Abstract
The selective functionalization of C–H bonds at the arene para position is highly challenging using transition metal catalysis. Iridium-catalyzed borylation has emerged as a leading technique for arene functionalization, but there are only a handful of strategies for para-selective borylation, which operate on specific substrate classes and use bespoke ligands or catalysts. We describe a remarkably general protocol which results in para-selectivity on some of the most common arene building blocks (anilines, benzylamines, phenols, benzyl alcohols) and uses standard borylation ligands. Our strategy hinges upon the facile conversion of the substrates into sulfate or sulfamate salts, wherein the anionic arene component is paired with a tetrabutylammonium cation. We hypothesize that the bulk of this cation disfavors meta-C–H borylation, thereby promoting the challenging para-selective reaction.
Use of transition metals to catalytically functionalize arene C–H bonds has undergone tremendous development. While most advances have dealt with ortho-selective functionalization, the past decade has seen many advances in meta-selective reactions.1 Many of these approaches have been based on templates or ligands with extended architectures, or on strategies which proceed via metalation at the ortho position. Considering this, it is not surprising that methods to direct transition metals to the para position remain rather fewer, given the greater distance from existing functionality.2 Although SEAr mechanisms give the para isomer when electrophiles react with electron rich arenes, it is uncommon for transition metals to perform C–H bond functionalization via SEAr mechanisms.3 Exceptions are several examples using gold catalysis4 and a smaller number of examples using high-valent copper5 or palladium.6 Yet such selectively metalated intermediates are extremely versatile and new methods to access them are urgently required.7,8
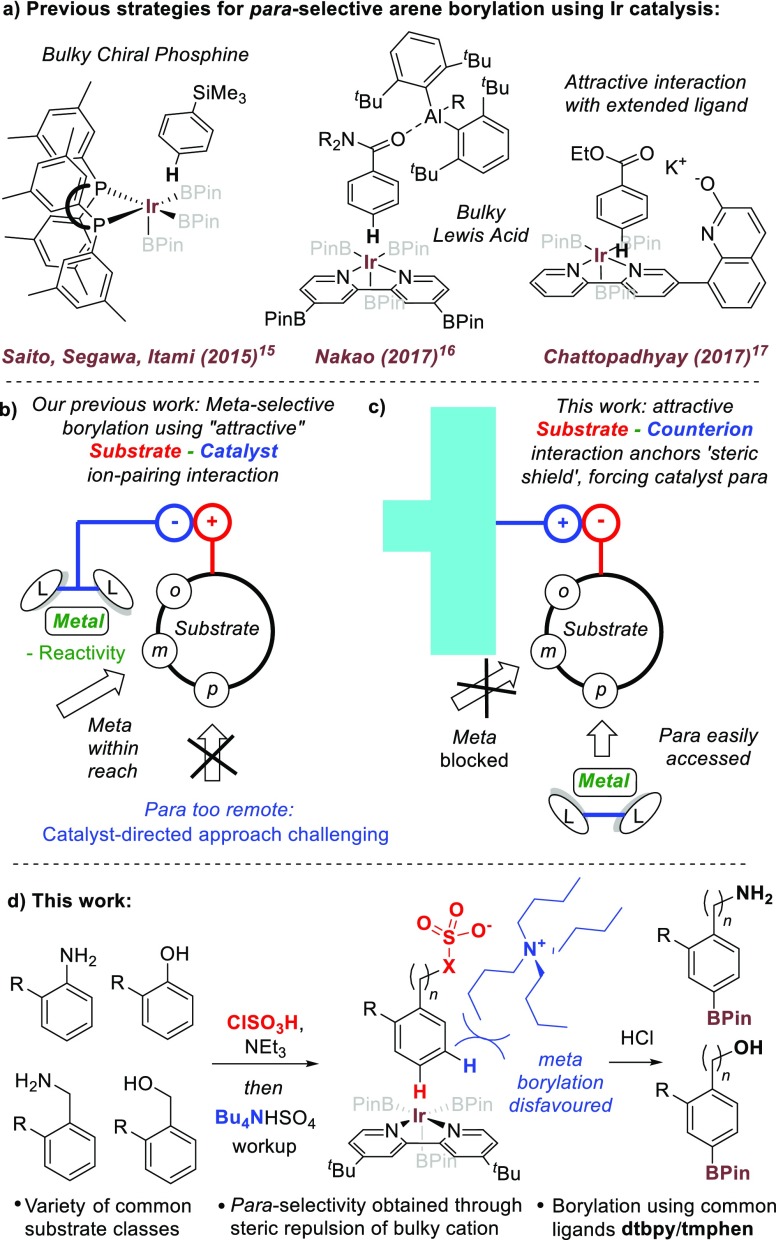
Iridium-catalyzed borylation has emerged to become one of the most versatile and intensively used of modern arene functionalization methods.9,10 While regioselectivity is typically determined by steric considerations, numerous modifications can direct borylation to the ortho position11 and, to a lesser extent, the meta position.12 In contrast, there are only three instances where para-selective iridium catalyzed C–H borylation has been achieved (Figure 1a).13,14 Saito, Segawa and Itami used a bulky, chiral phosphine ligand to create a highly sterically congested environment at iridium.15 However, high para-selectivity was limited to substrates possessing a very bulky substituent, and 1,2-disubstituted arenes were unselective. Nakao and co-workers used a bulky Lewis acid catalyst to complex aromatic amides, both activating the substrate and forcing borylation to occur at the most remote position due to steric interactions.16 Most recently, Chattopadhyay and co-workers disclosed an “L-shaped” bipyridine ligand proposed to direct borylation to the para position by virtue of interaction between the potassium salt of the ligand and an ester in the substrate.17
Figure 1.
Previous para-selective Ir-catalyzed borylation approaches and that taken in this work.
Despite the inventiveness of these approaches, two aspects limit wider application. First, they are restricted to relatively narrow substrate classes. Second, they require bespoke or noncommercially available ligands or catalysts, presenting a barrier to those looking to use “off-the-shelf” reagents, such as end-users in the pharmaceutical industry. At the outset of this work, we sought to develop a general and practical strategy that would enable para-selective borylation of a range of common substrate classes using readily available catalysts and ligands. We have previously utilized attractive ion-pairing interactions between catalyst and substrate to direct the iridium metal to the arene meta position (Figure 1b).12c,12f,12h We were concerned that adopting a similar strategy to target the para isomer would be very challenging due to the distance that the ligand would be required to reach. A design able to achieve this would likely have a complex, extended structure necessitating lengthy synthesis and reduced practical utility. We envisaged an alternative ion-pairing strategy in which the counterion of the substrate does not deliver the reactive catalyst, but is unfunctionalized and bulky, acting as a “steric shield” to obstruct borylation at the meta position, thereby resulting in para-selectivity with standard “off-the-shelf” borylation catalysts (Figure 1c).
We envisaged that the ubiquitous tetrabutylammonium cation may consitute an ideal “steric shield”; the alkyl chains project outward at all angles from the tetrahedral nitrogen, occupying a large area. This bulky cation could be paired with a variety of common arene building blocks (anilines, benzylamines, phenols, benzyl alcohols) which can all be rendered temporarily anionic in a single simple step through conversion to the corresponding sulfate (X = O) or sulfamate (X = N) salts (Figure 1d). Herein, we demonstrate the realization of this approach, which we believe represents the most general strategy for para-selective borylation developed to date and indeed provides complementarity to existing strategies. In contrast to our earlier work, the key ion-pairing interaction in this system is not being exploited in an attractive sense between substrate and catalyst, but rather to temporarily anchor the “steric shield” to the substrate. The resulting repulsive steric interactions with the incoming catalyst thereby guide the latter to the remote position that would be the most challenging to reach using a strategy invoking attractive catalyst-substrate interactions.
We began by converting 2-chloroaniline (1a) to the corresponding tetrabutylammonium sulfamate salt 2a by treatment with chlorosulfonic acid followed by cation exchange using Bu4NHSO4 (Scheme 1a). To our delight, iridium-catalyzed borylation of 2a under standard conditions with 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbpy) as a ligand resulted in >20:1 selectivity for the para isomer. Simply treating the crude reaction mixture with HCl in methanol revealed the parent aniline, which give 85% yield of para-borylated aniline 3a over two steps and only a single purification procedure. A similar outcome was achieved for the electronically different 2-trifluoromethylaniline (Scheme 1a, 1b to 3b). In contrast, direct borylation of parent anilines 1a and 1b results in m:p ratios of 1:3.9 and 1:1, respectively (Scheme 1b). The moderate para-selectivity in direct borylation of 1a echoes a similar observation made by Krska, Maleczka, Smith and co-workers, the origins of which are not obvious.18 Accordingly, we examined 4a and 4b, control substrates much closer in electronic nature to 2a and 2b, and found both of these give poor selectivity (Scheme 1c). This outcome suggests that the high para-selectivity obtained with sulfamate salts 2a and 2b does not have electronic origins and that the salt formation and association with the tetrabutylammonium cation are crucial factors.
Scheme 1. (a) Initial Results; (b, c) Control Reactions.
With these initial results in hand, we next examined a selection of 2-substituted anilines and were pleased to find that the high levels of para-selectivity were observed for a range of useful building blocks (Scheme 2A). Bromide and iodide substituents were well tolerated (3c, 3d), both giving >20:1 para-selectivity. The latter is notable given the incompatibility of many other C–H activation methods with iodide substituents. Arenes bearing trifluoromethoxy (3e) and difluoromethoxy (3g) substituents are also compatible, as is the difluorinated 3h. Interestingly, the para-selectivity was slightly reduced with a resonance withdrawing substituent (2-CN, 3f), in-line with the precedent that this substituent has a small para-directing (relative to itself) electronic effect in borylation.19 Substrates bearing electron-donating substituents (isopropyl, methoxy) gave low conversion, albeit with excellent para-selectivity (see SI).
Scheme 2. Scope of para C–H Borylation of Aniline (A), Benzylamine (B), Phenol (C) and Benzyl Alcohol (D) Derivatives, and Aryl and Benzylsulfonates (E),
Typically: substrate (0.25 mmol), B2Pin2 (0.25–0.5 mmol), [Ir(COD)OMe]2 (1.5–2.5 mol %), dtbpy (3–5 mol %), 1,4-dioxane (0.33–0.5 M), 70 °C (see SI for full details).
Isomeric ratios are para:meta taken from analysis of crude 1H NMR spectra after borylation, unless otherwise stated. Yields shown are isolated and typically include meta and para isomers which are generally inseparable.
Selectivity assessed after HCl step.
Selectivity assessed after trifluoroacetate protection step.
3,4,7,8-Tetramethyl-1,10-phenanthroline used instead of dtbpy.
Oxidized to corresponding phenol for isolation.
Given the ubiquity of benzylamines as aromatic building blocks, we next sought to test our strategy on benzylamine-derived sulfamates. As with the aniline variant, these tetrabutylammonium salts are easily synthesized from cheap materials. This class proved highly amenable, in most cases giving >20:1 regioselectivity (Scheme 2B). A range of useful halide substituents was tolerated, including Cl (7a), Br (7b), I (7c) and F (7g). Trifluoromethyl (7d), trifluoromethoxy (7e) and difluoromethoxy (7f) substituted variants also performed well. A methoxy-substituted variant gave excellent selectivity but with poor conversion (7h). It was necessary to derivatize the free amine products to enable purification on silica; isolated yields are following borylation/hydrolysis/trifluoroacetylation.
Encouraged by the effectiveness of the strategy thus far, we next extrapolated this to the ubiquitous oxygen-containing building blocks, phenols and benzyl alcohols.20 The tetrabutylammonium sulfate salts of these compounds are also easily obtained and cleaved. Gratifyingly, high para-selectivity was observed for a number of ortho-substituted phenol-derived sulfate salts, encompassing Cl (9a), Br (9b), I (9c), CF3 (9d), OCF3 (9e), iPr (9f) and a difluorinated analogue (9h) (Scheme 2C). Control borylations on 2-chlorophenol and on a 2-chlorophenol sulfate ester gave poor selectivity.21 Interestingly, selectivity (by crude 1H nuclear magnetic resonance (NMR) analysis following borylation) was somewhat reduced compared with the aniline-derived sulfamates. Particularly, substrates possessing conjugatively withdrawing groups such as nitro (9g) exhibited only moderate para-selectivity. This is likely due to the substituent electronic influence on the desired para-selectivity (vide supra).
Remarkably, the extension to a fourth common substrate class, benzyl alcohols, was well tolerated (Scheme 2D, 11a–11h). As in the phenol-derived sulfates, regioselectivity was adversely affected by resonance withdrawing groups (11h), but positively reinforced by resonance donors (11f). These results suggest that in substrate classes when the para-directing influence from the associated cation is weaker, substituents electronic effects can have greater impact on selectivity.
Finally, to further reinforce the generality and practicality of our strategy for the selective synthesis of complex, multifunctional building blocks, we targeted aryl sulfonates and benzyl sulfonates, substrates which inherently bear a negative charge (Scheme 2E). The former are readily accessed from commercially available aryl sulfonyl chlorides by treatment with tetrabutylammonium hydroxide, and the latter by the action of sodium sulfite on benzyl halides, followed by cation exchange. Furthermore, through treatment with POCl3, both sulfonates can be easily converted to sulfonyl chlorides, valuable precursors to sulfonamides. Both of these substrate classes result in para-selective borylation, in line with our hypothesis. Aryl sulfonates bearing ortho Cl (13a), Br (13b), CF3 (13c) and OCF3 (13d) gave excellent para-selectivity, as did the analogously substituted benzyl sulfonates (13e–13h). Following borylation, the crude borylated sulfonates were either converted through to the corresponding piperidine sulfonamides (13a–13d) or were isolated as sulfonyl chlorides (13e–13h).
At the outset, we advanced the tentative hypothesis that the bulk of the associated tetrabutylammonium cation may occupy sufficient space around the arene such that borylation at the normally accessible meta positions becomes disfavored (Figure 1C). To probe this hypothesis, we synthesized the 2-Cl member of the first four substrate classes with the smaller tetraethylammonium cation (synthesis of the tetramethyl variant for all members proved problematic) to gauge the impact on selectivity (Scheme 3a). These experiments showed that for three of the four classes, para-selectivity was significantly reduced, in line with our hypothesis (in the fourth, selectivity remained excellent at >20:1). We next selected the benzyl sulfonate substrate class to carry out a complete evaluation of sequential modulation of cation size on a single substrate. These experiments showed a clear and consistent trend to higher para-selectivity as the cation grows larger (Scheme 3b).
Scheme 3. Experiments To Probe Origin of Selectivity.
Finally, in order to visualize the steric effect of the associated tetrabutylammonium cation, we were able to obtain X-ray structures of the starting materials for three substrate classes (Figure 2). These give a visual impression of the bulk provided by the cation and support our central tenet that it could feasibly act as a noncovalent “shield” to block the arene meta-position. Accordingly, one might expect that removal of the ortho substituent incorporated into all substrates thus far may reduce para-selectivity as both meta positions would be open to borylation and the cation would not be able to block both at any one time. Indeed, this is the case; the para-selectivity was drastically reduced for all unsubstituted variants providing further support for our hypothesis.21 We also extended the chain length of all three classes of substrate as the 2-Cl variant but found that with two methylenes between the arene and the anionic group, selectivity was reduced to around 3:1 p:m, presumably due to the much greater flexibility.21
Figure 2.
X-ray crystal structures of starting materials allowing visualization of the bulk of the tetrabutylammonium cation.
In conclusion, we have developed a general method for the para-selective C–H borylation of a broad range of the most useful arene building blocks encompassing anilines, benzylamines, phenols and benzyl alcohols as well as several classes of arenesulfonate, valuable precursors to sulfonamides.22 Our method produces a variety of complex trisubstituted arene cores often with three functional handles for further elaboration that in many cases would require numerous steps to obtain by other methods. We provide evidence to suggest that this is due to ion-pairing of a bulky tetrabutylammonium cation with the substrate, which is rendered temporarily anionic. The bulky cation blocks the meta position, promoting borylation at the most remote and typically most challenging para position. Our approach provides a practical and general solution to the challenge of para-selective C–H borylation and demonstrates a new strategy for the utilization of ion-pairing interactions in catalysis.
Acknowledgments
We are grateful to AstraZeneca for a Ph.D. studentship through the AZ-Cambridge Ph.D. program (M.T.M.), the Cambridge Trust and Sidney Sussex College, Cambridge for a Ph.D. studentship (B.D.W.), the Royal Society for a University Research Fellowship (R.J.P.), the EPSRC (EP/N005422/1) and the European Research Council (EU Horizon 2020, Starting Grant 757381). We thank the EPSRC UK National Mass Spectrometry Facility at Swansea University. We thank Dr. Andrew Bond acquiring and solving the X-ray data. Thanks to Iain Cumming (AstraZeneca) for useful discussion.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b07267.
The authors declare no competing financial interest.
Supplementary Material
References
- a Li J.; De Sarkar S.; Ackermann L.. meta- and para-Selective C–H Functionalization by C–H Activation. In C-H Bond Activation and Catalytic Functionalization I; Dixneuf P. H., Doucet H., Eds.; Springer International Publishing: Cham, 2016; pp 217–257. [Google Scholar]; b Dey A.; Agasti S.; Maiti D. Palladium catalysed meta-C–H functionalization reactions. Org. Biomol. Chem. 2016, 14, 5440–5453. 10.1039/C6OB00395H. [DOI] [PubMed] [Google Scholar]; c Mihai M. T.; Genov G. R.; Phipps R. J. Access to the meta position of arenes through transition metal catalysed C–H bond functionalisation: a focus on metals other than palladium. Chem. Soc. Rev. 2018, 47, 149–171. 10.1039/C7CS00637C. [DOI] [PubMed] [Google Scholar]
- Dey A.; Maity S.; Maiti D. Reaching the south: metal-catalyzed transformation of the aromatic para-position. Chem. Commun. 2016, 52, 12398–12414. 10.1039/C6CC05235E. [DOI] [PubMed] [Google Scholar]
- For selected recent examples of para-selective functionalization via radical mechanisms, see:; a Romero N. A.; Margrey K. A.; Tay N. E.; Nicewicz D. A. Site-selective arene C-H amination via photoredox catalysis. Science 2015, 349, 1326–1330. 10.1126/science.aac9895. [DOI] [PubMed] [Google Scholar]; b Boursalian G. B.; Ham W. S.; Mazzotti A. R.; Ritter T. Charge-transfer-directed radical substitution enables para-selective C–H functionalization. Nat. Chem. 2016, 8, 810. 10.1038/nchem.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Berger F.; Plutschack M. B.; Riegger J.; Yu W.; Speicher S.; Ho M.; Frank N.; Ritter T. Site-selective and versatile aromatic C–H functionalization by thianthrenation. Nature 2019, 567, 223–228. 10.1038/s41586-019-0982-0. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Ball L. T.; Lloyd-Jones G. C.; Russell C. A. Gold-Catalyzed Direct Arylation. Science 2012, 337, 1644–1648. 10.1126/science.1225709. [DOI] [PubMed] [Google Scholar]; b Brand J. P.; Waser J. Para-Selective Gold-Catalyzed Direct Alkynylation of Anilines. Org. Lett. 2012, 14, 744–747. 10.1021/ol203289v. [DOI] [PubMed] [Google Scholar]; c Cambeiro X. C.; Boorman T. C.; Lu P.; Larrosa I. Redox-Controlled Selectivity of C-H Activation in the Oxidative Cross-Coupling of Arenes. Angew. Chem., Int. Ed. 2013, 52, 1781–1784. 10.1002/anie.201209007. [DOI] [PubMed] [Google Scholar]; d Yu Z.; Ma B.; Chen M.; Wu H.-H.; Liu L.; Zhang J. Highly Site-Selective Direct C–H Bond Functionalization of Phenols with α-Aryl-α-diazoacetates and Diazooxindoles via Gold Catalysis. J. Am. Chem. Soc. 2014, 136, 6904–6907. 10.1021/ja503163k. [DOI] [PubMed] [Google Scholar]; e Cambeiro X. C.; Ahlsten N.; Larrosa I. Au-Catalyzed Cross-Coupling of Arenes via Double C-H Activation. J. Am. Chem. Soc. 2015, 137, 15636–15639. 10.1021/jacs.5b10593. [DOI] [PubMed] [Google Scholar]
- Ciana C.-L.; Phipps R. J.; Brandt J. R.; Meyer F.-M.; Gaunt M. J. A Highly Para-Selective Copper(II)-Catalyzed Direct Arylation of Aniline and Phenol Derivatives. Angew. Chem., Int. Ed. 2011, 50, 458–462. 10.1002/anie.201004703. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Wang X.; Leow D.; Yu J.-Q. Pd(II)-Catalyzed para-Selective C–H Arylation of Monosubstituted Arenes. J. Am. Chem. Soc. 2011, 133, 13864–13867. 10.1021/ja206572w. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Luan Y.-X.; Zhang T.; Yao W.-W.; Lu K.; Kong L.-Y.; Lin Y.-T.; Ye M. Amide-Ligand-Controlled Highly para-Selective Arylation of Monosubstituted Simple Arenes with Arylboronic Acids. J. Am. Chem. Soc. 2017, 139, 1786–1789. 10.1021/jacs.6b12907. [DOI] [PubMed] [Google Scholar]; c Naksomboon K.; Poater J.; Bickelhaupt F. M.; Fernandez-Ibanez M. A. para-Selective C-H Olefination of Aniline Derivatives via Pd/S,O-Ligand Catalysis. J. Am. Chem. Soc. 2019, 141, 6719–6725. 10.1021/jacs.9b01908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For examples of Pd-catalyzed para-functionalization, see:; a Bag S.; Patra T.; Modak A.; Deb A.; Maity S.; Dutta U.; Dey A.; Kancherla R.; Maji A.; Hazra A.; Bera M.; Maiti D. Remote para-C–H Functionalization of Arenes by a D-Shaped Biphenyl Template-Based Assembly. J. Am. Chem. Soc. 2015, 137, 11888–11891. 10.1021/jacs.5b06793. [DOI] [PubMed] [Google Scholar]; b Patra T.; Bag S.; Kancherla R.; Mondal A.; Dey A.; Pimparkar S.; Agasti S.; Modak A.; Maiti D. Palladium-Catalyzed Directed para C–H Functionalization of Phenols. Angew. Chem., Int. Ed. 2016, 55, 7751–7755. 10.1002/anie.201601999. [DOI] [PubMed] [Google Scholar]; c Maji A.; Guin S.; Feng S.; Dahiya A.; Singh V. K.; Liu P.; Maiti D. Experimental and Computational Exploration of para-Selective Silylation with a Hydrogen-Bonded Template. Angew. Chem., Int. Ed. 2017, 56, 14903–14907. 10.1002/anie.201708449. [DOI] [PubMed] [Google Scholar]; d Maji A.; Dahiya A.; Lu G.; Bhattacharya T.; Brochetta M.; Zanoni G.; Liu P.; Maiti D. H-bonded reusable template assisted para-selective ketonisation using soft electrophilic vinyl ethers. Nat. Commun. 2018, 9, 3582. 10.1038/s41467-018-06018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For examples of Ru-catalyzed para-functionalization, see:; a Leitch J. A.; McMullin C. L.; Paterson A. J.; Mahon M. F.; Bhonoah Y.; Frost C. G. Ruthenium-Catalyzed para-Selective C–H Alkylation of Aniline Derivatives. Angew. Chem., Int. Ed. 2017, 56, 15131–15135. 10.1002/anie.201708961. [DOI] [PubMed] [Google Scholar]; b Yuan C.; Zhu L.; Chen C.; Chen X.; Yang Y.; Lan Y.; Zhao Y. Ruthenium(II)-enabled para-selective C–H difluoromethylation of anilides and their derivatives. Nat. Commun. 2018, 9, 1189. 10.1038/s41467-018-03341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yuan C.; Zhu L.; Zeng R.; Lan Y.; Zhao Y. Ruthenium(II)-Catalyzed C–H Difluoromethylation of Ketoximes: Tuning the Regioselectivity from the meta to the para Position. Angew. Chem., Int. Ed. 2018, 57, 1277–1281. 10.1002/anie.201711221. [DOI] [PubMed] [Google Scholar]
- a Cho J.-Y.; Tse M. K.; Holmes D.; Maleczka R. E.; Smith M. R. Remarkably Selective Iridium Catalysts for the Elaboration of Aromatic C-H Bonds. Science 2002, 295, 305–308. 10.1126/science.1067074. [DOI] [PubMed] [Google Scholar]; b Ishiyama T.; Takagi J.; Ishida K.; Miyaura N.; Anastasi N. R.; Hartwig J. F. Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate. J. Am. Chem. Soc. 2002, 124, 390–391. 10.1021/ja0173019. [DOI] [PubMed] [Google Scholar]; c Mkhalid I. A. I.; Barnard J. H.; Marder T. B.; Murphy J. M.; Hartwig J. F. C–H Activation for the Construction of C–B Bonds. Chem. Rev. 2010, 110, 890–931. 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]
- a Hartwig J. F. Regioselectivity of the borylation of alkanes and arenes. Chem. Soc. Rev. 2011, 40, 1992–2002. 10.1039/c0cs00156b. [DOI] [PubMed] [Google Scholar]; b Preshlock S. M.; Ghaffari B.; Maligres P. E.; Krska S. W.; Maleczka R. E.; Smith M. R. High-Throughput Optimization of Ir-Catalyzed C–H Borylation: A Tutorial for Practical Applications. J. Am. Chem. Soc. 2013, 135, 7572–7582. 10.1021/ja400295v. [DOI] [PubMed] [Google Scholar]
- Ros A.; Fernandez R.; Lassaletta J. M. Functional group directed C-H borylation. Chem. Soc. Rev. 2014, 43, 3229–3243. 10.1039/C3CS60418G. [DOI] [PubMed] [Google Scholar]
- a Kuninobu Y.; Ida H.; Nishi M.; Kanai M. A meta-selective C–H borylation directed by a secondary interaction between ligand and substrate. Nat. Chem. 2015, 7, 712–717. 10.1038/nchem.2322. [DOI] [PubMed] [Google Scholar]; b Bisht R.; Chattopadhyay B. Formal Ir-Catalyzed Ligand-Enabled Ortho and Meta Borylation of Aromatic Aldehydes via in Situ-Generated Imines. J. Am. Chem. Soc. 2016, 138, 84–87. 10.1021/jacs.5b11683. [DOI] [PubMed] [Google Scholar]; c Davis H. J.; Mihai M. T.; Phipps R. J. Ion Pair-Directed Regiocontrol in Transition-Metal Catalysis: A Meta-Selective C–H Borylation of Aromatic Quaternary Ammonium Salts. J. Am. Chem. Soc. 2016, 138, 12759–12762. 10.1021/jacs.6b08164. [DOI] [PubMed] [Google Scholar]; d Davis H. J.; Genov G. R.; Phipps R. J. meta-Selective C–H Borylation of Benzylamine-, Phenethylamine-, and Phenylpropylamine-Derived Amides Enabled by a Single Anionic Ligand. Angew. Chem., Int. Ed. 2017, 56, 13351–13355. 10.1002/anie.201708967. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Bisht R.; Hoque M. E.; Chattopadhyay B. Amide Effects in C–H Activation: Noncovalent Interactions with L-Shaped Ligand for meta Borylation of Aromatic Amides. Angew. Chem., Int. Ed. 2018, 57, 15762–15766. 10.1002/anie.201809929. [DOI] [PubMed] [Google Scholar]; f Mihai M. T.; Davis H. J.; Genov G. R.; Phipps R. J. Ion Pair-Directed C–H Activation on Flexible Ammonium Salts: meta-Selective Borylation of Quaternized Phenethylamines and Phenylpropylamines. ACS Catal. 2018, 8, 3764–3769. 10.1021/acscatal.8b00423. [DOI] [Google Scholar]; g Yang L.; Uemura N.; Nakao Y. meta-Selective C-H Borylation of Benzamides and Pyridines by an Iridium-Lewis Acid Bifunctional Catalyst. J. Am. Chem. Soc. 2019, 141, 7972–7979. 10.1021/jacs.9b03138. [DOI] [PubMed] [Google Scholar]; h Lee B.; Mihai M. T.; Stojalnikova V.; Phipps R. J. Ion-Pair-Directed Borylation of Aromatic Phosphonium Salts. J. Org. Chem. 2019, 10.1021/acs.joc.9b00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For leading examples of noncatalyzed electrophilic borylation, see:; a Bagutski V.; Del Grosso A.; Carrillo J. A.; Cade I. A.; Helm M. D.; Lawson J. R.; Singleton P. J.; Solomon S. A.; Marcelli T.; Ingleson M. J. Mechanistic Studies into Amine-Mediated Electrophilic Arene Borylation and Its Application in MIDA Boronate Synthesis. J. Am. Chem. Soc. 2013, 135, 474–487. 10.1021/ja3100963. [DOI] [PubMed] [Google Scholar]; b Del Grosso A.; Ayuso Carrillo J.; Ingleson M. J. Regioselective electrophilic borylation of haloarenes. Chem. Commun. 2015, 51, 2878–2881. 10.1039/C4CC10153G. [DOI] [PubMed] [Google Scholar]; c Yin Q.; Klare H. F. T.; Oestreich M. Catalytic Friedel–Crafts C–H Borylation of Electron-Rich Arenes: Dramatic Rate Acceleration by Added Alkenes. Angew. Chem., Int. Ed. 2017, 56, 3712–3717. 10.1002/anie.201611536. [DOI] [PubMed] [Google Scholar]
- For rhodium-catalyzed silylation which has enhanced sensitivity to existing sterics, see:Cheng C.; Hartwig J. F. Rhodium-Catalyzed Intermolecular C–H Silylation of Arenes with High Steric Regiocontrol. Science 2014, 343, 853–857. 10.1126/science.1248042. [DOI] [PubMed] [Google Scholar]
- a Saito Y.; Segawa Y.; Itami K. para-C–H Borylation of Benzene Derivatives by a Bulky Iridium Catalyst. J. Am. Chem. Soc. 2015, 137, 5193–5198. 10.1021/jacs.5b02052. [DOI] [PubMed] [Google Scholar]; b Haines B. E.; Saito Y.; Segawa Y.; Itami K.; Musaev D. G. Flexible Reaction Pocket on Bulky Diphosphine–Ir Complex Controls Regioselectivity in para-Selective C–H Borylation of Arenes. ACS Catal. 2016, 6, 7536–7546. 10.1021/acscatal.6b02317. [DOI] [Google Scholar]
- Yang L.; Semba K.; Nakao Y. para-Selective C–H Borylation of (Hetero)Arenes by Cooperative Iridium/Aluminum Catalysis. Angew. Chem., Int. Ed. 2017, 56, 4853–4857. 10.1002/anie.201701238. [DOI] [PubMed] [Google Scholar]
- Hoque M. E.; Bisht R.; Haldar C.; Chattopadhyay B. Noncovalent Interactions in Ir-Catalyzed C–H Activation: L-Shaped Ligand for Para-Selective Borylation of Aromatic Esters. J. Am. Chem. Soc. 2017, 139, 7745–7748. 10.1021/jacs.7b04490. [DOI] [PubMed] [Google Scholar]
- a Preshlock S. M.; Plattner D. L.; Maligres P. E.; Krska S. W.; Maleczka R. E.; Smith M. R. A Traceless Directing Group for C-H Borylation. Angew. Chem., Int. Ed. 2013, 52, 12915–12919. 10.1002/anie.201306511. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Smith M. R.; Bisht R.; Haldar C.; Pandey G.; Dannatt J. E.; Ghaffari B.; Maleczka R. E.; Chattopadhyay B. Achieving High Ortho Selectivity in Aniline C–H Borylations by Modifying Boron Substituents. ACS Catal. 2018, 8, 6216–6223. 10.1021/acscatal.8b00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajuddin H.; Harrisson P.; Bitterlich B.; Collings J. C.; Sim N.; Batsanov A. S.; Cheung M. S.; Kawamorita S.; Maxwell A. C.; Shukla L.; Morris J.; Lin Z.; Marder T. B.; Steel P. G. Iridium-catalyzed C-H borylation of quinolines and unsymmetrical 1,2-disubstituted benzenes: insights into steric and electronic effects on selectivity. Chem. Sci. 2012, 3, 3505–3515. 10.1039/c2sc20776a. [DOI] [Google Scholar]
- a Boebel T. A.; Hartwig J. F. Silyl-Directed, Iridium-Catalyzed ortho-Borylation of Arenes. A One-Pot ortho-Borylation of Phenols, Arylamines, and Alkylarenes. J. Am. Chem. Soc. 2008, 130, 7534–7535. 10.1021/ja8015878. [DOI] [PubMed] [Google Scholar]; b Chattopadhyay B.; Dannatt J. E.; Andujar-De Sanctis I. L.; Gore K. A.; Maleczka R. E.; Singleton D. A.; Smith M. R. Ir-Catalyzed ortho-Borylation of Phenols Directed by Substrate–Ligand Electrostatic Interactions: A Combined Experimental/in Silico Strategy for Optimizing Weak Interactions. J. Am. Chem. Soc. 2017, 139, 7864–7871. 10.1021/jacs.7b02232. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Li H.-L.; Kanai M.; Kuninobu Y. Iridium/Bipyridine-Catalyzed ortho-Selective C–H Borylation of Phenol and Aniline Derivatives. Org. Lett. 2017, 19, 5944–5947. 10.1021/acs.orglett.7b02936. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for full details.
- After submission of our work, we became aware that the groups of Maleczka and Smith had developed a similar approach to para-selective borylation. We are grateful to them for agreeing to publish their results in a back-to-back fashion with our own:Montero Bastidas J. R.; Oleskey T. J; Miller S. L.; Smith M. R.; Maleczka R. E. Para-Selective, Iridium-Catalyzed C−H Borylations of Sulfated. J. Am. Chem. Soc. 2019, 10.1021/jacs.9b08464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.