, we propose a one-step thermal conversion of waste toner powder into carbon/Fe3O4 nanocomposites for energy storage applications. Recovered toner carbon (RTC) and toner carbon calcined at 300 °C (RTC-300) were characterized using various analytical tools. From the FE-SEM analysis, the presence of carbon particles with uniformly decorated Fe3O4 nanoparticles was confirmed. RTC-300 carbon was used as an electrode material for supercapacitors, and it exhibited a high specific capacitance of 536 F/g at a current density of 3 A/g, which is almost six times higher than that of the commercial mesoporous graphitized carbon black. RTC-300 showed excellent electrochemical stability of 97% over 5000 cycles at a high current density of 20 A/g. The fabricated symmetric cell using RTC-300 electrode materials in an aqueous electrolyte with a cell voltage of 1.8 V delivered a high energy and high-power density of 42 W h/kg and 14.5 kW/kg, respectively. The fabricated device is stable up to 20,000 cycles at a high current density of 20 A/g with a loss of 23% capacitance.
1. Introduction
At present, environmental pollution is one of the utmost problems that the world is facing, which is causing serious and long-lasting damage to natural resources and living things.1,2 Pollution occurs by many forms of waste that end up in landfills such as chemicals in the form of solids, liquids, and gases that affect the environment either directly or indirectly.3 According to a Planet Ark report in 2006, approximately 500 million ink cartridges (toner) end up in a landfill site around the world every year.4 This would create waste of 3000 tons of plastic, 2500 tons of ferrous metals, 400 tons of aluminum, and 26 kg of precious metals5 (Figure S1). An ″empty″ cartridge contains 8% unused residual powder by weight, and it may be more, depending on the types of printer models.6Table S1 summarizes the major chemical compounds present in HP black toner powder based on the literature. Approximately, 6000 tons of carbon powder is ejected into the atmosphere every year that would essentially pollute the atmosphere, water and soil.1,7,8
Previously, some investigation has been shown on transforming waste toner carbon into potentially useful materials like synthetic oils,9 nano-SiO2, SiO2–carbon, nano-Fe3O4,7,8 fillers,6 and colorants6 in the rubber manufacturing process. For instance, Gaikwad et al. reported a thermal transformation of waste toner powder into value-added ferrous materials.7 At a high temperature of 1550 °C, there is a complete transformation of toner powder into ferrous materials (98%) where the carbon was used as an inherent reducing agent. Most recently, Li et al. reported a single-step thermal conversion of toner powder for use as anode materials for Li-ion battery applications.8 The heat-treated carbon-coated ferric oxides exhibited a discharge specific capacity of 1029 mA h/g. In all of the above methods, all the copolymers are thermally converted into carbon, carbon monoxide, and CO2, which again leads to an increase in the environmental pollution. Here, we demonstrated a one-step thermal conversion of toner powder at 300 °C into a carbon/Fe3O4 nanocomposite for symmetric supercapacitor applications. From the cyclic voltammetry (CV) and galvanostatic charge–discharge (CD) analysis, the prepared RTC-300 electrode materials exhibited a high specific capacitance of 536 F/g at a current density of 3 A/g, which is six times higher than that of the commercial mesoporous graphitized carbon black (MGCB).10
2. Results and Discussion
A single-step carbonization process for conversion of waste toner cartridge-derived RTC to RTC-300 is schematically shown in Figure 1a. The thermogravimetric analysis (TGA) profile of RTC shows a well-distinguished weight loss for adsorbed moisture and decomposition of the polymer and activated carbon (Figure 1b).8 From the profile, a slight weight loss of 1% was observed up to 150 °C due to the removal of physically and chemically adsorbed moisture. Further, when the temperature increased, a significant amount of weight loss (47%) is observed between 200 and 380 °C owing to the loss of the polymer backbone as carbon and CO2.11 Usually, the decomposition of the polymer backbone took place at temperatures higher than 300 °C.12 Additionally, a 6% weight loss is observed in the temperature region of 380 to 500 °C, which is mainly due to the decomposition of fixed carbon like an activated carbon block into CO and CO2.2,13 Above 500 °C, the TGA profile is stable up to 1000 °C, which clearly reveals that ∼46% of the Fe3O4 nanoparticles in the recovered material are from the waste toner.6,14
Figure 1.
(a) Schematic representation of RTC preparation from a waste toner cartridge; (b) TGA profile of RTC and (c) XRD patterns of RTC, RTC-300, and RTC-500 with a cubic Fe3O4 standard pattern (ICDD no. 01-088-0866).
The powder X-ray diffraction (XRD) patterns of RTC, RTC-300, and RTC-500 are shown in Figure 1c. All the three samples show highly intense peaks, and the observed peaks are indexed to the standard pattern of crystalline cubic Fe3O4 (ICDD card no: 01-088-0866). The diffraction peaks that appeared at 2θ values of 18.3, 30.1, 35.4, 37.1, 43.1, 53.5, 57.1, 62.6, and 74.1° are assigned to the (111), (220), (311), (222), (400), (422), (511), (440), and (533) planes of Fe3O4, respectively. During the heat treatment, the intensity of the XRD lines increased while the temperature increased, which reflects the highly crystalline nature of the Fe3O4 nanoparticles with high-temperature treatment. However, when increasing the temperature, the width of the XRD peaks decreased, which indicates the large crystallite size of the nanoparticles. From the XRD pattern, the average crystallite size of the particles is calculated using the Debye–Scherrer equation.15 The calculated average crystallite sizes of Fe3O4 nanoparticles in RTC, RTC-300, and RTC-500 are 45, 56, and 62 nm, respectively.
The field-emission scanning electron microscopy (FE-SEM) images of as-received RTC at low and high magnifications are shown in Figure 2a–c. It could be clearly seen that the sphere-shaped toner powder with an average size of 2–5 μm consisted of a few nanometer-sized Fe3O4 nanoparticles decorated on polymeric compounds (Figure 2c). The FE-SEM images of RTC-300 at low and high magnifications (Figure 2d–f and Figure S2) demonstrated that after the heat treatment, the sphere-shaped toner particles are significantly transformed into carbon/Fe3O4 nanocomposites. The micron-sized sphere-like RTC particles are converted into nanosized particles (RTC-300 and RTC-500) where 50–100 nm Fe3O4 nanoparticles are decorated on carbon surfaces (Figure 2f). This is due to the thermal decomposition of the styrene acrylate-based copolymer into carbon-based materials.16,17 Furthermore, the elemental mapping of RTC-300 materials shown in Figure 2g–j confirmed the presence of C, O, and Fe elements, which are uniformly distributed on the entire surface. This observation evidently confirms the presence of carbon and Fe3O4 nanoparticles in the RTC-300 nanocomposite materials. In addition, scanning electron microscopy (SEM) images of RTC-500 are shown in Figure 3. When the carbonization temperature increased up to 500 °C, the carbon materials decomposed as CO/CO2 and resulted in aggregated Fe3O4 nanoparticles with trace amounts of carbon. The elemental composition analysis of RTC-300 and RTC-500 by EDX confirmed the above statement (Figure S3). The drastic reduction in the carbon content in RTC-500 compared to RTC-300 clearly confirmed the decomposition of carbon.
Figure 2.
FE-SEM images of (a–c) RTC and (d–f) RTC-300 at different magnifications and elemental mapping of (g) all elements mixed (Mix), (h) carbon (C), (i) oxygen (O), and (j) iron (Fe) in RTC-300 samples.
Figure 3.
(a–c) SEM images of RTC-500 at different magnifications.
The microstructure of the recovered RTC-300 and RTC-500 materials was investigated using Brunauer–Emmett–Teller (BET) N2 adsorption/desorption isotherm analysis (Figure 4). According to the International Union of Pure and Applied Chemistry (IUPAC) classification, the resulting isotherm of RTC-300 and RTC-500 exhibited a typical type-IV isotherm, which is the characteristic of porous materials.18 The narrow silt-like pore shape (H4 classification) was observed for RTC-300 and RTC-500 (Figure 4). RTC-300 showed a high specific surface area (SSA) and pore volume of 16.4 m2/g and 0.111 cm3/g, respectively. However, RTC-500 showed a relative low SSA and pore volume of 3.2 m2/g and 0.018 cm3/g, respectively. The Barrett–Joyner–Halenda (BJH) pore size distribution study designates that the recovered RTC-300 and RTC-500 have a pore diameter in the range of 3.0 to 5.6 and 3.0 to 9.6 nm, respectively (Figure 4, inset). The average pore radii of the RTC-300 and RTC-500 materials are 1.5 and 1.52 nm, respectively. The combination of micro- and mesoporous with high SSA and pore volume will enable the fast access to the electrode surface through the pores at the electrode–electrolyte interface, which is of huge benefits during the charge–discharge process.
Figure 4.
BET adsorption–desorption isotherm of (a) RTC-300 and (b) RTC-500 and their corresponding BJH pore size distribution curve (inset).
Further, the prepared RTC-300 and RTC-500 electrode materials were tested for energy storage applications in supercapacitor devices. Thus, specific electrochemical characterization tools such as cyclic voltammetry (CV), galvanostatic charge–discharge (CD), and electrochemical impedance spectroscopy (EIS) analysis were carried out for supercapacitor applications. CV was carried out between −1.2 and 0.6 V (vs Hg/HgO) at various scan rates in 3.5 M KOH electrolyte. The typical CV profiles of RTC-300 and RTC-500 electrode materials at 50 mV/s scan rates are shown Figure 5a. The area of the CV profile is larger for RTC-300 than that for RTC-500. This is due to the presence of carbon in the RTC-300 nanocomposite materials. The CV profiles of RTC-300 and RTC-500 electrode materials at different scan rates (5–50 mV/s) are displayed in Figure 5b,c, respectively. A nonsymmetrical shape with a set of redox peaks appears for all the scan rates for both electrode materials. It clearly confirms the faradaic nature of the electrodes due to the presence of redox-active Fe3O4 nanoparticles.19−22 The observed oxidation and reduction peaks are attributed to the reversible faradaic process for Fe2+ ⇌ Fe3+ in alkaline OH anions.20
Figure 5.
(a) CV profiles of RTC-300 and RTC-500 at a 50 mV/s scan rate, CV profile of (b) RTC-300 and (c) RTC-500 at different scan rates, (d) plots of specific capacity (Cq) vs reciprocal of the square root of scan rate (v-1/2), and (e) reciprocal of specific capacity (Cq–1) vs the square root of scan rate (v1/2). (f) Bar diagram of the specific capacity (Cq) of RTC-300 and RTC-500 electrodes with capacitive (EDLC, red) and diffusive (PC, blue) charging derived from Trasatti’s method.
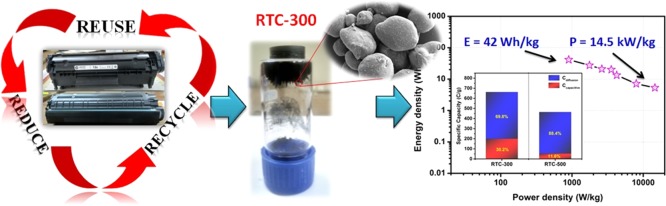
The capacitive contribution of electrical double layer capacitance (EDLC) and pseudocapacitive (PC) reactions of electrode materials are evaluated by the Trasatti method.23 Using this method, we have demonstrated the quantitative isolation of the capacitive elements (EDLC) from the diffusion controlled insertion processes (PC). In these methods, plotting the specific capacity as a function of scan rate reveals the charge-storing mechanism of the electrode materials.24,25 In general, the specific capacity decreased when the scan rate increased correspondingly. Assuming ion diffusion follows a semi-infinite linear diffusion ν → 0 (i.e., giving sufficient time for ions to diffuse and react from bulk electrolyte to the electrode–electrolyte interface), the possibility of an accessible “inner surface” area of the material gives the maximum specific capacity of the electrode materials (q → qT).26 Similarly, assuming that ion diffusion follows a semi-infinite linear diffusion ν → α (i.e., just allowing surface processes to happen), the only accessible area is the “outer surface” of the electrode–electrolyte interface, which gives the specific capacity of the electrode materials (q → qo).27 The specific capacity as a function of ν1/2 and ν–1/2 is shown in Figure 5 d,e. Linear fitting of the plot and extrapolating the fitted line to the y axis gives the Co, and subtraction of Co from CT yields the maximum pseudocapacitance (Ci).24 The detailed Trasatti method of analysis is given in the Supporting Information. Bar diagrams of the specific capacity of the RTC-300 and RTC-500 electrode materials with the contribution of the EDLC (red) and PC (blue) are displayed in Figure 5f. RTC-300 exhibited a 30.2 and 69.8% capacity derived from the EDLC and PC contributions while RTC-500 delivered a 11.6 and 88.4% capacity derived from the EDLC and PC contributions, respectively. High-temperature activation of RTC-500 leads to the removal of activated carbon and the presence of more Fe3O4 for the reason of PC contributing more than EDLC. In the case of RTC-300, the combination of nonfaradaic and faradaic processes helps enhance electrochemical activities for the charge storage.28,29
The typical CD profiles of RTC-300 and RTC-500 electrode materials at a constant current density of 3 A/g are shown Figure 6a. The specific capacitance of the electrode material was calculated from the discharge profile (eq 1 in the Supporting Information). RTC-300 and RTC-500 exhibited high specific capacitances of 536 and 323 F/g at a current density of 3 A/g, respectively. The CD profile of RTC-300 (Figure 6b) and RTC-500 (Figure 6c) electrodes at different current densities shows a typical nonlinear profile corresponding to the faradaic nature, which confirms the presence of Fe3O4 nanoparticles.20 The specific capacitance of the electrode material was calculated from the discharge profile, and RTC-300 exhibited specific capacitances of 536, 465, 397, 354, 255, and 175 F/g at current densities of 3, 4, 5, 6, 10, and 20 A/g, respectively. Similarly, RTC-500 exhibited specific capacitances of 323, 273, 249, 221, 151, and 79 F/g at current densities of 3, 4, 5, 6, 10, and 20 A/g, respectively. When the current density was increased up to 20 A/g, RTC-300 exhibited a high rate performance of 36%, which is higher than that of the RTC-500 (24%) (Figure 6d). The high specific capacitance of the RTC-300 electrode is better than that of the commercial MGCB materials (Figure S4). Moreover, there is no obvious internal resistance (iR drop) that could be observed in the charge–discharge profile, which indicates the good electrical conductivity of RTC-300 due to the synergistic effect of carbon and Fe3O4 nanoparticles. This combination of nonfaradaic and faradaic materials with a high electrical conductivity might be enhanced by the charge transfer reaction at the electrode–electrolyte interface for the fast surface reaction.30,31 The long-term electrochemical stability of the electrode materials is a key for viable applications. In order to check the electrochemical stability of the RTC-300 electrode materials, CD cycles were carried out for 5,000 charge–discharge cycles at a high current density of 20 A/g (Figure 6e). The capacitance retention of 97% was found and retained a constant Coulombic efficiency of 99% for 5,000 charge–discharge cycles, which reveals the high reversibility of the electrode materials during the electrochemical process.
Figure 6.
(a) CD profile of RTC-300 and RTC-500 at 3 A/g current density, CD profile of (b) RTC-300 and (c) RTC-500 at different current densities, (d) specific capacitance of all the materials as a function of current density curve, (e) specific capacitance and Coulombic efficiency of RTC-300 electrode materials as a function of cycle number, first and last few charge–discharge cycles (inset), and (f) Nyquist plot of RTC-300 with corresponding equivalent circuit (inset).
To understand the charge transfer process at the electrode–electrolyte interface of RTC-300 and the resistance associated with the diffusion of charge carriers, EIS was performed, and the resulting Nyquist plot is shown in Figure 6f. The EIS profile was fitted with a corresponding equivalent circuit, which consists of various R and C components (Figure 6f, inset). The Nyquist plot of RTC-300 showed a bulk resistance or solution resistance of Rs = 0.4 Ω, and the semicircle appeared in the high-frequency region due to the combination of charge-transfer resistance (Rct = 0.6 Ω) and the double layer capacitance (Cdl). The observed straight line is more vertical in the low-frequency region and has a smaller imaginary part, indicating the low diffusion resistance (Warburg impedance, W).32 Additionally, the presence of a pseudocapacitive component (CF) is due to the electrochemical faradaic nature of Fe3O4 in alkali medium.33 The low Rs and Rct and the combination of nonfaradaic and faradaic processes help enhance electrochemical activities with a good charge-transfer process at the electrode–electrolyte interface.
Nowadays, activated carbon-based electrode materials are widely used as electrode materials for supercapacitors with nonaqueous-based expensive organic and ionic liquid electrolytes.2,30,34 While supercapacitors have momentous advantages like high power, high electrochemical stability, and wider operating temperature, it has some disadvantages such as low energy density and high cost that limit them as an alternative for conventional energy storage devices.35 A supercapacitor with high cell voltage, high energy, high power density, and low cost is required for future modern energy technology. Thus, supercapacitor performance studies at the cell or device level are highly essential for commercial applications. For the full-cell fabrication, the symmetric supercapacitor cell is fabricated with RTC-300 electrodes in aqueous 3.5 M KOH as the electrolyte. The electrochemical performance of the fabricated symmetric cell was evaluated using CV and CD analyses. Figure 7a reveals the CV profile of a fabricated symmetric supercapacitor with a cell voltage of 1.8 V at different scan rates. The nonsymmetrical shape of the CV profiles with a set of redox peaks at all the scan rates clearly confirms the contribution of faradaic capacitance from Fe3O4 nanoparticles.
Figure 7.
(a) CV profile of the fabricated RTC-300 symmetric cell at different scan rates, (b) CD profile of the fabricated RTC-300 symmetric cell at different current densities, (c) specific capacitance and Coulombic efficiency of the fabricated RTC-300 symmetric cell as a function of cycle number, and (d) Ragone plot for the fabricated RTC-300 symmetric cell.
The calculated specific capacitances of the symmetric supercapacitor cell from the discharge profile (Figure 7b) are 368, 256, 200, 164, 140, 80, and 68 F/g at current densities of 1, 2, 3, 4, 5, 10, and 20 A/g, respectively. At a high current density of 20 A/g, the CD analysis was carried out for 20,000 charge–discharge cycles (Figure 7c), and after 20,000 charge–discharge cycles, 87% of the initial capacitance was retained. Also, a high Coulombic efficiency of 99.8% was maintained for 20,000 charge–discharge cycles, which reveals the high reversibility of the electrode materials during the electrochemical charge–discharge process. To the best of our knowledge, this is one of the best values and stable electrodes among the reported aqueous supercapacitors (Table S2).
The real performance of a supercapacitor is evaluated in terms of energy and power density of the fabricated devices. The typical Ragone plot of a symmetric supercapacitor cell with a cell voltage of 1.8 V clearly indicates that the fabricated device has a wide range of energy and power densities (Figure 7d). The fabricated symmetric supercapacitor cell exhibited a remarkable high energy and power density of 42 W h/kg and 900 W/kg at 1 A/g current density, respectively. Even at a high current density of 20 A/g, the fabricated symmetric supercapacitor cell delivered a high energy density of 5.3 W h/kg with a high power density 14.5 kW/kg. It could be clearly seen from the Ragone plot that the symmetric supercapacitor cell can provide a high energy density without sacrificing much the power density. The above-observed high energy and power densities are significantly higher than the reported values for carbon-based nanocomposite materials in symmetric and asymmetric supercapacitors using aqueous electrolyte. For instance, Saha et al. reported a hydrothermal method for the preparation of Fe3O4/RGO nanocomposite for a supercapacitor that exhibited a high energy and power density of 39.1 W h/kg and 1800 W/kg, respectively.36 Lim et al. reported hydrothermally derived porous 3D carbon-decorated Fe3O4 nanocomposite electrode materials that exhibited a high energy and power density of 29.2 W h/kg and 1.2 kW/kg, respectively.37 Also, some Fe3O4-based nanocomposites like graphene/Fe3O4 nanocomposites (9 W h/kg and 3 kW/kg)38 and Fe3O4/carbon nanotube/polyaniline ternary films (28 W h/kg and 5.3 kW/kg)19 delivered lower energy density than the RTC-300 symmetric cell.
3. Conclusions
Altogether, ∼6000 tons of carbon powder is ejected into the atmosphere in the form of waste toner powder every year that is essentially polluting the atmosphere. Here, one-step thermal conversion of waste toner powder into carbon/Fe3O4 nanocomposites was demonstrated for energy storage applications. The prepared RTC-300 exhibited a high specific capacitance of 536 F/g at a current density of 3 A/g with an excellent electrochemical stability of 97% for 5000 cycles. The fabricated symmetric cell in aqueous electrolyte with a cell voltage of 1.8 V delivered a high energy and high power density of 42 W h/kg and 14.5 kW/kg, respectively. The fabricated device is stable up to 20,000 cycles at a current density of 20 A/g with a loss of 23% of the initial capacitance.
4. Experimental Section
4.1. Materials
Waste cartridges (HP LaserJet 12A) are collected from the e-waste accumulated at our office. Activated conductive carbon (mesoporous graphitized carbon black, 99.95%), poly(vinylidene fluoride) (PVDF), and potassium hydroxide pellets (KOH, 98 wt %) were purchased from Sigma-Aldrich, India. Ethanol (CH3CH2OH, 99 wt %) was purchased from SRL Pvt. Ltd., India. Graphite foil (0.13 mm thick, 99.8%) was purchased from Alfa Aesar India Pvt. Ltd., India. All purchased chemicals and reagents were of analytical grade and used as received without any further purification. Deionized (DI) water was obtained through the MILLIPORE water system.
4.2. Preparation of Carbon/Fe3O4 Nanocomposite
Here, we demonstrated a one-step thermal conversion of toner powder at 300 °C into carbon/Fe3O4 nanocomposites for symmetric supercapacitor applications. In detail, the print toner cartridge (model no. HP LaserJet 12A) was recovered and dismantled to get residual toner, plastic, metal, and so on, with proper safety precautions. The residual toner powder was taken out and named recovered toner carbon (RTC). The RTC was kept in a furnace at 300 °C for 3 h. After the thermal decomposition of RTC, the recovered material was washed with water several times and named RTC-300 for further discussion. For the comparison analysis, RTC was heated at 500 °C for 3 h, and the obtained material is named RTC-500. The detailed material characterization and preparations of electrode materials and related specific capacitance, energy density, and power density calculations are discussed in the Supporting Information.
Acknowledgments
The authors thank the Science & Engineering Research Board, Department of Science and Technology (DST-SERB, File No EMR/2016/006807, GAP 25/17), India for financial support. K.S. (IF131153) thanks DST for INSPIRE Fellowship. The authors thank Central Instrumentation Facility, CSIR-CECRI, Karaikudi.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01337.
Characterization and detailed preparation of the working electrode for supercapacitor applications, chemical composition in print cartridges and comparison table of major chemical compounds present in HP black toner powder based on the literature, comparison table for carbon/Fe3O4 nanocomposites with recent reports and comparison of CV and CD profiles of RTC-300 and commercial activated carbon, EDX spectrum of RTC-300 and RTC-500 materials, and detailed Trasatti method analysis (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Awasthi A. K.; Zeng X.; Li J. Environmental Pollution of Electronic Waste Recycling in India: A Critical Review. Environ. Pollut. 2016, 211, 259–270. 10.1016/j.envpol.2015.11.027. [DOI] [PubMed] [Google Scholar]
- Subramani K.; Sudhan N.; Karnan M.; Sathish M. Orange Peel Derived Activated Carbon for Fabrication of High-Energy and High-Rate Supercapacitors. ChemistrySelect 2017, 2, 11384–11392. 10.1002/slct.201701857. [DOI] [Google Scholar]
- Natarajan S.; Aravindan V. Burgeoning Prospects of Spent Lithium-Ion Batteries in Multifarious Applications. Adv. Energy Mater. 2018, 1802303. 10.1002/aenm.201802303. [DOI] [Google Scholar]
- European Toner and Inkjet Remanufacturers Association . Remanufacturing cartridges is environmental-friendly. http://www.etira.org/environment/ (accessed January 9, 2019).
- Koseki H. Study and Countermeasure of Hazard of Dust Explosion of Various Toner Cartridges. Procedia Eng. 2014, 84, 273–279. 10.1016/j.proeng.2014.10.434. [DOI] [Google Scholar]
- Yordanova D.; Angelova S.; Dombalov I. Utilisation Options for Waste Toner Powder. J. Environ. Sci. 2014, 3, 140–144. [Google Scholar]
- Gaikwad V.; Kumar U.; Pahlevani F.; Piadasa A.; Sahajwalla V. Thermal Transformation of Waste Toner Powder into a Value-Added Ferrous Resource. ACS Sustainable Chem. Eng. 2017, 5, 11543–11550. 10.1021/acssuschemeng.7b02875. [DOI] [Google Scholar]
- Li Y.; Mao J.; Xie H.; Li J. Heat-Treatment Recycling of Waste Toner and Its Applications in Lithium Ion Batteries. J. Mater. Cycles Waste Manage. 2018, 20, 361–368. 10.1007/s10163-017-0599-z. [DOI] [Google Scholar]
- Ruan J.; Dong L.; Huang J.; Huang Z.; Huang K.; Dong H.; Zhang T.; Qiu R. Vacuum-Gasification-Condensation of Waste Toner to Produce Industrial Chemicals and Nanomaterials. ACS Sustainable Chem. Eng. 2017, 5, 4923–4929. 10.1021/acssuschemeng.7b00328. [DOI] [Google Scholar]
- Subramani K.; Sudhan N.; Divya R.; Sathish M. All-Solid-State Asymmetric Supercapacitors Based on Cobalt Hexacyanoferrate-Derived CoS and Activated Carbon. RSC Adv. 2017, 7, 6648–6659. 10.1039/C6RA27331A. [DOI] [Google Scholar]
- Zhao G.; Chen C.; Yu D.; Sun L.; Yang C.; Zhang H.; Sun Y.; Besenbacher F.; Yu M. One-Step Production of O-N-S Co-Doped Three-Dimensional Hierarchical Porous Carbons for High-Performance Supercapacitors. Nano Energy 2018, 47, 547–555. 10.1016/j.nanoen.2018.03.016. [DOI] [Google Scholar]
- Guimarães D. H.; Brioude M. D. M.; Fiúza R. D. P.; Prado L. A. S. D. A.; Boaventura J. S.; José N. M. Synthesis and Characterization of Polyesters Derived from Glycerol and Phthalic Acid. Mater. Res. 2007, 10, 257–260. 10.1590/S1516-14392007000300007. [DOI] [Google Scholar]
- Sudhan N.; Subramani K.; Karnan M.; Ilayaraja N.; Sathish M. Biomass-Derived Activated Porous Carbon from Rice Straw for a High-Energy Symmetric Supercapacitor in Aqueous and Non-Aqueous Electrolytes. Energy Fuels 2017, 31, 977–985. 10.1021/acs.energyfuels.6b01829. [DOI] [Google Scholar]
- Yufanyi D. M.; Agwara M. O.; Foba-Tendo J.; Ketcha J. M. Effect of Decomposition Temperature on the Crystallinity of α-Fe2O3 (Hematite) Obtained from an Iron(III)-Hexamethylenetetramine Precursor. Am. J. Chem. 2015, 5, 1–9. 10.5923/j.chemistry.20150501.01. [DOI] [Google Scholar]
- Cullity B. D.Elements of X-Ray Diffraction; Addison-Wesley: 1956. [Google Scholar]
- Sun Y.; Zhao J.; Wang J.; Tang N.; Zhao R.; Zhang D.; Guan T.; Li K. Sulfur-Doped Millimeter-Sized Microporous Activated Carbon Spheres Derived from Sulfonated Poly(Styrene-Divinylbenzene) for CO2 Capture. J. Phys. Chem. C 2017, 121, 10000–10009. 10.1021/acs.jpcc.7b02195. [DOI] [Google Scholar]
- Singh A.; Lal D. Preparation and Characterization of Activated Carbon Spheres from Polystyrene Sulphonate Beads by Steam and Carbon Dioxide Activation. J. Appl. Polym. Sci. 2010, 115, 2409–2415. 10.1002/app.31340. [DOI] [Google Scholar]
- Kaipannan S.; Marappan S. Fabrication of 9.6 V High-Performance Asymmetric Supercapacitors Stack Based on Nickel Hexacyanoferrate-Derived Ni(OH)2 Nanosheets and Bio-Derived Activated Carbon. Sci. Rep. 2019, 9, 1104. 10.1038/s41598-018-37566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Lu W.; Yan Y.; Chou T.-W. High Performance Solid-State Flexible Supercapacitor Based on Fe3O4/Carbon Nanotube/Polyaniline Ternary Films. J. Mater. Chem. A 2017, 5, 11271–11277. 10.1039/C7TA02008B. [DOI] [Google Scholar]
- Lin J.; Liang H.; Jia H.; Chen S.; Guo J.; Qi J.; Qu C.; Cao J.; Fei W.; Feng J. In Situ Encapsulated Fe3O4 Nanosheet Arrays with Graphene Layers as an Anode for High-Performance Asymmetric Supercapacitors. J. Mater. Chem. A 2017, 5, 24594–24601. 10.1039/C7TA07628B. [DOI] [Google Scholar]
- Lian C.; Wang Z.; Lin R.; Wang D.; Chen C.; Li Y. An Efficientfficient, Controllable and Facile Two-Step Synthesis Strategy: Fe3O4@RGO Composites with Various Fe3O4 Nanoparticles and Their Supercapacitance Properties. Nano Res. 2017, 10, 3303–3313. 10.1007/s12274-017-1543-8. [DOI] [Google Scholar]
- Vignesh V.; Subramani K.; Sathish M.; Navamathavan R. Electrochemical Investigation of Manganese Ferrites Prepared via a Facile Synthesis Route for Supercapacitor Applications. Colloids Surf., A 2018, 538, 668–677. 10.1016/j.colsurfa.2017.11.045. [DOI] [Google Scholar]
- Ardizzone S.; Fregonara G.; Trasatti S. “INNER” AND “OUTER” ACTIVE SURFACE OF RuO2, ELECTRODES. Electrochim. Acta 1990, 35, 263–267. 10.1016/0013-4686(90)85068-X. [DOI] [Google Scholar]
- Huang Z.-H.; Liu T.-Y.; Song Y.; Li Y.; Liu X.-X. Balancing the Electrical Double Layer Capacitance and Pseudocapacitance of Hetero-Atom Doped Carbon. Nanoscale 2017, 9, 13119–13127. 10.1039/c7nr04234e. [DOI] [PubMed] [Google Scholar]
- Zhi J.; Reiser O.; Huang F. Hierarchical MnO2 Spheres Decorated by Carbon-Coated Cobalt Nanobeads: Low-Cost and High-Performance Electrode Materials for Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 8452–8459. 10.1021/acsami.5b12779. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Wang Y.; Zhang X.; Wang G.; Ji P.; Yin F. WSe 2 /Reduced Graphene Oxide Nanocomposite with Superfast Sodium Ion Storage Ability as Anode for Sodium Ion Capacitors. J. Electrochem. Soc. 2018, 165, A3642–A3647. 10.1149/2.0231816jes. [DOI] [Google Scholar]
- Shao J.; Zhou X.; Liu Q.; Zou R.; Li W.; Yang J.; Hu J. Mechanism Analysis of the Capacitance Contributions and Ultralong Cycling-Stability of the Isomorphous MnO2@MnO2 Core/Shell Nanostructures for Supercapacitors. J. Mater. Chem. A 2015, 3, 6168–6176. 10.1039/c4ta06793b. [DOI] [Google Scholar]
- Amutha B.; Subramani K.; Reddy P. N.; Sathish M. Graphene-Polymer//Graphene-Manganese Oxide Nanocomposites-Based Asymmetric High Energy Supercapacitor with 1.8 V Cell Voltage in Aqueous Solution. ChemistrySelect 2017, 2, 10754–10761. 10.1002/slct.201701979. [DOI] [Google Scholar]
- Vignesh V.; Subramani K.; Oh M.-S.; Sathish M.; Navamathavan R. Synthesis of GNS-MnS Hybrid Nanocomposite for Enhanced Electrochemical Energy Storage Applications. Mater. Chem. Phys. 2019, 230, 249–257. 10.1016/j.matchemphys.2019.03.070. [DOI] [Google Scholar]
- Rana M.; Subramani K.; Sathish M.; Gautam U. K. Soya Derived Heteroatom Doped Carbon as a Promising Platform for Oxygen Reduction, Supercapacitor and CO2 Capture. Carbon 2017, 114, 679–689. 10.1016/j.carbon.2016.12.059. [DOI] [Google Scholar]
- Subramani K.; Sathish M. Facile Synthesis of ZnO Nanoflowers/Reduced Graphene Oxide Nanocomposite Using Zinc Hexacyanoferrate for Supercapacitor Applications. Mater. Lett. 2019, 236, 424–427. 10.1016/j.matlet.2018.10.111. [DOI] [Google Scholar]
- Karnan M.; Subramani K.; Sudhan N.; Ilayaraja N.; Sathish M. Aloe Vera Derived Activated High-Surface-Area Carbon for Flexible and High-Energy Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 35191–35202. 10.1021/acsami.6b10704. [DOI] [PubMed] [Google Scholar]
- Lu K.; Jiang R.; Gao X.; Ma H. Fe3O4 /Carbon Nanotubes/Polyaniline Ternary Composites with Synergistic Effects for High Performance Supercapacitors. RSC Adv. 2014, 4, 52393–52401. 10.1039/C4RA11088A. [DOI] [Google Scholar]
- Karnan M.; Subramani K.; Srividhya P. K.; Sathish M. Electrochemical Studies on Corncob Derived Activated Porous Carbon for Supercapacitors Application in Aqueous and Non-Aqueous Electrolytes. Electrochim. Acta 2017, 228, 586–596. 10.1016/j.electacta.2017.01.095. [DOI] [Google Scholar]
- Borenstein A.; Hanna O.; Attias R.; Luski S.; Brousse T.; Aurbach D. Carbon-Based Composite Materials for Supercapacitor Electrodes: A Review. J. Mater. Chem. A 2017, 5, 12653–12672. 10.1039/C7TA00863E. [DOI] [Google Scholar]
- Saha S.; Jana M.; Samanta P.; Chandra Murmu N.; Kim N. H.; Kuila T.; Lee J. H. Hydrothermal Synthesis of Fe3O4/RGO Composites and Investigation of Electrochemical Performances for Energy Storage Applications. RSC Adv. 2014, 4, 44777–44785. 10.1039/c4ra07388f. [DOI] [Google Scholar]
- Lim Y. S.; Lai C. W.; Abd Hamid S. B. Porous 3D Carbon Decorated Fe3O4 NanocompositeV Electrode for Highly Symmetrical Supercapacitor Performance. RSC Adv. 2017, 7, 23030–23040. 10.1039/C7RA00572E. [DOI] [Google Scholar]
- Karthikeyan K.; Kalpana D.; Amaresh S.; Lee Y. S. Microwave Synthesis of Graphene/Magnetite Composite Electrode Material for Symmetric Supercapacitor with Superior Rate Performance. RSC Adv. 2012, 2, 12322–12328. 10.1039/c2ra21715e. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.