Abstract
Carbon-based materials, as electrodes for supercapacitors, have attracted tremendous attention. Therefore, nitrogen-doped porous carbons (NPCs) were prepared through a facile carbonization/activation strategy by treating different mass ratios of melamine–urea–formaldehyde resin and KOH. It is clearly demonstrated that because of the introduction of KOH, the resulting NPCs were shown to have increased specific surface area and a rich pore structure, and the best sample possessed a large specific surface area of 2248 m2 g–1 and high N content, which contributed to the good electrochemical performance for supercapacitors. Accordingly, a three-electrode system assembles NPCs as an electrode using aqueous KOH solution; the specific capacitance was 341 F g–1 under the current density of 1 A g–1 and retained a specific capacitance of almost 92% after 5000 cycles. The maximum energy output for a symmetrical solid-state supercapacitor with NPCs as the electrode material was 9.60 W h kg–1 at 1 A g–1. NPCs have promising applications on high-performance supercapacitors and other energy-storage devices.
1. Introduction
The energy crisis poses a huge challenge to the sustainable development of human society, forcing humans to consider researching and developing efficient, clean, safe, green, and high-performance energy storage and conversion materials and equipment.1−5 Supercapacitors, as a kind of green energy-storage device, have attracted extensive attention because of its high power density, excellent rate performance, and good reversibility along with long durability.6−10 Furthermore, the electrode materials play a vital role in supercapacitors.11 Porous carbons of excellent thermochemical stability, low toxicity, good electrical conductivity, and large specific surface area have received extensive attention in electrode materials.12−15 However, porous carbons generally have defects such as poor energy density and lower specific capacitance, which hinder the widespread application of carbon materials in commercial supercapacitors.16−18 Therefore, developing high-performance porous carbon electrode materials is still a huge challenge for researchers.
Many studies have demonstrated that the electrochemical capability of porous carbons can be promoted effectively by introducing functional groups such as nitrogen,19 phosphorus,20 and sulfur.21 Incorporating N atoms not only significantly promotes surface wettability, electrical conductivity, and cycle stability of carbon materials but also increases the pseudocapacitance through the Faraday reaction.22,23 In general, a common strategy for preparing nitrogen-doped porous carbons (NPCs) is by utilizing reactive nitrogen sources to passivate carbon surfaces;24 another strategy is carbonization and activation of N-containing precursors.25 N-containing compounds are used as precursors to synthesize NPCs, thereby improving its electrochemical performance, for example, polyaspartic acid,26ortho-aminophenol/formaldehyde resin,27 polypyrrole,28 polyacrylonitrile,29 and so forth. Zhou et al.30 used m-aminophenol formaldehyde resin as a carbon and N source to obtain N-doped hierarchical porous carbon materials through precarbonization and KOH activation. When applied as a supercapacitor electrode material, it exhibited good electrochemical performance with a specific capacitance of 271.5 F g–1 under 0.2 A g–1 and a high specific capacitance retention of 94.1% after 10 000 cycles. Wang et al.31 synthesized activated mesoporous carbons (AMCs) by using KOH to activate phenolic-resin-based carbon. By changing the mass ratio of KOH/carbon, AMCs with 1118 m2 g–1 of specific area could be obtained, and in 1 M H2SO4, it showed a high specific capacitance of 260 F g–1 under 1 A g–1 and the capacitance was 163 F g–1 under 20 A g–1., and furthermore, the capacitance did not decrease after 10 000 cycles at 2 A g–1.
In this paper, an effective strategy was introduced to prepare NPCs. It involved two processes, precarbonization of the resin and an activation reaction process. By adjusting the activation temperature, the amount of activator, and the activated carbon, NPCs with abundant micropores were successfully synthesized. The highest specific capacitance of 341 F g–1 under 1 A g–1 was achieved in 6 M KOH. Furthermore, the electrode maintained a capacitance of 92% after 5000 cycles, which was conducted at a high current density of 10 A g–1. Symmetrical solid-state supercapacitor of NPCs-2-700 had a significant energy density as well as functional density, and the results of light-emitting diode (LED) bulb illuminated by two series-connected symmetric solid-state supercapacitors showed the potential of the electrode materials in actual application. The low cost and excellent performance of the electrode materials may be further advantageous for supercapacitors and have potential applications on other energy-storage devices.
2. Results and Discussion
The morphology and pore structure of the obtained samples were studied using field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM), as shown in Figure 1. SEM image (Figure 1a,b) showed that after activation with KOH at 700 °C, the structure of AC-500 opened up, resulting in a rough surface and pore structures of the carbon material. Moreover, the energy-dispersive X-ray spectroscopy (EDX) mapping images of NPCs-2-700 are exhibited in Figure 1e, which indicate that numerous N atoms were successfully in situ codoped in the carbon nanosheets. High-resolution TEM showed that the sheet had abundant micropores, and the amorphous microporous structure of samples could be clearly observed in Figure 1c,d. An important factor with regard to the electrical conductivity and surface wettability was the unique microporous structure of carbon materials, which suggested their promising applications in supercapacitors.32
Figure 1.

(a,b) FESEM images of NPCs-2-700, (c,d) TEM images of NPCs-2-700, and (e) EDX mapping images of NPCs-2-700.
Typical X-ray diffraction (XRD) patterns of NPCs-2-y ascertained the crystallographic structure of the product, as shown in Figure 2a. These carbonization products all displayed two diffraction peaks at 2θ around 25 and 44°, and it could be concluded that these carbonized products all possessed a porous carbon (002) crystal face as well as a (100) amorphous structure. Synchronously, we observed a decrease in the intensity of the peak at 25° with the increase of activation temperature; this could be ascribed to the fact that the structures of the graphitized samples were severely damaged at high temperatures during activation.30
Figure 2.
(a) XRD patterns, (b) Raman spectra, (c) nitrogen adsorption/desorption isotherms, and (d) corresponding pore size distributions of NPCs-2-600, NPCs-2-700, and NPCs-2-800.
The specific nature could be further elucidated by Raman spectroscopy. The curves of Raman spectroscopic analysis in Figure 2b indicated two characteristic broad peaks of the NPCs corresponding to the disordered carbon structures (D-band) and graphitic layer structures (G-band) at 1337 and 1595 cm–1, respectively. The degree of graphitization was expressed by the ID/IG ratio; the higher the ID/IG ratio, the larger the defect of the structure.33 The values of ID/IG were 0.94, 0.97, and 1.04 for NPCs-2-600, NPCs-2-700, and NPCs-2-800, respectively, proving that the graphitization degree decreased with the increase of temperature. Besides, the results were consistent with the observation from XRD.
The N2 adsorption/desorption isotherms along with the corresponding pore size distribution for the three samples of the NPCs are shown in Figure 2c,d. Obviously, in Figure 2c, NPCs-2-600, NPCs-2-700, and NPCs-2-800 underwent micropore filling under a relatively low pressure (P/P0 < 0.2), and the amount of gas adsorption increased sharply. Subsequently, a horizontal or near-horizontal curve appeared, indicating that the micropores were totally full of N2, with little or no further adsorption occurring, and their curves showed the characteristics of type I adsorption isotherm. The pore size distribution curve in Figure 2d further confirmed the micropore property. When the activation temperature increased, the specific surface area and the total pore volume of the NPCs increased first and then decreased; when the pyrolysis temperature increased to 700 °C, the specific surface area and the total pore volume of the NPCs increased from 373 m2 g–1, 0.21 cm3 g–1 to 2248 m2 g–1, 4.57 cm3 g–1. However, the specific surface area and total pore volume at 800 °C were 1815 m2 g–1 and 1.14 cm3 g–1, which might be due to the collapse of pores during carbonization.34 In addition, more detailed pore parameters obtained from the N2 adsorption isotherm are given in Table 1.
Table 1. Texture Properties of the NPCs.
| textural parameter |
|||||
|---|---|---|---|---|---|
| sample | SBETa (m2 g–1) | St-plotb (m2 g–1) | Vmicroc (cm3 g–1) | Vtotald (cm3 g–1) | Ce (F g–1) |
| NPCs-2-600 | 373 | 375 | 0.158 | 0.21 | 252 |
| NPCs-2-700 | 2248 | 1565 | 3.65 | 4.57 | 341 |
| NPCs-2-800 | 1815 | 679 | 0.465 | 1.14 | 236 |
Specific surface areas.
Micropore specific surface areas.
Micropore volume.
Total pore volume at P/P0 ≈ 0.99.
The specific capacitance of the samples at the current density of 1 A g–1.
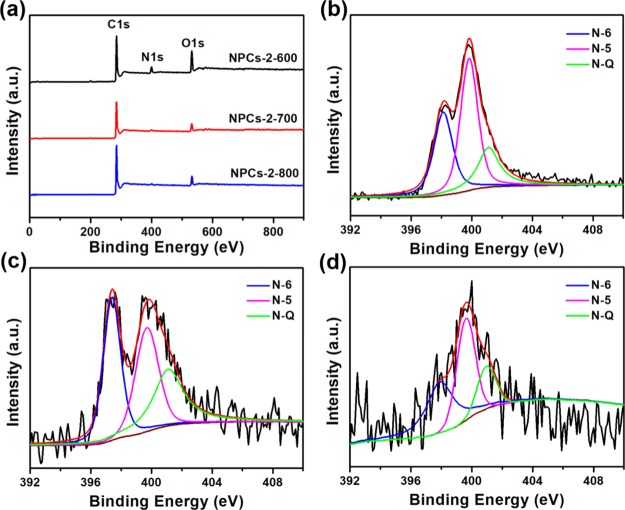
The surface element content and the chemical bond changes of NPCs were obtained using X-ray photoelectron spectroscopy (XPS). Three peaks of C, N, and O could be found in all of the spectrum in Figure 3a as a result of successful incorporation of N. In addition, as shown in Figure 3b–d, the N 1s spectrum could be divided into three separated peaks centered at 397.81, 399.72, and 401.06 eV, which were referred to pyridine-N (N-6), pyrrolic/pyridinic-N (N-5), and quaternary-N (N-Q), respectively. The strength of these peaks varied with the activation temperature, indicating that the contents of N-6, N-5, and N-Q also changed with temperature. The N contents of samples NPCs-2-600, NPCs-2-700, and NPCs-2-800 were 6.69, 2.18, and 1.07%, respectively, implying that the N content gradually decreased with the increasing activation temperature. The various relative contents of different N corresponded to the ratio of the fitted peak areas (Table 2). According to the data in Table 2, the relative contents of the elements were more significantly affected by temperature. The N-6 and N-5 contents in all samples were more than 70% of the total N atoms; furthermore, N-6 and N-5 could contribute a significant additional pseudocapacitance of the material because they were deemed to be electrochemically active in alkaline solution, and it was vital for increasing the capacitance of NPCs.35 It is worth noting that the contents of N-5 and N-6 accounted for 83.9% of the total N of NPCs-2-700, contributing to the better capacitance performance.
Figure 3.
(a) XPS spectra of NPCs-2-600, NPCs-2-700, and NPCs-2-800; (b–d) N 1s spectra for t NPCs-2-600, NPCs-2-700, and NPCs-2-800, respectively.
Table 2. Content of the C, O, and N Elements and Different Nitrogen Species.
| XPSa |
nitrogen
speciesb |
|||||
|---|---|---|---|---|---|---|
| sample | C | O | N | N-6 | N-5 | N-Q |
| NPCs-2-600 | 78.56 | 14.75 | 6.69 | 33.2 | 45.5 | 21.3 |
| NPCs-2-700 | 90.10 | 7.72 | 2.18 | 43.2 | 40.7 | 16.1 |
| NPCs-2-800 | 91.63 | 7.30 | 1.07 | 41.3 | 41.5 | 17.2 |
Weight percent of elements obtained from XPS data.
The relative percent of different nitrogen species obtained from the integral area of the fitted peaks.
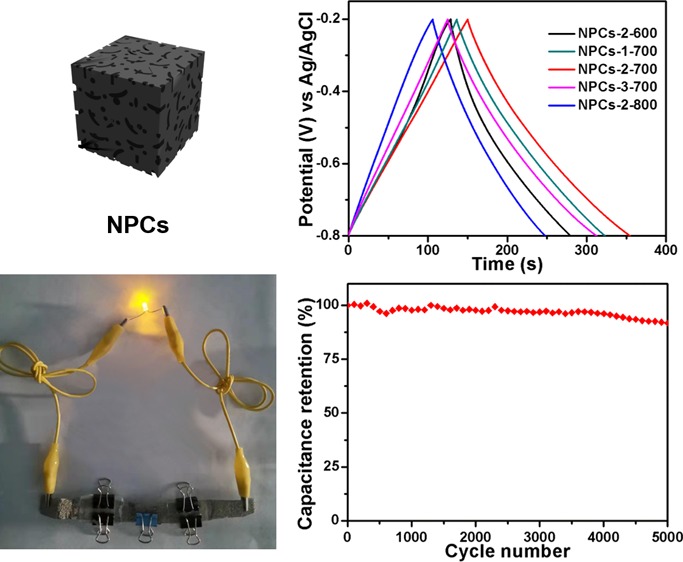
The electrochemical capability study of NPCs for supercapacitors was carried out on a three-electrode system by cyclic voltammetry (CV) and galvanostatic charge/discharge (GCD) tests as well as electrochemical impedance spectroscopy (EIS) in 6 M KOH. The CV curves of NPC electrodes at a scanning speed of 20 mV/s and a voltage range of −0.8 to −0.2 V are shown in Figure 4a; all CVs showed a regular rectangle, indicating that NPCs had an ideal double capacitance behavior. In general, the capacitance of a capacitor was measured by comparing the area of the CV curve within the same potential window. As shown in Figure 4a, NPCs-2-700 had the largest CV curve area, implying that its capacitance was the largest of all samples; identical conclusions were obtained from the results of GCD of NPCs in Figure 4b, and the discharge time of NPCs-2-700 was the longest. Specifically, using formula 1, the maximum capacitance of NPCs-2-700 at 1 A g–1 was calculated to be 341 F g–1, which was higher than most of the reported NPCs (Table S1). In addition, the capacitance values of NPCs-2-600, NPCs-2-800, NPCs-1-700, and NPCs-3-700 were 252, 236, 312—, and 314 F g–1, respectively. The CVs of NPCs-2-700 under different sweep speeds (10–100 mV/s) are revealed in Figure 4c. It could be observed in the curves that the current of NPCs-2-700 increased with the increase of the sweep speed. Moreover, all CV curves showed similar rectangular shapes, indicating that NPCs-2-700 had excellent capacitance characteristics.36 As shown in Figure 4d, the GCD curves of NPCs-2-700 were obtained at 1–10 A g–1. Excitingly, even at high current densities, all curves maintained a good isosceles triangle shape. This result was consistent with the CV test results and demonstrated that NPCs-2-700 possessed ideal supercapacitor behavior and excellent electrochemical reversibility. According to formula 1, the Cm values of NPCs-2-700 were 341, 146, 93, 54, and 25.5 F g–1 at a current density of 1, 2, 3, 5, and 10 A g–1, respectively. When the current density increased, the capacitance reduced significantly; the reason may be that interfacial electrolytes adsorbed a great deal of electrolyte ions, and consequently, the concentration of the electrolyte ion at the interface decreased rapidly and the polarization increased.37 Moreover, as can be seen from Figure 4e, the GCD cycle stability of NPCs-2-700 was studied at 10 A g–1, and the capacitance remained at almost 92% after 5000 cycles, which showed no significant capacity loss, indicating that NPCs-2-700 had outstanding cycle stability as an electrode material.
Figure 4.
Electrochemical performance of the NPCs in 6 M KOH electrolyte: (a) CV curves of the NPCs at the scanning rate of 20 mV/s; (b) GCD curves of the NPCs at the current density of 1 A g–1; (c) CV curves of NPCs-2-700 at 10, 20, 30, 50, and 100 mV/s; (d) GCD curves of NPCs-2-700 at 1, 2, 3, 5, and 10 A g–1; (e) capacitive retention curve of NPC-2-700 at a current density of 10 A g–1; and (f) Nyquist plots of NPCs-2-600, NPCs-2-700, and NPCs-2-800.
The EIS of the NPCs were obtained in the frequency range from 100 to 0.01 kHz, and this analysis could provide an insight into the resistance and capacitance behavior of the symmetrical supercapacitor. The semicircle in the high-frequency range and the vertical line in the low-frequency range of the Nyquist curves indicate the capacitive performance of the material.38Figure 4f shows the EIS diagram of NPCs-2-600, NPCs-2-700, and NPCs-2-800 porous carbon materials tested at open circuit potential. Among the three curves, the semicircle diameter of the NPCs-2-700 curve was the smallest in the high-frequency region, and additionally, the oblique line of the NPCs-2-700 curve was closest to the vertical axis in the low-frequency region, indicating that the contact resistance of NPCs-2-700 was the lowest and the electrical conductivity was excellent. This was supported by the CV and GCD test results.
For the purpose of satisfying the actual application, it is necessary to further explore the performance of these samples in symmetric solid-state supercapacitors. A symmetrical solid-state supercapacitor based on NPCs-2-700 was assembled (denoted as NPCs-2-700//NPCs-2-700). It can be seen from Figure 5a that in different potential windows (between 1.0 and 1.5 V), the CV curves of the NPCs-2-700//NPCs-2-700 at 30 mV/s showed a quasi-rectangular shape. When the potential window reached 1.4 V, the curve shape remained unchanged, indicating that the solid-state supercapacitor exhibited an outstanding capacitive behavior and fast charge/discharge characteristics. Nevertheless, when the potential window reached 1.5 V, the electrode current increased sharply, and the shape of the curve changed significantly because the polarization of the electrode material at a high potential decomposed the electrolyte. Therefore, the potential window of the device was chosen between 0 and 1.4 V (Figure 5b), and all CVs showed almost typical rectangular shapes under different scan rates, even under 100 mV/s, which imply that it owned excellent rate capability and double-layer capacitance effect. The result could be attributed to the high porosity and N content, which are beneficial to the rapid diffusion as well as conversion of electrolyte ions. With constant current charging, symmetric capacitors were tested for charge and discharge at various current densities. Figure 5c shows the GCD curves at different current densities (1–10 A g–1). All curves showed the typical shape of a symmetrical triangle, which was typical of double-layer capacitors. Calculated by formula 2, we obtained specific capacitances of 98.70, 45.39, 28.59, 15.58, 8.81, and 6.39 F g–1 for current densities of 1, 2, 3, 5, 8, and 10 A g–1. Moreover, Figure 5d presents the Ragone plots for NPCs-2-700//NPCs-2-700, which imply the correlation between energy density (E, W h kg–1) and power density (P, W kg–1). E and P for symmetric NPCs-2-700//NPCs-2-700 were calculated from he formulas 3 and 4. The results showed that the energy density of the symmetric supercapacitor changed from 9.60 W h kg–1 at 1 A g–1 to 6.21 W h kg–1 at 10 A g–1 with the increase of current density, and the power density varied from 350.15 W kg–1 at 1 A g–1 to 3349.59 W kg–1 at 10 A g–1, and the retention of E is 64.7%, which is higher than the value reported in previous literature based on carbon material as the symmetric supercapacitor electrode.30,39−42 What is more, as shown in Figure 5e, because of the excellent capacitive performance, the two charged symmetric solid-state supercapacitors could power one LED for more than 60 s.
Figure 5.
Electrochemical performance of the NPCs-2-700//NPCs-2-700 symmetric supercapacitor: (a) CV curves within different potential windows from 1.0 to 1.5 V at 30 mV/s; (b) CV curves at different scanning rates in a potential window between 0 and 1.4 V; (c) GCD curves of the symmetric cell at different current densities; (d) Ragone plot and performance comparison with the previous reported works; (e) photograph of LED powered by the NPCs-2-700//NPCs-2-700 supercapacitors (photograph courtesy of “Yu Jing”. Copyright 2019).
3. Conclusions
In conclusion, carbon-based material has attracted a lot of interest in supercapacitor electrodes, and we successfully synthesized NPCs with a porous structure by carbonizing melamine–urea–formaldehyde resins and KOH activation, possessing a large specific surface area (2248 m2 g–1) along with a narrow pore size distribution. The obtained NPCs showed a high charge-storage capacity in the three-electrode system, and NPCs-2-700 had a specific capacitance of 341 F g–1 under 1 A g–1 and also exhibited outstanding cycle stability (remain almost 92% specific capacitance). Moreover, the assembled symmetric capacitor NPCs-2-700//NPCs-2-700 possessed the highest energy density of 9.6 W h kg–1 under 1 A g–1, and the power density was 350.15 W kg–1. Increasing N content and micropore volume are the key to improving the performance of electrode materials. These results offer new possibilities for developing high-performance supercapacitor electrode materials and can also be explored in other electrochemical energy storage and conversion.
4. Experimental Section
4.1. Synthesis of NPCs
As shown in Scheme 1, the NPCs were synthesized by utilizing melamine–urea–formaldehyde resins as the carbon and nitrogen source and KOH as the activator. The synthetic steps of the resins were as follows: 4.8 g of urea, 13.01 g of formaldehyde aqueous solution (37%), and 30 mL of deionized water were added to a three-necked flask and fully dissolved by ultrasonication; then, triethanolamine was added dropwise to adjust the pH to 8.5–9.5. The solution was then magnetically stirred in a 70 °C water bath, and 3.4 g of melamine was added. The reaction was carried out for 3 h. Finally, the solution was allowed to stand, naturally cooled, suction filtered, and washed with deionized water. The obtained sample was dried at 85 °C in vacuum for 24 h to obtain dried resins. The dried resins were carbonized at 550 °C for 2 h under a N2 condition, and the activated carbon obtained by carbonizing at 550 °C (AC-550) was ground into a powder for use.
Scheme 1. Synthetic Schematic of NPCs.
In the KOH activation, AC-550 and KOH (the weight ratios of KOH and AC-550 are 3, 2, and 1) were added to an appropriate amount of ethanol solution The mixture was thoroughly mixed under magnetic stirring and then dried at 65 °C for over 24 h. The obtained dry mixture was activated at 600, 700, and 800 °C under a N2 atmosphere with a heating rate of 5 °C min–1 for 2 h. The resulting NPCs were removed of impurities with 1 M HCl and washed with deionized water at room temperature, and then the powder was dried in a vacuum oven to give the final NPCs. The final samples were abbreviated as NPCs-x-y (the mass ratio of KOH to AC-550 is represented by x; x = 1, 2, and 3; the temperature of activation is represented by y; y = 600, 700, and 800).
4.2. Electrochemical Tests
To gain more insight into the electrochemical performance of the NPCs, the electrochemical tests were implemented in a three-electrode system under ambient condition and were conducted in an electrochemical working station (CHI760E). The Pt wire and Ag/AgCl were used as the counter electrode and reference electrode, respectively. The preparation method of the working electrode (WE) was as follows:43 NPCs (80 wt %), acetylene black (10 wt %), polyvinylidene difluoride (10 wt %), and appropriate amount of ethanol were completely ground by a mortar, and the mixture was pasted evenly on a 1 × 1 cm nickel foam. Then, the nickel foam was compacted under a pressure of 10 MPa and dried overnight at 60 °C. The CV tests of NPCs were investigated at different scanning rates between −0.8 and −0.2 V. GCD tests were performed between 1 and 10 A g–1. EIS tests of NPCs were performed at an open circuit voltage between 100 kHz and 10 mHz, and the amplitude of ac voltage was 5 mV. From the GCD tests, the specific capacitance of samples in the three-electrode system could be calculated according to the following formula44
| 1 |
where I (A) is the discharge current, Δt (s) is discharge time, m (g) is the mass of the NPCs, and ΔV (V)is the discharge voltage. In a symmetrical solid-state supercapacitor, the mass of the active material on two WEs was the same. The two electrodes were immersed in poly(vinyl alcohol)/KOH, and a diaphragm was placed between the two WEs to assemble a symmetrical solid-state supercapacitor. The electrochemical properties of the NPC electrode material were evaluated by CV and GCD, and the specific capacitance of the individual electrode was obtained by the following formula44
| 2 |
where the discharge current, discharge time, and total mass of NPCs on the two electrodes and the voltage window are represented by I (A), Δt (s), m (g), and ΔV (V), respectively. Energy density (E, W h kg–1) was calculated by the following formula44
| 3 |
where Cs (F g–1) is the specific capacitance of a single electrode in the two-electrode system and V (V) is the operating voltage. Power density (P, W kg–1) was received from the equation44
| 4 |
where t (s) is the discharge time.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (nos. 51622805 and U1633116), the opening fund for the subject of Transportation Engineering in Tongji University (2016J012306), and the Research Project of Yunan Department of Transportation (grant no. 2016(A)16).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01916.
FESEM images, Raman spectra, XRD patterns, CV curves of samples at different scan rates, GCD curves of samples at different current densities, and comparison of specific capacitance of the NPCs-2-700 electrode with previously reported carbon materials (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Zhang L. L.; Zhao X. S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. 10.1039/b813846j. [DOI] [PubMed] [Google Scholar]
- Genc R.; Alas M. O.; Harputlu E.; Repp S.; Kremer N.; Castellano M.; Colak S. G.; Ocakoglu K.; Erdem E. High-Capacitance Hybrid Supercapacitor Based on Multi-Colored Fluorescent Carbon-Dots. Sci. Rep. 2017, 7, 11222. 10.1038/s41598-017-11347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.; Fu K.; Yu J.; Shi X.; Zhou P.; Cheng Z. Facile preparation of nanoporous C60/P3HT thin films from PLA-b-C60-b-P3HT triblock copolymers. Appl. Surf. Sci. 2018, 458, 70–76. 10.1016/j.apsusc.2018.07.076. [DOI] [Google Scholar]
- Miao L.; Qian X.; Zhu D.; Chen T.; Ping G.; Lv Y.; Xiong W.; Liu Y.; Gan L.; Liu M. From interpenetrating polymer networks to hierarchical porous carbons for advanced supercapacitor electrodes. Chin. Chem. Lett. 2019, 30, 1445–1449. 10.1016/j.cclet.2019.03.010. [DOI] [Google Scholar]
- Colherinhas G.; Malaspina T.; Fileti E. E. Storing Energy in Biodegradable Electrochemical Supercapacitors. ACS Omega 2018, 3, 13869–13875. 10.1021/acsomega.8b01980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi M.; Yang F.; Meng F.; Li M.; Manivannan A.; Wu N. Effects of Pore Structure on Performance of An Activated-Carbon Supercapacitor Electrode Recycled from Scrap Waste Tires. ACS Sustainable Chem. Eng. 2014, 2, 1592–1598. 10.1021/sc500336h. [DOI] [Google Scholar]
- Zhou M.; Pu F.; Wang Z.; Guan S. Nitrogen-doped porous carbons through KOH activation with superior performance in supercapacitors. Carbon 2014, 68, 185–194. 10.1016/j.carbon.2013.10.079. [DOI] [Google Scholar]
- Zhang Q.-Z.; Zhang D.; Miao Z.-C.; Zhang X.-L.; Chou S.-L. Research Progress in MnO2-Carbon Based Supercapacitor Electrode Materials. Small 2018, 14, 1702883. 10.1002/smll.201702883. [DOI] [PubMed] [Google Scholar]
- Song Z.; Li L.; Zhu D.; Miao L.; Duan H.; Wang Z.; Xiong W.; Lv Y.; Liu M.; Gan L. Synergistic design of a N, O co-doped honeycomb carbon electrode and an ionogel electrolyte enabling all-solid-state supercapacitors with an ultrahigh energy density. J. Mater. Chem. A 2019, 7, 816–826. 10.1039/c8ta10406a. [DOI] [Google Scholar]
- Lin X.-Q.; Lü Q.-F.; Li Q.; Wu M.; Liu R. Fabrication of Low-Cost and Ecofriendly Porous Biocarbon Using Konjaku Flour as the Raw Material for High-Performance Supercapacitor Application. ACS Omega 2018, 3, 13283–13289. 10.1021/acsomega.8b01718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.-F.; Lu Y.; Yu L.; Lou X. W. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ. Sci. 2017, 10, 1777–1783. 10.1039/c7ee00488e. [DOI] [Google Scholar]
- Long C.; Zhuang J.; Xiao Y.; Zheng M.; Hu H.; Dong H.; Lei B.; Zhang H.; Liu Y. Nitrogen-doped porous carbon with an ultrahigh specific surface area for superior performance supercapacitors. J. Power Sources 2016, 310, 145–153. 10.1016/j.jpowsour.2016.01.052. [DOI] [Google Scholar]
- Hsia B.; Kim M. S.; Carraro C.; Maboudian R. Cycling characteristics of high energy density, electrochemically activated porous-carbon supercapacitor electrodes in aqueous electrolytes. J. Mater. Chem. A 2013, 1, 10518. 10.1039/c3ta11670k. [DOI] [Google Scholar]
- Xue D.; Zhu D.; Xiong W.; Cao T.; Wang Z.; Lv Y.; Li L.; Liu M.; Gan L. Template-Free, Self-Doped Approach to Porous Carbon Spheres with High N/O Contents for High-Performance Supercapacitors. ACS Sustainable Chem. Eng. 2019, 7, 7024–7034. 10.1021/acssuschemeng.8b06774. [DOI] [Google Scholar]
- Han L.-N.; Wei X.; Zhu Q.-C.; Xu S.-M.; Wang K.-X.; Chen J.-S. Nitrogen-doped carbon nets with micro/mesoporous structures as electrodes for high-performance supercapacitors. J. Mater. Chem. A 2016, 4, 16698–16705. 10.1039/c6ta05607e. [DOI] [Google Scholar]
- Chen X.; Zhang J.; Zhang B.; Dong S.; Guo X.; Mu X.; Fei B. A novel hierarchical porous nitrogen-doped carbon derived from bamboo shoot for high performance supercapacitor. Sci. Rep. 2017, 7, 7362. 10.1038/s41598-017-06730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z.; Zhu D.; Li L.; Chen T.; Duan H.; Wang Z.; Lv Y.; Xiong W.; Liu M.; Gan L. Ultrahigh energy density of a N, O codoped carbon nanosphere based all-solid-state symmetric supercapacitor. J. Mater. Chem. A 2019, 7, 1177–1186. 10.1039/c8ta10158b. [DOI] [Google Scholar]
- Ramesh T.; Rajalakshmi N.; Dhathathreyan K. S.; Reddy L. R. G. Hierarchical Porous Carbon Microfibers Derived from Tamarind Seed Coat for High-Energy Supercapacitor Application. ACS Omega 2018, 3, 12832–12840. 10.1021/acsomega.8b01850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H.; Chen H.; Fu N.; Chen J.; Lan G.; Qian W.; Liu Y.; Lin H.; Han S. Excellent electrochemical properties and large CO2 capture of nitrogen-doped activated porous carbon synthesised from waste longan shells. Electrochim. Acta 2017, 231, 403–411. 10.1016/j.electacta.2017.01.194. [DOI] [Google Scholar]
- Huang C.; Sun T.; Hulicova-Jurcakova D. Wide electrochemical window of supercapacitors from coffee bean-derived phosphorus-rich carbons. ChemSusChem 2013, 6, 2330–2339. 10.1002/cssc.201300457. [DOI] [PubMed] [Google Scholar]
- Yu X.; Kang Y.; Park H. S. Sulfur and phosphorus co-doping of hierarchically porous graphene aerogels for enhancing supercapacitor performance. Carbon 2016, 101, 49–56. 10.1016/j.carbon.2016.01.073. [DOI] [Google Scholar]
- Wickramaratne N. P.; Xu J.; Wang M.; Zhu L.; Dai L.; Jaroniec M. Nitrogen Enriched Porous Carbon Spheres: Attractive Materials for Supercapacitor Electrodes and CO2 Adsorption. Chem. Mater. 2014, 26, 2820–2828. 10.1021/cm5001895. [DOI] [Google Scholar]
- Li Y.; Zheng S.; Liu X.; Li P.; Sun L.; Yang R.; Wang S.; Wu Z.-S.; Bao X.; Deng W.-Q. Conductive Microporous Covalent Triazine-Based Framework for High-Performance Electrochemical Capacitive Energy Storage. Angew. Chem., Int. Ed. Engl. 2018, 57, 7992–7996. 10.1002/anie.201711169. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Wang M.; Li X. Promising biomass-derived nitrogen-doped porous carbon for high performance supercapacitor. J. Porous Mater. 2019, 26, 99–108. 10.1007/s10934-018-0622-3. [DOI] [Google Scholar]
- Sun T.; Wang C.; Jiao D.; Zhu M.; Lv S.; Xiang J.; Qin C. Facile preparation of porous N-doped carbon via a one-step carbonization/activation treatment of polyvinylpyrrolidone/melamine formaldehyde resin with ammonium carbonate and its enhanced electrochemical performances for supercapacitors. J. Mater. Sci.: Mater. Electron. 2017, 28, 8993–9002. 10.1007/s10854-017-6630-2. [DOI] [Google Scholar]
- Ma G.; Wu Y.; Sun K.; Peng H.; Wang H.; Lei Z. High performance nitrogen-doped carbon for supercapacitor obtained by carbonizing eco-friendly and cheap polyaspartic acid. Mater. Lett. 2014, 132, 41–44. 10.1016/j.matlet.2014.06.028. [DOI] [Google Scholar]
- Li Y.; Zhang S.; Song H.; Chen X.; Zhou J.; Hong S. New insight into the heteroatom-doped carbon as the electrode material for supercapacitors. Electrochim. Acta 2015, 180, 879–886. 10.1016/j.electacta.2015.09.039. [DOI] [Google Scholar]
- Zhu J.; Xu Y.; Zhang Y.; Feng T.; Wang J.; Mao S.; Xiong L. Porous and high electronic conductivity nitrogen-doped nano-sheet carbon derived from polypyrrole for high-power supercapacitors. Carbon 2016, 107, 638–645. 10.1016/j.carbon.2016.06.063. [DOI] [Google Scholar]
- Yang X.; Wu D.; Chen X.; Fu R. Nitrogen-Enriched Nanocarbons with a 3-D Continuous Mesopore Structure from Polyacrylonitrile for Supercapacitor Application. J. Mater. Chem. C 2010, 114, 8581–8586. 10.1021/jp101255d. [DOI] [Google Scholar]
- Zhou J.; Zhang Z.; Xing W.; Yu J.; Han G.; Si W.; Zhuo S. Nitrogen-doped hierarchical porous carbon materials prepared from meta-aminophenol formaldehyde resin for supercapacitor with high rate performance. Electrochim. Acta 2015, 153, 68–75. 10.1016/j.electacta.2014.11.075. [DOI] [Google Scholar]
- Wang Z.; Zhou M.; Chen H.; Jiang J.; Guan S. Hierarchical activated mesoporous phenolic-resin-based carbons for supercapacitors. Chem. Asian J. 2014, 9, 2789–2797. 10.1002/asia.201402338. [DOI] [PubMed] [Google Scholar]
- Wei T.; Wei X.; Gao Y.; Li H. Large scale production of biomass-derived nitrogen-doped porous carbon materials for supercapacitors. Electrochim. Acta 2015, 169, 186–194. 10.1016/j.electacta.2015.04.082. [DOI] [Google Scholar]
- Kim D. K.; Bong S.; Jin X.; Seong K.-D.; Hwang M.; Kim N.-D.; You N.-H.; Piao Y. Facile In Situ Synthesis of Multiple-Heteroatom-Doped Carbons Derived from Polyimide Precursors for Flexible All-Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2018, 11, 1996–2005. 10.1021/acsami.8b15162. [DOI] [PubMed] [Google Scholar]
- Ma G.; Yang Q.; Sun K.; Peng H.; Ran F.; Zhao X.; Lei Z. Nitrogen-doped porous carbon derived from biomass waste for high-performance supercapacitor. Bioresour. Technol. 2015, 197, 137–142. 10.1016/j.biortech.2015.07.100. [DOI] [PubMed] [Google Scholar]
- Xie L.; Sun G.; Su F.; Guo X.; Kong Q.; Li X.; Huang X.; Wan L.; Song W.; Li K.; Lv C.; Chen C.-M. Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications. J. Mater. Chem. A 2016, 4, 1637–1646. 10.1039/c5ta09043a. [DOI] [Google Scholar]
- Tian W.; Gao Q.; Zhang L.; Yang C.; Li Z.; Tan Y.; Qian W.; Zhang H. Renewable graphene-like nitrogen-doped carbon nanosheets as supercapacitor electrodes with integrated high energy–power properties. J. Mater. Chem. A 2016, 4, 8690–8699. 10.1039/c6ta02828d. [DOI] [Google Scholar]
- Liu J.; Wang X.; Gao J.; Zhang Y.; Lu Q.; Liu M. Hollow porous carbon spheres with hierarchical nanoarchitecture for application of the high performance supercapacitors. Electrochim. Acta 2016, 211, 183–192. 10.1016/j.electacta.2016.05.217. [DOI] [Google Scholar]
- Zeng D.; Dou Y.; Li M.; Zhou M.; Li H.; Jiang K.; Yang F.; Peng J. Wool fiber-derived nitrogen-doped porous carbon prepared from molten salt carbonization method for supercapacitor application. J. Mater. Sci. 2018, 53, 8372–8384. 10.1007/s10853-018-2035-8. [DOI] [Google Scholar]
- Ling Z.; Wang Z.; Zhang M.; Yu C.; Wang G.; Dong Y.; Liu S.; Wang Y.; Qiu J. Sustainable Synthesis and Assembly of Biomass-Derived B/N Co-Doped Carbon Nanosheets with Ultrahigh Aspect Ratio for High-Performance Supercapacitors. Adv. Funct. Mater. 2016, 26, 111–119. 10.1002/adfm.201504004. [DOI] [Google Scholar]
- Du Z.; Peng Y.; Ma Z.; Li C.; Yang J.; Qin X.; Shao G. Synthesis of nitrogen-doped carbon cellular foam with ultra-high rate capability for supercapacitors. RSC Adv. 2015, 5, 10296–10303. 10.1039/c4ra14395g. [DOI] [Google Scholar]
- Song P.; Shen X.; He W.; Kong L.; He X.; Ji Z.; Yuan A.; Zhu G.; Li N. Protein-derived nitrogen-doped hierarchically porous carbon as electrode material for supercapacitors. J. Mater. Sci.: Mater. Electron. 2018, 29, 12206–12215. 10.1007/s10854-018-9329-0. [DOI] [Google Scholar]
- Li M.; Xue J. Integrated Synthesis of Nitrogen-Doped Mesoporous Carbon from Melamine Resins with Superior Performance in Supercapacitors. J. Mater. Chem. C 2014, 118, 2507–2517. 10.1021/jp410198r. [DOI] [Google Scholar]
- Wang Y.; Xuan H.; Lin G.; Wang F.; Chen Z.; Dong X. A melamine-assisted chemical blowing synthesis of N-doped activated carbon sheets for supercapacitor application. J. Power Sources 2016, 319, 262–270. 10.1016/j.jpowsour.2016.04.069. [DOI] [Google Scholar]
- Liu M.; Niu J.; Zhang Z.; Dou M.; Wang F. Potassium compound-assistant synthesis of multi-heteroatom doped ultrathin porous carbon nanosheets for high performance supercapacitors. Nano Energy 2018, 51, 366–372. 10.1016/j.nanoen.2018.06.037. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.