Abstract
With the development of multifunctional imaging, gadolinium (Gd)-bearing inorganic nanoparticles (NPs), which were doped with trivalent lanthanide (Ln3+), have been applied in magnetic resonance imaging (MRI) and optical imaging owing to their high payload of Gd3+ ions and specific optical characteristics. In this study, we chose GdVO4 codoped with Eu3+ and Bi3+ as the host material to generate a highly efficient contrast agent (CA) for MRI and long-term luminescence imaging. The new CA emits strong and stable luminescence because of its strong characteristic emissions, resulting from the energy-transfer process from the vanadate groups (VO43–) to the Eu3+ and Bi3+ dopants. Additionally, these NPs provided conspicuous T1 and T2 relaxation time-shortening characteristics, which result in MRI enhancement. GdVO4:Eu3+,Bi3+ NPs were tested on liver tumor-bearing nude mice, and showed improved liver tumor contrast in T2-weighted MR images (T2WI). The dual-modal imaging probe exhibited no cytotoxicity or organ toxicity, reflecting its excellent biocompatibility. Thus, GdVO4:Eu3+,Bi3+ has the potential to be used for bioassays in vitro and liver tumor targeting in vivo. The results reveal the great promise of using the designed GdVO4:Eu3+,Bi3+ NPs as luminescent and MRI dual-mode bioprobes for clinical bioimaging applications.
1. Introduction
Molecular imaging has been applied in the development of precision medicine, where it has improved the accuracy of clinical diagnoses and treatments.1−4 Contrast agents (CAs) composed of nanomaterials and organic materials are extensively applied to molecular imaging.5−8 Using magnetic resonance imaging (MRI) to diagnose liver tumors is a good clinical choice.9,10 Compared to routine MRI, current clinical MRI techniques for detecting hepatocellular carcinoma (HCC) achieve higher mean sensitivity and specificity (89.7% and 92%, respectively, compared with 84.9% and 89.7% for routine MRI) with standard small-molecule Gd chelates.11 The optical imaging technique has significant advantages, such as excellent sensitivity, high resolution, contrast, instrument portability, and low cytotoxicity.5 Moreover, multimodal imaging probes can provide more precise information for disease diagnosis and can be used to shuttle drugs into tumor tissue.12
Most of the available reports on different rare earth (RE) orthovanadate nanomaterials have focused on the YVO4 and LaVO4 platforms.13−16 Because of the lackness of significant magnetic characteristics, the nanophosphors have been used only for biomedical applications, but they have not been used as MRI CAs.16 Actually, gadolinium vanadate (GdVO4) is an essential host matrix, which is widely used in near-infrared (NIR) light and activated RE oxide phosphors.17,18 However, concerns have recently been raised about brain deposition of the small-molecule Gd chelates currently used in clinical practice.19,20 Compared with commercial small-molecule Gd chelate complexes, Gd-based inorganic nanoparticles (NPs) incorporate Gd3+ ions into a solid structure rather than into organic molecules, effectively avoiding the possibility of dissociation and the consequent leakage of Gd3+ while minimizing related toxicity and increasing the proton relaxivity.21 A series of inorganic Gd-based compounds have been used for MRI contrast enhancement, including gadolinium oxides (Gd2O3),22,23 gadolinium fluorides (GdF3, NaGdF4),24−26 gadolinium hydroxides (Gd(OH)3),27 and gadolinium oxysalts (GdVO4, GdPO4).28−30 Furthermore, in multifunctional imaging fields, these NPs are promising vehicles for carrying luminescence-imaging cations, especially trivalent lanthanide ions (Ln3+). Notably, some of these new MRI CAs demonstrate both T1 and T2 relaxation time-shortening effects, and Gd-based NPs have a high r2/r1 ratio, with dominant T2 signal enhancement.31 GdVO4 NPs have strong potential for multimodal MRI and luminescence imaging.
RE-based luminescent NPs, consisting of a host material which is doped with Ln ions, lack fluorescence scintillation, have excellent photostability, long lives, low cytotoxicity, and extraordinary narrow emission lines.32 Gadolinium orthovanadate (GdVO4)-based NPs can be doped with RE ions for luminescent displays because of their equal valences and similar ionic radii. When GdVO4 is used as a host material, the [VO4]3– groups strongly absorb UV light. In addition, trivalent europium (Eu3+) ion-doped materials, with excellent red luminescence resulting from the 5D0 to 7F2 (620 nm) electric dipole emission transition in the visible light range, are highly suitable photoluminescent probes because of the long photoluminescence (PL) lifetime of Eu3+.33 However, the red emission of Eu3+ is primarily produced via downshifting luminescence (DSL) processes, which depend on excitation by UV radiation with short wavelengths (normally <300 nm), which limits the applications of such materials for bioimaging.34,35 Bi3+ (bismuth) sensitizers improve the luminescence properties by shifting the excitation peak toward longer wavelengths.36 In addition, Bi3+ functions as an activator when incorporated into the [VO4]3– host material. With the 6s2 electronic configuration, Bi3+ ions doped into REVO4 materials doped with Bi3+ ions host active electrons that are first transferred from Bi3+ to the V5+ 3d0 configuration and then to Eu3+.37,38
Here, we present GdVO4:Eu3+,Bi3+ NPs in which europium (Eu3+) and bismuth (Bi3+) are codoped into GdVO4 NPs for detection with MRI and DSL bioimaging. The purpose of this study was to determine both the in vitro and in vivo bimodal imaging characteristics of this new CA.
2. Results and Discussion
2.1. Synthesis and Structural Data
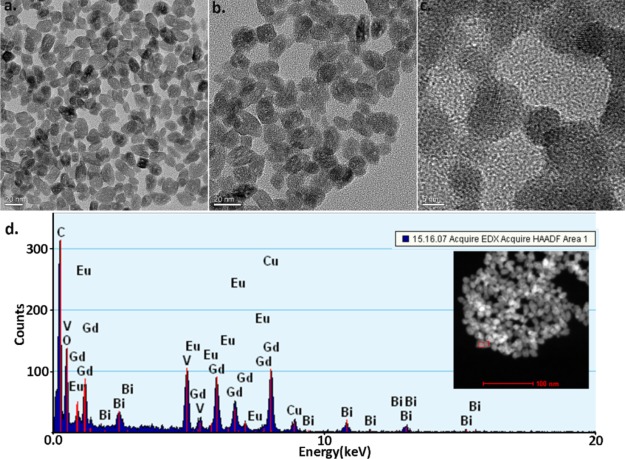
Figure 1a–c represents transmission electron microscopy (TEM) micrographs of the GdVO4:Eu3+,Bi3+ NPs. The low-magnification TEM images (Figure 1a,b) displayed that the NPs exhibit an oval shape with a uniform size and are uniformly dispersed. The length of each particle is approximately 20–30 nm, and the width is approximately 10 nm. The structure of an individual NP that exhibits well-defined lattice fringes was depicted via high-magnification TEM micrograph (Figure 1c).
Figure 1.
(a,b) TEM micrographs of GdVO4:Eu3+,Bi3+ NP samples, scale bar = 20 nm. (c) High-magnification TEM micrograph of the NPs, scale bar = 5 nm. (d) EDS exhibiting the NPs elementary composition of the NPs.
The purity of the NPs was investigated using energy-dispersive spectroscopy (EDS). Figure 1d presents an EDS spectrum of the sample. This result shows that the sample contains the elements of Gd, Bi, Eu, vanadium (V), and oxygen (O) (in Figure 1d, the elemental mapping image is shown in the small red box on the bottom-left of the inset), while the carbon (C) and copper (Cu) peaks emanate from the TEM carbon-coated copper grid. These EDS measurements demonstrate the successful incorporation of a high content of Eu3+ and Bi3+ ions into the nanocrystals.
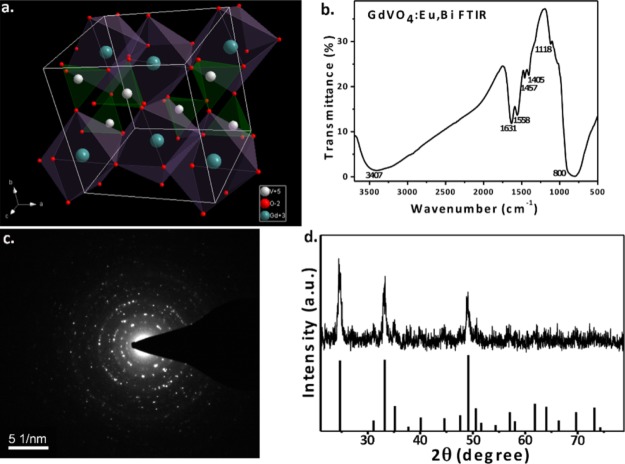
Figure 2a is a graphic of the GdVO4 crystal structure and the coordination mode for the tetragonal structure in which the Gd3+ and O2– ions tetrahedrally coordinate with the V5+ cations in the spaces among the isolated VO4 tetrahedral units. Therefore, Gd3+ ions and the surrounding eight O2– ions create a distorted GdO8 dodecahedron. VO4 tetrahedra and GdO8 dodecahedra alternate and share edges, with Gd3+ and V5+ ions in a direct line that is parallel to the c-axis.39,40
Figure 2.
(a) Schematic of the tetragonal-phase GdVO4 structure (coordination mode). (b) FTIR spectrum of GdVO4:Eu3+,Bi3+ NPs. (c) SAED pattern of GdVO4:Eu3+,Bi3+ NPs. (d) XRD pattern of the GdVO4:Eu3+,Bi3+ sample.
In Figure 2c, the selected area electron diffraction (SAED) pattern displays a bright diffraction rosette are due to the (200), (220), and (400) crystal phases. Therefore, the NPs showed high crystallinity, forming a typical and pure tetragonal phase. In addition, in Figure 2d, the X-ray diffraction (XRD) patterns of the GdVO4:Eu3+,Bi3+ consisting of peaks that follow the normal pattern of GdVO4 (JCPDS, card no. 72-0277), including the presence of peaks corresponding to the GdVO4, which has a cubic zircon crystal structure (I41/amd space group).37 The NPs are pure-phase GdVO4 because no other peaks are observed.
A Fourier transform infrared (FTIR) spectroscopy absorption spectrum is shown in Figure 2b. The characteristic peak arising from the vanadium-oxygen stretching of tetrahedra appears at 800 nm; and this peak is known to appear in the region from 800 to 1050 cm–1.41 Peaks of 3400 and 1600 cm–1 result from the widening absorptions of water.
2.2. Optical Properties and Spectroscopic Data
Unfortunately, using UV excitation for in vivo imaging is limited by its uncaging process, which is very harmful, and it is difficult to apply this method to deep tissues because of the poor tissue penetration depth.4,42 Downshifting emission excited by UV radiation (360 nm) cannot be effectively used for in vivo bioimaging limited by the greatly absorption of biological tissue in the UV spectral region (the quite short penetration length for the excitation radiation). In contrast, because of the advantages of NIR light, such as its tissue penetrability and low damage to cells and tissues, NIR light bands are ideal for biomedical applications.43 The upconversion luminescence of Nd3+ can be excited and emitted at 808 nm, which is in the NIR range. It can achieve a higher signal-to-noise ratio and deeper tissue penetration when applied to small animal imaging.44 Fortunately, phosphors could be excited by near-UV radiation, as the excitation peak of GdVO4:Eu3+ can be shifted toward longer wavelengths if doped with Bi3+.34
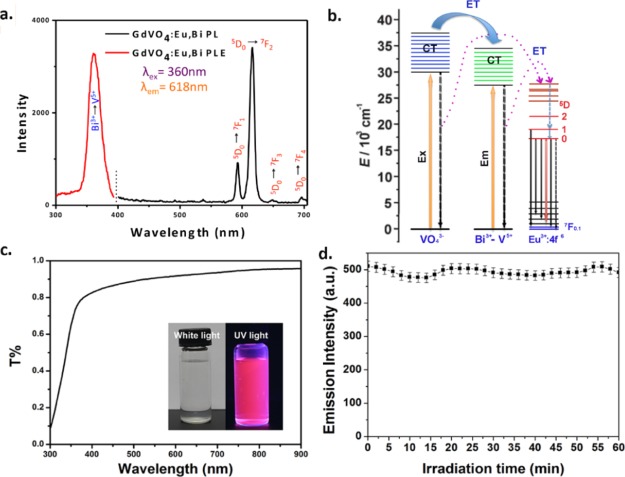
For luminescence imaging, the emission spectra and PL excitation of GdVO4:Eu3+,Bi3+ were examined. GdVO4 is a superb host material for downconversion because of its efficient energy transfer to the excited states of RE3+ dopants and strong absorption in the UV range.40 The 280 nm excitation band is well known as the characteristic excitation spectral peak of GdVO4:Eu3+, on account of charge-transfer band from O2– to V5+ within [VO4]3– groups; subsequently energy is transferred to the Eu3+ cations.34,43−46 The excitation spectra were because of the main emission line of Eu3+ ions in REVO4 at 618 nm, and they were broadly examined in the near-UV range. In Figure 3a, the Bi3+ cations were introduced into the host lattice that leads to a new energy band, the maximum location of the wide absorption band, which is centered at approximately 360 nm. This shift can be explained by the appearance of the Bi3+ to V5+ CT process, following by energy transfer to Eu3+ cations.47
Figure 3.
(a) PL excitation and emission spectrum (Ex = 360 nm, Em = 618 nm) of GdVO4:Eu3+,Bi3+ NPs. (b) Schematic diagram of the proposed energetic processes occurring in the GdVO4:Eu3+,Bi3+ samples. (c) Transmissivity spectrum of the samples; the inset shows the sample under near-UV-light and white-light excitation. (d) Luminescence time traces of the GdVO4:Eu3+,Bi3+ NPs under UV-light irradiation for 1 h.
In Figure 3a, under UV excitation at 360 nm, emission spectra of the suspensions of the NPs displayed the characteristic transitions of Eu3+ cations from 5D0 to 7FJ (J = 1–4), and the 5D0 to 7F2 transition induces the highest intensity emission line at 618 nm.33 The electric dipole transition 5D0 to 7F2 leads to the strong red light of GdVO4:Eu3+,Bi3+ NPs. In addition, the schematic diagram of energy-transfer processes of different ions shown in the emission spectrum are displayed in Figure 3b.
The transmissivity (T) of the GdVO4:Eu3+,Bi3+ NP solution is shown in Figure 3c, indicating that the light wavelength is broadened. Furthermore, under visible-light irradiation, the transmissivity of the material solution increased, and the T value approached 1. In accordance with the T value, the material solutions absorb near-UV light and emit red visible light (inset of Figure 3c).
To determine the photostability of the NPs, luminescence time traces of GdVO4:Eu3+,Bi3+ NPs were obtained with 2 min bins under irradiation for 1 h. The result shows that no photobleaching was observed after constant irradiation, demonstrating the good photostability of the GdVO4:Eu3+,Bi3+ NPs for long-term bioimaging (Figure 3d).
2.3. Cell Imaging
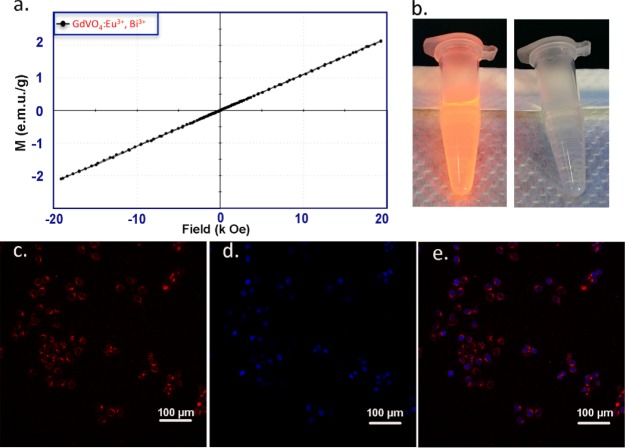
In Figure 4c–e, the uptake characteristics of the prepared NPs by HepG2 cells (human liver HCC cells) were tested by confocal laser-scanning microscopy, which was recorded by the tetramethylrhodamine (TRITC) channel. In the interior of the cells, a bright red emission was observed. This suggests that the HepG2 cells can be effectively taken up by the NPs. The results indicate that the NPs are suitable CAs for fluorescence imaging in vitro. However, the low emission wavelength of the NPs is not suitable for in-depth in vivo fluorescence imaging.48
Figure 4.
Magnetic properties of GdVO4:Eu3+,Bi3+ NPs: (a) RT magnetization field (M–H) curve. (b) DLS fluorescence imaging under UV light (the right photo shows the control sample under visible light). Fluorescence images of HepG2 incubated with NPs: (c) TRITC channel, (d) DAPI channel, and (e) merged channels.
2.4. Magnetic Properties and MRI Applications
To make the GdVO4:Eu3+,Bi3+ NPs sufficiently compatible for further MRI applications, their magnetic properties were investigated with a vibrating sample magnetometer (VSM). As shown in Figure 6a, the room-temperature (RT) magnetization curve was linearly correlated with the magnetization intensity of 1.28 emu/g of NPs at 10 kOe, indicating that the NPs have potential applications in MRI.
Figure 6.
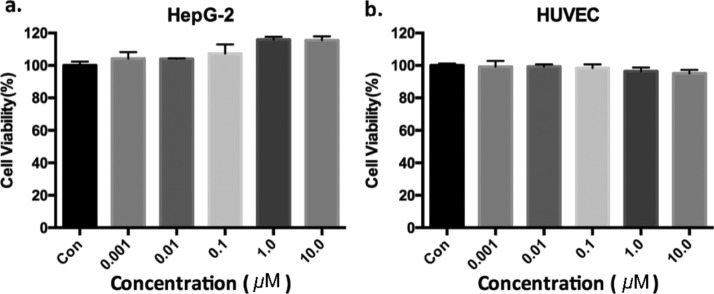
Viability of HepG2 cells (a) and HUVECs (b) after incubation with GdVO4:Eu3+,Bi3+ NPs at increasing Gd concentrations. (a,b) Cell viability was measured by a CCK-8 array using the means and SD of the number of experiments per experimental group.
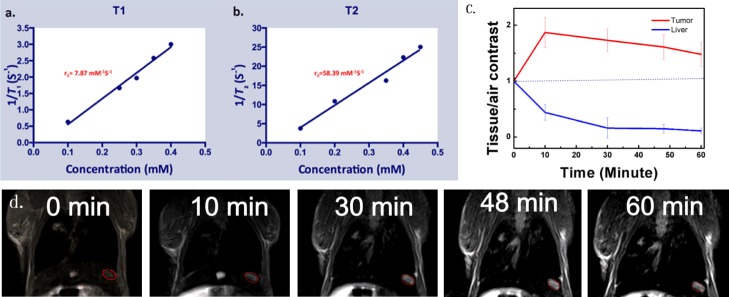
Figure 5a,b shows in vitroT1 and T2 measurements for Gd3+-doped RE NPs in aqueous suspensions with different Gd3+ concentrations. The literature contains many reports showing that Gd3+-doped RE NPs have extremely low relaxivity, which limits their applications. In fact, most reports have focused on the T1 contrast effect but not the T2 contrast effect. Paramagnetic Gd-based MRI CAs exhibit increased signal intensity in the T1-weighted images, so they are defined as “positive CAs”.50 Actually, Gd3+ ions have seven unpaired electrons, which can generate large magnetic fluctuations, resulting in the shortening of the T1 and T2 values of the surrounding protons. In general, a low concentration of Gd3+ shortens the T1 values, whereas extreme T2 shortening occurs at high Gd3+ concentrations. The GdVO4:Eu3+,Bi3+ NPs exhibit a high signal intensity, with an r1 value of r1 = 7.87 mM–1 s–1 and an r2 value of 58.39 mM–1 s–1. The r2/r1 ratio of the NPs was calculated as 7.42, indicating that the GdVO4:Eu3+,Bi3+ NPs have potential applications as dual-weighted MRI CAs (both T1 and T2) for in vivo MRI,49 although the high r1 (r1 = 7.87 mM–1 s–1) of the GdVO4:Eu3+,Bi3+ NPs was also observed. Obviously, the NPs’ r2/r1 ratio is higher than the clinical value (r2/r1 ratio close to 1), primarily due to the T2-shortening effect. Additionally, GdVO4-related NPs have been mostly used as T1-weighted CAs in vitro and in vivo,28,51 and few studies on the applications of these NPs for T2-weighted MRI have been conducted.
Figure 5.
(a,b) Curves of longitudinal and transverse vs metal concentration of GdVO4:Eu3+,Bi3+ NPs at 3.0 T. (c) Contrast intensities of the tumors, normal hepatic tissues compared with air. (d) T2WI of tumor-bearing nude mice in vivo before (left: t = 0 min) and after (right) the injection of GdVO4:Eu3+,Bi3+ NPs into the tail vein. The red circle shows the tumor site.
Therefore, for in vivo MRI studies, we concentrated on the function of the NPs as T2 MRI CAs. As shown in Figure 5d, tumor-bearing BALB/c nude mice were intravenously injected at a dose of 10 mg of Gd per kg of mice body weight before recording by T2WI. After injecting the CA, a significant decrease of approximately 44% at 10 min (instead of an increase) was observed in the signal strength of the hepatic parenchyma regions in T2WI by measuring the signal intensities of the regions of tumor and liver regions, possibly due to the effective uptake of NPs. Meanwhile, the tumor tissue showed an enhancement of 27% in the tumor at 10 min (Figure 5c), suggesting an obvious T1 effect of the tumor tissue. This finding is consistent with previous reports of a lack of Kupffer cells in HCCs.52,53
2.5. Biocompatibility and Toxicity
Before GdVO4:Eu3+,Bi3+ NPs can be used for in vivo multimodal imaging, toxicity testing is crucial. The NPs’ cytotoxicity was evaluated by a Cell Counting Kit-8 (CCK-8) assay on HepG2 cells and human umbilical vein endothelial cells (HUVECs). HepG2 cells and HUVECs were incubated for 24 h in NP suspensions with NP concentrations ranging between 0 and 10 μM. In Figure 6, the change in proliferation was negligible for the treated cells.
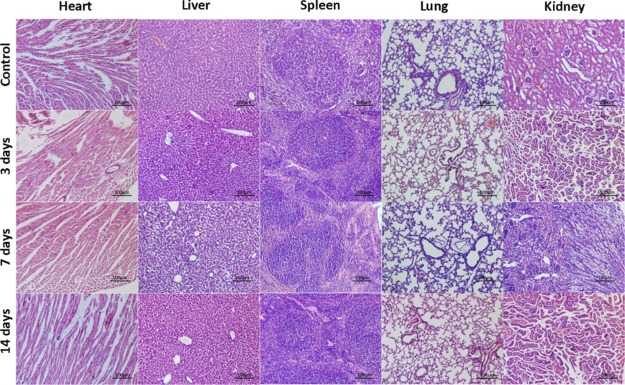
To further evaluate the long-term safety of the GdVO4:Eu3+,Bi3+ NPs, 20 healthy BALB/c mice were sacrificed and dissected at 0, 3, 7, and 14 days after intravenous administration with GdVO4:Eu3+,Bi3+ NP suspension (n = 5, respectively). Histological analysis (in Figure 7) revealed that the organs (heart, spleen, liver, lung, and kidney) were normal. In addition, no deaths or behavioral changes were observed among the animals after NP administration, indicating that the GdVO4:Eu3+,Bi3+ NPs are biocompatible.
Figure 7.
H&E-stained images of different organs after intravenous administration of GdVO4:Eu3+,Bi3+ NPs into normal mice at 0, 3, 7, and 14 days (scale bar = 100 μm).
3. Experimental Section
3.1. Materials
Fetal bovine serum (FBS) was purchased from Invitrogen (Carlsbad, CA). CCK-8 was acquired from Dojindo, Japan. Trypsin, phosphate-buffered saline (PBS) and RPMI 1640 medium were supplied by HyClone (Thermo, USA). Other reagents were obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). All chemicals were used as acquired without further purification. Ultrapure water was obtained from a Milli-Q water system.
3.2. NPs Synthesis
The GdVO4:Eu3+,Bi3+ NPs were synthesized using our group’s previously established method for preparing layered RE hydroxides.53,54 During GdVO4:Eu3+,Bi3+ NPs’ synthesis, suitable amounts of the RE precursor LnCl3 (Ln = Gd, Eu, Bi) were dissolved in ultrapure water to a concentration of 0.5 M. Under vigorous agitation, 1 mL of PEI solution (10% by weight) was added. The resulting solution was purged with nitrogen for 1 min to eliminate oxygen after stirring for another 5 min. The mixture was sealed into a 50 mL Teflon-lined autoclave, which was maintained at 200 °C for 2.5 h. The reaction mixture was stirred magnetically to remove the supernatant via centrifugation at 10 000 rpm for 5 min, after naturally cooling to RT. The precipitate was washed twice by ethanol and once with water and then redispersed with 5.0 mL Milli-Q water. Powder was obtained after the precipitate was dried for 12 h at 40 °C.
3.3. Characterization of the NPs
A transmission electron microscope (Tecnai G2 F20 S-TWIN, FEI, USA) was used to obtain the TEM micrographs of the GdVO4:Eu3+,Bi3+ NPs. EDS coupled with TEM was used to analyze the element compositions of the NPs. An X’Pert Pro diffractometer (X’Pert Pro MPD DY129, PANalytical, Netherlands) was used to conduct XRD. The crystal structure was determined through SAED (XRD, AXIS Ultra DLD, UK). FTIR (Nicolet 6700, Thermo Fisher Scientific, USA) was used to characterize the samples. Fluorescence emission spectra of the NPs were observed on a Hitachi F-4500 fluorescence spectrophotometer equipped with a Xe lamp excitation source. The NPs’ photostability was assessed by using a Cary 5000 UV–vis–NIR spectrometer to analyze the absorption spectra of immobilized NPs under continuous UV irradiation.
3.4. Cell Culture and Cytotoxicity Test
The HepG2 cell line and HUVEC line were supplied from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were incubated at 37 °C under 5% CO2 atmosphere, with high-glucose Dulbecco’s modified Eagle’s medium (HyClone), within 1% streptomycin/penicillin (HyClone) and 10% FBS. A CCK-8 assay (Dojindo, Japan) was used to determine the in vitro cytotoxicity of the NPs. Briefly, 5 × 103 cells of the HepG2 cells or HUVECs were planted into 96-well plates. After seeding for 24 h, the cells were then exposed to NP solutions at different concentrations and cultured overnight. The gadolinium concentrations were 0, 0.001, 0.01, 0.1, 1.0, and 10.0 μM. The cells were then washed by PBS twice and tested by CCK-8 assay at 37 °C. The absorbance at 450 nm was measured using a Varioskan Flash microplate reader. Every result is reported as the average of six samples; the data are shown as the mean ± standard deviation (SD).
3.5. Cellular Uptake and Imaging
HepG2 cells were used to demonstrate the suitability of GdVO4:Eu3+,Bi3+ NPs for bioimaging. Typically, the 2 × 105 HepG2 was incubated in each well of a 6-well plate with cover glasses for 24 h. Following, 1000 μg/mL NPs were added to each chamber after refreshing the growth medium. Then, cocultured for 2 h at 37 °C under a moist atmosphere of 5% CO2. The cells were fixed in 4% paraformaldehyde solution for 15 min at RT after washing three times in PBS and then stained with a DAPI solution. The stained cells were washed and detected via confocal laser-scanning microscope (Ni-E, Nikon, Japan) with a 20× lens to obtain the luminescence images.
3.6. Establishment of a Liver Orthotopic Transplantation Tumor-Bearing Mouse Model
The animal experiment was conducted following the guidelines of the Animal Care and Use Committee of Sichuan University. Twenty male 7-week-old BALB/c nude mice, each weighed approximately 20 g, were acquired from the Institute of Experimental Animals, Sichuan Academy of Medical Sciences. HepG2 cells were harvested and resuspended in sterile PBS. For the orthotopic xenograft tumor model, mice were anesthetized via an intraperitoneal administration of chloral hydrate (10 wt %), and 1 × 106 HepG2 cells in 10 μL were injected slowly in the lower left lobe of the hepar (the largest one) after laparotomy directly.55
3.7. MR Relaxometry and in Vivo Imaging
The magnetization values of the NPs were measured by a VSM (Lake Shore 7410) at RT under an applied field ranging between 0 and 2.0 T. A 3.0 T clinical MRI instrument (Siemens Trio Tim) was performed to study in vitro MR relaxometry. To measure the MR relaxivity r1 and r2 values, the GdVO4:Eu3+,Bi3+ NP solutions of different Gd3+ concentrations (0.1, 0.2, 0.3, 0.4, and 0.5 mM) in 4.0 mL test tubes in the PBS buffer containing 1% agarose were prepared.
Five mice with liver tumor xenografts underwent MRI with the rat coil on a 3.0 T MRI system (Siemens Trio Tim). The relevant MR parameters are as follows: T2-weighted fast spin-echo, sequence: echo time (TE): 83 ms; repetition time (TR): 3000 ms; slice thickness: 1 mm; field of view: 60 × 60 cm2; matrix: 320 × 320 pixels. After the tumor models were established, the mice were anesthetized with 10 wt % chloral hydrate, and 1 mmol Gd/mL of GdVO4:Eu3+,Bi3+ NPs was injected intravenously via the tail at a concentration of 10 mg Gd/kg mouse. Each mouse was scanned 0, 10, 30, 48, and 60 min after injection.
To quantify the contrast enhancement, ImageJ software was used to measure the signal intensity of interest in tumors and normal hepatic tissues. The signal intensities were compared after normalization.
3.8. In Vivo Toxicity Studies and Histological Analysis
Healthy male BALB/c mice were divided into four groups (n = 5 for each group) randomly, and 10 mg of Gd per kg mouse weight GdVO4:Eu3+,Bi3+ NP suspension was intravenously injected into the BALB/c mice through the tail vein for the three test groups (n = 15), and the control group (n = 5) was injected with 0.9 wt % NaCl.
To study the histologic changes, mice were sacrificed at 0, 3, 7, and 14 days after administration. The internal organs (liver, heart, spleen, lungs, and kidneys) were collected, fixed in 4% paraformaldehyde, and embedded in paraffin. The 5 μm-thick sections were evaluated with hematoxylin and eosin (H&E).
3.9. Statistical Analysis
All data are expressed as the mean ± SD. Every in vitro experiment was conducted in triplicate. In vitro data of different experimental groups were compared via a one-way analysis of variance and Student’s t-test. In vivo data of different experimental groups were similarly compared. Statistical analysis was performed using Prism 6.0 software with P < 0.05, which indicates significant differences between experimental groups.
4. Conclusions
In conclusion, GdVO4 NPs containing Eu3+ and Bi3+ ions are multimodal imaging agents that enable efficient MR T2-weighted imaging and DSL luminescence imaging. The NPs can be excited in the near-UV and visible spectra ranges, and they have an excitation peak at 360 nm caused by the introduction of Bi3+ ions and an emission peak at 618 nm that is characteristic of the spectral profile of Eu3+. Photostability experiments indicated that the NPs can provide luminescence images or enable cell monitoring in vivo in the long term. The relaxivity of these NPs demonstrated a 7.42 mM–1 s–1 high r2/r1 ratio for 3.0 T MRI, indicating that the NPs are suitable as a negative MRI CA. This observation conforms to the T2WI. The negative contrast enhances the tumor-to-liver contrast in HCC-bearing mice. Furthermore, the good biocompatibility and low cytotoxicity of the NPs indicate their potential for biomedical applications.
Acknowledgments
Thanks to Professor Heike E. Daldrup-Link for academic suggestions and revisions. Financial support from the Natural Science Foundation of China (Grant 81501462), the Sichuan Science and Technology Program (Grant 19YYJC2577), and the Functional and Molecular Imaging Key Laboratory of Sichuan Province (FMIKLSP, Grant 2012JO0011) is also acknowledged.
Author Contributions
¶ G.Z., L.C., F.Z. contributed equally.
The authors declare no competing financial interest.
References
- Yang T.; Sun Y.; Liu Q.; Feng W.; Yang P.; Li F. Cubic sub-20 nm NaLuF4-based upconversion nanophosphors for high-contrast bioimaging in different animal species. Biomaterials 2012, 33, 3733–3742. 10.1016/j.biomaterials.2012.01.063. [DOI] [PubMed] [Google Scholar]
- Kwon O. S.; Song H. S.; Conde J.; Kim H.-i.; Artzi N.; Kim J.-H. Dual-color emissive upconversion nanocapsules for differential cancer bioimaging in vivo. ACS Nano 2016, 10, 1512–1521. 10.1021/acsnano.5b07075. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Liu F.; Liu X.; Xing B. NIR light controlled photorelease of siRNA and its targeted intracellular delivery based on upconversion nanoparticles. Nanoscale 2013, 5, 231–238. 10.1039/c2nr32835f. [DOI] [PubMed] [Google Scholar]
- Jayakumar M. K. G.; Idris N. M.; Zhang Y. Remote activation of biomolecules in deep tissues using near-infrared-to-UV upconversion nanotransducers. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 8483–8488. 10.1073/pnas.1114551109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Ou H.; Liu R.; Ding D. Regulating the photophysical property of organic/polymer optical agents for promoted cancer phototheranostics. Adv. Mater. 2019, 31, 1806331. 10.1002/adma.201806331. [DOI] [PubMed] [Google Scholar]
- Qi J.; Chen C.; Zhang X.; Hu X.; Ji S.; Kwok R. T. K.; Lam J. W. Y.; Ding D.; Tang B. Z. Light-driven transformable optical agent with adaptive functions for boosting cancer surgery outcomes. Nat. Commun. 2018, 9, 1848. 10.1038/s41467-018-04222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X.; Zhang X.; Duan X.; Zheng H.-L.; Xue X.-S.; Ding D. Near-infrared afterglow luminescent aggregation-induced emission dots with ultrahigh tumor-to-liver signal ratio for promoted image-guided cancer surgery. Nano Lett. 2019, 19, 318–330. 10.1021/acs.nanolett.8b03936. [DOI] [PubMed] [Google Scholar]
- Gao H.; Zhang X.; Chen C.; Li K.; Ding D. Unity makes strength: how aggregation-induced emission luminogens advance the biomedical field. Adv. Biosyst. 2018, 2, 1800074. 10.1002/adbi.201800074. [DOI] [Google Scholar]
- Mi P.; Kokuryo D.; Cabral H.; Wu H.; Terada Y.; Saga T.; Aoki I.; Nishiyama N.; Kataoka K. A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nat. Nanotechnol. 2016, 11, 724. 10.1038/nnano.2016.72. [DOI] [PubMed] [Google Scholar]
- Shimada K.; Isoda H.; Hirokawa Y.; Arizono S.; Shibata T.; Togashi K. Comparison of gadolinium-EOB-DTPA-enhanced and diffusion-weighted liver MRI for detection of small hepatic metastases. Eur. Radiol. 2010, 20, 2690–2698. 10.1007/s00330-010-1842-3. [DOI] [PubMed] [Google Scholar]
- Ahn S. S.; Kim M.-J.; Lim J. S.; Hong H.-S.; Chung Y. E.; Choi J.-Y. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology 2010, 255, 459–466. 10.1148/radiol.10091388. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Yin T.; Gao G.; Shapter J. G.; Lai W.; Huang P.; Qi W.; Song J.; Cui D. Multifunctional core@shell magnetic nanoprobes for enhancing targeted magnetic resonance imaging and fluorescent labeling in vitro and in vivo. ACS Appl. Mater. Interfaces 2017, 9, 17777–17785. 10.1021/acsami.7b04288. [DOI] [PubMed] [Google Scholar]
- Wang F.; Xue X.; Liu X. Multicolor tuning of (Ln, P)-doped YVO4 nanoparticles by single-wavelength excitation. Angew. Chem., Int. Ed. 2008, 47, 906–909. 10.1002/anie.200704520. [DOI] [PubMed] [Google Scholar]
- Liu J.; Li Y. General synthesis of colloidal rare earth orthovanadate nanocrystals. J. Mater. Chem. 2007, 17, 1797–1803. 10.1039/b617959b. [DOI] [Google Scholar]
- Liu J.; Li Y. D. Synthesis and self-assembly of luminescent Ln3+-doped LaVO4 uniform nanocrystals. Adv. Mater. 2007, 19, 1118–1122. 10.1002/adma.200600336. [DOI] [Google Scholar]
- Gupta B. K.; Rathee V.; Narayanan T. N.; Thanikaivelan P.; Saha A.; Govind S.; Singh S. P.; Shanker V.; Marti A. A.; Ajayan P. M. Probing a bifunctional luminomagnetic nanophosphor for biological applications: a photoluminescence and time-resolved spectroscopic study. Small 2011, 7, 1767–1773. 10.1002/smll.201100441. [DOI] [PubMed] [Google Scholar]
- Anitha M.; Ramakrishnan P.; Chatterjee A.; Alexander G.; Singh H. Spectral properties and emission efficiencies of GdVO4 phosphors. Appl. Phys. A: Mater. Sci. Process. 2002, 74, 153–162. 10.1007/s003390100981. [DOI] [Google Scholar]
- Xin H.; Lin L.-X.; Wu J.-H.; Yan B. Hydrothermal synthesis and multi-color photoluminescence of GdVO4: Ln3+ (Ln= Sm, Dy, Er) sub-micrometer phosphors. J. Mater. Sci.: Mater. Electron. 2011, 22, 1330–1334. 10.1007/s10854-011-0308-y. [DOI] [Google Scholar]
- Bolles G. M.; Yazdani M.; Stalcup S. T.; Creeden S. G.; Collins H. R.; Nietert P. J.; Roberts D. R. Development of high signal intensity within the globus pallidus and dentate nucleus following multiple administrations of gadobenate dimeglumine. Am. J. Neuroradiol. 2018, 39, 415–420. 10.3174/ajnr.a5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald R. J.; McDonald J. S.; Kallmes D. F.; Jentoft M. E.; Paolini M. A.; Murray D. L.; Williamson E. E.; Eckel L. J. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology 2017, 285, 546–554. 10.1148/radiol.2017161595. [DOI] [PubMed] [Google Scholar]
- Dong H.; Du S.-R.; Zheng X.-Y.; Lyu G.-M.; Sun L.-D.; Li L.-D.; Zhang P.-Z.; Zhang C.; Yan C.-H. Lanthanide nanoparticles: from design toward bioimaging and therapy. Chem. Rev. 2015, 115, 10725–10815. 10.1021/acs.chemrev.5b00091. [DOI] [PubMed] [Google Scholar]
- Ahmad M. W.; Kim C. R.; Baeck J. S.; Chang Y.; Kim T. J.; Bae J. E.; Chae K. S.; Lee G. H. Bovine serum albumin (BSA) and cleaved-BSA conjugated ultrasmall Gd2O3 nanoparticles: Synthesis, characterization, and application to MRI contrast agents. Colloids Surf., A 2014, 450, 67–75. 10.1016/j.colsurfa.2014.03.011. [DOI] [Google Scholar]
- Fang J.; Chandrasekharan P.; Liu X.-L.; Yang Y.; Lv Y.-B.; Yang C.-T.; Ding J. Manipulating the surface coating of ultra-small Gd2O3 nanoparticles for improved T1-weighted MR imaging. Biomaterials 2014, 35, 1636–1642. 10.1016/j.biomaterials.2013.11.032. [DOI] [PubMed] [Google Scholar]
- Tian Y.; Yang H.-Y.; Li K.; Jin X. Monodispersed ultrathin GdF3 nanowires: oriented attachment, luminescence, and relaxivity for MRI contrast agents. J. Mater. Chem. 2012, 22, 22510–22516. 10.1039/c2jm34987f. [DOI] [Google Scholar]
- Xing H.; Zhang S.; Bu W.; Zheng X.; Wang L.; Xiao Q.; Ni D.; Zhang J.; Zhou L.; Peng W.; Zhao K.; Hua Y.; Shi J. Ultrasmall NaGdF4 nanodots for efficient MR angiography and atherosclerotic plaque imaging. Adv. Mater. 2014, 26, 3867–3872. 10.1002/adma.201305222. [DOI] [PubMed] [Google Scholar]
- Hou Y.; Qiao R.; Fang F.; Wang X.; Dong C.; Liu K.; Liu C.; Liu Z.; Lei H.; Wang F.; Gao M. NaGdF4 nanoparticle-based molecular probes for magnetic resonance imaging of intraperitoneal tumor xenografts in vivo. ACS Nano 2013, 7, 330–338. 10.1021/nn304837c. [DOI] [PubMed] [Google Scholar]
- Huang S.; Liu J.; Liu D.; Yuan Q. Facile and large-scale synthesis of Gd(OH)3 nanorods for MR imaging with low toxicity. New J. Chem. 2012, 36, 1335–1338. 10.1039/c2nj21009f. [DOI] [Google Scholar]
- Dong K.; Ju E.; Liu J.; Han X.; Ren J.; Qu X. Ultrasmall biomolecule-anchored hybrid GdVO4 nanophosphors as a metabolizable multimodal bioimaging contrast agent. Nanoscale 2014, 6, 12042–12049. 10.1039/c4nr03819c. [DOI] [PubMed] [Google Scholar]
- Yin W.; Zhou L.; Gu Z.; Tian G.; Jin S.; Yan L.; Liu X.; Xing G.; Ren W.; Liu F.; Pan Z.; Zhao Y. Lanthanide-doped GdVO4 upconversion nanophosphors with tunable emissions and their applications for biomedical imaging. J. Mater. Chem. 2012, 22, 6974–6981. 10.1039/c2jm16152d. [DOI] [Google Scholar]
- Ren W.; Tian G.; Zhou L.; Yin W.; Yan L.; Jin S.; Zu Y.; Li S.; Gu Z.; Zhao Y. Lanthanide ion-doped GdPO4 nanorods with dual-modal bio-optical and magnetic resonance imaging properties. Nanoscale 2012, 4, 3754–3760. 10.1039/c2nr30683b. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Liviano S.; Nuñez N. O.; Rivera-Fernández S.; De la Fuente J. M.; Ocaña M. Ionic liquid mediated synthesis and surface modification of multifunctional mesoporous Eu:GdF3 nanoparticles for biomedical applications. Langmuir 2013, 29, 3411–3418. 10.1021/la4001076. [DOI] [PubMed] [Google Scholar]
- Bouzigues C.; Gacoin T.; Alexandrou A. Biological applications of rare-earth based nanoparticles. ACS Nano 2011, 5, 8488–8505. 10.1021/nn202378b. [DOI] [PubMed] [Google Scholar]
- Yi S. S.; Bae J. S.; Shim K. S.; Moon B. K.; Seo H. J.; Jeong J. H.; Kim J. H. Photoluminescence behaviors of Eu-doped GdVO4 thin film phosphors grown by pulsed laser ablation. J. Alloys Compd. 2006, 408–412, 890–893. 10.1016/j.jallcom.2004.12.107. [DOI] [Google Scholar]
- Escudero A.; Carrillo-Carrión C.; Zyuzin M. V.; Ashraf S.; Hartmann R.; Núñez N. O.; Ocaña M.; Parak W. J. Synthesis and functionalization of monodisperse near-ultraviolet and visible excitable multifunctional Eu3+, Bi3+:REVO4 nanophosphors for bioimaging and biosensing applications. Nanoscale 2016, 8, 12221–12236. 10.1039/c6nr03369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W.; Jung M.; Yoon D. Influence of Eu3+, Bi3+ co-doping content on photoluminescence of YVO4 red phosphors induced by ultraviolet excitation. Sens. Actuators, B 2007, 126, 324–327. 10.1016/j.snb.2007.02.033. [DOI] [Google Scholar]
- Xu Q.; Lin B.; Mao Y. Photoluminescence characteristics of energy transfer between Er3+ and Bi3+ in Gd2O3: Er3+, Bi3+. J. Lumin. 2008, 128, 1965–1968. 10.1016/j.jlumin.2008.06.007. [DOI] [Google Scholar]
- Takeshita S.; Isobe T.; Niikura S. Low-temperature wet chemical synthesis and photoluminescence properties of YVO4: Bi3+, Eu3+ nanophosphors. J. Lumin. 2008, 128, 1515–1522. 10.1016/j.jlumin.2008.02.012. [DOI] [Google Scholar]
- Lenczewska K.; Gerasymchuk Y.; Vu N.; Liem N. Q.; Boulon G.; Hreniak D. The size effect on the energy transfer in Bi3+–Eu3+ co-doped GdVO4 nanocrystals. J. Mater. Chem. C 2017, 5, 3014–3023. 10.1039/c6tc04660f. [DOI] [Google Scholar]
- Szczeszak A.; Grzyb T.; Śniadecki Z.; Andrzejewska N.; Lis S.; Matczak M.; Nowaczyk G.; Jurga S.; Idzikowski B. Structural, spectroscopic, and magnetic properties of Eu3+-doped GdVO4 nanocrystals synthesized by a hydrothermal method. Inorg. Chem. 2014, 53, 12243–12252. 10.1021/ic500354t. [DOI] [PubMed] [Google Scholar]
- Gavrilović T. V.; Jovanović D. J.; Lojpur V.; Dramićanin M. D. Multifunctional Eu3+-and Er3+/Yb3+-doped GdVO4 nanoparticles synthesized by reverse micelle method. Sci. Rep. 2015, 4, 4209. 10.1038/srep04209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalley B. N.; Pode R. B.; Gupta P. K. Synthesis of GdVO4: Bi, Eu red phosphor by combustion process. Phys. Status Solidi A 2000, 177, 293–302. . [DOI] [Google Scholar]
- Wang C.; Tao H.; Cheng L.; Liu Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154. 10.1016/j.biomaterials.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Li X.; Zhang F.; Zhao D. Highly efficient lanthanide upconverting nanomaterials: progresses and challenges. Nano Today 2013, 8, 643–676. 10.1016/j.nantod.2013.11.003. [DOI] [Google Scholar]
- Yang Q.; Li X.; Xue Z.; Li Y.; Jiang M.; Zeng S. Short-wave near-infrared emissive GdPO4:Nd3+ theranostic probe for in vivo bioimaging beyond 1300 nm. RSC Adv. 2018, 8, 12832–12840. 10.1039/c7ra12864a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W.; Wang Y.; Bai X.; Dong B.; Liu Q.; Chen J.; Song H. Controllable Synthesis and Size-Dependent Luminescent Properties of YVO4:Eu3+ Nanospheres and Microspheres. J. Phys. Chem. C 2010, 114, 14018–14024. 10.1021/jp1048666. [DOI] [Google Scholar]
- Riwotzki K.; Haase M. Wet-chemical synthesis of doped colloidal nanoparticles: YVO4:Ln (Ln = Eu, Sm, Dy). J. Phys. Chem. B 1998, 102, 10129–10135. 10.1021/jp982293c. [DOI] [Google Scholar]
- Chen D.; Yu Y.; Huang P.; Lin H.; Shan Z.; Zeng L.; Yang A.; Wang Y. Color-tunable luminescence for Bi3+/Ln3+: YVO4 (Ln=Eu, Sm, Dy, Ho) nanophosphors excitable by near-ultraviolet light. Phys. Chem. Chem. Phys. 2010, 12, 7775–7778. 10.1039/c003678a. [DOI] [PubMed] [Google Scholar]
- Wu M.; Li L.; Yu X.; Zhang D.; Sun T.; Li X.; Sun L.; Lui S.; Huang X.; Bi F.; Wang H.; Zhu H.; Gong Q. Multifunctional layered gadolinium hydroxide nanoplates for ultrahigh field magnetic resonance imaging, computed tomography and fluorescence bioimaging. J. Biomed. Nanotechnol. 2014, 10, 3620–3630. 10.1166/jbn.2014.2035. [DOI] [PubMed] [Google Scholar]
- Chen F.; Bu W.; Zhang S.; Liu J.; Fan W.; Zhou L.; Peng W.; Shi J. Gd3+-ion-doped upconversion nanoprobes: relaxivity mechanism probing and sensitivity optimization. Adv. Funct. Mater. 2013, 23, 298–307. 10.1002/adfm.201201469. [DOI] [Google Scholar]
- Lee M.-J.; Kim M.-J.; Yoon C.-S.; Song S. Y.; Park K.; Kim W. S. The T2-shortening effect of gadolinium and the optimal conditions for maximizing the CNR for evaluating the biliary system: a phantom study. Korean J. Radiol. 2011, 12, 358–364. 10.3348/kjr.2011.12.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.; Liu S.; Li D.; Wang M.; Moats R.; Shan H.; Conti P. S.; Li Z. The synthesis of lanthanide-doped GdVO4 ultrathin nanosheets with great optical and paramagnetic properties for FRET biodetection and in vivo MR imaging. J. Mater. Chem. B 2014, 2, 3998–4007. 10.1039/c4tb00144c. [DOI] [PubMed] [Google Scholar]
- Yim H.; Yang S.-G.; Jeon Y. S.; Park I. S.; Kim M.; Lee D. H.; Bae Y. H.; Na K. The performance of gadolinium diethylene triamine pentaacetate-pullulan hepatocyte-specific T1 contrast agent for MRI. Biomaterials 2011, 32, 5187–5194. 10.1016/j.biomaterials.2011.03.069. [DOI] [PubMed] [Google Scholar]
- Liu C.; Hou Y.; Gao M. Are rare-earth nanoparticles suitable for in vivo applications?. Adv. Mater. 2014, 26, 6922–6932. 10.1002/adma.201305535. [DOI] [PubMed] [Google Scholar]
- Xiang Y.; Yu X.-F.; He D.-F.; Sun Z.; Cao Z.; Wang Q.-Q. Synthesis of highly luminescent and anion-exchangeable cerium-doped layered Yttrium hydroxides for sensing and photofunctional applications. Adv. Funct. Mater. 2011, 21, 4388–4396. 10.1002/adfm.201101808. [DOI] [Google Scholar]
- Song K. D.; Choi D.; Lee J. H.; Im G. H.; Yang J.; Kim J.-H.; Lee W. J. Evaluation of tumor microvascular response to brivanib by dynamic contrast-enhanced 7-T MRI in an orthotopic xenograft model of hepatocellular carcinoma. Am. J. Roentgenol. 2014, 202, W559–W566. 10.2214/ajr.13.11042. [DOI] [PubMed] [Google Scholar]