Abstract
Many species of cyanobacteria are capable of producing toxins and causing nuisance blooms, however response to environmental conditions is likely taxon-specific. Environmental factors influencing cyanobacterial composition and toxin production in lakes have been examined in many studies; yet are often confined to individual water bodies, or to a small number of systems within the same region. Here, data from the 2012 USEPA National Lakes Assessment are used to examine relationships between biovolume of common potentially-toxigenic cyanobacteria (Aphanizomenon spp., Cylindrospermopsis spp., Dolichospermum spp., Microcystis spp. and Planktothrix spp.) and environmental variables across the entire conterminous United States, and results are compared across nine distinct ecoregions. Total phosphorus and water clarity were identified as the most influential environmental factors correlated with phytoplankton community composition. The Northern, Southern and Temperate Plains ecoregions displayed the highest biovolumes of potentially toxigenic taxa on average, as well as highest mean concentrations of microcystin. In those three ecoregions, samples with microcystin concentrations greater than 1 ppb were primarily dominated by Planktothrix spp. while in all other ecoregions Dolichospermum spp. was the dominant genus. Canonical Correlation Analysis revealed a strong association between high microcystin concentrations and high nutrient concentrations (total nitrogen and total phosphorus), and between high microcystin concentrations and low percentage of watershed forest cover. Results from this study indicate that the likely occurrence of potentially toxigenic taxa in lakes and reservoirs is predictable on a biogeographical basis, depending on morphological and water quality characteristics. Data from this study may be useful to regional managers attempting to prevent or mitigate nuisance cyanobacterial blooms.
Introduction
Phytoplankton community composition in lakes and reservoirs (hereinafter, lakes) is strongly influenced both spatially and temporally by variability in water quality factors such as nutrient concentrations, hydrodynamics and water temperature (Tillman et al., 1982; Watson et al., 1997; de Souza Cardoso and da Motta Marques, 2009; Beaver et al., 2012, 2013). Generally, phytoplankton communities tend to be dominated by cyanobacteria in systems with high concentrations of nutrients, low water clarity and warmer surface temperatures – conditions that are characteristic of eutrophic water bodies (Smith, 1986; Downing et al., 2001; Kosten et al., 2012). Planktonic species of cyanobacteria can produce toxins as secondary metabolites, which can negatively impact lake ecosystems at higher trophic levels and cause adverse effects on human health and recreation. Both climate warming and eutrophication are predicted to increase the frequency and severity of toxic cyanobacterial blooms worldwide (Heisler et al., 2008; Paerl and Huisman, 2008; O’Neil et al., 2012; Paerl, 2017). Thus, determining which environmental conditions are most likely to lead to cyanobacterial blooms (and subsequent toxin production) is of great interest to lake managers and natural resource professionals (Paerl, 2017).
Most studies that monitor phytoplankton community structure and occurrence of toxins over space and time focus on a single lake or a small region, as large-scale and prolonged sampling can be difficult and costly. Consequently, conflicting results have been reported regarding which factors are most likely to lead to toxic bloom events. It is also likely that toxin production in specific species and strains of cyanobacteria is triggered by different environmental conditions (Marmen et al., 2016; Wood et al., 2017). The USEPA’s National Lakes Assessment (NLA), which surveys over 1000 randomly-selected lakes across the U.S. every five years is a relevant public resource for broad-scale limnological research (Pollard et al., 2018) and provides a unique opportunity to examine cyanobacterial communities over multiple ecoregions and a gradient of water quality conditions.
The current study seeks to determine which water quality variables are related to phytoplankton community composition and what environmental conditions promote the prevalence of potentially-toxigenic cyanobacteria in lakes in the conterminous U.S. Analysis of water quality and land use data from the 2007 NLA (Beaver et al., 2014) showed that microcystin concentrations >1 ppb (above the World Health Organization recommended threshold for safe drinking water) were primarily located in the upper Midwest region of the U.S., and were positively associated with agricultural land-use practices, high total nitrogen (TN) and high dissolved organic carbon (DOC). The current study utilizes data from the 2012 NLA and extends upon previous analyses to determine whether microcystin concentrations in those regions and elsewhere in the U.S. are associated with particular cyanobacterial taxa, and how those taxa are related to specific water quality variables. Based on the finding from Beaver et al. (2014) that similar land-use practices in different regions of the country did not yield similar toxicity, it is expected that high microcystin concentrations are correlated to high biovolumes of toxigenic taxa, and that those taxa are strongly influenced by water quality parameters associated with agriculture-dominated watersheds (high nutrients and DOC). Recent studies have used data from the NLA to examine the relationship between water quality variables and total cyanobacterial abundance (Beaver et al., 2014) or biomass (Beaulieu et al., 2013). This study expands on previous records by utilizing individually derived biovolumes to examine relationships between specific taxa and microcystin concentrations on a national scale.
Methods
Study sites
1038 lakes were comprehensively sampled throughout the conterminous U.S. from May through September of 2012 as part of the U.S. Environmental Protection Agency’s 2012 National Lakes Assessment (USEPA, 2016). Lakes greater than 1 ha in area and 1 m in depth, with at least a quarter-acre of open water area were selected, using unbiased stratified random sampling, from the USGS/USEPA National Hydrography Dataset (NHDPlus) version 2 (see Simley and Carswell, 2009). Both natural and man-made lakes were included in the survey; however the Laurentian Great Lakes, the Great Salt Lake, commercial treatment/disposal ponds, brackish lakes and ephemeral lakes were excluded. A subset of lakes were sampled twice within the study period, and each sampling event was treated as an independent sample.
Sample collection
Each lake was sampled for water quality, biological condition, habitat conditions, and recreational suitability, however collection methodology described here applies only to the variables included in this study. Phytoplankton, microcystin and water quality samples were taken from an index site in each lake. The index site was considered an open water area up to 50 m deep or at the mid-point in reservoirs. Lake surface area was also recorded, and a ratio of area:depth was calculated using a modified version the equation for dynamic ratio (i.e. potential for disturbance) described in Håkansan (1982):
Vertical temperature profiles were conducted at the index site; however in this study only mean water temperatures from the upper 5 m of the water column were evaluated. Secchi depth was recorded using a standard Secchi disk on the shady side of the boat. An integrated sampler was used to collect whole water grab samples from within the euphotic zone, generally within the top 2 m of the water column. Water was transferred from the sampler into a rinsed 4 L cubitainer and this process was repeated until the cubitainer was filled. Subsamples were then taken from the cubitainer for nutrients, phytoplankton and microcystin. A 500 ml subsample for microcystin was immediately frozen following collection. A 1000 ml subsample for phytoplankton was preserved with Lugol’s iodine solution. Microcystin and phytoplankton samples were then shipped to BSA Environmental Services, Inc. (Beachwood, OH) for analysis. A 250 ml subsample for nutrient analysis was acidified and shipped overnight to processing labs. Microcystin and phytoplankton were also sampled in the littoral zone of all lakes, however in this study only open-water samples were analyzed in order to be consistent with water quality variables. For more details on sample collection, see USEPA (2012a).
Laboratory analyses
Total nitrogen (mg L−1) and total phosphorus (μg L−1) were determined using automated colorimetric analysis following persulfate digestion. Dissolved organic carbon (mg L−1) was determined using UV promoted persulfate oxidation to CO2 with infrared detection. Total microcystin concentrations (ppb) were determined using a Microcystins/Nodularins enzyme-linked immunosorbent assay (Abraxis, detection limit 0.1 μg L−1, -ADDA specific) following three freeze-thaw cycles to lyse cyanobacteria cells (Graham et al., 2010). Phytoplankton were examined under inverted light microscopes (Leica DMLB) at 400X using pre-concentrated Utermöhl sedimentation chambers that had been allowed to settle for a minimum of 8 h. Taxonomists identified organisms to the lowest possible taxonomic level, usually species, and enumerated at least 400 natural algal units (colonies, filaments and unicells). Up to 10 individual cells for each taxon were measured, and biovolume (μm3 L−1) was calculated using formulae for geometric shapes closely resembling specific taxa (Hillebrand et al., 1999). Total biovolume for each taxon was calculated by multiplying mean individual biovolume by cell abundance (# L−1). More information on laboratory analyses can be found in USEPA (2012b).
Five cyanobacterial genera including Aphanizomenon Morren, Cylindrospermopsis (Woloszynska) Seenayya and Subba Raju, Dolichospermum (Ralfs ex Bornet et Flahault), Microcystis Lemmermann, and Planktothrix (Gomont) constitute the focus of this study. Aphanizomenon, Cylindrospermopsis, Dolichospermum and Planktothrix were chosen because they were the most frequent potentially toxigenic taxa observed in the 2012 NLA survey. Microcystis was also included due to the extensive degree of previous scientific study regarding its occurrence and toxicity. While all five genera contain species and strains capable of cyanotoxin production, only Dolichospermum, Microcystis, and Planktothrix are capable of producing microcystins (Carmichael, 2001). As such, only those three genera are analyzed in the context of microcystin production. All species originally identified under the genus Anabaena are referred to and treated as Dolichospermum in this report, following recent nomenclatural changes that occurred between the time of initial identification and present data analysis (Komárek, 2016). Specimens identified as Planktothrix spp. may have been previously classified as Oscillatoria (Anagnostidis and Komárek, 1988).
Mapping, land use and trophic state determination
Using the latitude and longitude for all sample sites and the absolute value for each criterion (individual species biovolume, microcystin concentration), the data were plotted onto a map using Arc GIS® software. Quantitative data were overlaid onto a background map detailing 9 distinct ecoregions. For the purposes of the National Lakes Assessment and other components of the EPA’s National Aquatic Resource Surveys (NARS), the 85 Level III ecoregions of the conterminous U.S. (Omernik, 1987) were agglomerated into 9 broader ecoregions (Herlihy et al., 2008), which were assessed on the basis of uniformity in reference-site quality and naturally occurring variation in stream macroinvertebrate assemblages. Hereinafter the term “ecoregion” will refer to the 9 agglomerated ecoregions outlined by Herlihy et al. (2008). Percentage land use for each lake’s watershed was determined following methods outlined in Beaver et al. (2014). After all biological and chemical analyses were complete, trophic state was determined using chlorophyll-a, Secchi depth and total phosphorus data (USEPA, 2012c).
Niche centroids
Relationships between environmental variables and microcystin concentration and between environmental variables and five individual cyanobacteria genera (Aphanizomenon, Cylindrospermopsis, Dolichospermum, Microcystis and Planktothrix) were quantified using niche centroid analysis (ter Braak and Verdonschot, 1995). In this analysis, the weighted mean biovolume or microcystin concentration and the environmental variable of interest are used to determine the optimum value at which the maximal concentration/biovolume may be observed. Using biovolume of an individual species as an example, the niche centroid optima were calculated using:
where:
yik = the biovolume of species k in the ith sample,
y+k = the summed biovolume of species k in all samples, and
xi = the environmental variable in the ith sample.
1.1. Canonical correlation analysis
A canonical correlation analysis (CCA) was performed using the Canonical Analysis of Principal Coordinates (CAP) function of the PERMANOVA + add-on in PRIMER 6 (Clarke and Gorley, 2006) between standardized phytoplankton biovolume and seven normalized environmental variables (total nitrogen, total phosphorus, water temperature, Secchi depth, dissolved organic carbon, index site depth and area:depth ratio). Prior to analysis, all phytoplankton biovolumes were agglomerated by genus, or family when necessary. Taxa not contributing at least 5% biovolume to any sample were excluded from the analysis to reduce noise. Subsequent to agglomeration and noise reduction, a data matrix of 201 phytoplankton taxa from 1092 samples was prepared detailing the total biovolume for each taxon in every sample. A sample-matched matrix of water quality variables was also prepared. A Bray-Curtis resemblance matrix was computed for the standardized phytoplankton biovolume matrix, which was analyzed against the sample-matched, normalized water quality matrix using the CAP routine. A second CCA was performed between the standardized phytoplankton data and a sample-matched matrix of untransformed percentage land-use data (n = 1221) using the same process described above. Subsequent to running the CCAs, concentrations of microcystin were superimposed over the resultant ordination plots. Additional details on the statistical methodology are provided in Beaver et al. (2012, 2013).
Results
Ecoregion
Water quality factors varied by ecoregion, with the Temperate Plains, Northern Plains and Southern Plains tending to have poorer water quality (higher nutrients, lower water clarity) on average (Table 1). Secchi depth was lowest on average in the Coastal Plains (1.0 m), Temperate Plains (1.2 m), Northern Plains (1.2 m) and Southern Plains (1.0 m), indicating low water clarity and reduced photic zone; Secchi depth was highest on average (indicating high water clarity) in the Western Mountains region (3.6 m). Both mean total nitrogen and mean total phosphorus were highest in the Temperate Plains (1.64 mg L−1 and 173 μg L−1, respectively), Northern Plains (2.92 mg L−1 and 332 μg L−1, respectively) and Southern Plains (2.59 mg L−1 and 245 μg L−1, respectively), and lowest in the Northern Appalachians (0.41 mg L−1 and 25 μg L−1, respectively). Lakes in the three Plains ecoregions were also relatively shallow on average, with the Northern, Southern and Temperate Plains having mean index site depths of 5.55, 4.28 and 5.39 m respectfully. Dissolved organic carbon was highest on average in the Northern and Southern Plains (18.50 and 19.99 mg L−1 respectfully), although concentrations in the Southern Plains were highly variable. The Coastal Plains had the lowest average index site depth (3.92 m) and the highest average area:depth ratio (0.63), indicating that lakes in that region tended to be larger and shallower when compared to other ecoregions.
Table 1.
Mean values for water quality parameters by ecoregion (±SE). Abbreviations for ecoregions are as follows: NAP = Northern Appalachians, SAP = Southern Appalachians, CPL = Coastal Plains, TPL = Temperate Plains, NPL = Northern Plains, SPL = Southern Plains, UMW = Upper Midwest, WMT = Western Mountains, XER = Xeric.
| Secchi Depth (m) | TN (mg L−1) | TP (μg L−1) | Temp (°C) | DOC (mg L−1) | Index Site Depth (m) | Area:Depth | |
|---|---|---|---|---|---|---|---|
| NAP | 2.9 (± 0.2) | 0.41 (± 0.03) | 25 (± 3) | 23.2 (± 0.6) | 4.58 (± 0.21) | 9.57 (± 0.98) | 0.15 (± 0.02) |
| SAP | 2.0 (± 0.1) | 0.57 (± 0.05) | 58 (± 9) | 25.1 (± 0.3) | 3.69 (± 0.21) | 8.88 (± 0.73) | 0.17 (± 0.02) |
| CPL | 1.0 (± 0.1) | 1.24 (± 0.12) | 125 (± 24) | 28.1 (± 0.2) | 8.41 (± 0.65) | 3.92 (± 0.29) | 0.63 (± 0.13) |
| TPL | 1.2 (± 0.1) | 1.64 (± 0.10) | 173 (± 19) | 24.8 (± 0.5) | 9.43 (± 0.48) | 5.39 (± 0.35) | 0.27 (± 0.03) |
| NPL | 1.2 (± 0.1) | 2.92 (± 0.69) | 332 (± 68) | 20.4 (± 0.4) | 18.50 (± 1.50) | 5.55 (± 0.90) | 0.33 (± 0.05) |
| SPL | 1.0 (± 0.3) | 2.59 (± 0.58) | 245 (± 47) | 25.2 (± 0.5) | 19.99 (± 6.20) | 4.28 (± 0.34) | 0.38 (± 0.04) |
| UMW | 2.3 (± 0.1) | 0.87 (± 0.06) | 42 (± 4) | 22.9 (± 0.3) | 8.66 (± 0.38) | 8.25 (± 0.51) | 0.14 (± 0.01) |
| WMT | 3.6 (± 0.3) | 0.42 (± 0.04) | 52 (± 7) | 17.8 (± 0.3) | 4.09 (± 0.31) | 13.41 (± 1.08) | 0.12 (± 0.01) |
| XER | 1.9 (± 0.2) | 0.77 (± 0.10) | 130 (± 18) | 20.4 (± 0.4) | 5.91 (± 0.53) | 9.66 (± 1.08) | 0.25 (± 0.03) |
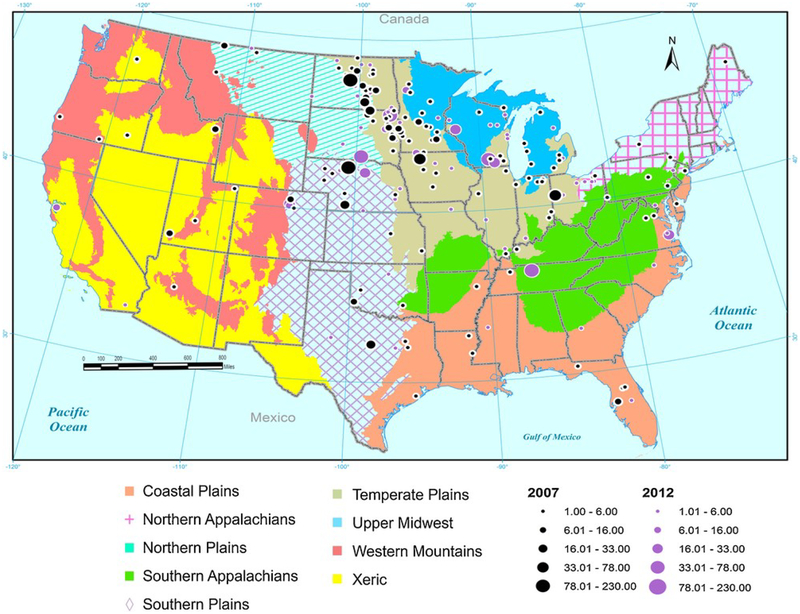
Out of the 1038 lakes sampled for microcystin, only 95 had concentrations >1 ppb in the open-water zone. Within that group of 95 lakes, 72% were located in the Northern, Temperate and Southern Plains (Table 2). The Northern, Temperate and Southern Plains ecoregions had higher microcystin on average (2.05, 1.74 and 1.06 ppb) than the other six ecoregions (all <0.6 ppb, Table 2, Fig. 1). Notably, there were no samples containing >1 ppb microcystin from the Western Mountains ecoregion.
Table 2.
Mean microcystin concentrations (ppb) (±SE) and number of lakes with microcystin concentrations greater than 1 ppb by ecoregion. Abbreviations for ecoregions are as follows: NAP = Northern Appalachians, SAP = Southern Appalachians, CPL = Coastal Plains, TPL = Temperate Plains, NPL = Northern Plains, SPL = Southern Plains, UMW = Upper Midwest, WMT = Western Mountains, XER = Xeric.
| n | Microcystin (ppb) | # Lakes >1 ppb | |
|---|---|---|---|
| 120 | NAP | 0.12 (±0.04) | 3 |
| 103 | SAP | 0.57 (±0.38) | 4 |
| 137 | CPL | 0.33 (±0.14) | 8 |
| 156 | TPL | 1.74 (±0.53) | 39 |
| 78 | NPL | 2.05 (±0.72) | 17 |
| 93 | SPL | 1.06 (±0.36) | 12 |
| 156 | UMW | 0.32 (±0.12) | 9 |
| 187 | WMT | 0.06 (±0.01) | 0 |
| 100 | XER | 0.31 (±0.15) | 3 |
Fig. 1.
Map of the U.S. showing 9 ecoregions and distribution and relative concentrations (ppb) of microcystin in samples containing at least 1 ppb from the 2007 (black) and 2012 (purple) NLA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
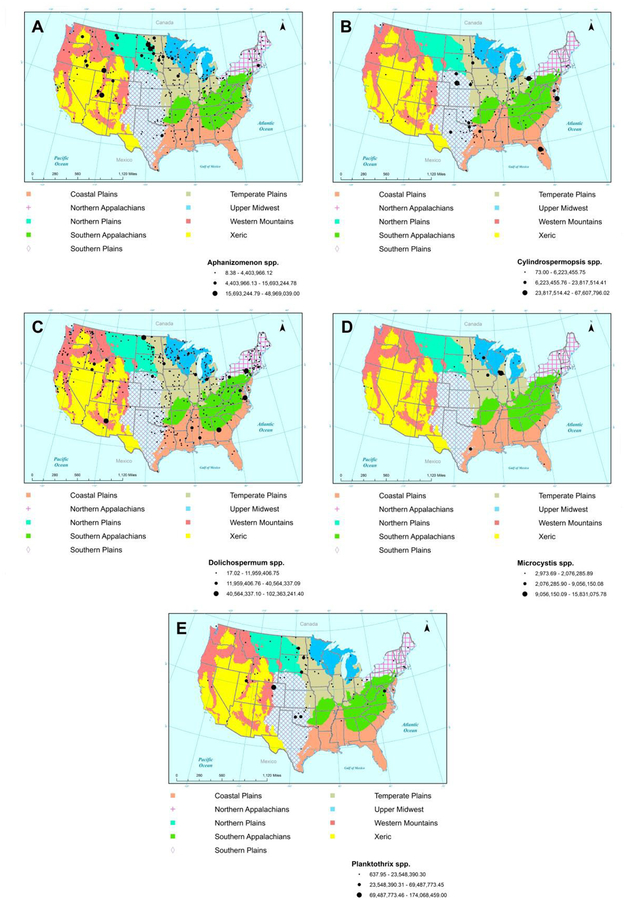
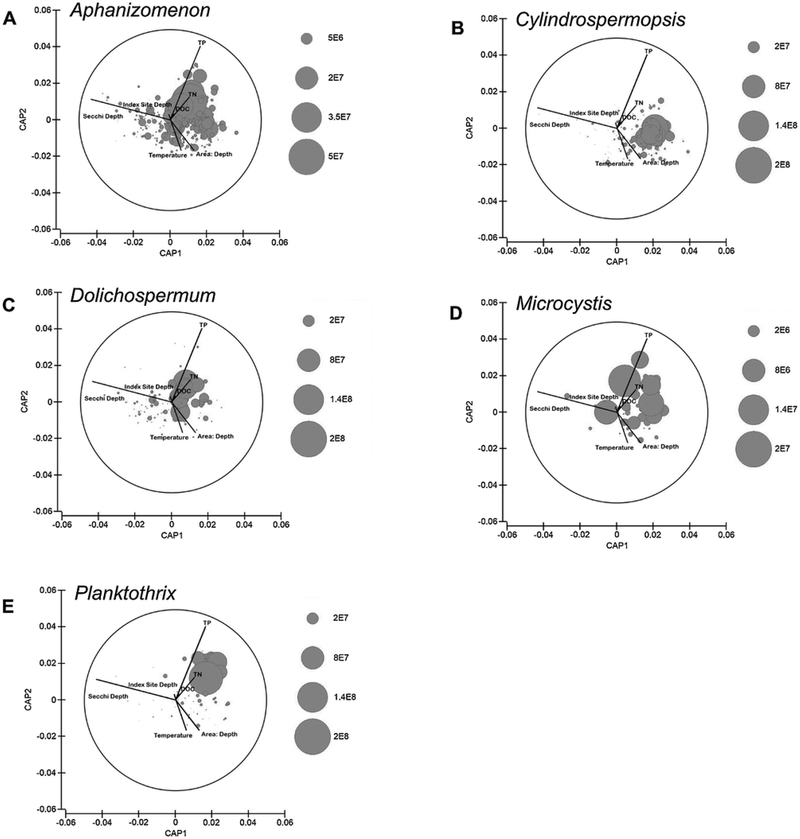
Relative cyanobacterial taxa biovolume also showed distinct ecoregional patterns. Highest mean biovolumes of Aphanizomenon spp. were observed in the Northern and Temperate Plains (1.64 * 106 and 6.67 * 105 μm3 L−1), as well as in the Xeric region (6.53 * 105 μm3 L−1) (Fig. 2a). High biovolumes of Cylindrospermopsis spp. occurred in a few instances in the Southern and Temperate Plains (means of 1.14 * 106 and 1.12 * 106 μm3 L−1), but were notably higher in the Coastal Plains (mean of 1.47 * 106 μm3 L−1) compared to other cyanobacterial taxa (Fig. 2b). Of the five taxa examined, Dolichospermum spp. had the least clear ecoregional distribution, with relatively similar biovolumes across all ecoregions (Fig. 2c). Occurrences of Microcystis spp. were observed primarily in lakes in the eastern portion of the U.S., with few observations in the Northern Plains, Southern Plains, Southern Appalachians or Xeric ecoregions (Fig. 2d). No instances of Microcystis spp. were observed in open-water regions in lakes of the Western Mountains ecoregion. Biovolume was lowest overall for Microcystis spp. compared to other common toxigenic taxa. Highest biovolumes for Planktothrix spp. were found in the Northern, Southern and Temperate Plains ecoregions (1.67 * 106, 3.15 * 106 and 9.11 * 105 μm3 L−1 respectfully, Fig. 2e).
Fig. 2.
Map of the U.S. showing 9 ecoregions and distribution and relative biovolume (μm3 L−1) of common potentially toxigenic cyanobacterial genera in samples from the 2012 NLA. A)Aphanizomenon spp. B) Cylindrospermopsis spp. C) Dolichospermum spp. D) Microcystis spp. E) Planktothrix spp
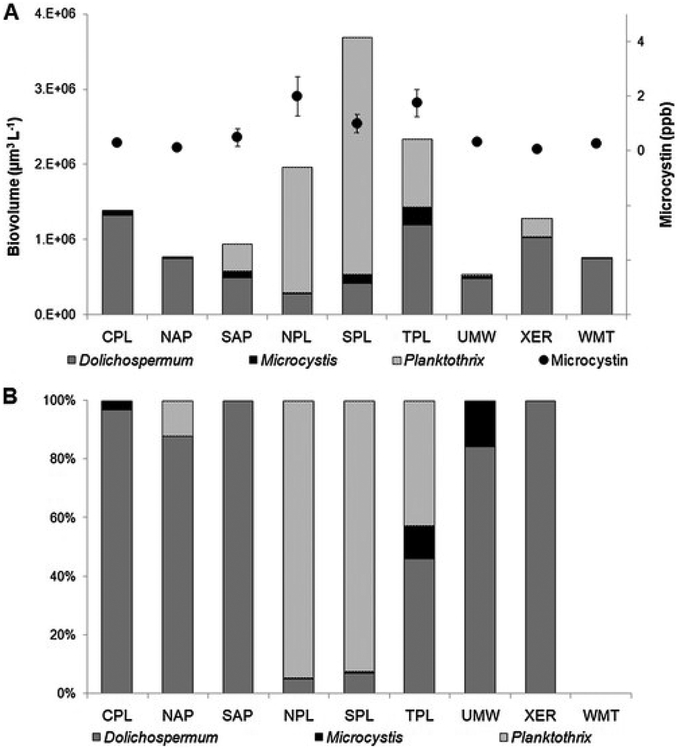
Amongst genera capable of producing microcystin, Planktothrix spp. had higher mean biovolumes in ecoregions where microcystin was highest (Northern, Southern and Temperate Plains, Fig. 3a), while Dolichospermum spp. was the dominant microcystin-producing taxa in all other ecoregions. A similar pattern was observed in lakes with >1 ppb microcystin (Fig. 3b). In the Northern and Southern Plains, Planktothrix spp. contributed the majority of the biovolume amongst potentially-toxigenic taxa. In the Temperate Plains, both Planktothrix spp. and Dolichospermum spp. contributed appreciably to total biovolume of potential microcystin producers. In all other ecoregions Dolichospermum spp. tended to be dominant in samples >1 ppb microcystin, while Microcystis spp. contributed less to total biovolume in those samples. Samples in which microcystin concentrations were >1 ppb but no specimens of Microcystis spp., Dolichospermum spp. or Planktothrix spp. were observed were excluded from this analysis. As such, it is possible that high concentrations of microcystin in lakes in the Plains ecoregions and elsewhere in the U.S. could be caused by other potentially-toxigenic taxa (see Paerl, 2018), or may have been sampled after bloom die-off.
Fig. 3.
A) Mean biovolume (μm3 L−1) by ecoregion for three common, potential microcystin-producing taxa, and mean microcystin (ppb) by ecoregion. Error bars represent standard error. B) relative biovolume of potential microcystin-producing taxa in samples containing >1 ppb microcystin by ecoregion. No samples in the Western Mountains ecoregion contained >1 ppb microcystin. Abbreviations for ecoregions are as follows: NAP = Northern Appalachians, SAP = Southern Appalachians, CPL = Coastal Plains, TPL = Temperate Plains, NPL = Northern Plains, SPL = Southern Plains, UMW = Upper Midwest, WMT = Western Mountains, XER = Xeric
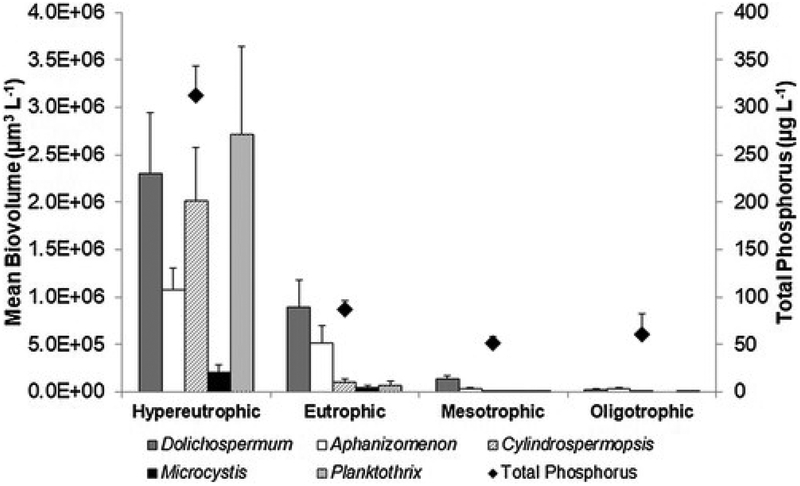
A subset of lakes in the 2012 NLA (n = 1030) for which key chemical and biological data were collected were classed into four trophic states: hypereutrophic, eutrophic, mesotrophic or oligotrophic. Mean biovolume was determined for common potentially toxigenic taxa for each trophic state (Fig. 4). For both mesotrophic and oligotrophic lakes, mean biovolumes for all potentially toxigenic cyanobacteria were low (>1.31 * 105 μm3 L−1). In eutrophic lakes, Dolichospermum spp. (8.88 *105 μm3 L−1) and Aphanizomenon spp. (5.19 * 105 μm3 L−1) had highest biovolume, while biovolume for other potentially toxigenic taxa was comparatively low on average. Mean biovolumes for both Dolichospermum spp. and Aphanizomenon spp. were higher in hypereutrophic lakes than in eutrophic lakes (2.30 * 106 μm3 L−1 and 1.07 * 106 μm3 L−1 respectfully). Mean biovolumes for Microcystis spp. and Planktothrix spp. were highest in hypereutrophic lakes (2.01 * 105 and 2.71 * 106 μm3 L−1 respectfully) and constituted over an order of magnitude increase from mean biovolumes in eutrophic lakes (4.15 * 104 and 6.67 * 104 μm3 L−1 respectively).
Fig. 4.
Mean biovolume of common potentially toxigenic taxa in lakes in four trophic categories: Hypereutrophic (n = 236), Eutrophic (n = 306), Mesotrophic (n = 325) and Oligotrophic (n = 163). Error bars represent standard error.
Niche centroids
Niche centroid optima for the five common toxigenic taxa were determined for Secchi depth, TN, TP, water temperature, DOC, N:P, index site depth and area:depth (Table 3). Results indicate highest Dolichospermum spp. biovolume would be found in lakes with relatively higher surface temperatures and lower relative concentrations of DOC, while Microcystis spp. biovolume is predicted to be highest at relatively lower temperatures. Lakes with higher N:P values would favor Aphanizomenon spp. over other potentially toxigenic taxa, but Cylindrospermopsis spp. and Planktothrix spp. would have higher biovolumes at higher TP concentrations. Optima for Secchi depth show that Planktothrix spp. biovolume tends to be highest in lakes with lower water clarity and low area:depth ratios. Niche centroid optima for microcystin indicate that higher concentrations tend to occur under conditions of warmer temperatures and low area:depth ratios.
Table 3.
Locations of niche centroids (ter Braak and Verdonschot, 1995) showing the relationship between toxigenic taxa and microcystin to water quality variables.
| Dolichospermum | Aphanizomenon | Cylindrospermopsis | Microcystis | Planktothrix | Microcystin | |
|---|---|---|---|---|---|---|
| Secchi Depth (m) | 1.6 | 2.1 | 1.7 | 1.6 | 1.7 | 1.9 |
| Total Nitrogen (mg L−1) | 1.17 | 0.98 | 1.68 | 1.30 | 1.24 | 1.26 |
| Total Phosphorus (μg L−1) | 127 | 104 | 182 | 113 | 182 | 79 |
| Surface Temp (°C) | 24.0 | 22.8 | 23.5 | 18.6 | 22.9 | 23.6 |
| Dissolved Organic Carbon (mg L−1) | 7.00 | 7.43 | 10.74 | 8.82 | 9.69 | 9.87 |
| N:P | 18.17 | 20.32 | 15.37 | 13.36 | 11.67 | 19.78 |
| Index Site Depth (m) | 6.66 | 5.41 | 5.73 | 6.80 | 5.76 | 7.84 |
| Area:Depth | 0.24 | 0.41 | 0.28 | 0.48 | 0.18 | 0.16 |
Canonical correlation analyses
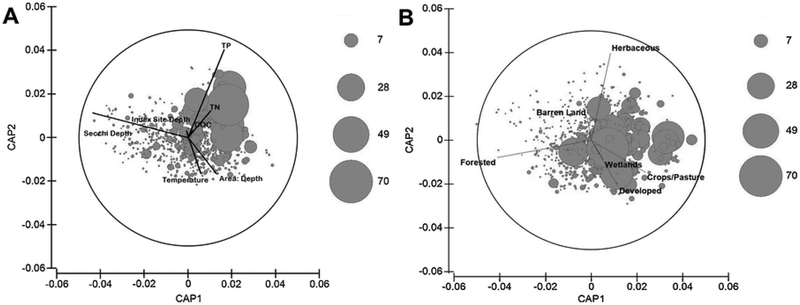
The CCA comparing phytoplankton taxa and environmental variables produced two pairs of canonical axes explaining 49% (CAP1, first axis) and 30% (CAP2, second axis) of the variance amongst correlations between phytoplankton biovolume and environmental variables. Secchi depth (water clarity) displayed a strong negative correlation with the first axis (−0.874), while total phosphorus displayed a strong positive correlation (0.810) with the second axis. All other environmental variables were weakly correlated with phytoplankton biovolume. Secchi depth was inversely correlated with area:depth. High biovolumes of Aphanizomenon spp. appeared on the positive side of the second axis, implying an association with higher nutrients (Fig. 5a). High biovolumes of Cylindrospermopsis spp. showed a positive correlation with the first axis, indicating that highest biovolumes occurred in sites with low water clarity and higher area:depth (Fig. 5b). Both Dolichospermum spp. and Microcystis spp. did not show a clear association with any water quality variables (Figs. 5c, d), while for Planktothrix spp. there was a strong, positive correlation with the positive sides of the first and second axes, implying a strong correlation with higher nutrients (Fig. 5e). Superimposition of microcystin concentrations showed an association between higher microcystin concentrations and higher nutrients (TN, TP and DOC), and between higher microcystin concentrations and lower Secchi depths (reduced water clarity) (Fig. 6a).
Fig. 5.
Ordination diagrams from the CCA comparing phytoplankton biovolume with environmental variables. Each bubble represents one sample, while bubble size represents relative biovolume (μm3 L−1) for A) Aphanizomenon spp., B) Cylindrospermopsis spp., C) Dolichospermum spp., D) Microcystis spp. and E) Planktothrix spp.
Fig. 6.
Ordination diagrams from A) the CCA comparing phytoplankton biovolume with environmental variables and B) the CCA comparing phytoplankton biovolume with percentage land-use. Each bubble represents one sample, while bubble size represents relative microcystin concentration (ppb).
The CCA comparing phytoplankton taxa and land-use categories produced two pairs of canonical axes explaining 47% (CAP1, first axis) and 38% (CAP2, second axis) of the variance amongst correlations between phytoplankton biovolume and percentage land-use. Superimposition of microcystin concentrations showed a negative relationship between microcystin and forested area (Fig. 6b), indicating that lakes with low forest cover in their watersheds tended to have increased concentrations of microcystin.
Discussion
Studies focusing on cyanobacterial biogeography have found conflicting evidence for the ubiquitous distribution of potentially toxigenic species, indicating that some species may be truly cosmopolitan and equally likely to appear anywhere on earth if conditions are suitable (e.g. Microcystis aeruginosa, Neilan et al., 1997; van Gremberghe et al., 2011; Harke et al., 2016) while others have biogeographically distinct populations (e.g. Cylindrospermopsis raciborskii, Moreira et al., 2011). Strain-specific identification of cyanobacteria was beyond the scope of this study, and as such it is difficult to draw definitive conclusions about the distribution of cyanobacteria species within the U.S. Nevertheless, the observed regional trends may prove valuable for environmental managers interested in prevention of harmful algal blooms in specific systems. Differential distributions of potentially toxigenic cyanobacterial taxa were observed within the U.S., which seems to be driven primarily by regional trends in water quality, particularly in terms of water clarity and total phosphorus.
A recent study using the 2012 NLA data found that nation-wide assessments of cyanobacteria and microcystin did not show clear trends, and more-focused, regional assessments were necessary to draw significant conclusions (Marion et al., 2017). Likewise, similar findings that the Plains ecoregions showed poorest water quality as assessed during the 2012 NLA (Marion et al., 2017) agreed with both the findings of this study and with findings from the 2007 NLA (Beaver et al., 2014; Doubek et al., 2015; Taranu et al., 2017). Previous studies have also found that individual cyanobacteria taxa are differentially influenced by fluctuations in nutrient concentrations and temperature (Rigosi et al., 2014). The findings from this study add to a growing body of evidence (Brookes and Carey, 2011; Carey et al., 2012; Beaver et al., 2012, 2014; Doubek et al., 2015; Mantzouki et al., 2016; Paerl et al., 2016; Marion et al., 2017; Taranu et al., 2017) suggesting that the long-term control and mitigation of harmful cyanobacteria blooms must be tailored to meet specific water quality challenges that vary by region and by dominant taxa.
Since the cyanobacteria are a group that varies greatly in terms of morphology, habitat-preference and ecophysiology (Chorus and Bartram, 1999; Carey et al., 2012; Mantzouki et al., 2016), different mitigation strategies may be more or less effective depending on the dominant taxa and hydrological features of individual lakes. Many cyanobacteria are able to form gas vesicles, allowing cells and colonies to migrate vertically through the water column. Those taxa that form large, floating colonies, such as Microcystis spp. and Dolichospermum spp., possess superior vertical migration velocities which may lend an advantage in highly stratified lakes where nutrients are often trapped in the darker layers of the hypolimnion (Wagner and Adrian, 2009; Carey et al., 2012). Nitrogen-fixation capability, exhibited by Dolichospermum spp., Aphanizomenon spp. and Cylindrospermopsis spp. is advantageous under nitrogen-deplete conditions (Wood et al., 2010), while non-N-fixing cyanobacteria such as Microcystis spp. and Planktothrix spp. are generally considered indicators of nitrogen enrichment (Paerl, 2018). In systems where N-fixers and non-N-fixing cyanobacteria occur together, mitigation may be especially challenging and reduction of both nitrogen and other nutrients (i.e. phosphorus, iron) may be necessary to deter nuisance blooms. Low N:P ratios (<23) have been found in association with a greater potential for microcystin production (Orihel et al., 2012); results from this study support this idea, as niche centroid analysis showed an N:P optima of 19.78 for microcystin.
The genus Cylindrospermopsis is historically a tropical taxon that has slowly expanded its range into the northern latitudes of North America (Padisák, 1997; Sinha et al., 2012), and is generally thought to be adapted to warmer systems; results of this study support this notion and show a strong association between Cylindrospermopsis spp. biovolume and warmer surface temperatures. Many species of Microcystis, particularly toxic strains (Davis et al., 2009), have also been shown to exhibit relatively higher growth rates at warmer temperatures and to be limited in growth rate at lower temperatures, compared to other cyanobacteria (Robarts and Zohary, 1987). In contrast, niche centroid analysis predicted that Microcystis spp. biovolume would be highest at relatively lower temperatures compared to other potentially toxigenic taxa. Likewise, a toxic bloom of Microcystis recently appeared in Lake Mead (Beaver et al., 2018) when surface temperatures were sub-maximal (approximately 22 °C). Despite an association between high growth rates and warmer temperatures, Cylindrospermopsis spp. blooms rarely produce toxins (saxitoxin and cylindrospermopsin) in northern latitudes (Sinha et al., 2012; Antunes et al., 2015). It is important to note that while higher growth rate for all potentially toxigenic taxa is often an indication of excess nutrients and eutrophic conditions, toxin production is still poorly understood and is not always correlated with high cyanobacterial biovolume (Hollister and Kreakie, 2016).
Several studies (Post et al., 1985; Scheffer et al., 1997) have shown that Planktothrix spp. has a high tolerance for reduced light availability, likely allowing it to outcompete other cyanobacteria in shallow lakes prone to frequent mixing. The fact that lakes in the Plains ecoregions have, on average, lower Secchi depths (reduced visibility and shallower photic zone depths) may be linked to the increased prevalence of Planktothrix spp. in those regions. In this study, niche-centroid analysis predicted highest biovolumes of Planktothrix spp. at relatively low visibilities; however other common toxigenic taxa also showed visibility optima within a similar range, suggesting that Planktothrix spp. would not necessarily have an advantage under low visibility conditions. The CCA showed that Planktothrix spp. biovolume in this study was more strongly associated with increased nutrient concentrations than reduced water clarity. Similar results showing that environmental factors other than light availability (i.e. nutrients, temperature) have a stronger influence on Planktothrix spp. have been seen in Lake Okeechobee (Florida, U.S.A.) – a shallow, well-mixed lake where one would expect to find low-light adapted species positively associated with turbid conditions (Havens et al., 1998). These results indicate that compounding factors likely influence the dominance of certain cyanobacterial taxa, and that ecophysiological adaptations may be speciesor strain-specific.
Hypereutrophic lakes in the Plains that experience extraordinarily high biovolumes of Planktothrix spp. on an internannual basis (Buckeye Lake and Grand Lake Saint Mary’s, Ohio, USA), Planktothrix spp. biovolume is strongly associated with reduced light availability and high area:depth ratio (J. Beaver, unpublished data). In contrast, niche centroid optima in this study indicated that highest biovolumes of Planktothrix spp. were observed in lakes that had comparatively low area:depth ratios, which lends further evidence that other environmental factors (likely nutrient concentrations) may be better predictors for the occurrence of Planktothrix spp. Although the Coastal Plains had highest area:depth ratios on average, Planktothrix spp. were rarely seen there, and were more commonly observed at high biovolumes in lakes in the Northern, Southern and Temperate Plains. Kokociński et al. (2010) found that Planktothrix spp. and Cylindrospermopsis spp. can co-occur in eutrophic lakes, and may compete with each other for dominance based on shifting environmental conditions. When light availability is low and phosphorus is high, Planktothrix spp. tends to be dominant, while Cylindrospermopsis spp. may have a competitive advantage at higher temperatures and lower phosphorus concentrations due to its ability to store phosphorus intracellularly (Isvánovics et al., 2000). Warmer temperatures and lower total phosphorus concentrations likely favor Cylindrospermopsis spp. in the Coastal Plains ecoregion, which may interfere with potential dominance by Planktothrix spp. – despite the abundance of large, shallow and turbid lakes in that region. The fact that Planktothrix spp. biovolume was over an order of magnitude higher in hypereutrophic lakes regardless of ecoregion implies that certain environmental conditions (namely low area:depth combined with high nutrient concentrations) could lead to dominance by Planktothrix spp., and associated toxin risk, anywhere in the country. Notably, however, 67% of hypereutrophic lakes were reported from the Northern, Southern and Temperate Plains, which makes it difficult to separate ecoregion patterns with overall water quality trends. The results of this study imply that the prevalence of Planktothrix spp. may be predictable on a spatial (ecoregion) scale within the U.S.; however spatial trends in distribution and relative magnitude of this taxon are also closely tied to water quality.
Much attention has been focused on determining the environmental factors that influence bloom formation and toxicity of Microcystis spp., and in particular Microcystis aeruginosa (e.g. Watanabe and Oishi, 1985; Jiang et al., 2008; Jähnichen et al., 2011; Harke et al., 2016; Geada et al., 2017). This may be due to the fact that Microcystis spp. is one of the most common producers of microcystin worldwide (Harke et al., 2016), and blooms regularly in many high-profile, well-studied eutrophic lakes including Lake Taihu (China) (Paerl et al., 2011), Lake Okeechobee (Florida, USA) (Havens et al., 1998) and Lake Erie (Ohio, USA) (Michalak et al., 2013). Interestingly, in this study, Microcystis spp. was found at much lower biovolumes than either Dolichospermum spp. or Planktothrix spp., particularly in the Plains ecoregions, which saw highest mean microcystin concentrations. While this does not necessarily indicate lower potential for toxicity in Microcystis-dominated lakes, it does suggest that future studies should focus on determining environmental factors more likely to influence Dolichospermum spp. or Planktothrix spp. in order to better understand how lakes respond to eutrophication and the development of chronic toxicity.
It is widely agreed-upon that warming climate scenarios are likely to favor the frequency and duration of harmful cyanobacteria blooms (Paerl and Huisman, 2008, 2009; Wagner and Adrian, 2009; Carey et al., 2012; O’Neil et al., 2012; Paerl and Paul, 2012). Climatically induced short term warming of the water column has been shown to lead to toxic bloom events in lakes that are considered meso-oligotrophic (Beaver et al., 2018), while steady increases in ambient air and water temperatures over the course of decades may lead to the establishment of persistent and annually recurring blooms (Wagner and Adrian, 2009). Unfortunately, there is likely not a “one size fits all” solution to effectively control cyanobacterial growth or prevent toxic bloom events (Paerl, 2018). This is especially true given that the degree and extent of toxicity from harmful algal blooms has historically been difficult to predict, even when high biovolumes of potential toxin producers are present.
Knowledge of region-specific patterns may allow for wide-scale collaborations amongst environmental and water-quality management agencies in certain areas. Of particular interest is high frequency of toxicity, high average nutrient concentrations and low average light availability in lakes of the Plains ecoregions. Although certainly not all lakes in the Plains experience these conditions, it appears that lakes in these ecoregions are more susceptible to such characteristics than lakes in other ecoregions of the U.S. The analysis relating land-use practices to phytoplankton biovolume suggests that lack of forest cover is associated with increased risk of cyanobacterial blooms, especially involving Planktothrix spp. A similar spatial trend showing increased risk of cyanobacteria blooms in the Plains ecoregions was observed by Marion et al., (2017) using county-level land use data. That study found that low deciduous forest cover and higher cultivated crop cover were associated with increased cyanobacteria bloom area, and speculated that denitrification in forest ecosystems could play a role in slowing or preventing eutrophication. Over half of the samples with microcystin concentrations >1 ppb fell within the boundaries of the most recent North American glaciated extent (data not shown), which includes large portions of the Northern Plains, Temperate Plains and Upper Midwest ecoregions. Soils in those regions are mineraland nutrientrich, and are prone to high rates of erosion (Karlen et al., 2010). Those properties, along with high agricultural activity, likely make lakes in those ecoregions especially susceptible to nutrient runoff and eutrophication. Further research that aims at determining specific landscape and/or anthropogenic factors that influence the trend of poor water quality in those ecoregions is needed.
Analysis of the 2012 NLA data is limited by the fact that plankton samples were collected only once or twice over a relatively short time period, which does not capture the full range of environmental conditions that any particular lake may experience over the course of a year (Hayes and Vanni, 2018). It is likely that some lakes were sampled during peak productivity, while other systems were sampled during periods of relatively low productivity. Additionally, environmental factors that could impact cyanobacterial growth and toxicity such as fish abundance and presence of cyanophages were not collected. Despite these limitations, the NLA provides a comprehensive picture of regional trends in water quality in the U.S. due to the broad spatial extent of sampled lakes and the non-targeted nature of site selection. For researchers wishing to investigate drivers of toxic blooms on a site-specific or regional scale, the NLA has identified particular lakes in which blooms are known to occur, and this study outlines a framework of environmental factors likely affecting particular taxa. Future studies using comparable data from the 2017 NLA will help to put this study and others into the context of environmental change over time and may highlight the potential impacts of climate warming on lake water quality.
Acknowledgments
The authors acknowledge the hard work put in by all personnel involved in sample collection and analysis for the 2012 National Lakes Assessment, as well as Benjamin Vitanye for his assistance with land use data and map files. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. [CG]
References
- Anagnostidis K, Komárek J, 1988. Modern approach to the classification system of cyanophytes. 3 – Oscillatoriales. Arch. Hydrobiol. Suppl 80, 327–472. [Google Scholar]
- Antunes JT, Leão PN, Vasconcelos VM, 2015. Cylindrospermopsis raciborskii: review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front. Microbiol 6, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu M, Pick F, Gregory-Eaves I, 2013. Nutrients and water temperature are significant predictors of cyanobacterial biomass in a 1147 lakes data set. Limnol. Oceanogr 58 (5), 1736–1746. [Google Scholar]
- Beaver JR, Scotese KC, Minerovic AD, Buccier KM, Tausz CE, Clapham WB, 2012. Land use patterns, ecoregion and phytoplankton relationships in productive Ohio reservoirs. Inland Waters 2 (2), 101–108. [Google Scholar]
- Beaver JR, Casamatta DA, East TL, Havens KE, Rodusky AJ, James RT, Tausz CE, Buccier KM, 2013. Extreme weather events influence the phytoplankton community structure in a large lowland subtropical lake (Lake Okeechobee, Florida, USA). Hydrobiologia 709 (1), 213–226. [Google Scholar]
- Beaver JR, Manis EE, Loftin KA, Graham JL, Pollard AI, Mitchell RM, 2014. Land use patterns, ecoregion, and microcystin relationships in US lakes and reservoirs: a preliminary evaluation. Harmful Algae 36, 57–62. [Google Scholar]
- Beaver JR, Kirsch JE, Tausz CE, Samples EE, Renicker TR, Scotese KC, McMaster HA, Blasius-Wert BJ, Zimba PV, Casamatta DA, 2018. Long-term trends in seasonal plankton dynamics in Lake Mead (Nevada-Arizona, USA) and implications for climate change. Hydrobiologia 10.1007/s10750-018-3638-4. [DOI] [Google Scholar]
- Brookes JD, Carey CC, 2011. Resilience to blooms. Science 334 (6052), 46–47. [DOI] [PubMed] [Google Scholar]
- Carey CC, Ibelings BW, Hoffmann EP, Hamilton DP, Brookes JD, 2012. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res 46 (5), 1394–1407. [DOI] [PubMed] [Google Scholar]
- Carmichael WW, 2001. Health effects of toxin-producing cyanobacteria: the “cyanoHABs”. Hum. Ecol. Risk Assess 7 (5), 1393–1407. [Google Scholar]
- Chorus I, Bartram J, 1999. Toxic Cyanobacteria in Water: a Guide to their Public Health Consequences, Monitoring and Management St. Edmunsbury Press, Suffolk. [Google Scholar]
- Clarke KR, Gorley RN, 2006. User Manual/Tutorial PRIMER-E Ltd., Plymouth. [Google Scholar]
- Davis TW, Berry DL, Boyer GL, Gobler CJ, 2009. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8 (5), 715–725. [Google Scholar]
- de Souza Cardoso L, da Motta Marques D, 2009. Hydrodynamics-driven plankton community in a shallow lake. Aquat. Microb. Ecol 43 (1), 73–84. [Google Scholar]
- Doubek JP, Carey CC, Cardinale BJ, 2015. Anthropogenic land use is associated with N-fixing cyanobacterial dominance in lakes across the continental United States. Aquat. Sci 77 (4), 681–694. [Google Scholar]
- Downing JA, Watson SB, McCauley E, 2001. Predicting cyanobacteria dominance in lakes. Can. J. Fish. Aquat. Sci 58 (10), 1905–1908. [Google Scholar]
- Geada P, Pereira RN, Vasconcelos V, Vicente AA, Fernandes BD, 2017. Assessment of synergistic interactions between environmental factors on Microcystis aeruginosa growth and microcystin production. Algal Res 27, 235–243. [Google Scholar]
- Graham JL, Loftin KA, Meyer MT, Ziegler AC, 2010. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the Midwestern U.S. Environ. Sci. Technol 44 (19), 7361–7368. [DOI] [PubMed] [Google Scholar]
- Håkansan L 1982. Lake bottom dynamics and morphometry: the dynamic ratio. Water Resour. Res 18 (5), 1444–1450. [Google Scholar]
- Harke MJ, Steffen MM, Gobler CJ, Otten TG, Wilhelm SW, Wood SA, Paerl HW, 2016. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp.. Harmful Algae 54, 4–20. [DOI] [PubMed] [Google Scholar]
- Havens KE, Phlips EJ, Cichra MF, Li B-L, 1998. Light availability as a possible regulator of cyanobacteria species composition in a shallow subtropical lake. Freshw. Rev 39, 547–556. [Google Scholar]
- Heisler J, Glibert PM, Burkholder JM, Anderson DM, Cochlan W, Dennison WC, Dortch Q, Gobler CJ, Heil CA, Humphries E, Lewitus A, Magnien R, Marshall HG, Sellner K, Stockwell DA, Stoecker DK, Suddleson M, 2008. Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlihy AT, Paulsen SG, Sickle JV, Stoddard JL, Hawkins CP, Yuan LL, 2008. Striving for consistency in a national assessment: the challenges of applying a reference-condition approach at a continental scale. J. N. Am. Benthol. Soc 27 (4), 860–877. [Google Scholar]
- Hillebrand H, Dürselen CD, Kirschtel D, Pollingher U, Zohary T, 1999. Biovolume calculation for pelagic and benthic microalgae. J. Phycol 35 (2), 403–424. [Google Scholar]
- Hollister JW, Kreakie BJ, 2016. Associations between chlorophyll a and various microcystin health advisory concentrations. F1000Research 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isvánovics V, Shafik HM, Présing M, Juhos S, 2000. Growth and phosphate uptake kinetics of the cyanobacterium, Cylindrospermopsis raciborskii (Cyanophyceae) in throughflow cultures. Freshw. Rev 43 (2), 257–275. [Google Scholar]
- Jähnichen S, Long BM, Petzoldt T, 2011. Microcystin production by Microcystis aeruginosa: direct regulation by multiple environmental factors. Harmful Algae 12, 95–104. [Google Scholar]
- Jiang Y, Ji B, Wong RNS, Wong MH, 2008. Statistical study on the effects of environmental factors on the growth and microcystins production of bloom-forming cyanobacterium—microcystis aeruginosa. Harmful Algae 7 (2), 127–136. [Google Scholar]
- Karlen DL, Dinnes DL, Singer JW, 2010. Midwest Soil and Water Conservation: Past, Present, and Future, Soil and Water Conservation Advances in the US: Past Effects–Future Outlook Soil Science Society of America, Inc., Madison, pp. 131–162. [Google Scholar]
- Kokociński M, Stefaniak K, Mankiewicz-Boczek J, Izydorczyk K, Soininen J, 2010. The ecology of the invasive cyanobacerium Cylindrospermopsis raciborskii (Nostocales, Cyanophyta) in two hypereutrophic lakes dominated by Planktothrix aghardii (Oscillatoriales, Cyanophyta). Eur. J. Phycol 45 (4), 365–374. [Google Scholar]
- Komárek J, 2016. Review of the cyanobacterial genera implying planktic species after recent taxonomic revisions according to polyphasic methods: state as of 2014. Hydrobiologia 764 (1), 259–270. [Google Scholar]
- Kosten S, Huszar VL, Bécares E, Costa LS, Donk E, Hansson LA, Jeppesen E, Kruk C, Lacerot G, Mazzeo N, Meester L, 2012. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Change Biol. Bioenergy 18 (1), 118–126. [Google Scholar]
- Mantzouki E, Visser PM, Bormans M, Ibelings BW, 2016. Understanding the key ecological traits of cyanobacteria as a basis for their management and control in changing lakes. Aquat. Ecol 50 (3), 333–350. [Google Scholar]
- Marion JW, Zhang F, Cutting D, Lee J, 2017. Associations between county-level land cover classes and cyanobacteria blooms in the United States. Ecol. Eng 108B, 556–563. [Google Scholar]
- Marmen S, Aharonovich D, Grossowicz M, Blank L, Yacobi YZ, Sher DJ, 2016. Distribution and habitat specificity of potentially-toxic Microcystis across climate, land, and water use gradients. Front. Microbiol 7, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak AM, Anderson EJ, Beletsky D, Boland S, Bosch NS, Bridgeman TB, Chaffin JD, Cho K, Confesor R, Daloğlu I, DePinto JV, Evans MA, Fahnenstiel GL, He L, Ho JC, Jenkins L, Johengen TH, Kuo KC, LaPorte E, Liu X, McWilliams MR, Moore MR, Posselt DJ, Richards P, Scavia D, Steiner AL, Verhamme E, Wright DM, Zagorski MA, 2013. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. U. S. A 110 (16), 6448–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira C, Fathalli A, Vasconcelos V, Antunes A, 2011. Genetic diversity and structure of the invasive toxic cyanobacterium Cylindrospermopsis raciborskii. Curr. Microbiol 62 (5), 1590–1595. [DOI] [PubMed] [Google Scholar]
- Neilan BA, Jacobs D, Blackall LL, Hawkins PR, Cox PT, Goodman AE, 1997. rRNA sequences and evolutionary relationships among toxic and nontoxic cyanobac-teria of the genus Microcystis. Int. J. Syst. Evol. Microbiol 47 (3), 693–697. [DOI] [PubMed] [Google Scholar]
- Neil JM, Davis TW, Burford MA, Gobler CJ, 2012. The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae 14, 313–334. [Google Scholar]
- Omernik JM, 1987. Ecoregions of the conterminous United States. Ann. Assoc. Am. Geogr 77 (1), 118–125. [Google Scholar]
- Padisák J, 1997. Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju, an expanding, highly adaptive cyanobacterium: worldwide distribution and review of its ecology. Arch. Hydrobiol. Suppl. Algol. Stud 107 (4), 563–593. [Google Scholar]
- Paerl HW, 2017. Controlling harmful cyanobacterial blooms in a climatically more extreme world: management options and research needs. J. Plankton Res 39 (5), 763–771. [Google Scholar]
- Paerl HW, 2018. Mitigating toxic planktonic cyanobacterial blooms in aquatic ecosystems facing increasing anthropogenic and climatic pressures. Toxins 10 (2), 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl HW, Huisman J, 2008. Blooms like it hot. Science 320 (5872), 57–58. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Huisman J, 2009. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep 1 (1), 27–37. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Paul VJ, 2012. Climate change: links to global expansion of harmful cyanobacteria. Water Res 46 (5), 1349–1363. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Xu H, McCarthy MJ, Zhu G, Qin B, Li Y, Gardner WS, 2011Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N & P) management strategy. Water Res 45 (5), 1973–1983. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Gardner WS, Havens KE,Joyner AR, McCarthy MJ, Newell SE, Qin B, Scott JT, 2016. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 54, 213–222. [DOI] [PubMed] [Google Scholar]
- Pollard AI, Hampton SE, Leech DM, 2018. The promise and potential of continental-scale limnology using the U.S. Environmental Protection Agency’s National Lakes Assessment. Limnol. Oceanogr. Bull 27 (2), 36–41. [Google Scholar]
- Post AF, de Wit R, Mur LR, 1985. Interactions between temperature and light intensity on growth and photosynthesis of the cyanobacterium Oscillatoria agardhii. J. Plankton Res 7 (4), 487–495. [Google Scholar]
- Rigosi A, Carey CC, Ibelings BW, Brookes JD, 2014. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr 59 (1), 99–114. [Google Scholar]
- Robarts RD, Zohary T, 1987. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom‐forming cyanobacteria. N. Z. J. Mar. Freshw. Res 21 (3), 391–399. [Google Scholar]
- Scheffer M, Rinaldi S, Gragnani A, Mur LR, van Nes EH, 1997. On the dominance of filamentous cyanobacteria in shallow, Turbid lakes. Ecology 78 (1), 272–282. [Google Scholar]
- Simley JD, Carswell WJ Jr., 2009. The National Map – Hydrography: U.S. Geological Survey Fact Sheet 2009–3054; pp. 4. [Google Scholar]
- Sinha R, Pearson LA, Davis TW, Burford MA, Orr PT, Neilan BA, 2012. Increased incidence of Cylindrospermopsis raciborskii in temperate zones–is climate change responsible?. Water Res 46 (5), 1408–1419. [DOI] [PubMed] [Google Scholar]
- Smith VH, 1986. Light and nutrient effects on the relative biomass of blue-green algae in lake phytoplankton. Can. J. Fish. Aquat. Sci 43 (1), 148–153. [Google Scholar]
- Taranu ZE, Gregory‐Eaves I, Steele RJ, Beaulieu M, Legendre P, 2017. Predicting microcystin concentrations in lakes and reservoirs at a continental scale: a new framework for modelling an important health risk factor. Glob. Ecol. Biogeogr 26 (6), 625–637. [Google Scholar]
- ter Braak CJ, Verdonschot PF, 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci 57 (3), 255–289. [Google Scholar]
- Tillman D, Kilham SS, Kilham P, 1982. Phytoplankton community ecology: the role of limiting nutrients. Ann. Rev. Ecol. Syst 13 (1), 49–72. [Google Scholar]
- U.S. Environmental Protection Agency, 2012. 2012 National Lakes Assessment Field Operations Manual. EPA 841-B-11-003, U.S. Environmental Protection Agency, Office of Water, Washington, DC, pp. 234. [Google Scholar]
- U.S. Environmental Protection Agency, 2012. 2012 National Lakes Assessment Laboratory Operations Manual. EPA 841-B-11-004, U.S. Environmental Protection Agency, Office of Water, Washington, DC, pp. 113. [Google Scholar]
- U.S. Environmental Protection Agency, 2012. 2012 National Lakes Assessment Quality Assurance Project Plan. EPA 841-B-11-006, U.S. Environmental Protection Agency, Office of Water, Washington, DC, pp. 77. [Google Scholar]
- U.S. Environmental Protection Agency, 2016. National Lakes Assessment 2012: A Collaborative Survey of Lakes in the United States. EPA 841-R-16–113 U.S. Environmental Protection Agency, Washington, DC, pp. 35. [Google Scholar]
- van Gremberghe I, Leliaert F, Mergeay J, Vanormelingen P, Van der Gucht K, Debeer A-E, Lacerot G, De Meester L, Vyverman W, 2011. Lack of phylogenetic structure in the freshwater Cyanobacterium Microcystis aeruginosa suggests global dispersal. PLoS One 6 (5), ee19561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C, Adrian R, 2009. Cyanobacteria dominance: quantifying the effects of climate change. Limnol. Oceanogr 54 (6), 2460–2468. [Google Scholar]
- Watanabe MF, Oishi S, 1985. Effects of environmental factors on toxicity of a cyanobacterium (Microcystis aeruginosa) under culture conditions. Appl. Environ. Microbiol 49 (5), 1342–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SB, McCauley E, Downing JA, 1997. Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnol. Oceanogr 42 (3), 487–495. [Google Scholar]
- Wood SA, Prentice MJ, Smith K, Hamilton DP, 2010. Low dissolved inorganic nitrogen and increased heterocyte frequency: precursors to Anabaena planktonica blooms in a temperate, eutrophic reservoir. J. Plankton Res 32 (9), 1315–1325. [Google Scholar]
- Wood SA, Borges H, Puddick J, Biessy L, Atalah J, Hawes I, Dietrich DR, Hamilton DP, 2017. Contrasting cyanobacterial communities and microcystin concentrations in summers with extreme weather events: insights into potential effects of climate change. Hydrobiologia 785 (1), 71–89. [Google Scholar]