Abstract

A new class of blue light-emitting bowl-shaped mesogens with the thiacalix[4]arene core appended with 1,3,4-thiadiazole derivatives having peripheral alkoxy side chains have been synthesized and well characterized. The liquid crystalline behavior of present synthesized derivatives was examined by optical polarizing microscopy, differential scanning calorimetry, and X-ray diffraction studies. It was observed that these thiacalix[4]arene derivatives were capable of stabilizing the observed Colh phase with a higher temperature range. The cone-shaped thiacalix[4]arene-based liquid crystals with peripheral alkoxy side chains able to pack into the columns with enriched intermolecular interactions and thermal behavior. All derivatives showed blue luminescence in solution, solid thin-film, and gelation state. The hexagonal columnar phase and emissive nature of thiadiazole-based thiacalixarene compounds having xerogel behavior make them favorable in the application of emissive electronic display devices. The electrochemical properties of these thiacalixarene-based compounds demonstrate the effect of alkyl side chain on the highest occupied molecular orbital–lowest unoccupied molecular orbital energy levels and also exhibited lower electron band gaps. The electroluminescence behavior of the compound 10c was examined as emissive layers in the fabrication of organic light-emitting diodes.
Introduction
In recent years, the liquid crystal of self-organizing molecular systems with multifunctional properties is one of the most attractive and active fields of current research. Liquid crystals with inherent properties like fluidity and stability have been already used in several commercial applications.1 There are several types of liquid crystalline compounds, one of the most fruitful compound is the supramolecular columnar liquid crystal. Supramolecular assembly composed of disc-shaped aromatic molecules has gained significant attention, as it is potentially viable to the formation of columnar-type structures with the presence of Π–Π stacking interactions.2 Presently, the demand of organic semiconductors with high mobility and strong luminescence has grown manifolds as they facilitate in the development of various electronic display applications such as organic light-emitting diodes (OLEDs), organic light-emitting transistors (OLETs), and organic lasers.3,4
OLEDs are monolithic solid-state devices that typically consist a series of organic thin films sandwiched between two thin-film conductive electrodes which display a substantial part in the growth of new flat-panel displays.5,6 Small OLED panels are used for the displays of mobile phones, while large OLED panels are popular in displays of television, mirror display, transparent display, and signage.7−9 Nowadays, it is accepted that OLEDs will become the governing technology in the display market. For the fabrication of OLED products, three phosphorescent emitters are required, red, green, and blue emitters.10−12 Literature reports several materials that emit red and green light; however, those with blue light emission are rare. Additionally, the lifetime of green and red phosphorescent devices is higher as compared to the blue fluorescent device. Nowadays, among these three light-emitting phosphorescent compounds, blue light-emitting compounds are getting much more attention because of their indispensable requirement for the fabrication of white light-emitting diodes and other devices, respectively.13−17 In addition, columnar hexagonal liquid crystals that are formed by the packing of central benzene cores one above the other with substitution of the peripheral side chain which behave as molecular wires that help to stabilize the Colh phase in one-dimension.18−21
From the previous study, there are various kinds of liquid crystalline compounds based on 1,3,4-oxadiazole which were reported in the literature. Basically, 1,3,4-oxadiazoles are one of the class of heterocyclic compounds which are known for their many advantages like high-fluorescence quantum yield, thermal stability, luminescence efficiency, hydrolytic stability, electron-transporting behavior. Therefore, they found more applications in the fabrication of n-type electroluminescent layers in OLEDs.22−24 In addition to the number of advantages of oxadiazole core-based liquid crystals, there are certain drawbacks like narrow temperature range, lower solubility, and higher melting and clearing temperatures which limit their applications in various fields.25 In present investigation, we have focus on 1,3,4-thiadizole derivatives which have similarities with 1,3,4-oxadiazole expect hetero atoms in their core structure. The oxygen atom in oxadiazole is interchanged by sulfur atoms which enhance its properties like higher melting and clearing temperatures, dipole moments, higher viscosity, and mesophase temperature range.26 In the literature, different shapes of fluorescent liquid crystals based on 1,3,4-thiadiazole are reported till date but very few with polycatenar LCs, star-shaped LCs, and supramolecular LCs.27−33 In the present study, we report the OLED device performance by using newly synthesized supramolecular columnar hexagonal mesogens based on thiacalix[4]arene derivatives substituted with 1,3,4-thidiazoles derivatives. Its photophysical behavior suggests that the grating of thiadiazole derivatives on two sides of the thiacalix[4]arene core, which having a sulfur-rich environment can help to disperse the emitting nature of thiadiazole derivatives in the thiacalixarene core, respectively.
Calixarenes are the class of cyclic oligomers in supramolecular chemistry formed via condensation of phenol and aldehydes which currently belongs to the part of third generation after crown ethers and cyclodextrins.34 The first liquid crystalline compounds based on the calixarene core were reported by Dalcanale et al.35 In recent years, calixarenes have been successfully introduced as a central tetra cyclic core with hydrophobic and hydrophilic sides to design different types of mesogens.36,37 Menon et al. introduced an aliphatic side chain inbuilt with different linking groups on the calixarene core.38,39 Yang and his co-workers reported various calixarene-based liquid crystalline materials with different temperature range and thermal stability.40−44 Among them, our research group also reported various calix[4]arene-based hexagonal columnar LCs with good photophysical behavior.45,46 Marcos et al. reported calix[4]arene-based mesogens inbuilt with the Schiff base linking unit.47
In contrast, new functions remain to be developed through the substitution of bridge methylene groups by hetero atoms.48 Thiacalix[4]arene, the new member of the calixarene family has now become a robust scaffold in supramolecular chemistry and material science.49 They possess many fascinating features such as large cavity size, binding ability toward anion, cation and transition metals with the presence of larger cavity, and also the possibility of multiple chemical modifications on their morphology. Different functionalizations on the thiacalixarene core have been extensively used in different applications like mimicking of molecular logic gates, separating biological important citations, display, and photoactive emissive compounds.50−52
Supramolecular gels from Π-conjugated low-molecular weight gelators have attracted intense interest because of their advantages to create various superstructures, diversity, and forming self-assembly.53 Specifically, low-molecular Π-conjugated gelators based on macrocyclic structures into the oligomers like crown ethers, cyclodextrin, and calixarene effectively prevents the close packing of the molecules.54 Among various macrocyclic compounds, calix[4]arenes are of more interest because of their ability to be functionalized on lower and upper rim, Π–Π interactions, and formation of self-assembly. From the literature, it can be noted that thiacalix[4]arene-based compounds which can act as a gelators are rarely reported.55 Formation of gels via H-bonding consisting of the functional groups like amide, aroyl hydrozone, peptide, and sugars is well reported.56,57 An extensive literature survey that revealed no supramolecular thiacalix[4]arene-based columnar mesogens with blue luminescence and gelation properties have been reported. In present investigation, we report four new cone- or bowl-shaped luminescent supramolecular LCs based on thiacalix[4]arene and 1,3,4-thiadiazole Schiff base group inbuilt with a variable peripheral alkyl chain to determine their structure–property-relationship. Furthermore, their electrochemical, density functional theory (DFT), and photophysical behaviors were implemented to shed light on their electronic molecular structure. Further, the bowl-shaped di-substituted thiadiazole thiacalix[4]arene-based columnar liquid crystalline derivatives (10c, 10d) also showed gelation properties in nonpolar solvents which are formed by Π–Π interactions and also the presence of H-bonding.
Result and Discussion
Synthesis and Characterization
The synthetic strategy to prepare thiacalixarene derivatives based on 1,3,4-thiadiazole and thiacalix[4]arene derivatives is presented in given Schemes 1 and 2. Compound 2 was prepared by refluxing the reaction mixture of compound 1 with hydrazine hydrate in pyridine.46 Further, compound 2 is dissolved in pyridine and reacted with 3,4,5-trimethoxy benzoyl chloride to achieve compound 3. The obtained compound 3 is further reacted with phosphorous pentasulfide (P2S5) to give the thiadiazole derivative (4).46 On further heating to compound 4 in the presence of anhy. AlCl3 in benzene resulted in compound 5.46 Compound 5 was coupled with different alkyl bromides to get compound 6a–6c.46 Further, the oxidation of compound (6a–6d) was carried out with chromyl chloride in the presence of pyridine and dichloromethane (DCM) to form compounds (7a–7d).46 The Schiff-base derivatives (8a–8d) were formed by the reaction of compound (7a–7d) with 2,4-dihydroxy aniline.47 Base-catalyzed condensation of 4-tert-butyl phenol and sulphur powder (S8) in the presence of tetraethylene glycol dimethyl ether in a single step to obtain 4-tert-butyl thiacalixarene (9) is depicted in Scheme 2.58 Finally, the target bi-substituted supramolecular derivatives (10a–10d) were synthesized by the reaction of Schiff-base thiadiazole intermediates (8a–8d) with bis-alkyl bromide thiacalix[4]arene (9) in dimethylformamide (DMF).59 The obtained final solid derivatives were further purified through column chromatography with chloroform and methanol as eluent in the ratio of 4:1. Molecular structural characterizations of intermediates and final target compounds were carried out by using 1H NMR, 13C NMR, IR, and ESI-HRMS which are presented in the Supporting Information (see in Supporting Information). From the 1H NMR, compounds 10a–10d exhibited a pair of singlets for the −C(CH3)3 group on the thiacalixarene core with the presence of two singlets for aromatic hydrogen indicates the substitution of thiadiazole Schiff-base derivatives on lower rim to form stable cone confirmation that displays columnar hexagonal liquid crystalline properties.48,60 Literature reports confirm the substitution of thiadiazole on two sides of thiacalixarene-favored cone confirmation.48 Additionally, the difference of the observed two singlets of the aromatic proton in final target compounds is nearly 0.37 ppm, which again confirms the cone confirmation of synthesized supramolecular derivatives.49−52
Scheme 1.
Reagents and conditions: (i) ethanol, NH2NH2·H2O; (ii) trimethoxy benzoyl chloride, pyridine, lower temp; (iii) P2S5, pyridine; (iv) AlCl3, benzene; (v) R–Br, anhydrous K2CO3, acetone; (vi) CrO2Cl2, pyridine, DCM; (vii) 2,4-dihydroxy aniline, EtOH, 3 h.
Scheme 2.
Reagents and conditions: (i) elemental sulfur (S8), NaOH, MeO(CH2CH2O)4Me; (ii) dibromo ethane, anhydrous K2CO3, DMF, reflux, 6 h; (iii) 8a–8d, anhydrous K2CO3, DMF.
TGA Analysis
Thermogravimetric analyses (TGA) of the compounds (10a–10d) were performed to verify thermal stability under the nitrogen atmosphere. The TGA curves for all four derivatives show similar decomposition behavior (Figure S1, see in Supporting Information). All the compounds (10a–10d) displayed thermal stability up to ≈291 °C with decomposition temperatures above 480 °C. Additionally, there was no loss of mass up to 180 °C suggesting the absence of water or any additional solvent molecules trapped during the exposition of the mesophase. Moreover, the degradation of the prepared thiacalixarene-based derivatives is in the range of 240–310 °C, which indicates its good thermal stability behavior.
DSC Analysis
The phase transition temperature of synthesized compounds 10a–10d was preliminarily studied by the differential scanning calorimetry (DSC) technique. The observed transition temperature range of target compounds was investigated by DSC on first heating and cooling rates of 10 °C (Figure 1). The corresponding data of transition temperature and enthalpy change were summarized in Table 1. Upon heating and cooling conditions, all four derivatives exhibited two endothermic peaks on heating and cooling conditions corresponding to crystal to columnar hexagonal and columnar hexagonal to isotropic phase (Figure S2). Compound 10a with lower alkyl chain showed two endothermic peaks at 101.2 and 197.6 °C on heating conditions. On cooling, they traced two endothermic peaks at 198.8 and 103.2 °C. Likewise, compound 10b showed two endothermic peaks at 96.4 and 161.9 °C on heating and similar broad endothermic peaks traced at 162.9 and 98.1 °C for cooling conditions. Similarly, compound 10c revealed Cr–Colh phase transition at 77.4 °C and second endothermic peak at 143.8 °C upon heating, and on cooling it showed Colh–Cr phase transition at 79.2 °C and another endothermic peak corresponding to Colh–I traced at 146.4 °C as evident from the DSC scans. Compound 10d presented two broad endothermic peaks at 65.2 and 141.5 °C because of the presence of Cr–Colh and Colh–I phase transition upon heating condition, and on cooling it traced at 143.6 and 66.8 °C which is nearer to the observed phase transition on heating condition. Upon heating, compound 10d exhibited first transition peak at 65.2 °C and second endothermic peak at 141.2 °C, and on cooling condition the two endothermic peaks located at 66.8 and 143.6 °C, respectively. It is observed that compound 10a displays higher temperature range of the mesophase as compared to other derivatives. The changes observed in phase transition temperature of the mesophase due to the substitution of the variable peripheral alkyl chain in which the methylene group is linked to each other leads to change in its properties like flexibility, molecular length, dipole moment, adhesive forces, and polarity, respectively.53,54
Figure 1.

Thermal behavior of the compound (10a–10d) with the Schiff-base group shown in the bar graph upon heating (a) and cooling (b) conditions.
Table 1. Phase Transitions and Corresponding Thermodynamic Dataa.
| phase
sequence |
||
|---|---|---|
| compound | heating | cooling |
| 10a | Cr 101.2 (12.8) Colh 197.6 (2.9) I | I 198.8 (1.9) Colh 103.2 (30.6) |
| 10b | Cr 96.4 (21.6) Colh 161.9 (5.6) I | I 162.9 (2.9) Colh 98.1 (24.1) |
| 10c | Cr 77.4 (38.7) Colh 143.8 (7.3) I | I 146.4 (5.6) Colh 79.2 (13.2) |
| 10d | Cr 65.2 (17.4) Colh 141.5 (3.9) I | I 143.6 (3.6) Colh 66.8 (12.4) |
Abbreviations: Cr = solid crystalline state, Colh = hexagonal columnar mesophase, I = isotropic liquid.
POM Study
The phase transition behaviors of supramolecular LCs based on the thiacalixarene core appended with thiadiazole Schiff base derivatives were investigated by polarizing optical microscopy (POM) upon heating and cooling conditions. From Figure S3, it is apparent that there was no significant difference in the observed texture characteristic of compound (10a–10d). The defect texture characteristic in all four derivatives confirms the presence of the columnar hexagonal mesophase. All the thiadiazole-based thiacalix[4]arene compounds (10a–10d) showed lower transition and clearing temperatures. The mesophase temperature ranges for supramolecular compounds (10a–10d) were observed at 101.3, 77.8, 73.1, and 76.9 °C, respectively. Thus, it is clear that the presence of the sulfur atom in the thiadiazole and thiacalix[4]arene core increases the lateral dipole moment that causes S···S interactions and is responsible for the higher mesophase temperature range.48 The substitution of short peripheral alkyl chain length exhibited a higher mesophase range as compared to other synthesized derivatives. The phase transition temperatures for all of the compounds (10a–10d) were observed at 102.8, 94.8, 75.2, and 61.6 °C upon heating and on cooling the similar textural pattern obtained for the columnar phase was noticed at 101.6, 93.6, 73.2, and 62.7 °C, respectively. One can see that, with an increase in the alkoxy side chain, the temperature range of the phase transition decreased and the probability of stereo heterogeneity increased, which affect its flexibility and weakens the core–core interaction of the self-assembly of thiacalix[4]arene-based thiadiazole derivatives. Consequently, an enantiotropic columnar LCs perceived a good temperature range for the prepared thiacalix[4]arene derivatives.
XRD Analysis
The liquid crystalline properties of compounds (10a–10d) were further explored by using X-ray diffraction (XRD) examination. All supramolecular derivatives were examined for their liquid crystalline properties at their transition temperature observed in POM and DSC analysis. The sample was melted and filled in the Lindemann capillary tube as an isotropic state and cooled to their mesomorphic state and scanned for X-ray studies. The X-ray profile of oxadiazole- and thiadiazole-based thiacalix[4]arene showed three reflection peaks in the lower angle region and two other reflections peaks in the higher angle region shown in Figure S4a–d. The X-ray traces of compound 10a at 104.0 °C displayed reflections at 2.62°, 6.20°, 7.62°, 19.92°, and 22.61° in small angle and wide angle regions. In the lower angle region, compound 10b showed three reflections at 2.46°, 6.26°, and 7.64°, compound 10c at 2.18°, 6.13°, and 7.69°, and compound 10d at 2.11°, 5.62°, and 7.71° while in the wide angle region compound 10b showed two reflections at 19.96° and 22.67°, compound 10c at 19.86° and 22.63°, compound 10d at 19.92° and 22.54°. The d-spacing value of compound 10a at the lower angle region was 33.69, 14.26 and 11.61 Å. Likewise, 35.89, 14.12, and 11.58 Å for compound 10b, 40.52, 14.43, and 11.50 Å for compound 10c, 41.84, 15.73, and 11.48 Å for compound 10d, respectively. The ratio of appeared reflections of all the derivatives were 1:1/√3:1/√4, signifying the Miller indices [100], [110], and [200] planes. In the wide angle region, two small diffuse peaks are found which are corresponding to the packing of alkoxy side chain shaking of core–core interactions within the column in the self-assembly. From the literature, similar XRD data were reported for various columnar liquid crystals based the on calixarene core.40−44 The estimated length of the target molecules is closer to the observed length of target compounds. Noticeably, the presence of a broad peak wide angle region indicates definite order in the peripheral alkoxy-trisubstituted alkyl chain-substituted thiadiazole derivatives inbuilt with the thiacalix[4]arene core. The possible arrangement of supramolecular compounds to form the columnar hexagonal shape is depicted in Figure 2. Based on the POM, DSC, and XRD analysis, it can be noted that all the supramolecular two side-substituted thiadiazole derivatives adopted a stable hexagonal columnar phase.
Figure 2.

Proposed schematic arrangement of the columnar hexagonal phase in compound 10c.
Photophysical Behaviors
We have investigated the photophysical behavior of compounds (10a–10d) in solution and solid thin films. The absorption and fluorescence spectra of compounds 10a and 10d were measured in different solvents as given in Figures S5 and S6, respectively. It can be noted that all derivatives showed the intensities of absorbance and fluorescence higher in tetrahydrofuran (THF) as compared to other organic solvents. The moderate behavior of tetrahydro furan can dissolve a wide range of polar and nonpolar compounds. Thus, the solutions of all bowl-shaped thiacalix[4]arene derivatives were made in THF to measure the absorption and fluorescent properties. Compounds 10a–10d revealed the same absorption band at 448, 451, 454, and 451 nm in THF, respectively. The corresponding maximum emission was observed at 459, 461, 464, and 478 nm as shown in Figure 3a,b. Changes in fluorescence intensity and absorption peaks were dependent on the solvent polarity.61 The highly delocalized systems with the presence of Π–Π* transitions and also the presence of sulfur environment of the thiacalixarene core showed higher absorption coefficient (ε = 24.9 to 26.3 × 106 L mol–1 cm–1) (Table 2). The solid thin film of compounds 10a–10d presented higher absorption at 451, 454, 459, and 462 nm and higher emission at 467, 464, 476, and 498 nm as mentioned in Figure 3c,d. The intramolecular charge-transfer efficiency of synthesized compounds 10c–10d was higher as compared to compounds 10a–10b suggesting that the higher alkyl chain substitution with the electron-donating oxygen atom on the phenyl ring and conjugation with aromatic rings inbuilt with thiadiazole derivatives on the lower rim of thiacalixarene. All the synthesized derivatives (10a–10d) showed dark blue fluorescence in long UV (Figure 3b). The material emitting blue light are not only limited in blue color but also their energy levels become very high and they transport an effective fine-tuning emissive wavelength by combining with an additional dopant emitter to fabricate OLEDs.62−64 All the compounds showed a stable emission band-centered maxima at 459–478 nm. The present supramolecular thiadiazole Schiff-base thiacalix[4]arene compounds demonstrated red shift in the absorption and emission maxima. It has been observed that the variable alkyl chain on the terminal phenyl ring had no effect on the influence of absorption and emission spectra. The quantum yields of synthesized bowl-shaped columnar liquid crystalline compounds in solution and solid thin-film state were observed in the range of 0.28–0.57 (Table 2). Furthermore, all the compounds in the solid thin-film state showed lower quantum yield as compared to the solution state, this is due to the presence of aggregation in the solid state. The presence of trisubstituted alkyl chain length decreased the aggregation-caused quenching effect and enhanced the fluorescence intensity.65
Figure 3.
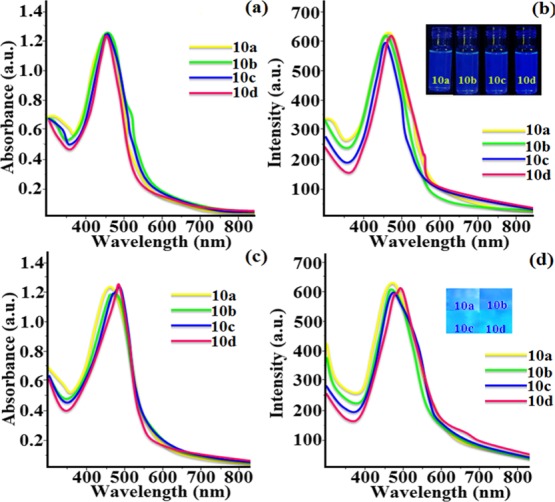
Absorption (a–c) and fluorescence (b–d) spectra of compounds 10a–10d in micromolar THF solution and solid thin-film state.
Table 2. Photophysical Properties of Compounds (10a–10d) in THFc.
| comp. | absorptiona (nm) | emissiona (nm) | Stoke shift (nm) | quantum yielda | (ε/106 L mol–1 cm–1) | absorptionb (nm) | emissionb (nm) | quantum yieldb |
|---|---|---|---|---|---|---|---|---|
| 10a | 448 | 459 | 11 | 0.28 | 24.9 | 451 | 467 | 0.21 |
| 10b | 451 | 463 | 12 | 0.32 | 25.7 | 454 | 464 | 0.27 |
| 10c | 454 | 469 | 15 | 0.57 | 25.9 | 459 | 476 | 0.38 |
| 10d | 451 | 478 | 27 | 0.47 | 26.3 | 462 | 498 | 0.41 |
Absorption and emission maxima in the solution state.
Absorption and emission spectra in the solid thin-film state.
Relative quantum yield of these compounds in the solution and solid state was calculated with respect to the solution of quinine sulphite in 0.1 M H2SO4 as the standard (Qf = 0.54).
Electrochemical Behavior
Cyclic voltammetry (CV) is a type of potentiodynamic electrochemical measurement generally used to study the electrochemical properties of the molecule that adsorbed onto the electrode. All the synthesized thiacalixarene-based compounds were examined for their electrochemical behavior by carrying out CV studies in anhydrous micromolar THF. Cyclic voltammograms of compounds 10a and 10b with computationally observed highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels are mentioned in Figures 4 and S7, respectively. The calculated band gaps, energy level with oxidation, and reduction potential are mentioned in Table S1.
Figure 4.

Cyclic voltammogram of compound 10a (a); HOMO, LUMO energy levels of compound 10a by DFT calculation at the B3LYP/3-21G* level (b,c).
The oxidation and reduction waves of all the synthesized compounds become similar in cyclic voltammograms. The auxiliary electrode used in the present study is 0.1 M solution of tetrabutylammonium perchlorate in deoxygenated THF equipped with Ag/AgNO3 (0.1 M) reference, platinum, and carbon working electrode.66 From Figures 4 and S7, it is clearly observed that the compounds 10a and 10b exhibited definite irreversible waves of oxidation and reduction. The synthesized thiacalix[4]arene derivatives displayed a lower band gap, which clearly indicates the higher reactivity of the compounds. This band gap observed for thiacalix[4]arene derivatives is much less compared to previously reported compounds based on the 4-tert-butyl calix[4]arene core.45,46 The energy levels of LUMO were calculated by ELUMO = −(4.8 – E1/2, Fc,Fc+ + Ered, onset) eV, whereas the energy levels of HOMO were calculated by EHOMO = −(4.8 – E1/2, Fc,Fc+ + Eoxd, onset) eV. Compounds (10a, 10b) with lower alkyl side spacer showed LUMO and HOMO levels as −2.71, −2.73, −5.82, and −5.80 eV, while higher alkyl chain-substituted thiacalixarene-based compounds (10c,10d) revealed the LUMO and HOMO energy levels as −2.74, −2.69, −5.81, and −5.84 eV (Table S1). The energy level stabilization of HOMO and LUMO has little influence by the trisubstituted peripheral alkoxy side chain on the lower rim of thiacalixarene, respectively. Among all four synthesized bowl-shaped thiacalix[4]arene liquid crystals, compound 10d becomes more stable because of the existence of a greater band gap. The calculated values of band gaps by CV were nearly supported by the theoretical values obtained from DFT. Further, the cyclic voltammograms of compounds 10c and 10d are presented in Figures S8 and S9 (Supporting Information).
Cone Conformation Study
It is identified that thiacalix[4]arene derivatives have four main analogues, namely, cone, partial cone, 1,2-alternate, and 1,3-alternate. Compared to normal calix[4]arene, the four sulfur bridges replacing methylene groups provide various new characteristics like large cavity inside the core, more flexibility, greater scope for chemical modifications, and sensor capability.67 Thiacalixarene always adopt the cone shape because of the formation of the hydrogen bonding between the phenolic groups on the lower rim and further the crystal structure data are well supported by XRD studies.68,69 The 1H NMR spectra of thiacalix[4]arene in CDCl3 suggests weaker intramolecular hydrogen bonding compared to normal 4-tert-butyl calixarene because of its enlarged framework. Further, it is difficult to distinguish exact confirmation of thiacalix[4]arene from cone and 1,3-alternate caused by the absence of methylene bridges in the thiacalix[4]arene core, respectively. All synthesized thiacalix[4]arene-based compounds showed cone conformation, which is further confirmed by the 1H NMR technique. All derivatives exhibited two singlets (1:1) for the −C(CH3)3 group of thiacalix[4]arene and presence of two singlets (1:1) for the aromatic protons suggesting the cone conformation adopted by all derivatives. However, the 1H NMR exhibited two other singlets (1:1) for −OCH2- and −CH2O-disubstituted groups on the lower rim nearly at 4.21 and 4.83 ppm that confirms the existence of the cone shape by all newly synthesized derivatives.69 The geometries of 1,3,4-thiadiazole thiacalix[4]arene compounds were evaluated at the DFT level (B3LYP/3-21G*), as given in Figures S10–S16 (see in Supporting Information). This evidently confirms that all the synthesized supramolecular derivatives are in cone confirmation. The calculated energies, dipole moments, and band gaps of compounds 10a–10d are given in Table 3. One can see that, the dipole moment and band gap of compound 10d is lower, which indicates its higher reactivity as compared to other derivatives (10a–10c).
Table 3. Energetics Properties of Thiacalixarenes Derivativesa.
| comp. | HOMO (a.u) | LUMO (a.u) | energy gap a.u (eV) | total energy (a.u) | dipole moment (D) |
|---|---|---|---|---|---|
| 10a | –0.19443 | –0.07669 | 3.2038 | –7998.026 | 15.3077 |
| 10b | –0.19419 | –0.07656 | 3.2009 | –8467.242 | 15.3169 |
| 10c | –0.19424 | –0.07652 | 3.2032 | –8936.458 | 15.4433 |
| 10d | –0.19235 | –0.07562 | 3.1763 | –9874.878 | 12.4985 |
1 a.u = 27.2116 eV = 627.509 kcal mol.
Gelation Studies
The gelation properties of synthesized compounds were examined in various organic solvents at different concentrations, respectively. From the four synthesized thiacalix[4]arene derivatives, compounds 10c and 10d were heated in a glass sample vial until the solid materials were completely dissolved and further cooled at room temperature to form gel. Compounds 10c and 10d having two side thiadiazole rings with alkyl chain inbuilt with Schiff-base thiacalixarenes formed organogels in dodecane and decane (Figure 5a,b, Table S2 in Supporting Information). This gel formation was possible because of multivalent Π–Π interactions between the bowl-shaped compounds and the organic solvent. These supramolecular thiacalix[4]arene-based derivatives were soluble in nonpolar solvents but formed precipitates in polar solvents, respectively. Compounds 10c and 10d showed critical a gelation concentration (CGC) of 1.1 and 1.6 wt % in dodecane while they showed CGC concentration of 1.5 and 1.9 wt % in decane. In the present study, it is observed that compounds with lower alkoxy peripheral side chain stabilized the gel at a lower CGC value as compared to the higher substituted alkoxy side on the terminal side of the phenyl ring. To explore the aggregation behavior, thiacalix[4]arene-based LCs in dodecane to the form network gelator. The further studies of the gel were carried out by using atomic force microscopy (AFM) and field-emission scanning electron microscopy (FE-SEM). From the AFM study, it is clearly seen that the aggregated molecules form network-type structures of the nanofiber at lower concentration in dodecane as mentioned in Figure S17a,b. The morphology of the obtained xerogels was further studied by FE-SEM. Figure S17c,d represents the SEM images of the prepared xerogel of compounds 10c and 10d, which exhibits micro and nano thin fibers to give a network structure of nanofibers. The realignment of the gelator fibers in the supramolecular structure of the bi-substituted thiacalix[4]arene derivatives in decane and dodecane clearly indicates the formation of the network structure in the organogels because of the presence of hydrogen bonding between −CH=N and −OH groups at the lower part of the thiacalixarene core. The additional interactions of the sulfur bridge with nearby hydroxyl groups may thereby induce alignment of fibers.
Figure 5.

(a,b) Solution of comp. 10c (a1), comp. 10d (b1) in day light, gel state in day light of comp. 10c (a2,b2), gel state in long UV light of comp. 10d (a3,b3).
The presence of peripheral alkoxy side chain (−OR) creates van der Waals forces in addition to the intermolecular Π–Π interaction between aromatic phenyl rings, which is the driving force for the formation of gel.70 The gel of compounds 10c (Figure 5a) and 10d (Figure 5b) in dodecane (a1,b1) solvent showed stable gelation (a2,b2) behavior at room temperature, which further showed fluorescence (a3,b3) on exposure to UV light, respectively. The formation of organogels was observed within 4.0 min during the cooling process. Compounds 10c and 10d showed higher emission intensity as temperature decreases from 70 °C (sol) to 18 °C (gel) (Figures S18a, S19a). The emission spectra of compounds 10c and 10d were transferred from 475 to 491 and 468 to 486 nm, respectively (Figures S18b and S19b). Upon heating and cooling conditions, compounds 10c, 10d showed the gelation process in a reversible manner for number of cycles as evident from Figures S18c,d and S19c,d.
Furthermore, the structure of xerogel in compounds 10c and 10d were studied by the XRD pattern. For the XRD analysis, the thin film of compounds 10c and 10d were made by the drop-casting method on a glass slide, respectively. The XRD pattern of xerogel of compound 10c exhibited several peaks at 2.89, 5.52, 9.08, 14.09, 19.96, 22.60, 24.05, and 27.03 (Figure S20a). In the same way, the xerogel compound 10d displayed peaks at 2.86, 4.64, 7.18, 11.56, 13.63, 15.98, 20.37, 25.11, and 28.82 (Figure S20b). Thiacalixarene-based xerogels showed reflections in the XRD state due to the presence of strong intermolecular interactions between the molecules. Compound 10c showed d-spacing values at 32.76, 20.26, 10.38, 7.05, 4.49, 3.77, and 3.18 Å for compound 10d, 34.06, 20.18, 10.38, 7.05, 4.69, 3.77, and 3.18 Å. The thiacalixarene-based supramolecules in the xerogel state formed a lamellar type of arrangement confirmed from the ratio of d-spacing and clearly suggest the rectangular type of arrangement of the bowl-shaped thiadiazole molecules. The lattice parameters “a” and “b” of compounds 10c and 10d in the rectangular cell were found to be 65.22, 28.97, 63.72, and 28.85 Å (Tables S3, and S4). The columnar arrangement of supramolecules in the xerogel state is supported by the birefringent texture pattern, which represents the occurrence of hierarchical self-assembly in presently synthesized bowl-shaped mesogens (Figure S21, see in Supporting Information). These results support the organization of thiacalix[4]arene derivatives arranged in hierarchical self-assembly given in Figure 6. Here, in the present study, we have first time demonstrated thiadiazole-substituted thiacalix[4]arene derivatives as a gelator in the absence of any metal doping to induce gelation properties on account of the H-bonding interaction.
Figure 6.

Proposed representation of columnar self-assembly in solution and organogels (10c–10d).
Electroluminescence Study
The fluorescent blue light-emitting thiacalix[4]arene-based compounds were further studied for their electroluminescence (EL) performance in the OLED device. The synthesized supramolecular derivative 10c was selected to study its EL property. The fluorescence quantum yield of compound 10c was employed as the emitter with doped and nondoped solution-processed OLED devices was fabricated with simplified making of the device shown in Table S5. The organic supramolecular compounds were doped in bipolar CBP to permit host-to-guest energy transfer. Figure S22a represents the architecture design of the device with the energy level diagram. The EL spectra with current density–voltage–luminescence characteristics of the fabricated OLED devices were mentioned in Figure S22b,c.
OLED panels are made from carbon-based organic materials that emit light when electricity is passed through them. The emitting light from doped and nondoped emissive layers of the organic compound in OLEDs may be due to relaxation of electron transfer from the LUMO state to HUMO state in the molecules, respectively.71−73 The device with different % of CBP and neat state showed good EL performance. The fabricated device with 1 wt % displayed a power efficiency (PE) of 0.2 lm/W, luminescence of 438 cd/m2, current efficiency (CE) of 0.6 cd/A, and an external quantum efficiency (EQE) of 0.8%. By increasing the dopant with the device (6 wt %) showed a maximum luminescence of 314 cd/m2 at 6.9 V with CE of 0.5 cd/A, PE of 0.2 lm/W, and an EQE of 0.3%. The device inbuilt with a emissive layer of compound 10c (nondoped) showed a maximum luminescence of 286 cd/m2 at 7.4 V, CE of 0.6 cd/A, PE of 0.2 lm/W, and EQE of 0.4%, respectively. It can be observed that the dilution effect occurs with an increasing dopant ratio with a bipolar CBP host; it means the higher concentration increases the formation of crystallinity and self-aggregation organization of bowl-shaped derivatives in the devices which affect its morphology in the film. The device with 1 wt % doping concentration of dye shows higher maximum luminescence with higher power and CE as compared to other doping concentration (Figure S22c).
It is observed that the higher dopant concentration of blue dye shows relative low turn-on voltage and higher current densities for the device. However, the lower luminance value could be because of the unbalanced charge-transport ratio which results in the leakage of charge at the interface of electrodes without recombining inside the emitting layer. The current density versus voltage and luminance versus current density of all fabricated OLED devices are mentioned in Figure S23 (see in Supporting Information). All the CBP-based doped (1, 2, 5 and 6 wt %) devices show a bluish-green EL emission with peaks at 438, 407, 353, and 314 nm. Consequently, the Commission International de 1’E clairage coordinates (CIE) color coordinates were (0.24, 0.33), (0.24, 0.33), (0.24, 0.33), and (0.24, 0.32) while nondoped CBP-based device (neat) also showed the EL peak at 286 nm and CIE coordinates of (0.24, 0.31). From this, it can be noted that as the dopant ratio varies from 1 to 6 wt %, the dye did not display any variations in EL maxima and CIE coordinates probably suggest that the bowl-shaped molecules become aggregate to repel uniformly at higher concentrations.74
Conclusions
In conclusion, we have designed, synthesized, and characterized bowl-shaped thiacalixarene-based supramolecular LCs that self-assembles to form a columnar hexagonal phase with a good temperature range. All the compounds displayed blue fluorescence in the solution and solid thin-film state. The self-assembly of supramolecular thiacalixarene-based mesogens stabilize columnar hexagonal phase with broad mesophase range and good thermal stability. These supramolecular compounds (10c, 10d) are the first examples of liquid crystals based on the thiacalix[4]arene core to form columnar self-assembly, respectively. Also, they possess emissive nature in the gelation state which makes this research applicable in the fabrication of emissive displays. The selected supramolecular compound 10c with trisubstituted octyloxy side chain was used in the fabrication of OLEDs as doped and nondoped with emitter bipolar CBP. Therefore, these supramolecular thiacalixarenes derivatives can serve as a good platform for the development of bowl-shaped LCs used to fabricate OLEDs. Such multifunctional supramolecular materials should have great potentials in the search for new thermally stable luminescent columnar LCs and its applications in displays, photochemical molecular switches, and gelation, and as chemosensors.
Experimental Section
Materials and Methods
All the starting raw materials and chemical reagents were obtained as analytical grade and used without purification. The required solvents were distilled and purified prior to use in the synthesis work. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were collected on the Bruker ADVANCE spectrometer (400 MHz). The IR spectra were collected on Shimadzu in the range of 3600–500 cm–1. Elemental analyses (C,H,N) were carried out with the PE (CHN) 2400 analyzer. The CV experiment data were carried out on CH Instruments electrochemical workstation. The reference electrode used was calibrated with the ferrocene/ferrocenium redox couple. The POM images were performed on a polarized pptical microscope equipped under heating and cooling conditions. The mass spectra of target compounds were carried out by using high-resolution mass spectrometer. The phase-transition temperatures were observed on Shimadzu DSC-50. TGA were performed on a PerkinElmer-STA 6000 apparatus under the nitrogen atmosphere. The samples were heated at room temperature to 550 °C at 10 °C/min. XRD studies were recorded on a Rigaku-Ultima IV powder diffractometer equipped with a Cu kα source (λ = 1.54 Å, 40 kV). The absorption and fluorescence spectra were studied by using Jasco V-570 UV–vis and Jasco FP-6500 spectrofluorometer at different wavelengths.
General Method for the Preparation of Compound 8a
Compound (8a) is prepared by the reaction of compound (7a) and 2,4-dihydroxy aniline with the presence of few drops of glacial acetic acid in ethanol.4 The reaction mixture is refluxed for 3 h and monitored by using thin-layer chromatography (TLC) followed by the methanol/chloroform system (1:4). Yield: 76%; IR (KBr pellet) in cm–1: 3440, 2940, 2841, 1610, 1460, 1340, 1331, 1240, 1122, 986, 834, 704; 1H NMR (CDCl3, 400 MHz): δ 8.97 (s, 2H, −OH), 8.12 (s, 1H, −CH=N), 6.41 (s, 2H, Ar), 6.80 (s, 1H, Ar), 7.58 (d, 2H, Ar), 7.18 (d, 2H, Ar), 6.97 (s, 2H, Ar), 4.06 (t, 6H, −OC4H9), 0.88 (t, 6H, −OC4H9), 1.46 (sext, 6H, −OC4H9), 1.75 (p, 6H, −OC4H9); 13C NMR: 171.1, 159.81, 156.34, 147.64, 136.17, 128.35, 125.46, 115.53, 109.86, 106.54, 69.51, 31.08, 19.04, 14.10.
General Method for the Preparation of Compound 8b
Compound (8b) is prepared by the reaction of compound (7b) and 2,4-dihydroxy aniline with the presence of few drops of glacial acetic acid in ethanol.4 The reaction mixture is refluxed for 3 h and monitored by using TLC followed by methanol: chloroform system (1:4). Yield: 79%; IR (KBr pellet) in cm–1: 3446, 2946, 2841, 1620, 1560, 1460, 1340, 1331, 1240, 1210, 1122, 986, 834, 760; 1H NMR (CDCl3, 400 MHz): δ 8.94 (s, 2H, −OH), 8.12 (s, 1H, −CH=N), 6.46 (s, 2H, Ar), 6.80 (s, 1H, Ar), 7.52 (d, 2H, Ar), 7.18 (d, 2H, Ar), 6.84 (s, 2H, Ar), 4.06 (t, 6H, −OC6H13), 0.88 (t, 6H, −OC6H13), 1.26–1.28 (m, 12H, −OC6H13), 1.46 (sext, 6H, −OC6H13), 1.75 (p, 6H, −OC6H13); 13C NMR: 171.1, 159.81, 156.34, 147.64, 136.17, 128.35, 125.46, 115.53, 109.86, 106.54, 69.51, 31.08, 19.04, 14.10.
General Method for the Preparation of Compound 8c
Compound (8c) is prepared by the reaction of compound (7c) and 2,4-dihydroxy aniline with the presence of few drops of glacial acetic acid in ethanol.4 The reaction mixture is refluxed for 3 h and monitored by using TLC followed by the methanol/chloroform system (1:4). Yield: 71%; IR (KBr pellet) in cm–1: 3446, 2946, 2841, 1620, 1560, 1460, 1340, 1331, 1240, 1210, 1122, 986, 834, 760; 1H NMR (CDCl3, 400 MHz): δ 8.94 (s, 2H, −OH), 8.16 (s, 1H, −CH=N), 6.46 (s, 2H, Ar), 6.80 (s, 1H, Ar), 7.69 (d, 2H, Ar), 7.18 (d, 2H, Ar), 6.89 (s, 2H, Ar), 4.06 (t, 6H, −OC8H17), 0.88–0.90 (t, 6H, −OC8H17), 1.26–1.28 (m, 18H, −OC8H17), 1.46 (sext, 6H, −OC8H17), 1.75 (p, 6H, −OC10H21); 13C NMR: 171.1, 159.81, 156.34, 147.64, 136.17, 128.35, 125.46, 115.53, 109.86, 106.54, 69.51, 31.08, 19.04, 14.10.
General Method for the Preparation of Compound 8d
Compound (8d) is prepared by the reaction of compound (7c) and 2,4-dihydroxy aniline with the presence of few drops of glacial acetic acid in ethanol.4 The reaction mixture is refluxed for 3 h and monitored by using TLC followed by the methanol/chloroform system (1:4). Yield: 69%; IR (KBr pellet) in cm–1: 3446, 2946, 2841, 1620, 1560, 1460, 1340, 1331, 1240, 1210, 1122, 986, 834, 760; 1H NMR (CDCl3, 400 MHz): δ 8.94 (s, 2H, −OH), 8.16 (s, 1H, −CH=N), 6.46 (s, 2H, Ar), 6.80 (s, 1H, Ar), 7.69 (d, 2H, Ar), 7.18 (d, 2H, Ar), 6.89 (s, 2H, Ar), 4.06 (t, 6H, −OC10H21), 0.88–0.90 (t, 6H, −OC10H21), 1.26–1.28 (m, 24H, −OC10H21), 1.46 (sext, 6H, −OC10H21), 1.75 (p, 6H, −OC10H21); 13C NMR: 171.1, 159.81, 156.34, 147.64, 136.17, 128.35, 125.46, 115.53, 109.86, 106.54, 69.51, 31.08, 19.04, 14.10.
General Method of the Preparation of p-tert-Butyl thiacalix[4]arene (9)
Parent p-tert-butyl thiacalix[4]arene (9) was synthesized by involving base-catalyzed cyclo-condensation of p-tert-butyl phenol and sulfur powder by the reported procedure.4 mp 318–322 °C; 1H NMR (CDCl3, 400 MHZ): δ 1.35 (36H, s, −CH3), 6.9–7.4 (8H, s, Ar), and 8.1 (4H, s, OH); 13C NMR: δ 31.35, 35.23, 122.53, 136.42, 144.72, and 156.63 (Ar); FT-IR (KBr): 3331 cm–1 for −OH stretching band, 2963 cm–1 aromatic C–H stretching band; ESI-MS (m/z): 719 (M – 1).
General Method for the Preparation of Compound 9a
p-tert-Butyl thiacalix[4]arene-di-ethoxy bromide (9a) is prepared by the condensation reaction of compound 9 with dibromo ethane in acetonitrile with the presence of anhydrous K2CO3 as a base.51H NMR (CDCl3, 400 MHz): 0.90 (t, 3H), 1.31 (s, 36H), 1.76 (sext, 2H), 4.08 (t, 2H), 6.84 (s, 4H), 7.64 (s, 4H), 8.42 (s, 2H); 13C NMR: 160.69, 156.64, 144.72, 136.42, 122.56, 77.46, 77.04, 67.51, 35.25, 31.35, 29.18.
General Method for the Preparation of Compound 10a
The compound has been prepared by refluxing the reaction of compound (9a) (0.0015 mol) and compound (8a) (0.0030 mol), anhydrous K2CO3 (0.0030 mol) in DMF (30 mL) for 6 h. The reaction mixture was extracted by using DCM; the combined organic layer was washed with water, brine solution. Evaporation of the solvent by using a rotary evaporator and purification of the residue by using column chromatography followed by the methanol/chloroform system (1:4).3 (10a): Yield 71%, elemental analysis: C110H130N6O14S6 calcd: C, 67.66; H, 6.71; N, 4.30; O, 11.47%. Found: C, 68.72; H, 6.78; N, 4.21; O, 12.42%. FT-IR (KBr) in cm–1: 2990, 1610, 1522, 1440, 1320, 1140, 1120, 981, 886. 1H NMR (CDCl3, 400 MHz): 0.88–0.92 (t, J = 6.3 Hz, 18H, −OC4H9), 1.31 (s, 36H, t-butyl group), 1.47 (sext, 12H, −OC4H9), 1.71 (p, 12H, −OC4H9), 4.04 (t, 12H, −OC4H9), 4.41 (s, 4H, −CH2−), 6.51 (d, J = 6.7 Hz, 4H, Ar), 7.41 (s, 2H, Ar), 7.81 (d, J = 6.3 Hz, 8H, Ar), 6.91 (d, 8H, Ar), 6.74 (d, J = 8.7 Hz, 8H, Ar), 7.02 (s, 2H, Ar), 6.64 (s, 2H, Ar), 8.64 (s, 2H, −CH=N), 8.03 (s, 2H, −OH). 13C NMR: 161.50, 160.42, 159.42, 147.83, 144.73, 138.62, 136.41, 135.81, 129.70, 127.61, 124.24, 108.18, 105.18, 103.64, 77.47, 77.04, 76.62, 69.11, 68.15, 35.21, 31.83, 31.27, 19.04, 14.14. MALDI Tof MS for compound 10a (M + 1): calcd, 1950.5713; found, 1951.803.
Compound 10b
It was obtained in 69% yield, elemental analysis: C122H154N6O14S6 calcd: C, 69.09; H, 7.32; N, 3.96; O, 10.56; S, 9.07%. Found: C, 68.93; H, 7.38; N, 4.07; O, 10.76%. FT-IR (KBr) in cm–1: 2990, 1610, 1522, 1440, 1320, 1140, 1120, 981, 886. 1H NMR (CDCl3, 400 MHz): 0.90–0.92 (t, J = 6.6 Hz, 18H, −OC6H13), 1.26 (m, 8H, −OC6H13), 1.31 (s, 36H, t-butyl group), 1.46 (sext, 12H, −OC6H13), 1.71 (p, 12H, −OC6H13), 4.04 (t, 12H, −OC6H13), 4.40 (s, 4H, −CH2−), 6.51 (d, J = 7.3 Hz, 4H, Ar), 7.43 (s, 2H, Ar), 7.85 (d, J = 6.4 Hz, 8H, Ar), 6.91 (d, J = 8.7 Hz, 8H, Ar), 6.74 (d, 8H, Ar), 7.02 (s, 2H, Ar), 6.63 (s, 2H, Ar), 8.64 (s, 2H, −CH=N), 8.04 (s, 2H, −OH). 13C NMR: 161.50, 160.42, 159.42, 147.83, 144.73, 138.62, 136.41, 135.81, 129.70, 127.61, 124.24, 108.18, 105.18, 103.64, 77.47, 77.04, 76.62, 69.11, 68.15, 35.21, 31.83, 31.27, 19.04, 14.14. MALDI Tof MS for compound 10b (M + 1): calcd, 2118.9846; found, 2119.994.
Compound 10c
It was obtained in 75% yield, elemental analysis: C134H178N6O14S6 calcd: C, 70.30; H, 7.84; N, 3.67; O, 9.78; S, 8.40%. Found, C, 69.98; H, 7.91; N, 3.75; O, 9.67%. FT-IR (KBr) in cm–1: 2990, 1610, 1522, 1440, 1320, 1140, 1120, 981, 886. 1H NMR (CDCl3, 400 MHz): 0.90–0.92 (t, J = 6.6 Hz, 18H, −OC8H17), 1.26–1.28 (m, 14H, −OC8H17), 1.31 (s, 36H, t-butyl group), 1.46 (sext, 12H, −OC8H17), 1.71 (p, 12H, −OC8H17), 4.04 (t, 12H, −OC8H17), 4.40 (s, 4H, −CH2−), 6.51 (d, J = 6.6 Hz, 4H, Ar), 7.43 (s, 2H, Ar), 7.85 (d, J = 7.4 Hz, 8H, Ar), 6.91 (d, J = 8.2 Hz, 8H, Ar), 6.74 (d, 8H, Ar), 7.02 (s, 2H, Ar), 6.63 (s, 2H, Ar), 8.72 (s, 2H, −CH=N), 8.03 (s, 2H, −OH). 13C NMR: 161.80, 160.42, 159.42, 147.83, 144.73, 138.62, 136.41, 135.81, 129.70, 127.61, 124.24, 108.18, 105.18, 103.64, 77.47, 77.04, 76.62, 69.11, 68.15, 35.21, 31.83, 31.27, 19.04, 14.14. MALDI Tof MS for compound 10c (M + 1): calcd, 2287.1760; found, 2288.184.
Compound 10d
It was obtained in 76% yield, elemental analysis: C146H202N6O14S6 calcd: C, 71.35; H, 8.29; N, 3.42; O, 9.11%; S, 7.83%. Found: C, 71.43; H, 8.23; N, 3.51; O, 9.04%. FT-IR (KBr) in cm–1: 2890, 1620, 1522, 1441, 1320, 1240, 1140, 1120, 981, 780. 1H NMR (CDCl3, 400 MHz): 0.90–0.92 (t, J = 6.6 Hz, 18H, −OC8H17), 1.26–1.28 (m, 18H, −OC10H21), 1.31 (s, 36H, t-butyl group), 1.46 (sext, 12H, −OC10H21), 1.71 (p, 12H, −OC10H21), 4.04 (t, 12H, −OC10H21), 4.40 (s, 4H, −CH2−), 6.51 (d, J = 8.1 Hz, 4H, Ar), 7.43 (s, 2H, Ar), 7.85 (d, J = 7.6 Hz, 8H, Ar), 6.91 (d, J = 7.4 Hz, 8H, Ar), 6.74 (d, 8H, Ar), 7.02 (s, 2H, Ar), 6.63 (s, 2H, Ar), 8.64 (s, 2H, −CH=N), 8.02 (s, 2H, −OH). 13C NMR: 161.80, 160.42, 158.42, 147.83, 143.73, 138.62, 136.41, 135.81, 129.70, 127.61, 124.24, 108.18, 105.18, 103.64, 77.47, 77.04, 76.62, 69.11, 68.15, 35.21, 31.83, 31.27, 19.04, 14.14. MALDI Tof MS for compound 10d (M + 1): calcd, 2255.3246; found, 2456.362.
Acknowledgments
V.S.S. acknowledge thanks to Chemistry Department, Madhav University for providing lab and other required facility. We are grateful to Center of Excellence (Rajkot) for providing spectral services. Fruitful discussions with Dr. Hemant Kumar Singh (Department of Chemistry, Indian Institute of Technology, Guwahati) in the high temperature XRD analysis are also gratefully acknowledged. One of the authors, P.A.S. gratefully acknowledges Human Resource Development Group-Council of Scientific & Industrial Research (CSIR), New Delhi for Research Associate Fellowship (file no.: 09/070(0058)2K18 EMR-I). We also thank MNIT Jaipur for providing SEM and AFM analysis and Dr. Ravindra B. Solanki for helpful discussions on fluorescence measurements.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01776.
Experimental, materials and methods, synthesis and structural characterization data (1H and 13C NMR spectra), MALDI-TOF mass spectra, POM, XRD, electrochemical, DSC, gelation, and DFT studies (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Bisoyi H. K.; Li Q. Light-Driven Liquid Crystalline Materials: From Photo-Induced Phase Transitions and Property Modulations to Applications. Chem. Rev. 2016, 116, 15089–15166. 10.1021/acs.chemrev.6b00415. [DOI] [PubMed] [Google Scholar]
- Hassheider T.; Benning S. A.; Kitzerow H.-S.; Achard M.-F.; Bock H. Color-Tuned Electroluminescence from Columnar Liquid Crystalline Alkyl Arenecarboxylates. Angew. Chem., Int. Ed. 2001, 40, 2060–2063. . [DOI] [PubMed] [Google Scholar]
- Wöhrle T.; Wurzbach I.; Kirres J.; Kostidou A.; Kapernaum N.; Litterscheidt J.; Haenle J. C.; Staffeld P.; Baro A.; Giesselmann F.; Laschat S. Discotic Liquid Crystal. Chem. Rev. 2016, 116, 1139–1241. 10.1021/acs.chemrev.5b00190. [DOI] [PubMed] [Google Scholar]
- Imrie C. T.; Henderson P. A. Liquid Crystal Dimers and Higher Oligomers: Between Monomers And Polymers. Chem. Soc. Rev. 2007, 36, 2096–2124. 10.1039/b714102e. [DOI] [PubMed] [Google Scholar]
- Loi M. A.; Como E. D.; Dinelli F.; Murgia M.; Zamboni R.; Biscarini F.; Muccini M. Supramolecular Organization in Ultra-Thin Films of α-Sexithiophene on Silicon Dioxide. Nat. Mater. 2005, 4, 81–85. 10.1038/nmat1279. [DOI] [Google Scholar]
- Schmidt-Mende L.; Fechtenkotter A.; Mullen K.; Moons E.; Friend R. H.; MacKenzie J. D. Self-Organized Discotic Liquid Crystals for High-Efficiency Organic Photovoltaics. Science 2001, 293, 1119–1122. 10.1126/science.293.5532.1119. [DOI] [PubMed] [Google Scholar]
- Baldo M. A.; O’Brien D. F.; You Y.; Shoustikov A.; Sibley S.; Thompson M. E.; Forrest S. R. Highly Efficient Phosphorescent Emission from Organic Electroluminescent Devices. Nature 1998, 395, 151–154. 10.1038/25954. [DOI] [Google Scholar]
- Uoyama H.; Goushi K.; Shizu K.; Nomura H.; Adachi C. Highly Efficient Organic Light-Emitting Diodes from Delayed Fluorescence. Nature 2012, 492, 234–238. 10.1038/nature11687. [DOI] [PubMed] [Google Scholar]
- Seed A. Synthesis of self-organizing mesogenic materials containing a sulfur-based five- membered heterocyclic core. Chem. Soc. Rev. 2007, 36, 2046–2069. 10.1039/b612666a. [DOI] [PubMed] [Google Scholar]
- Zhu M.; Yang C. Blue Fluorescent Emitters: Design Tactics and Applications in Organic Light-Emitting Diodes. Chem. Soc. Rev. 2013, 42, 4963–4976. 10.1039/c3cs35440g. [DOI] [PubMed] [Google Scholar]
- Kulkarni A. P.; Tonzola C. J.; Babel A.; Jenekhe S. A. Electron Transport Materials for Organic Light-Emitting Diodes. Chem. Mater. 2004, 16, 4556–4573. 10.1021/cm049473l. [DOI] [Google Scholar]
- Wang C.; Jung G.-Y.; Hua Y.; Pearson C.; Bryce M. R.; Petty M. C.; Batsanov A. S.; Goeta A. E.; Howard J. A. K. An efficient pyridine and oxadiazole-containing hole-blocking material for organic light-emitting diodes: synthesis, crystal structure, and device performance. Chem. Mater. 2001, 13, 1167–1173. 10.1021/cm0010250. [DOI] [Google Scholar]
- Chen S.; Deng L.; Xie J.; Peng L.; Xie L.; Fan Q.; Huang W. Recent Developments in Top-Emitting Organic Light-Emitting Diodes. Adv. Mater. 2010, 22, 5227–5239. 10.1002/adma.201001167. [DOI] [PubMed] [Google Scholar]
- Ma Z.; Sonar P.; Chen Z. K. Recent Progress in Fluorescent Blue Light-emitting Materials. Curr. Org. Chem. 2010, 14, 2034–2069. 10.2174/138527210793351562. [DOI] [Google Scholar]
- Kulkarni A. P.; Tonzola C. J.; Babel A.; Jenekhe S. A. Electron Transport Materials for Organic Light-Emitting Diodes. Chem. Mater. 2004, 16, 4556–4573. 10.1021/cm049473l. [DOI] [Google Scholar]
- Kalyani N. T.; Dhoble S. J. Organic light emitting diodes: Energy saving lighting technology-A review. Renew. Sustain. Energy Rev. 2012, 16, 2696–2723. 10.1016/j.rser.2012.02.021. [DOI] [Google Scholar]
- Im Y.; Byun S. Y.; Kim J. H.; Lee D. R.; Oh C. S.; Yook K. S.; Lee J. Y. Recent Progress in High-Efficiency Blue-Light-Emitting Materials for Organic Light-Emitting Diodes. Adv. Funct. Mater. 2017, 27, 1603007. 10.1002/adfm.201603007. [DOI] [Google Scholar]
- Pathak S. K.; Nath S.; De J.; Pal S. K.; Achalkumar A. S. Contrasting effects of heterocycle substitution and branched tails in the arms of star-shaped molecules. New J. Chem. 2017, 41, 4680–4688. 10.1039/c7nj00911a. [DOI] [Google Scholar]
- Pradhan B.; Vaisakh V. M.; Nair G. G.; Rao D. S. S.; Prasad S. K.; Sudhakar A. A. Effect of Atomic-Scale Differences on the Self-Assembly of Thiophene-based Polycatenars in Liquid Crystalline and Organogel States. Chem.—Eur. J. 2016, 22, 17843–17856. 10.1002/chem.201603678. [DOI] [PubMed] [Google Scholar]
- Bala I.; Gupta S. P.; De J.; Pal S. K. Room-Temperature Columnar Nematic and Soft Crystalline Columnar Assemblies of a New Series of Perylene-Centred Disc Tetramers. Chem.—Eur. J. 2017, 23, 12767–12778. 10.1002/chem.201702181. [DOI] [PubMed] [Google Scholar]
- Parra M.; Hidalgo P.; Alderete J. New supramolecular liquid crystals induced by hydrogen bonding between pyridyl-1,2,4-oxadiazole derivatives and 2,5-thiophene dicarboxylic acid. Liq. Cryst. 2005, 32, 449–455. 10.1080/02678290500075142. [DOI] [Google Scholar]
- Seed A. Synthesis of self-organizing mesogenic materials containing a sulfur-based five- membered heterocyclic core. Chem. Soc. Rev. 2007, 36, 2046–2069. 10.1039/b612666a. [DOI] [PubMed] [Google Scholar]
- Roy B.; De N.; Majumdar K. C. Advances in Metal-Free Heterocycle-Based Columnar Liquid Crystals. Chem.—Eur. J. 2012, 18, 14560–14588. 10.1002/chem.201200483. [DOI] [PubMed] [Google Scholar]
- Grimsdale A. C.; Leok Chan K.; Martin R. E.; Jokisz P. G.; Holmes A. B. Synthesis of Light- Emitting Conjugated Polymers for Applications in Electroluminescent Devices. Chem. Rev. 2009, 109, 897–1091. 10.1021/cr000013v. [DOI] [PubMed] [Google Scholar]
- Han J. 1,3,4-Oxadiazole based liquid crystals. J. Mater. Chem. C 2013, 1, 7779–7797. 10.1039/c3tc31458h. [DOI] [Google Scholar]
- Parra M. L.; Elgueta E. Y.; Ulloa J. A.; Vergara J. M.; Sanchez A. I. Columnar liquid crystals based on amino-1,3,4-thiadiazole derivatives. Liq. Cryst. 2012, 39, 917–925. 10.1080/02678292.2012.686635. [DOI] [Google Scholar]
- Pradhan B.; Gupta R. K.; Pathak S. K.; De J.; Pal S. K.; Achalkumar A. S. Columnar self- assembly of luminescent bent-shaped hexacatenars with a central pyridine core connected with substituted 1,3,4-oxadiazole and thiadiazoles. New J. Chem. 2018, 42, 3781–3798. 10.1039/c7nj04449f. [DOI] [Google Scholar]
- Elgueta E. Y.; Parra M. L.; Diaz E. W.; Barbera J. Synthesis of novel symmetrical tetra and hexacatenar di-amides containing 1,2,3-thiadiazole units and a study of their mesomorphic and luminescence properties. Liq. Cryst. 2014, 41, 861–871. 10.1080/02678292.2014.883446. [DOI] [Google Scholar]
- Lehmann M.; Seltmann J.; Auer A. A.; Prochnow E.; Benedikt U. Synthesis and mesomorphic properties of new V-shaped shape-persistent nematogens containing a thiazole or a thiadiazole bending unit. J. Mater. Chem. 2009, 19, 1978–1988. 10.1039/b818240j. [DOI] [Google Scholar]
- Peng X.; Gao H.; Xiao Y.; Cheng H.; Huang F.; Cheng X. Synthesis and self-assembly of photoresponsive and luminescent polycatenar liquid crystals incorporating an azobenzene unit interconnecting two 1,3,4-thiadiazoles. New J. Chem. 2017, 41, 2004–2012. 10.1039/c6nj02604d. [DOI] [Google Scholar]
- Pathak S. K.; Gupta M.; Pal S. K.; Achalkumar A. S. Hexacatenars Exhibiting π-π Driven Supergelation, Aggregation Induced Blue Light Emission and Thermochromism. ChemistrySelect 2016, 1, 5107–5120. 10.1002/slct.201600927. [DOI] [Google Scholar]
- Nath S.; Pathak S. K.; De J.; Pal S. K.; Achalkumar A. S. Star-shaped π-gelators based on oxadiazole and thiadiazoles: a structure–property correlation. Mol. Syst. Des. Eng. 2017, 2, 478–489. 10.1039/c7me00040e. [DOI] [Google Scholar]
- Yadav A. K.; Pradhan B.; Ulla H.; Nath S.; De J.; Pal S. K.; Satyanarayan M. N.; Achalkumar A. S. Tuning the self-assembly and photophysical properties of bi-1,3,4-thiadiazole derivatives through electron donor–acceptor interactions and their application in OLEDs. J. Mater. Chem. C 2017, 5, 9345–9358. 10.1039/c7tc01420a. [DOI] [Google Scholar]
- Sameni S.; Jeunesse C.; Matt D.; Harrowfield J. Calix[4]arene daisychains. Chem. Soc. Rev. 2009, 38, 2117–2146. 10.1039/b900183b. [DOI] [PubMed] [Google Scholar]
- Cometti G.; Dalcanale E.; Vosel A. D.; Levelut A. M. New bowl-shaped columnar liquid crystals. Columnar Phase. J. Chem. Soc., Chem. Commun. 1990, 163–165. 10.1039/c39900000163. [DOI] [Google Scholar]
- Komori T.; Shinkai S. A New Class of Mesomorphic Materials Designed from Calix[n]arenes. Chem. Lett. 1992, 21, 901–904. 10.1246/cl.1992.901. [DOI] [Google Scholar]
- Sutariya P. G.; Modi N. R.; Pandya A.; Rana V. A.; Menon S. K. Synthesis, mesomorphism and dielectric behaviour of novel basket shaped scaffolds constructed on lower rim azocalix[4]arenes. RSC Adv. 2013, 3, 4176–4180. 10.1039/c3ra22422h. [DOI] [Google Scholar]
- Yang F.; Zhang Y.; GuO H.; Bai X. Y. Novel supramolecular liquid crystals: cyclodextrin-triphenylene column liquid crystals based on click chemistry. New J. Chem. 2013, 37, 2275–2279. 10.1039/c3nj00474k. [DOI] [Google Scholar]
- Yang F.; Guo H.; Xie J.; Lin J. Synthesis of Calixarene-Linked Discotic TriphenyleneSynthesis of Calixarene-Linked Discotic Triphenylene. Eur. J. Org. Chem. 2011, 5141–5145. 10.1002/ejoc.201100685. [DOI] [Google Scholar]
- Fang X.; Guo H.; Yang F.; Wu Y. Novel gallic-calixarene liquid crystals: syntheses and conformation influences on mesomorphism. Tetrahedron Lett. 2015, 56, 6128–6131. 10.1016/j.tetlet.2015.09.093. [DOI] [Google Scholar]
- Hong B.; Yang F.; Guo H.; Jiao Z. Synthesis, complexation, and mesomorphism of novel calixarene-linked discotic triphenylene based on click chemistry. Tetrahedron Lett. 2014, 55, 252–255. 10.1016/j.tetlet.2013.11.008. [DOI] [Google Scholar]
- Tang H.; Guo H.; Yang F.; Zhu S. Synthesis and mesomorphic properties of calix[4]resorcinarene–triphenylene oligomers. Liq. Cryst. 2017, 44, 1566–1574. 10.1080/02678292.2017.1305459. [DOI] [Google Scholar]
- Han C.; Guo H.; Lai J.; Yang F. Calix[4]resorcinarene-cholesterol columnar liquid crystals: Synthesis, mesomorphism and the influence of spacers on liquid crystalline behaviors. J. Mol. Liq. 2017, 231, 220–224. 10.1016/j.molliq.2017.01.111. [DOI] [Google Scholar]
- Tang H.; Guo H.; Yang F.; Zhu S. Synthesis and mesomorphic properties of calix[4]resorcinarene–triphenylene oligomers. Liq. Cryst. 2017, 44, 1566–1574. 10.1080/02678292.2017.1305459. [DOI] [Google Scholar]
- Sharma V. S.; Sharma A. S.; Vekariya R. H. Columnar self-assembly of bowl shaped fluorescent liquid crystals based on calix[4]arene with Schiff base units. New J. Chem. 2018, 42, 15044–15051. 10.1039/c8nj01721b. [DOI] [Google Scholar]
- Sharma V. S.; Shah A. P.; Sharma A. S.; Athar M. Columnar self-assembly, gelation and electrochemical behavior of cone-shaped luminescent supramolecular calix[4]arene LCs based on oxadiazole and thiadiazole derivatives. New J. Chem. 2019, 43, 1910–1925. 10.1039/c8nj04922j. [DOI] [Google Scholar]
- Romero J.; Barberá J.; Blesa M.-J.; Concellón A.; Romero P.; Serrano J. L.; Marcos M. Liquid Crystal Organization of Calix[4]arene-Appended Schiff Bases and Recognition towards Zn+2. ChemistrySelect 2017, 2, 101–109. 10.1002/slct.201601826. [DOI] [Google Scholar]
- Kumar R.; Lee Y. O.; Bhalla V.; Kumar M.; Kim J. S. Recent developments of thiacalixarene based molecular motifs. Chem. Soc. Rev. 2014, 43, 4824–4820. 10.1039/c4cs00068d. [DOI] [PubMed] [Google Scholar]
- Zhao M.; Lv J.; Guo D.-S. Promising advances of thiacalix[4]arene in crystal structures. RSC Adv. 2017, 7, 10021–10050. 10.1039/c6ra25616c. [DOI] [Google Scholar]
- Dhir A.; Bhalla V.; Kumar M. Ratiometry of monomer/excimer emissions of dipyrenyl thiacalix[4]arene for Cu2+ detection: a potential Cu2+ and K+ switched INHIBIT logic gate with NOT and YES logic function. Tetrahedron Lett. 2008, 49, 4227–4230. 10.1016/j.tetlet.2008.04.155. [DOI] [Google Scholar]
- Kumar R.; Bhalla V.; Kumar M. Ratiometric/‘turn-on’ fluorescent chemosensor for CN–: mimicking XNOR logic function with Fe3+ ions. Dalton Trans. 2013, 42, 8808–8814. 10.1039/c3dt50142f. [DOI] [PubMed] [Google Scholar]
- Mutihac L.; Lee J. H.; Kim J. S.; Vicens J. Recognition of amino acid by functionalized Calixarenes. Chem. Soc. Rev. 2011, 40, 2777–2796. 10.1039/c0cs00005a. [DOI] [PubMed] [Google Scholar]
- Sangeetha N. M.; Maitra U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. 10.1039/b417081b. [DOI] [PubMed] [Google Scholar]
- Kawano S.-i.; Fujita N.; Shinkai S. Novel host–guest organogels as stabilized by the formation of crown–ammonium pseudo-rotaxane complexes. Chem. Commun. 2003, 1352–1353. 10.1039/b303061j. [DOI] [PubMed] [Google Scholar]
- Kim K. Y.; Park S.; Jung S. H.; Lee S. S.; Park K.-M.; Shinkai S.; Jung J. H. Geometric Change of a Thiacalix[4]arene Supramolecular Gel with Volatile Gases and Its Chromogenic Detection for Rapid Analysis. Inorg. Chem. 2014, 53, 3004–3011. 10.1021/ic402804p. [DOI] [PubMed] [Google Scholar]
- Wang G.; Cheuk S.; Yang H.; Goyal N.; Reddy P. V. N.; Hopkinson B. Synthesis and Characterization of Monosaccharide-Derived Carbamates as Low-Molecular-Weight Gelators. Langmuir 2009, 25, 8696–8705. 10.1021/la804337g. [DOI] [PubMed] [Google Scholar]
- Jung J. H.; John G.; Masuda M.; Yoshida K.; Shinkai S.; Shimizu T. Self-Assembly of a Sugar-Based Gelator in Water: Its Remarkable Diversity in Gelation Ability and Aggregate Structure. Langmuir 2001, 17, 7229–7232. 10.1021/la0109516. [DOI] [Google Scholar]
- Kumagai H.; Hasegawa M.; Miyanari S.; Sugawa Y.; Sato Y.; Hori T.; Ueda S.; Kamiyama H.; Miyano S. Facile Synthesis of p-tert-butyl thiacalix[4]arene by the reaction of p-tert-butyl phenol with elemental sulphur in the presence of base. Tetrahedron Lett. 1997, 38, 3971–3972. 10.1016/s0040-4039(97)00792-2. [DOI] [Google Scholar]
- Sharma V. S.; Patel R. B. Molecular structure and mesomorphism: Effect of tail/lateral group. Mol. Cryst. Liq. Cryst. 2016, 630, 58–68. 10.1080/15421406.2016.1146866. [DOI] [Google Scholar]
- Akdas H.; Bringel L.; Graf E.; Hosseini M. W.; Mislin G.; Pansanel J.; De Cian A.; Fischer J. Thiacalixarenes: Synthesis and structural analysis of thiacalix[4]arene and of p- tert-butylthiacalix[4]arene. Tetrahedron Lett. 1998, 39, 2311–2314. 10.1016/s0040-4039(98)00067-7. [DOI] [Google Scholar]
- Bozkurt E.; Gül H. İ.; Tuğrak M. Investigation of solvent effect on photophysical properties of some sulfonamides derivatives. Turk. J. Chem. 2017, 41, 282–293. 10.3906/kim-1604-61. [DOI] [Google Scholar]
- Adachi C.; Tsutsui T.; Saito S. Blue light-emitting organic electroluminescent devices. Appl. Phys. Lett. 1990, 56, 799–801. 10.1063/1.103177. [DOI] [Google Scholar]
- Antoniadis H.; Inbasekaran M.; Woo E. P. Blue-green organic light-emitting diodes based on fluorene-oxadiazole compounds. Appl. Phys. Lett. 1998, 73, 3055–3057. 10.1063/1.122670. [DOI] [Google Scholar]
- Tao S. L.; Peng Z. K.; Zhang X. H.; Wang P. F.; Lee C.-S.; Lee S.-T. Highly Efficient Non- Doped Blue Organic Light-Emitting Diodes Based on Fluorene Derivatives with High Thermal Stability. Adv. Funct. Mater. 2005, 15, 1716–1721. 10.1002/adfm.200500067. [DOI] [Google Scholar]
- Zhu M.; Zhuo Y.; Guo H.; Yang F.; Qiu J. Enhanced fluorescence in both solution and solid state for perylene liquid crystals with six peripheral alkyl substituents on 1,6,7,12-bay positions and imides positions. J. Lumin. 2018, 194, 264–270. 10.1016/j.jlumin.2017.10.049. [DOI] [Google Scholar]
- Pathak S. K.; Gupta R. K.; Nath S.; Rao D. S. S.; Prasad S. K.; Achalkumar A. S. Columnar self-assembly of star-shaped luminescent oxadiazole and thiadiazole derivatives. J. Mater. Chem. C 2015, 3, 2940–2952. 10.1039/c5tc00009b. [DOI] [Google Scholar]
- Shokova E. A.; Kovalev V. V. Thiacalixarenes-A New Class of Synthetic Receptors. Russ. J. Org. Chem. 2003, 39, 1–28. 10.1023/A:1023416409935. [DOI] [Google Scholar]
- Akdas H.; Bringel L.; Graf E.; Hosseini M. W.; Mislin G.; Pansanel J.; De Cian A.; Fischer J. Thiacalixarenes: Synthesis and structural analysis of thiacalix[4]arene and of p-tert-butylthiacalix[4]arene. Tetrahedron Lett. 1998, 39, 2311–2314. 10.1016/s0040-4039(98)00067-7. [DOI] [Google Scholar]
- Iki N.; Morohashi N.; Narumi F. Novel molecular receptors based on a thiacalix[4]arene platform. Preparations of the di- and tetracarboxylic acid derivatives and their binding properties towards transition metal ions. Tetrahedron Lett. 1999, 40, 7337–7341. 10.1016/s0040-4039(99)01503-8. [DOI] [Google Scholar]
- Singh H. K.; Gupta R. K.; Singh S. K.; Rao D. S. S.; Prasad S. K.; Achalkumar A. S.; Singh B. Synthesis and self-assembly of aroylhydrazone based polycatenars: A structure- property correlation. J. Mol. Liq. 2019, 284, 282–290. 10.1016/j.molliq.2019.04.003. [DOI] [Google Scholar]
- Gebler D. D.; Wang Y. Z.; Blatchford J. W.; Jessen S. W.; Fu D.-K.; Swager T. M.; MacDiarmid A. G.; Epstein A. J. Exciplex Emission in Bilayer Polymer Light-Emitting Devices. Appl. Phys. Lett. 1997, 70, 1644–1646. 10.1063/1.118657. [DOI] [Google Scholar]
- Cheng Y.-J.; Liao M.-H.; Shih H.-M.; Shih P.-I.; Hsu C.-S. Exciplex Electroluminescence Induced by Cross-Linked Hole-Transporting Materials for White Light Polymer Light- Emitting Diodes. Macromolecules 2011, 44, 5968–5976. 10.1021/ma2006969. [DOI] [Google Scholar]
- De J.; Gupta S. P.; Swayamprabha S. S.; Dubey D. K.; Bala I.; Sarkar I.; Dey G.; Jou J.-H.; Ghosh S.; Pal S. K. Blue Luminescent OLED Devices of a New Class of Star-shaped Mesogen Exhibiting Π-Π Driven Supergelation. J. Phys. Chem. C 2018, 122, 23659–23674. 10.1021/acs.jpcc.8b05811. [DOI] [Google Scholar]
- Jou J.-H.; Fu S.-C.; An C.-C.; Shyue J.-J.; Chin C.-L.; He Z.-K. High Efficiency Yellow Organic Light-Emitting Diodes with a Solution-Process Feasible Iridium Based Emitter. J. Mater. Chem. C 2017, 5, 5478–5486. 10.1039/c7tc01516j. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




