Abstract
We are seeking to identify molecular targets that are relevant to breast cancer cells with stem-like properties. There is growing evidence that cancer stem cells (CSCs) are supported by inflammatory mediators expressed in the tumor microenvironment. The chemokine receptor CXCR3 binds the interferon-γ-inducible, ELR-negative CXC chemokines CXCL9, CXCL10, and CXCL11 and malignant cells have co-opted this receptor to promote tumor cell migration and invasion. There are 2 major isoforms of CXCR3: CXCR3A and CXCR3B. The latter is generated from alternative splicing and results in a protein with a longer N-terminal domain. CXCR3 isoform A is generally considered to play a major role in tumor metastasis. When the entire tumor cell population is examined, CXCR3 isoform B is usually detected at much lower levels than CXCR3A and for this, and other reasons, was not considered to drive tumor progression. We have shown that CXCR3B is significantly upregulated in the subpopulation of breast CSCs in comparison with the bulk tumor cell population in 3 independent breast cancer cell lines (MDA-MB-231, SUM159, and T47D). Modulation of CXCR3B levels by knock in strategies increases CSC populations identified by aldehyde dehydrogenase activity or CD44+CD24− phenotype as well as tumorsphere-forming capacity. The reverse is seen when CXCR3B is gene-silenced. CXCL11 and CXCL10 directly induce CSC. We also report that novel CXCR3 allosteric modulators BD064 and BD103 prevent the induction of CSCs. BD103 inhibited experimental metastasis. This protective effect is associated with the reversal of CXCR3 ligand-mediated activation of STAT3, ERK1/2, CREB, and NOTCH1 pathways. We propose that CXCR3B, expressed on CSC, should be explored further as a novel therapeutic target.
Keywords: Chemokine receptor, chemokine ligands, cancer stem cells, allosteric receptor modulators
Introduction
There is an urgent need to identify molecular targets that are relevant to stem-like cancer cells (CSC). There is considerable evidence that the inflammatory milieu of the tumor microenvironment drives CSC. Several chemokine receptors, including CXCR3, contribute to malignant behavior of multiple cancers including breast, prostate, colon, and melanoma.1-7 The chemokine receptor CXCR3 binds the interferon-γ-inducible, ELR-negative CXC chemokines CXCL9/MIG, CXCL10/IP-10, CXCL11/I-TAC, as well as the more promiscuous ligand CXCL4/P4. CXCR3 acts to promote hematopoietic cell migration to sites of tissue injury or inflammation where CXCR3 ligands are expressed. Malignant cells have co-opted this receptor to promote migration and invasion. Evidence is growing for a subpopulation of malignant cells that possess stem cell properties that underlie tumor progression, resistance to therapy, and treatment relapse.8 We have recently provided evidence that CXCR3 plays a different role in support of these CSCs relative to the bulk tumor cell population.9 An additional level of complexity is provided by the discovery of multiple CXCR3 isoforms, ie, CXCR3 isoform A and CXCR3 isoform B, that play different roles in cancer.10,11 It is generally agreed that isoform CXCR3A drives cancer metastasis. CXCR3B is detected at much lower levels relative to CXCR3A when the bulk tumor cell population is examined and CXCR3B was therefore not considered to be a major component of malignant behavior; however, we recently reported that, in contrast to the bulk population, CSCs upregulate CXCR3B relative to CXCR3A.9 We propose that the 2 major isoforms of CXCR3, CXCR3A and CXCR3B, are differentially expressed in non-CSC and CSC subpopulations, and each isoform plays a unique role in determining malignant behavior.
Materials and Methods
Cell lines
Human breast cancer cell lines MCF7, MDA-MB-231, and T47D are grown in Dulbecco’s Modified Eagle Medium; SKBR3 cells are grown in McCoy’s 5A media; all media are supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products, Woodland, CA, USA), sodium bicarbonate, 2 mM l-glutamine, 100 units/mL penicillin, and 100 units/mL streptomycin. SUM159 cells are grown in Ham’s F-12 media supplemented with 10% FBS, hydrocortisone (1.0 µg/mL), insulin (5 µg/mL), amphotericin B (2.5 µg/mL), and gentamicin (15 µg/mL). All cells were maintained in 5% CO2 atmosphere.
CXCR3B-overexpressing and CXCR3B-gene-silenced MDA-MB-231 cells
CXCR3B-overexpressing cell lines were generated as described in detail by Li et al.9 Two clones stably expressing CXCR3B retroviral expression plasmid (CXCR3Bcl20 and CXCR3Bcl22) or neo-vector as control were previously characterized by quantitative real-time polymerase chain reaction and Western blotting. CXCR3B-silenced cells (CXCR3Bshcl38) or vector control cells were generated by transfection of lentiviral CXCR3B shRNA (short hairpin RNA) plasmids as previously described.9
Xenogen/metastasis assay
Luciferase-expressing MDA-MB-231 neo cells were detected by bioluminescence imaging (IVIS 200; Xenogen, Alameda, CA, USA) of anesthetized mice injected intraperitoneally with 100 μL of 7.5 mg/mL d-luciferin (PerkinElmer, Waltham, MA, USA). Bioluminescence from the regions of interest was defined manually and the data were expressed as photon flux (photons/s/cm2/steradian) and analyzed by IVIS software.
Western blot analysis
Protein extracts were analyzed by standard methods and antibodies to CXCR3 (R&D Systems, Minneapolis, MN, USA); CXCR3B (Creative BioMart, Shirley, NY, USA); ERK (Cell Signaling Technology, Danvers, MA, USA); p-ERK (Sigma-Aldrich, St. Louis, MO, USA); CREB, p-CREB, NOTCH 1 (Cell Signaling Technology); STAT3, p-STAT3, and β-actin (Sigma-Aldrich). Densitometry was performed using ImageJ software.
Proliferation assays
Cell proliferation in response to CXCL10 or CXCL11 (chemokine ligands 10 and 11; PeproTech, Rocky Hill, NJ, USA) was measured by PrestoBlue reagent (Invitrogen, Carlsbad, CA, USA). Cells were seeded into 96-well plates (Millipore Sigma, Burlington, MA, USA). The next day, CXCR3 ligands and/or CXCR3 allosteric modulators, BD106 or BD064, were added to the appropriate wells and, following a further 72-hour incubation, cell number was determined by PrestoBlue reagent and reported as relative fluorescence units.
CXCR3 allosteric modulators
The synthesis of N-{1-[3-(4-Ethoxyphenyl)-4-oxo-3,4-dihydropyrido[2,3-d]pyrimi-din-2-yl]ethyl}-4-(4-fluorobutoxy)-N-[(1-methylpiperidin-4-yl)me-thyl]butanamide (BD103) and {5-[(N-{1-[3-(4-ethoxyphenyl)-4-oxo-3,4-dihydropyrido[2,3-d]pyrimidin-2-yl]ethyl}-2-[4-fluoro-3-(trifluoromethyl)phenyl]acetamido)methyl]-2-fluorophenyl}boronic acid (BD064) was previously described.12
Tumorsphere formation assay
Tumorsphere assay as previously described13 in serum-free MammoCult medium (Stemcell Technologies, Vancouver, BC, Canada) and plated at 1 × 103 to 1 × 104 cells/well of a 24-well ultra-low attachment plate in triplicate (Corning, Lowell, MA, USA). After 24 hours, BD106 or BD064 was added to the appropriate wells. Tumorspheres cultured for 10 days and sphere counts were taken. Spheres were dissociated using trypsin and cell number/sphere was calculated.
Breast cancer stem cell phenotyping by ALDH assay
Aldefluor assay was performed using Aldefluor kit (Stemcell Technologies) following the company’s protocol. Fluorescence was analyzed by FACSCanto II cytometer and data were analyzed with FlowJo software in the Flow Cytometry Shared Service of the University of Maryland Greenebaum Comprehensive Cancer Center.
CD24, CD44, and CXCR3B Flow Cytometry
Cells were harvested using enzyme-free cell dissociation solution (Millipore). Cells were fixed using ice cold 70% ethyl alcohol, blocked with 3% FBS and stained with CXCR3B antibody (Proteintech, Rosemont, IL, USA) followed by APC-conjugated goat anti-mouse polyclonal antibody (R&D Systems). After extensive washing, cells were stained with FITC-conjugated anti-human CD44 (BD Pharmingen, San Jose, CA, USA) and PE-conjugated anti-human CD44 (BD Pharmingen). Flow cytometry analysis was performed on BD FACSCanto II cytometer. Data analysis was performed with FCS Express 6 software. The target cells were gated on FSC/SSC plot to remove debris, followed by a singlet gate on FSC-H/FSCW plot. CXCR3B+percentage was calculated from the CD44+CD24− population in the University of Maryland Greenebaum Comprehensive Cancer Center.
Results
CXCR3B is upregulated in Breast CSC
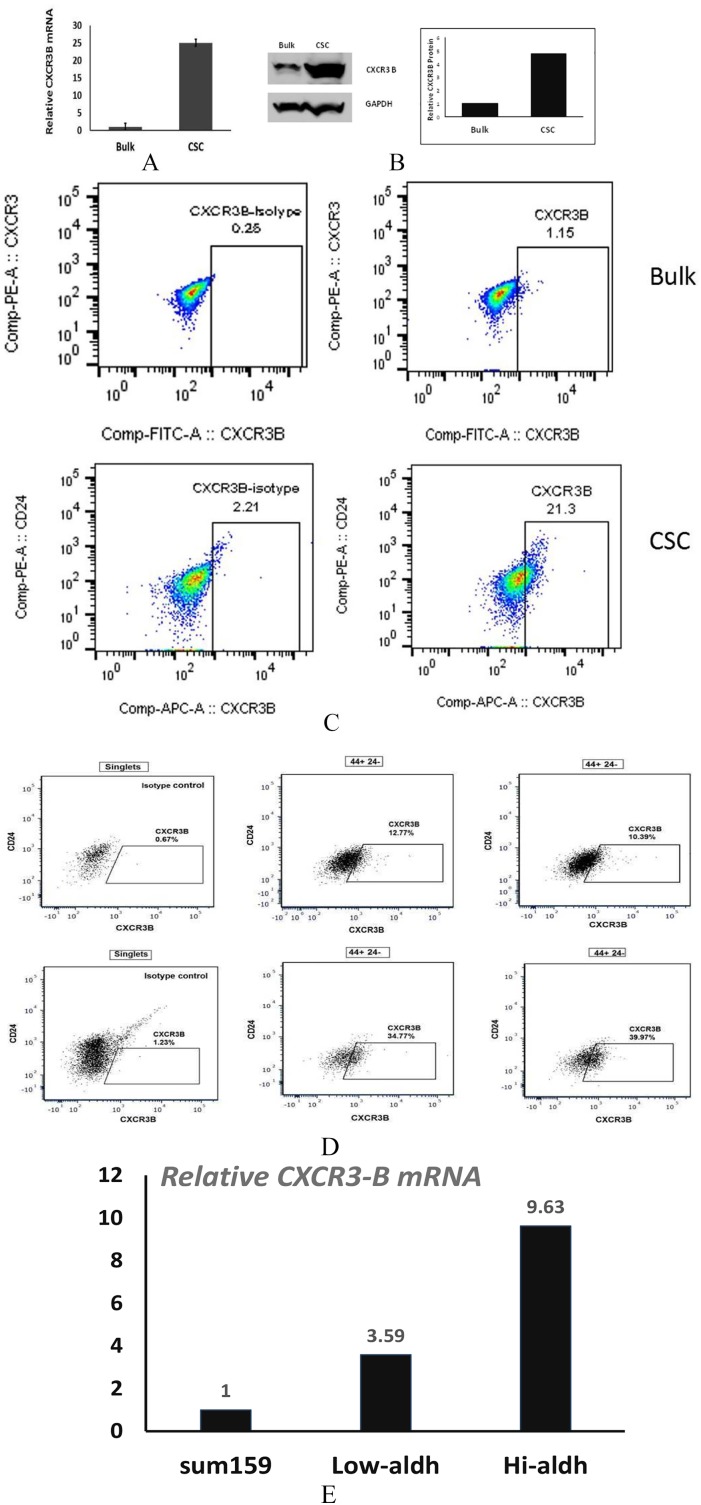
It is generally accepted that CXCR3-isoform A promotes metastasis.1-7,10 We sought to better understand the role of CXCR3-isoform B in breast cancer behavior. We reported previously that while CXCR3B expression is comparatively low in bulk populations relative to CXCR3A, the converse is true in CSC where the CXCR3B isoform is upregulated relative to CXCR3A.9 Using a model of triple-negative breast cancer, we now confirm that CXCR3B expression is upregulated in CSC-forming tumorspheres of MDA-MB-231 cells relative to the bulk (non-CSC plus CSC) population. By quantitative polymerase chain reaction, CXCR3B messenger RNA (mRNA) was increased by 25-fold in sphere-forming vs the bulk tumor cell population (Figure 1A). Western blotting confirmed an increase in total CXCR3B protein (4.8-fold) in sphere-forming cells vs the bulk population (Figure 1B). The positive correlation of CXCR3B expression with cancer stem cell properties is not unique to MDA-MB-231 cells. In the luminal breast cancer cell line T47D, 1% of the bulk population is CXCR3B-positive; however, 21.3% of the tumorsphere-forming cells express this isoform (Figure 1C). A subpopulation expressing high levels of CD44 and low CD24 is recognized as a breast CSC phenotype.14 Using the CD44+CD24− phenotype, we compared CXCR3B expression in the CSC vs non-CSC populations. From the bulk MDA-MB-231 cells, an average of 11.6 ± 0.8% of the CD44+CD24− population are CXCR3B+ (Figure 1D, upper panel), whereas 37.4 ± 1.8% of the tumorsphere-forming, CD44+CD24− population are CXCR3B+ (Figure 1D, lower panel). Aldehyde dehydrogenase activity is elevated in malignant breast CSCs.14 If basal-type SUM159 cells are sorted on the basis of ALDH1 (aldehyde dehydrogenase) positivity, the ALDH1 highly positive population expresses 9.63-fold more CXCR3B vs the unsorted population (Figure 1E). Thus, in 3 independent cell lines (2 basal-type; 1 luminal), CXCR3B is upregulated in the subpopulation with CSC properties (sphere formation; ALDH1+; CD44+/CD24−.
Figure 1.
CXCR3B isoform is more highly expressed in CSC vs the bulk population. (A) Relative CXCR3 mRNA expression by qPCR comparing tumorsphere-forming (CSC) or bulk population MDA-MB-231 cells. (B) Immunoblotting for CXCR3B of protein lysates obtained from CSC or bulk population tumor cells. (C) By flow cytometry, the CD44+CD24− population was identified from bulk population T47D cells (upper panel) or CSC tumorsphere (lower panel) cells and CXCR3B+ cells assessed in this population. CXCR3B+ gate was drawn based on the single-cell population stained with 3 isotope controls. Two replicates. (D) MDA-MB-231 bulk cells (upper panel) or CSC (lower panel) stained for CD44, CD24, and CXCR3B+ gate drawn as in C. Two replicates. (E) SUM159 cells analyzed by Aldefluor assay, sorted and analyzed for CXCR3B mRNA by RT-qPCR. CXCR3B expressed as fold increase relative to unsorted cells = 1.0. CSC indicates cancer stem cells; mRNA, messenger RNA; qPCR indicates quantitative PCR; RT-qPCR, quantitative reverse transcription PCR.
Overexpression of CXCR3B Increases CSC Numbers and Function
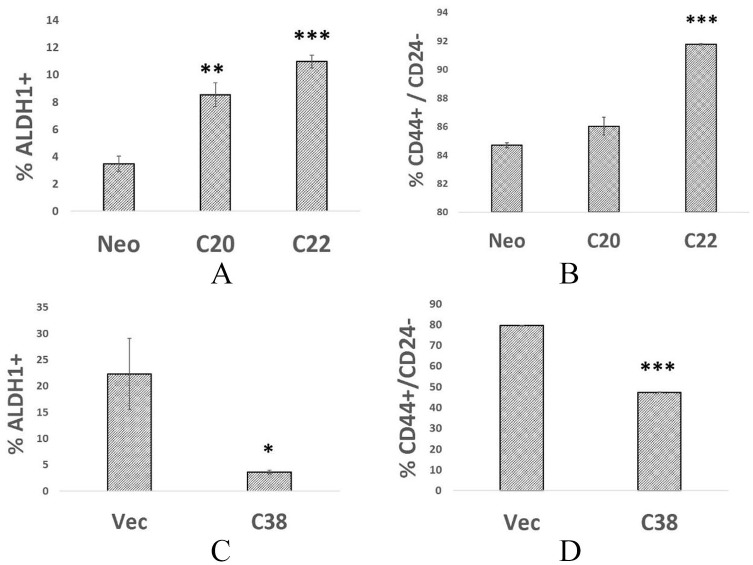
Taken together, these data identify a correlation between CXCR3B and CSC properties. To identify a functional relationship, we determined the effect of stable overexpression of CXCR3B on CSC properties. We compared the ALDH1+ fraction of MDA-MB-231 parental cells (expressing neo-vector) with 2 clones engineered to overexpress CXCR3B (hereafter referred to as clones 20 and 22). The fraction of ALDH1+ cells was increased by 2.5-fold and 3.2-fold in MDA-MB-231R3B clones 20 and 22, respectively, in comparison with MDA-MB-231-neo controls (Figure 2A). When the fraction of CD44+CD24− cells was compared, nearly all MDA-MB-231-neo cells have the phenotype CD44+CD24− (84.7% ± 0.3; Figure 2B). Nevertheless, a modest increase in this population was observed in 2 clones overexpressing CXCR3B (86.0% ± 1.1; 91.8% ± 0.06). For further validation of the data, we then asked whether CXCR3B gene silencing would reduce the CSC population identified by either ALDH1 positivity or the CD44+CD24− phenotype. In 2 independent experiments, ALDH1+ cells were decreased by 40% (data not shown; P = .05) or 84% (Figure 2C) in clone 38 cells expressing shRNA targeting CXCR3B. Similarly, only 47% of clone 38 cells express the CD44+CD24− phenotype vs 79% of MDA-MB-231-vector cells (Figure 2D).
Figure 2.
Altering the expression levels of CXCR3B changes the proportion of ALDH1+ and CD44+CD24− cells. (A). ALDH1+ fraction estimated by Aldefluor assay for MDA-MB-231-neo, MDA-MB-231R3B-overexpressing cells—clones 20 and 22 in triplicate. (B) CD44+CD24− fraction of same cells as in panel A. (C) ALDH1+ fraction of MDA-MB-231-vector cells or MDA-MB-231 R3B KD cells—clone 38. (D) CD44+CD24− fraction of MDA-MB-231-vector cells or MDA-MB-231—clone 38 cells.
*P < .05; **P < .02; ***P < .001.
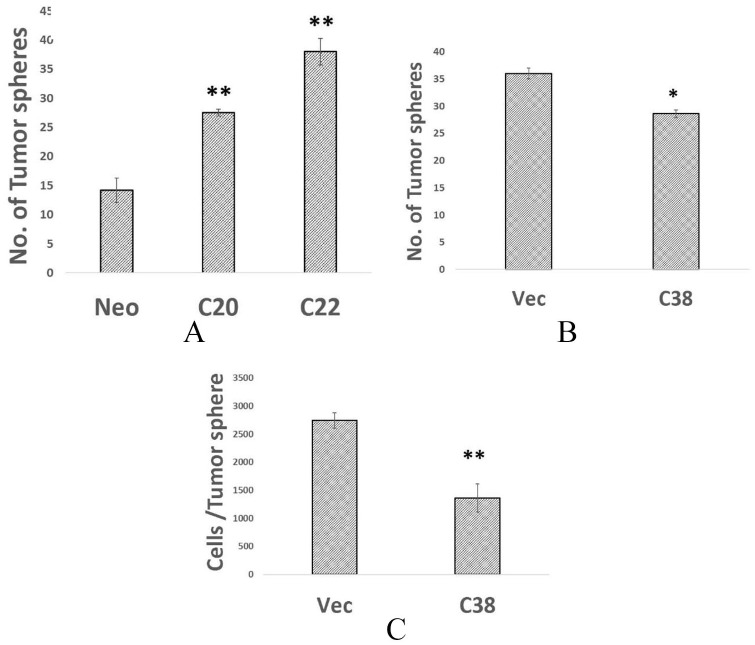
Another functional indicator of CSCs is the ability to form 3-dimensional spheres under low attachment conditions. MDA-MB-231 cells forcibly overexpressing CXCR3B have enhanced tumorsphere-forming capacity (1.9-fold, for clone 20; 2.7-fold increase for clone 22 (Figure 3A)). In contrast, gene silencing of CXCR3B decreased the tumorsphere-forming capacity by 21% and reduced the cellularity of spheres from an average of 2742 ± 138 to 1362 ± 249 cells/sphere (Figure 3B and C).
Figure 3.
(A) Altering the expression levels of CXCR3B modifies tumorsphere-forming capacity. 1 × 103 of MDA-MB-231-neo or MDA-MB-231R3B-overexpressing clones 20 or 22 plated in low attachment conditions and number of tumorpheres determined on day 10 in triplicate. (B) Tumorsphere-forming capacity of 1 × 104 MDA-MB-231-luc or MDA-MB-231-lucR3B knockdown clone 38 cells determined. (C) From the same cultures, tumorspheres were harvested and disassociated and number of cells/sphere was calculated in triplicate culture.
*P < .01; **P < .001.
CXCL11 and CXCL10 Induce CSC That is Blocked With CXCR3 Allosteric Modulators
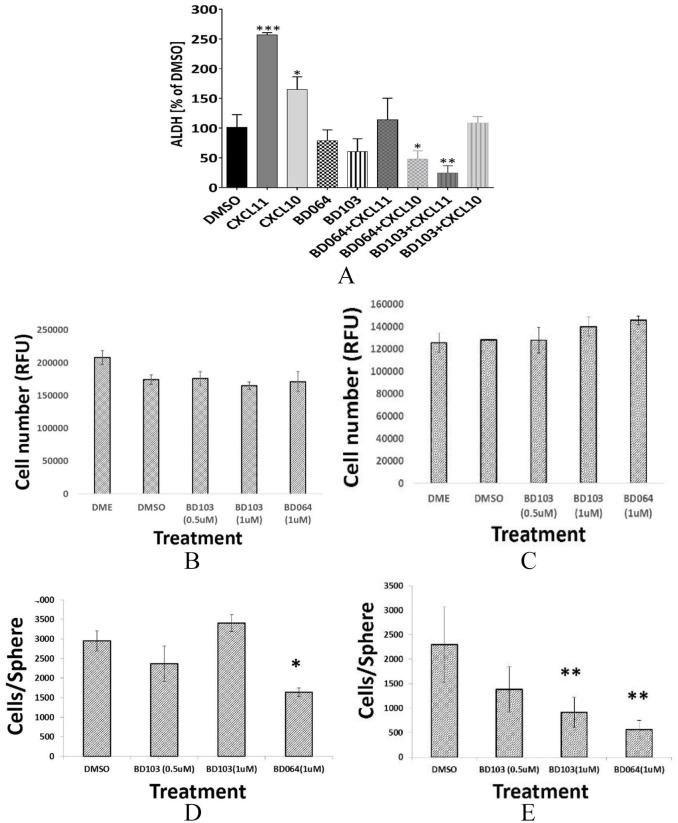
The chemokines CXCL10 and CXCL11 each bind CXCR3 with high affinity. We evaluated the ability of these CXCR3 ligands to induce CSC. Either CXCL11 or, to a lesser degree, CXCL10 increased the fraction of ALDH1+ cells (Figure 4A). A common strategy to inhibit ligand-mediated signaling is the application of small molecule receptor antagonists, including antagonists of CXCR3.2 AMG487, developed by Amgen, has been widely used for this purpose, but the relative ligand and isoform specificity of this compound was not known. In our hands, AMG487 showed no selectivity for G protein activation vs β-arrestin recruitment.15,16 Over the past 40 years, it has become obvious that G protein–coupled receptors contain alternative binding sites (allosteric sites) where small molecules exert their effects at locations that are topographically distinct from the orthosteric binding site.17-19 Therefore, these allosteric modulators gain therapeutic advantages including greater subtype selectivity and probe dependence. The modulator thereby is able to modify or completely inhibit some signals of the endogenous ligands while allowing others to pass unaltered. Recently, 2 functionally selective negative allosteric modulators were identified that exhibited probe-dependent inhibition of CXCR3 signaling.15,16 BD064 preferentially inhibits CXCL11-mediated β-arrestin 2 recruitment over G protein activation, whereas BD103 preferably blocks CXCL12-mediated activation of G proteins rather than β-arrestin 2 recruitment. We employed these 2 novel allosteric CXCR3 modulators developed by the Tschammer lab, to determine whether the induction of CSC by CXCL10 or CXCL11 could be blocked. Neither BD064 nor BD103 alone, in the absence of ligand, significantly affected the portion of ALDH1+ cells (Figure 4A); however, both compounds were able to inhibit CXCL10 or CXCL11-induced CSC. BD103 and BD064 both antagonize CXCR3; however, BD064 more potently inhibited CXCL10-mediated induction of CSC but BD103 preferentially blocked CSC induction by CXCL11. This observation of probe dependence is in agreement with previous reports.15,16
Figure 4.
(A) CXCL10 and CXCL11 induce CSC that is blocked by CXCR3 allosteric modulators. MDA-MB-231 cells incubated with DMSO, CXCL10, or CXCL11 (100 ng/mL); BD64 (10 µM/L); or BD103 (10 µM/L) and ALDH1+ cells determined by FACS analysis using Aldefluor assay. Triplicate determinations. *P < .05; **P < .01; ***P < .001 vs DMSO. (B) Bulk MDA-MB-231-neo-luc or (C) MDA-MB-231-luc R3B-overexpressing cells (Clone A) treated with BD103 (0.5, 1.0 µM/L), BD064 (1.0 µM/L), or DMSO and cell number determined at 48 hours by PrestoBlue reagent and expressed as relative fluorescent units (RFU). (D) Tumorspheres derived from MDA-MB-231-neo-luc or (E) MDA-MB-231-luc R3B-overexpressing cells treated as in panel B and effect on tumorsphere size determined. CSC indicates cancer stem cells; DMSO, dimethyl sulfoxide.
We examined the ability of BD064 and BD103 to inhibit bulk and CSC growth in vitro. Neither compound inhibited the proliferation of bulk tumor cell populations (Figure 4B and C), but BD064, and to a lesser (not significant) degree, BD103, inhibits the ability of CSC to form tumorspheres (Figure 4D). Consistent with potential CXCR3B-targeting, tumorspheres produced by CXCR3B-overexpressing cells were more sensitive to the inhibitory effects of either BD103 or BD064 than tumorspheres derived from vector control cells (Figure 4E).
CXCR3 Ligands Activate Pathways Associated With CXC and Growth Stimulation
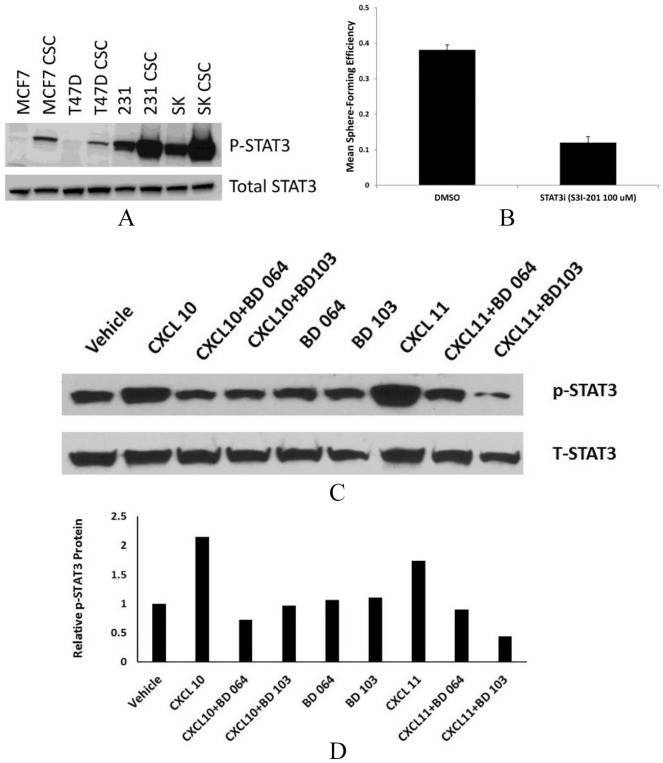
Ligand-mediated signaling through CXCR3A vs CXCR3B is distinct and also can vary by cell. Furthermore, each CXCR3 ligand can be coupled to different intracellular signaling pathways and can possess nonredundant roles in the same cells (biased agonism).20-22 We, therefore, investigated the effects of CXCR3 ligands and allosteric modulators on STAT3, ERK1/2, CREB, and Notch1 pathways. Emerging data suggest that STAT3 activation is important to the survival of breast CSC.14,23 We compared the relative expression of activated STAT3 in breast CSC vs bulk population cells of 4 different breast cancer cell lines representing luminal breast cancer (MCF7, T47D), triple-negative breast cancer (MDA-MB-231) and Her-2–amplified disease (SKBR3). In all 4 cell lines, phosphorylated STAT3 is elevated in tumorsphere-forming cells compared with the non-CSC pool (Figure 5A). Tumorsphere-forming ability of MDA-MB-231 cells was reduced by the STAT3 inhibitor STATTIC, confirming the functional importance of activated STAT3 in CSC (Figure 5B). We examined the ability of CXCL10 and CXCL11 to activate STAT3. Both CXCR3 ligands efficiently induced STAT3 phosphorylation (Figure 5C and D); this activation was effectively inhibited by either BD064 or BD103.
Figure 5.
CXCR3 ligands induce activated STAT3 that is blocked by BD064 or BD103. (A) Non-stem cell and CSC subpopulations of MCF7, T47D, MBA-MB-231, SKBr3 cancer cell lines probed for total and phospho-STAT3 by Western blotting. (B) Tumorspheres treated with DMSO or STAT3 inhibitor Stattic and tumor-forming efficiency determined. (C) MDA-MB-231 cells cultured in the presence of CXCL10 or CXCL11 (100 ng/mL) and/or BD64 or BD103 (1.0 µM/L) and probed for total STAT3 and p-STAT3 by Western blotting. (D) Expressed as fold expression relative to vehicle = 1.0.
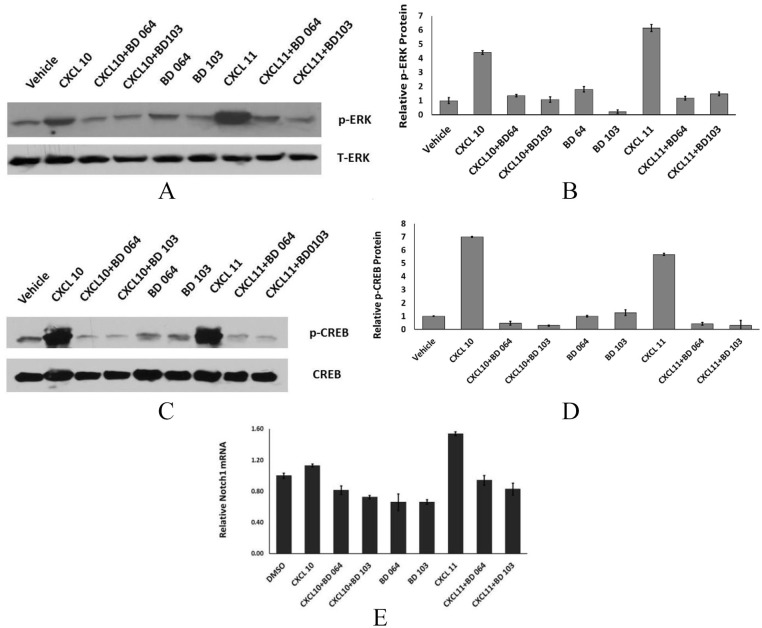
ERK1/2 activation is also linked to CXCR3, but the ability of individual ligands to activate ERK is not equal and is also cell context-dependent. For example, CXCL9 inhibits, rather than activates, JNK and ERK in endothelial cells.24 CXCL10, but not CXCL11, activates ERK in HEK cells. We determined the ability of CXCL10 or CXCL11 to activate ERK in breast cancer cells. CXCL11 was modestly more effective at inducing phosphorylated ERK, relative to CXCL10 (5.4-fold vs 2.96-fold, respectively, Figure 6A and B). Both BD064 and BD103 inhibited chemokine-mediated ERK activation.
Figure 6.
CXCR3 ligands induce ERK1/2, CREB, and Notch1. (A, B) MDA-MB-231 cells treated as in Figure 5 and protein immunoblotted with antibody to total ERK or phospho-ERK or C, D immunoblotted with antibody to total CREB or phospho-CREB or E. qPCR for Notch1 expression in cells treated as in Figure 5.
cAMP acts on cyclic AMP response elements (CREB) to regulate multiple target genes. Stimulation of cells with either CXCL10 or CXCL11 induces phosphorylated CREB, a response that is markedly inhibited in the presence of either BD103 or BD064 (Figure 6C and D). In preliminary studies, we had noted that Notch1 mRNA was increased in CXCR3B-overexpressing vs vector control cells (data not shown). We now show that CXCL11, but not CXCL10, markedly induces Notch1 mRNA (Figure 6E). Like ligand-induced ERK, STAT3, and CREB activation, Notch1 induction is blocked by BD064 or BD103.
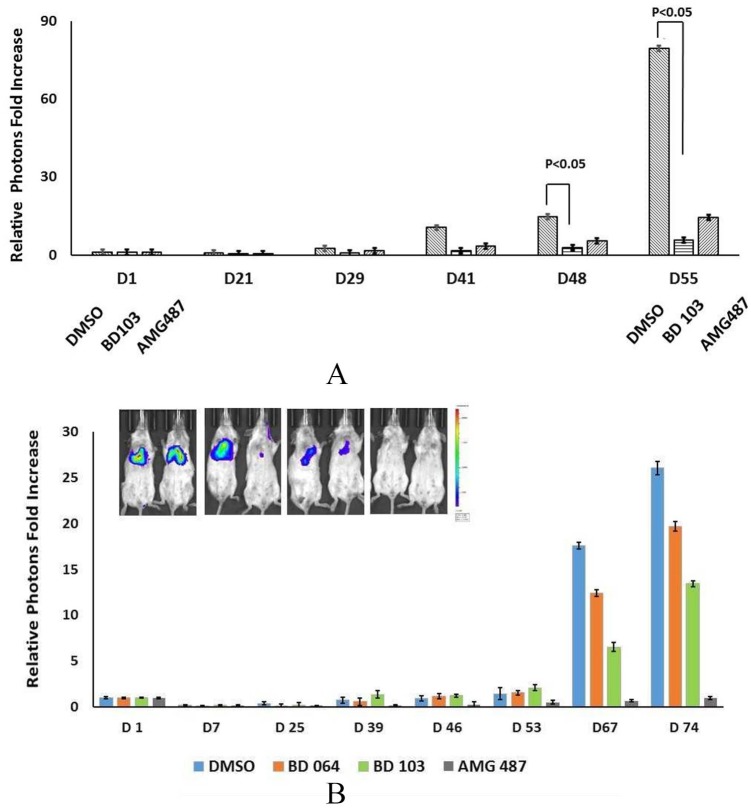
CXCR3 Allosteric Modulators Inhibit Experimental Metastasis
Finally, we evaluated the antimetastatic activity of BD103 and BD064 as well as AMG487, a widely used CXCR3 inhibitor. To focus this experiment on the effects of tumor cell–specific receptor antagonism, we pretreated tumor cells with AMG487, BD103, or BD064 prior to intravenous injection into Balb/SCID mice. In 3 independent experiments, CXCR3 antagonists were able to inhibit experimental metastasis (Figure 7A and B). The limited quantities of BD103 and BD064 restricted the number of mice that could be included in these studies, compromising the ability to obtain statistically robust conclusions; however, the ability of BD103 to inhibit metastasis was statistically significant (Figure 7A) and a similar trend was observed for BD064. These data suggest that all 3 receptor modulators reduced lung colonization (Figure 7A and B and data not shown). The rank order of effectiveness varied from experiment to experiment; however, in comparison with either BD103 or AMG487, BD064 was consistently less efficacious.
Figure 7.
CXCR3 allosteric modulators inhibit tumor cell colonization in the lung. (A) MDA-MB-231 tumor cells cultured in DMSO, BD103 (1 μM/L) or AMG487 (1 μM/L) for 24 hours, washed and 2.5 × 105 viable cells injected intravenous into Balb/c/SCID mice and bioluminescence determined on the indicated day. (B) MDA-MB-231 cells cultured in BD064 (1 µM/L), BD103 (1 µM/L), or AMG487 (1 μM/L), injected into mice as in A.
Discussion
The relationship of elevated CXCR3 to poor outcomes in breast cancer has been reported by several labs3,10,25 and is particularly striking in basal breast cancers26 which disproportionately affect African Americans. Interestingly, breast tumors from African American women are characterized by an IFN-γ signature of CXCR3 ligand expression.27 CXCR3 gene expression is increased in ER− breast cancer and relatively decreased in ER+ disease.28 The positive correlation of CXCR3 expression with poor outcomes is observed in many other cancers including prostate, melanoma, hepatocellular carcinoma, and renal cell carcinoma4,5,29-31 One exception is a report in gastric cancer in which CXCR3 expression was associated with better overall survival.32 Like receptor expression, increased ligand expression is associated with poor prognosis in some cancers.33,34 The utility of CXCR3 as a prognostic marker has been summarized in a recent meta-analysis.35
Based on these and many other studies, the relationship of CXCR3 to malignant behavior initially seemed straightforward that tumor cell autonomous expression of CXCR3 promotes invasion and metastasis and contributes to poor outcomes in many cancer types. The identification of multiple splice isoforms of human CXCR3 has made the picture more complicated. Human, but not murine, CXCR3 is expressed as 2 major splice isoforms CXCR3A and CXCR3B. CXCR3A is considered the classical isoform consisting of 368 amino acids; CXCR3B is generated through alternative splicing and results in a protein with a longer N-terminal domain. While many labs have reported that CXCR3 protein (by immunohistochemistry) is associated with more aggressive disease, the role of individual isoforms in clinical specimens is rarely examined because no antibody that specifically recognizes the CXCR3A isoform can be produced due to the absence of a unique A-specific sequence. Therefore, prior studies, showing a correlation of CXCR3 expression (isoform not specified) with poor clinical outcomes, cannot conclude that this is specifically due to high expression of CXCR3A. We previously examined CXCR3 isoform expression in both primary human breast cancer specimens and established breast cancer cell lines and observed that in all primary malignant samples, as well as cell lines, CXCR3A is more highly expressed than in normal cells.9 Furthermore, CXCR3A is also dominant in malignant cell lines relative to CXCR3B. Likewise, the CXCR3A isoform is more highly expressed than CXCR3B in malignant prostate5 or colon,36 but isoform B is favored in normal or precancerous epithelium. In contrast to most of the studies, a recent report describes higher levels of CXCR3B vs CXCR3A in gastric cancer and also that high CXCR3 expression (isoform not specified) was associated with a better prognosis.32 The reason for these different outcomes is not yet clear; these conclusions are in contrast to other studies, also in gastric cancer, in which the CXCL10/CXCR3 axis is linked to tumor cell invasion, migration, and worse outcomes.37 There are also conflicting data regarding the role of CXCR3 in promoting or inhibiting renal cell carcinoma.30,31,33,38
In addition to different expression levels, CXCR3A and CXCR3B often have opposing roles in the same cell. There is a consensus that CXCR3A promotes migration and invasion of many cancers, whereas CXCR3B either inhibits migration or has no role in chemotaxis. (The pro-metastatic role of CXCR3 in syngeneic mouse models is considered to mimic hu-CXCR3A.) Overexpression of CXCR3B in basal-like MDA-MB-231 cells inhibits CXCL10-stimulated proliferation,10,11 which is accompanied by reduced ligand-mediated activation of ERK1/2 and p38 kinases.
In contrast to the growing body of evidence regarding the role of CXCR3 in general tumor populations, very limited information is available regarding the role of any CXCR3 isoform in the behavior of CSC. Most of the studies examine the bulk tumor cell population in primary tissues or established cancer cell lines. The current working hypothesis is that CSCs represent a very rare cell type present within the bulk population that is responsible for therapy resistance and disease relapse. One distinguishing characteristic of both normal and malignant stem cells is the relatively low proliferative rate relative to the non-stem cell population. When considering the biology of CXCR3, we found an additional level of complexity present in the CSC vs the non-CSC population.9 We reported previously that, unlike the bulk population in which CXCR3B is markedly lower than CXCR3A, CXCR3B is elevated in CSC compared with the bulk population and this pattern is observed in 2 basal-type as well as a luminal breast cancer cell line. We now extend these observations to show that these patterns are functionally important. Tumorsphere-forming capacity is inhibited when CXCR3B is silenced. In addition, CXCR3B knockdown cells have a smaller ALDH1+ fraction and fewer cells with a CD44+CD24− phenotype, in comparison with CXCR3B-vec cells. Conversely, overexpressing CXCR3B further enhances tumorsphere-forming potential, increases the CD44+CD24− population, and doubles the fraction of ALDH1+ cells. This biology is not unique to breast CSCs. There is also evidence for a hepatic carcinoma stem cell, identified by high CD133 expression. Exposure of HepG2 cells to CXCL10 increases the number of CD133+ cells, enhances the tumorsphere-forming ability, and upregulates c-Myc.39 Thus, CSC of multiple cancer types may be supported by CXCR3 ligands.
Our studies have focused on the tumor cell–autonomous role of CXCR3. It is well established, however, that host immune cells, including cytotoxic T cells, T regulatory cells, and natural killer (NK) cells can express CXCR3. One unanswered question is whether antagonizing CXCR3 on the tumor cell, to inhibit growth, metastasis, and stem cell expansion, would compromise antitumor effector cells. An intriguing study in a preclinical model of breast cancer shows that, consistent with the literature, antagonism of tumor-CXCR3 prevents tumor cell migration and metastasis in vivo and, in fact, does not compromise host immunity.40 In fact, less metastatic disease is observed in CXCR3−/− hosts. These authors proposed that antagonizing host CXCR3 redirects myeloid cells to a type I polarization rather than to an immune-suppressive (high IL-4, IL-10, argininase) phenotype. These data are also consistent with our previous studies in which we demonstrated that the ability of CXCR3 antagonists to inhibit metastasis in a related syngeneic murine model of metastatic breast cancer is highly dependent on NK cells.2 A comparison of tumor-infiltrating lymphocyte (TIL) and programmed death ligand 1 (PD-L1) and other immune-related genes is primary vs metastatic clinical breast cancer samples detected fewer TILs and less PD-L1 expression in metastatic lesions suggesting that metastatic breast cancers are more immunologically inert than the parent tumor,41 an observation that is also consistent with prior preclinical studies. The CXCL9/10/11 axis acts on CXCR3 expressed on gastric cancer cell lines to upregulate PD-L1 through STAT and PI3K-Akt, and it would be expected that systemic CXCR3 antagonism would blunt the induction of this immune checkpoint pathway.42 Likewise, it was recently reported that CXCR3 present on regulatory T cells combined with CXCR3 ligands in the colon tumor microenvironment may act together to suppress tumor growth.43 Thus, it may be generally true that CXCR3 inhibition can result in both direct antitumor and anti-stem cell effects while simultaneously improving the efficacy of the antitumor immune response.
There is a growing understanding that even though CXCR3 ligands bind the same CXCR3 receptor with high affinity, each ligand can have redundant, collaborative, and even antagonistic functions vis-à-vis the other CXCR3 ligands. Thus, while CXCL10 interactions with certain immune effector cells may be critical, CXCL11 may be more important to intrinsic behavior of malignant cells. These complexities are described for CXCR3 expressed on T lymphocytes44,45 but are likely to be relevant to tumor cell autonomous functions as well. We also observed that CXCR3 ligands, CXCL10 and CXCL11, induce an ALDH1+ population and that the negative allosteric modulators, BD103 or BD064, reduced the ALDH1+ fraction. We now have a clearer picture of the ligand selectivity of these responses that is facilitated by the discovery of superior allosteric modulators that allow us to begin to tease out the mechanisms by which these ligands induce a CSC phenotype. We found that this response is driven more effectively by CXCL11 vs CXCL10 and this corresponds to a great extent with the enhanced ability of CXCL11 to activate ERK and NOTCH1. BD103 was reported to preferentially inhibit CXCL11-mediated activation of G proteins rather than recruitment of β-arrestin 2. In this study, BD103 more efficiently inhibited the induction of CSC by CXCL11 and was also more inhibitory to tumor metastasis in comparison with BD064.
Conclusions
Taken together, these results suggest several testable hypotheses: (1) that CXCL11 may more effectively drive CSC and tumor metastasis than CXCL10 and (2) the relevant mechanism may involve activation of G proteins. These hypotheses will be tested in future studies. Wu et al5 report higher CXCR3B in normal prostate epithelium compared with malignant prostate epithelium and we have observed the same relationship in normal MCF10A vs malignant breast cells.9 We propose that the CXCR3Bhigh population in malignant cells mimics normal mammary stem cells to maintain a relatively quiescent and therapy-resistant population. Taken together, these studies suggest that novel CXCR3 allosteric modulators should be examined further as potential cancer therapies.
Acknowledgments
Studies in the Fulton Lab supported in part by the U.S. Department of Defense Breast Cancer Research Program. R.B. and N.T. thank the Graduate Training School GRK 1910 of the German Research Foundation for financial support. N.T. participated in the European COST Action CM1207 GLISTEN: GPCR Ligand Interactions, Structures and Transmembrane Signaling: a European Research Network.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Design and conduct of laboratory studies (NK, XM, RB, TK, JR). Director of Flow Cytometry Shared Service and analysis of Flow Cytometry data (XF). Provision of and consultation regarding BD064, BD103 (NT). Design of all experiments and preparation of manuscript (AF).
References
- 1. Goldberg-Bittman L, Neumark E, Sagi-Assif O, et al. The expression of the chemokine receptor CXCR3 and its ligand, CXCL10, in human breast adenocarcinoma cell lines. Immunol Lett. 2004;92:171-178. [DOI] [PubMed] [Google Scholar]
- 2. Walser T, Rifat S, Ma X, et al. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res. 2006;66:7701-7707. [DOI] [PubMed] [Google Scholar]
- 3. Ma X, Norsworthy K, Kundu N, et al. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol Cancer Ther. 2009;8:490-498. [DOI] [PubMed] [Google Scholar]
- 4. Robledo MM, Bartolome RA, Longo N, et al. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem. 2001;276:45098-45105. [DOI] [PubMed] [Google Scholar]
- 5. Wu Z, Dhir R, Wells A. Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion. Mol Cancer. 2012;11:3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawada K, Hosogi H, Sonoshita M, et al. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene. 2007;26:4679-4688. [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Fulton AM. CXCR3/CXCL3 axis: friend or foe? In: Ben-Baruch A, ed. The Inflammatory Milieu of Tumors: Cytokines and Chemokines That Affect Tumor Growth and Metastasis. Sharjah, United Arab Emirates: Bentham Science; 2012:88-98. [Google Scholar]
- 8. Ginestier C, Liu S, Diebel ME, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y, Reader JC, Ma X, Kundu N, Kochel T, Fulton AM. Divergent roles of CXCR3 isoforms in promoting cancer stem-like cell survival and metastasis. Breast Cancer Res Treat. 2015;149:403-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Datta D, Flaxenburg JA, Laxmanan S, et al. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer. Cancer Res. 2006;66:9509-9518. [DOI] [PubMed] [Google Scholar]
- 11. Balan M, Pal S. A novel CXCR3-B chemokine receptor induced growth inhibitory signal in cancer cells is mediated through the regulation of Bach-1 protein and Nrf2 protein nuclear translocation. J Biol Chem. 2014;289:3126-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bernat V, Admas TH, Brox R, Heinemann FW, Tschammer N. Boronic acids as probes for investigation of allosteric modulation of the chemokine receptor CXCR3. ACS Chem Biol. 2014;9:2664-2677. [DOI] [PubMed] [Google Scholar]
- 13. Kundu S, Ma X, Kochel T, et al. Prostaglandin E receptor EP4 is a therapeutic target in breast cancer cells with stem-like properties. Breast Cancer Res Treat. 2014;143:19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin L, Hutzen B, Lee H-F, et al. Evaluation of STAT3 signaling in ALDH+ and ALDH+/− CD44+/CD24− subpopulations of breast cancer cells. PLoS ONE. 2013;8:e82821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernat V, Brox R, Heinrich MR, Auberson YP, Tschammer N. Ligand-biased and probe-dependent modulation of chemokine receptor CXCR3 signaling by negative allosteric modulators. ChemMedChem. 2015;10:566-574. [DOI] [PubMed] [Google Scholar]
- 16. Brox R, Milanos L, Saleh N, et al. Molecular mechanisms of biased and probe-dependent signaling at CXC-motif chemokine receptor CXCR3 induced by negative allosteric modulators. Mol Pharmacol. 2018;93:309-322. [DOI] [PubMed] [Google Scholar]
- 17. Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323-374. [DOI] [PubMed] [Google Scholar]
- 18. Kenakin T. New concepts in drug discovery. Collateral efficacy and permissive antagonism. Nat Rev Drug Discov. 2005;4:919-927. [DOI] [PubMed] [Google Scholar]
- 19. Kenakin T. Functional selectivity and biased receptor signaling. J Pharmacol Expt Ther. 2011;336:296-302. [DOI] [PubMed] [Google Scholar]
- 20. Kenakin T, Christopoulos A. Signaling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205-216. [DOI] [PubMed] [Google Scholar]
- 21. Berchiche YA, Sakmar TP. CXC chemokine receptor 3 alternative splice variants selectively activate different signaling pathways. Mol Pharmacol. 2016;90:483-495. [DOI] [PubMed] [Google Scholar]
- 22. Smith JS, Alagesan P, Desai NK, et al. CXC motif chemokine receptor 3 splice variants differentially activate beta-arrestins to regulate downstream signaling pathway. Mol Pharmacol. 2017;92:136-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chung SS, Giehl N, Wu Y, Vadgama JV. STAT activation in HER-2 -overexpressing breast cancer promotes epithelial-mesenchymal transition and cancer stem cell traits. Int J Oncol. 2014;44:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sahin H, Borkha-Kamphorst E, Kuppe C, et al. Chemokine CXCL9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatol. 2012;55:1610-1619. [DOI] [PubMed] [Google Scholar]
- 25. Bronger H, Karge A, Dreyer T, et al. Induction of cathepsin B by the CXCR3 chemokines CXCL9 and CXCL10 in human breast cancer cells. Oncol Lett. 2017;13:4224-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mulligan AM, Raitman I, Feeley L, et al. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin Cancer Res. 2013;19:336-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin DN, Boersma BJ, Yi M, et al. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS ONE. 2009;4:e4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao C, McDowell IC, Zhao S, Brown CD, Engelhardt BE. Context specific and differential gene co-expression networks via Bayesian biclustering. PLoS Comput Biol. 2016;12:e1004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ding Q, Xia Y, Ding S, Lu P, Sun L, Liu M. An alternatively spliced variant of CXCR3 mediates the metastasis of CD133+ liver cancer cells induced by CXCL9. Oncotarget. 2016;7:14405-14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun K-H, Sun G-H, Wu Y-C, Ko B-J, Hsu H-T, Wu S-T. TNF-α augments CXCR2 and CXCR3 to promote progression of renal cell carcinoma. J Cell Mol Med. 2016;20:2020-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Utsumi T, Suyama T, Imamura Y, et al. The association of CXCR3 and renal cell carcinoma metastasis. J Urol. 2014;192:567-574. [DOI] [PubMed] [Google Scholar]
- 32. Hu M, Li K, Maskey N, et al. Overexpression of the chemokine receptor CXCR3 and its correlation with favorable prognosis in gastric cancer. Hum Pathol. 2015;46:1872-1880. [DOI] [PubMed] [Google Scholar]
- 33. Liu W, Liu Y, Fu Q, et al. Elevated expression of IFN-inducible CXCR3 ligands predicts poor prognosis in patients with non-metastatic clear-cell renal cell carcinoma. Oncotarget. 2016;7:13976-13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou H, Wu J, Wang T, Zhang X, Liu D. CXCL10/CXCR3 axis promotes the invasion of gastric cancer via PI3K/AKT pathway-dependent MMPs production. Biomed Pharmacother. 2016;82:479-488. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Y, Xu L, Peng M. CXCR3 is a prognostic marker and a potential target for patients with solid tumors: a meta-analysis. Onco Targets Ther. 2018;11:1045-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li H, Rong S, Chen C, et al. Disparate roles of CXCR3A and CXCR3B in regulating progressive properties of colorectal cancer cells. Mol Carcinog. 2019;58: 171-184. [DOI] [PubMed] [Google Scholar]
- 37. Yang C, Zheng W, Du W. CXCR3A contributes to the invasion and metastasis of gastric cancer cells. Oncol Rep. 2016;36:1686-1692. [DOI] [PubMed] [Google Scholar]
- 38. Klatte T, Seligson DB, Leppert JT, et al. The chemokine receptor CXCR3 is an independent prognostic factor in patients with localized clear cell renal cell carcinoma. J Urol. 2008;179:61-66. [DOI] [PubMed] [Google Scholar]
- 39. Ouyang Y, Liu K, Hao M, et al. Radiofrequency ablation-increased CXCL10 is associated with earlier recurrence of hepatocellular carcinoma by promoting stemness. Tumour Biol. 2016;37:3697-3704. [DOI] [PubMed] [Google Scholar]
- 40. Zhu G, Yan HH, Pang Y, et al. CXCR3 as a molecular target in breast cancer metastasis: inhibition of tumor cell migration and promotion of host anti-tumor immunity. Oncotarget. 2015;6:43408-43419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Szekely B, Bossuyt V, Li X, et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018;29:2232-2239. [DOI] [PubMed] [Google Scholar]
- 42. Zhang C, Li Z, Xu L, et al. CXCL9/10/11, a regulator of PD-L1 expression in gastric cancer. BMC Cancer. 2018;18:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abron JD, Singh NP, Murphy AE, et al. Differential role of CXCR3 in inflammation and colorectal cancer. Oncotarget. 2018;9:17928-17936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Metzemaekers M, Vanheule V, Janssens R, Struyf S, Proost P. Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 ligands. Frontiers Immunol. 2018;8:1970. [DOI] [PMC free article] [PubMed] [Google Scholar]