Abstract
Background:
Urinary symptoms are common for people with neurogenic lower urinary tract dysfunction (NLUTD). No nonprescription approach has been proven safe and effective for self-management of urinary symptoms. Our objective was to describe the safety and tolerability of Lactobacillus rhamnosus GG (LGG®) instilled intravesically for self-management of inflammatory urinary symptoms in adults and children with NLUTD due to spinal cord injury or disease (SCI/D) and who use intermittent catheterization (IC).
Methods:
A total of 103 individuals with SCI/D enrolled in an 18-month study consisting of three 6-month stages: baseline (weekly observation of urinary symptoms); intervention (self-instilled intravesical LGG® in response to more cloudy or foul-smelling urine); and washout (weekly observation of urinary symptoms). Urinary symptoms were assessed using the Urinary Symptom Questionnaire for people with neurogenic bladder using intermittent catheters (USQNB-IC). Safety was based on serious adverse events and adverse events (S/AEs) and trends in symptoms. Tolerability was defined as the independence of AE experience and willingness to use/pay for this intervention.
Results:
A total of 74 (77%) adults and 6 (86%) of children completed the study, of whom 64 instilled LGG® for a total of 357 instillations (range 1–41 per person). There were 59 S/AEs, 44% (26/59) of which were categorized as infectious genitourinary. There was no statistical relationship between S/AEs and use or dose of the intervention.
Conclusions:
One or two doses of self-instilled intravesical LGG® in response to more cloudy or foul-smelling urine was safe and well tolerated among this sample of adults and children with SCI/D who have NLUTD and use IC.
Keywords: intravesical, lactobacillus, neurogenic bladder, paraplegia, probiotic, spina bifida, spinal cord injury, tetraplegia
Introduction
Urinary tract infection (UTI) is the most common outpatient infection worldwide, and the most common infection for people with neurogenic lower urinary tract dysfunction (NLUTD) due to spinal cord injury or disease (SCI/D).1–3 While mortality risk due to UTI in this population has declined with improved bladder management,4 UTI remains the most common secondary condition, most common cause of emergency room visits, and the most common infectious cause of rehospitalization.2,3,5,6
Despite its prevalence, the clinical definition of UTI among people with NLUTD is elusive.7,8 Diagnostic criteria include some combination of: symptoms;7–9 inflammatory reaction (typically white blood cells) differentiating UTI from asymptomatic bacteriuria;10,11 and bacterial load (count determined by urine culture).12,13 As a result of inconsistencies amongst the authoritative guidelines,8,14,15 diagnosis is largely subjective and highly variable,16 likely contributing to overprescription of antimicrobials.
To overcome the variability associated with ‘UTI’ as an outcome, our team developed a set of Urinary Symptom Questionnaires for people with neurogenic bladder (USQNBs) to advance care, self-management, and evidence. The first of these, the USQNB-IC, is for people with NLUTD and who use an intermittent catheter (IC),17,18 while two other USQNBs are in development (indwelling catheter and void versions). Each instrument has been developed following a model that prioritizes the patient experience, while integrating the clinical and research perspectives.17
Among the 29 urinary symptom items on the USQNB-IC, we identified the urinary symptoms ‘cloudy’ or ‘more foul-smelling’ urine as the most common symptoms reported by people with NLUTD,17 and selected these as triggers for self-management. These symptoms do not fit the definition of UTI according to the Infectious Diseases Society of America or European Association of Urology guidelines7,14; however, these symptoms may represent a pre-UTI state, and are often considered to be indicative of urinary inflammation in clinical practice. Because of their prevalence and potential association with inflammation, but not meeting the symptom requirement for infection, a first-in-human self-management trial was designed around these symptoms.
Our investigational agent for self-management is self-administered intravesical Lactobacillus rhamnosus GG (LGG®). This approach is supported by our prior work demonstrating that Lactobacillus is a predominant bacterial genus present in the urine of asymptomatic, healthy controls without NLUTD.19,20 We have also shown that Lactobacillus is the predominant organism in the urine of young to middle-aged females, and one of the four predominant organisms in males (along with Streptococcus, Veillonella, and Prevotella). In contrast, the urine of subjects with NLUTD is nearly devoid of Lactobacillus.12
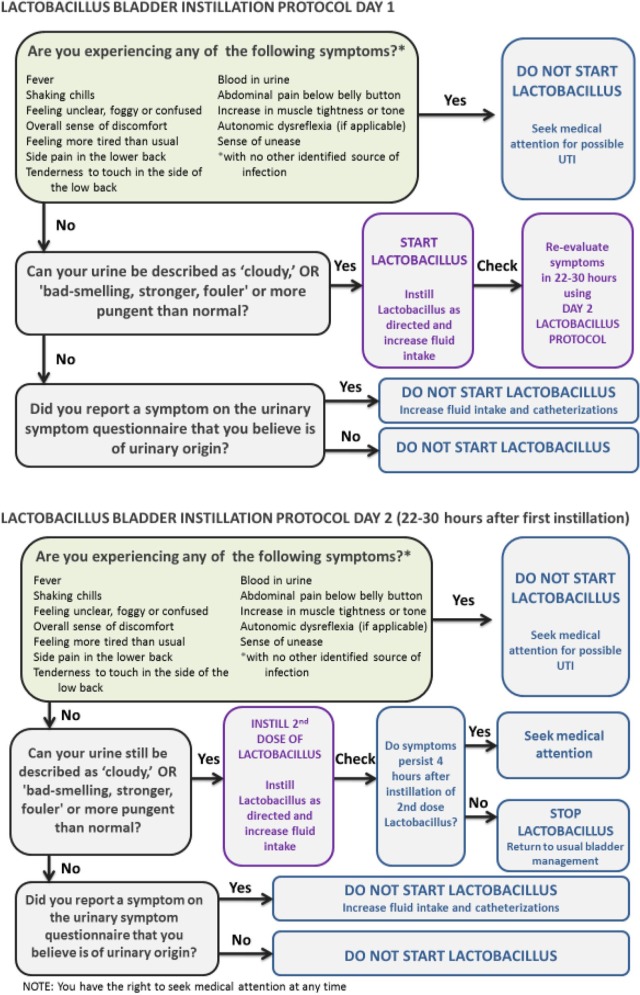
Integrating this preliminary work, we developed a Self-Management Protocol using Probiotics (SMP-Pro) for individuals with NLUTD (see Figure 1). The aim of the SMP-Pro is to provide people with NLUTD who frequently experience urinary symptoms and UTI a self-management approach to urinary symptoms, targeting the most commonly occurring urinary symptoms, and using a probiotic bacteria that has been shown to be present to a greater degree in the urine of healthy volunteers as opposed to those with NLUTD.
Figure 1.
Self-management protocol using probiotics (SMP-Pro).
The purpose of this first-in-human trial was to assess whether self-instilled intravesical LGG® is safe and well-tolerated, and whether urinary symptom burden is reduced. Prior to testing this hypothesis, we were required to file an investigational new drug (IND) application with the US Food and Drug Administration, and to demonstrate preliminary safety of this preparation via this novel administration method. The initial safety report of the first 10 subjects (5 adults and 5 children; one instillation each) is currently in press.21 Here we report on the safety and tolerability of one or two doses of self-administered intravesical LGG® instilled within a 22–30 h time period in response to cloudy or more foul-smelling urine.
Methods
The trial was approved by the MedStar Health Institutional Review Board (IRB) (#2014-11). All study personnel were certified in, and the study protocol conformed to, the ethical guidelines of the 1975 Declaration of Helsinki as reflected in approval by the MedStar Health IRB. The trial was registered with clinicaltrials.gov (Adults #2014-211, Children #5753) and an independent Data Safety Monitoring Board (DSMB) reviewed adverse events (AEs) and serious AEs (SAEs).
Design
The trial was a prospective 18-month, three-stage study (6 months each: baseline, intervention, washout), in which participants with NLUTD due to SCI/D, multiple sclerosis (MS), or spina bifida (SB) reported symptoms using the USQNB-IC weekly for 6 months (baseline); followed the SMP-Pro (Figure 1) to guide intravesical self-instillation of LGG® + reported symptoms weekly using the USQNB-IC for 6 months (intervention); then reported symptoms using the USQNB-IC weekly for 6 months (washout). Safety data included all AEs and SAEs experienced by any participant.
Participants
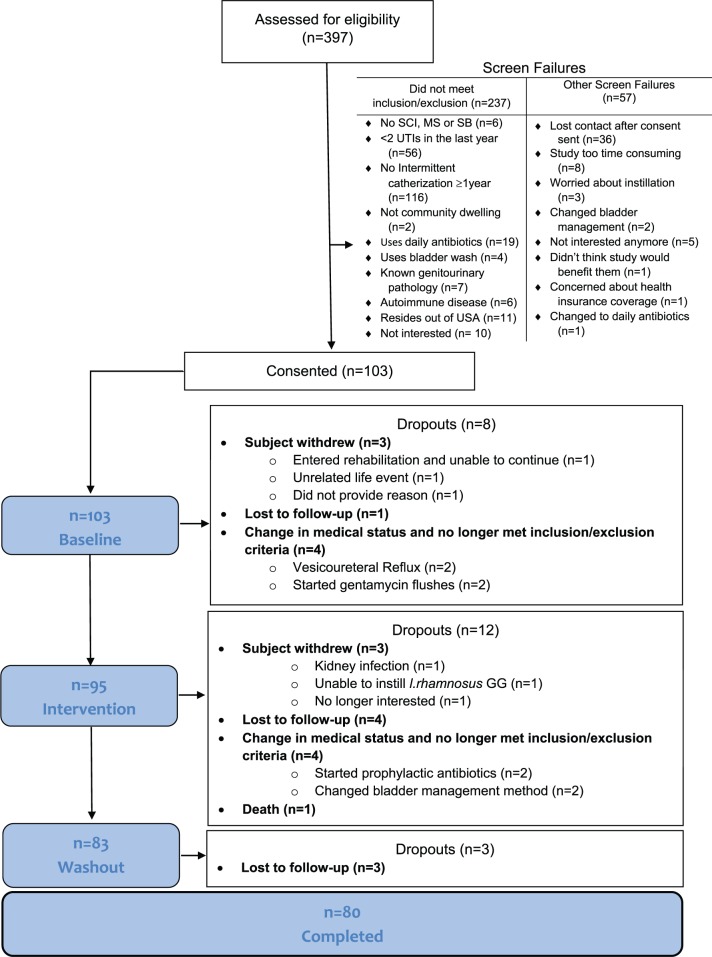
Subject eligibility criteria included: NLUTD managed with IC, living in the community (i.e. not within a long-term care facility), and presence of SCI/D, MS or SB ⩾1 year for adults. We excluded patients with known genitourinary (GU) pathology beyond NLUTD; instillation of other intravesical agents; psychological or psychiatric conditions influencing the ability to follow instructions; participation in a confounding study; pregnant/breastfeeding women; immunodeficiencies; cancer/autoimmune disorders; allergy to any component in the probiotic; change in neurologic status in the previous 2 weeks; antibiotic use in the previous 2 weeks; sensitivity to ampicillin or tetracycline; UTI within the previous 2 weeks;22 and residence outside of the US. Pediatric patients had to be between 6 and 18 years of age to be eligible, and had to have a caregiver that would assent to the child’s participation in the study. A total of 397 individuals were screened (see Figure 2 CONSORT diagram).23
Figure 2.
CONSORT diagram.
Study procedures
After informed consent, the USQNB-IC was reviewed with all participants and caregivers. Participants/caregivers were emailed the USQNB-IC weekly for 18 months. The weekly USQNB-IC first asked whether they had experienced any symptoms during the previous week. If they answered ‘no’, then no further action was necessary. If they answered ‘yes’, then the participant would choose which of the 29 USQNB-IC symptoms were experienced, and rate each symptom in terms of frequency, severity, and impact. During the first 6 months (baseline observation; phase I), participants completed the USQNB-IC weekly only.
During the final month of the baseline phase, the participant/caregiver was trained by a Consumer Expert (an individual with NLUTD who uses IC or caregiver) on the SMP-Pro, including LGG® (ATCC 53103, Culturelle, 20 billion live organisms) instillation, utilizing a standardized handbook and video. Participants were trained to measure and mix the contents of a LGG® capsule with a specified amount of normal saline that varied by participant size (estimating 10% of bladder capacity based on height) via face-to-face training. At the end of the training session, participants performed one LGG® instillation under the supervision of study personnel.
During months 7–12, as directed by the SMP-Pro, participants instilled LGG® when they experienced cloudier or more foul-smelling, stronger, fouler or more pungent than normal urine. One or both of these symptoms had to be experienced in the absence of the following USQNB-IC symptoms: fever, shaking chills, feeling unclear, foggy or confused, overall sense of discomfort, feeling more tired than usual, side pain in the lower back, tenderness to touch in the side of the low back, blood in urine, abdominal pain below belly button, increase in muscle tightness or tone, autonomic dysreflexia, or sense of unease, as these symptoms (according to guidelines) may be consistent with UTI.
Safety assessment
AE and SAE reporting followed the FDA IND safety reporting requirements outlined in 21 CFR 312.32.24 An AE [21 CFR 312.32(a)] was defined as ‘any untoward medical occurrence whether or not it was associated with the use of the study drug and whether or not it was considered drug related.’ An SAE [21 CFR 312.32(a)] was identified when any of the following occurred: death, a life-threatening AE, inpatient hospitalization (or prolongation of hospitalization), or a ‘persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions’, An independent DSMB was tasked with determining the relatedness of the SAEs to the intervention: ‘definitely unrelated’; ‘unlikely to be related’; or ‘possibly’, ‘probably’, and ‘definitely’ related. In addition, participants were asked to describe whether or not they considered an AE or SAE to be related to the study drug.
All AEs and SAEs were recorded by participant report and provided to the DSMB at 6-monthly intervals. Only SAEs that occurred at any time after LGG® instillation were reviewed by the DSMB for independent determination of relatedness to the intervention. The study clinician (SLG) evaluated all AEs and SAEs for body system classification. For all SAEs classified as GU infections that occurred at any time after LGG® instillation, urinalysis and urine culture results were obtained and provided to the DSMB. If the participant experienced an AE or SAE but had not instilled LGG®, then the event was classified by the study clinician as ‘definitely unrelated’ to the intervention.
Tolerability assessment
A four-item survey was administered at the end of each 6-month phase of the study. Participants used a Likert scale to rate their satisfaction with changes in the impact, frequency, and severity of their symptoms in the previous 6 months. As a patient-reported indicator of the tolerability of the intervention, we used their answers to the fourth item, ‘can you estimate, using a scale from 0 to 100%, whether or not you would seek out this intervention and pay for it yourself if insurance did not pay for it?’ Participants indicated their answer by moving a ‘slider’ with three anchors: 0% = would absolutely never do this; 50% = might do this; 100% = would absolutely do this.
Statistical analyses
Data was collected and managed using REDCap electronic data capture tools hosted at Children’s National Medical Center, and exported as a .csv file to SPSS v. 25 (IBM, Inc.) for analysis. Our safety analysis was based on the total number of AEs (AE+SAE) experienced by all participants before, and at any time after, their first instillation. For those who instilled at least once, paired t tests compared AE+SAE counts that were experienced prior to exposure to the intervention with counts experienced after exposure to the intervention. For the most conservative inferences possible, we included all AE+SAEs irrespective of whether or not they were deemed to be related to the intervention by the DSMB. AE+SAE experiences were considered separately for children and adults, but we planned to carry out all analyses collapsing across age. Individuals who did not instill were included in our considerations to represent the ‘expected’ level of events over time.
For our tolerability analyses, we sought to understand how, and whether, the likelihood to seek/pay for this intervention might be associated with any AEs. ‘Tolerability’ of the intervention was thus defined as statistical independence of this likelihood and the individual’s total AE experience. This definition naturally limited ‘tolerability’ analyses to those who had instilled at least once. We averaged participant ratings on the single item ‘Can you estimate, using a scale from 0 to 100%, whether or not you would seek out this intervention and pay for it yourself if insurance did not pay for it?’ from the end of the intervention (month 12) and washout (month 18) phases of the study only (tolerability of the intervention could not play a role in baseline/pre-instillation ratings). To estimate the relationship between AE+SAE experience and interest in seeking/paying for the intervention among those who instilled at least once, we created groups based on the number of S/AEs that were experienced (0, 1, 2+). ANOVAs were planned separately for the tolerability ratings from children and adults, however due to the small number of children, their data were analyzed together with the adults. To support a conclusion that LGG® was ‘well tolerated’, AE+SAE experience should be independent of these ratings; thus, we prespecified an equivalence analysis, defining ‘equivalence’ as differences between average ratings of AE+SAE experience groups (0, 1, 2+) of not more than 5% (d = ± 5%).
In all analyses, alpha was set at 0.05, and we did not correct for multiple comparisons because even marginally significant findings in any of these analyses could indicate either safety concerns or low tolerability. Missing data was not imputed, but, for average tolerability, if only one of these ratings was captured, it was used as the ‘average’, assuming no change.
Results
Study population
A total of 103 individuals, 96 adults (SCI = 81; SB = 12; MS = 3), and 7 children (SB = 6; SCI = 1) between 6 and 21 years of age, were enrolled. Of these, 74 (77%) adults and 6 (86%) children completed the 18-month study. Table 1 describes the study population.
Table 1.
Participant demographics.
| Adults | Children | |
|---|---|---|
| Number | 96 | 7 |
| Mean Age (range) | 43.3 (20–74) | 9.9 (8–11) |
| % Female | 36.5 | 57.1 |
| % SCI (n) | 84.4 (81) | 14.3 (1) |
| % SB (n) | 12.5 (12) | 85.7 (6) |
| % MS (n) | 3.1 (3) | 0 |
| Level of Injury (if applicable) | ||
| % Cervical (n) | 24.7 (23) | 0 |
| % Thoracic (n) | 61.3 (57) | 14.3 (1) |
| % Lumbar (n) | 12.9 (12) | 85.7 (6) |
| % Sacral (n) | 1.1 (1) | 0 |
| Completeness of Injury (if applicable) | ||
| % Complete (n) | 36.6 (34) | (0) |
| % Incomplete (n) | 58.1 (54) | 85.7 (6) |
| % Unknown (n) | 5.4 (5) | 14.3 (1) |
| Mean years (range) since injury or diagnosis | 18.5 (1–62) | 9.9 (8–11) |
MS, multiple sclerosis; SB, spina bifida; SCI, spinal cord injury.
Use of SMP-Pro intervention
Of the 103 participants enrolled, 95 (88 adults, 7 children) entered phase II (intervention), and 64 (67.4%) instilled LGG® at least once, for a total of 357 instillations. The 59/88 (67%) adults who entered phase II self-administered between 1 and 41 instillations, for a total of 324 (90.8%) instillations. Of the seven children, five (71%) children instilled LGG®, administering 3–9 doses per participant for a total of 33 (9.2%) instillations.
To characterize participant use of/exposure to the SMP-Pro directed intervention, we defined ‘Noninstillers’ as those who completed at least phase II but did not instill; ‘Dropouts’ as those who did not complete phase I, and so never had the opportunity to instill; and ‘Instillers’ as those who instilled at least once.
During the baseline phase (phase I), the range of weeks (of 24) that participants met instillation criteria was 0–23. That is, some participants never met trigger criteria, while others met criteria in 23/24 weeks. During the baseline phase, those who never instilled met instillation criteria an average of 2.1 (SD 3.1) weeks, those who (later) instilled met instillation criteria an average of 5.0 (SD 6.1) weeks, and those who dropped out met criteria an average of 0.5 (SD 1.2) weeks.
Safety
There were 59 AEs+SAEs (13 AEs and 46 SAEs) that occurred during the 18-month study. All (100%) of the 13 AEs occurred in 11 adult participants; no AEs were experienced by child participants, while 95.7% (44/46) of SAEs occurred in 27 adults and 4.3% (2/46) of SAEs were experienced by 2 child participants. Nearly half (26/59, 44%) of all AEs+SAEs were classified as GU infections. The 2 SAEs that occurred in children were in different participants and both occurred prior to the instillation phase; 4/13 AEs and 22/46 SAEs in adults occurred prior to the instillation phase. Because so few SAEs (and no AEs) were experienced by children, we combined their data with adults for the remaining analyses. Table 2 summarizes AEs and SAEs according to whether the participant went on to instill LGG® at least once during phase II (n = 64), or never did (n = 37). AE+SAE counts are given by study period and body system.
Table 2.
AEs+SAEs by study phase, instillation category, and body system.
| AEs and SAEs counts | |||
|---|---|---|---|
| Phase I (baseline) n = 103 | Phase II
(intervention) n = 95 |
Phase III (follow up) n = 83 | |
| Instillers n = 64 | |||
| Body system classification of AE and SAE | |||
| GU Infectious | 6 SAEs, 2 AEs | 2 SAEs, 3 AEs | 4 SAEs, 1 AE |
| GU Other | 0 SAE, 0 AE | 0 SAE, 3 AEs | 2 SAE, 0 AE |
| GI | 2 SAE, 0 AE | 0 SAE, 0 AE | 0 SAE, 0 AE |
| Other | 6 SAE, 2 AEs | 5 SAE, 2 AEs | 3 SAE, 0 AEs |
| Total | 14 SAE, 4 AEs | 7 SAE, 8 AEs | 9 SAE, 1 AE |
| Drop-outs before phase II intervention (n = 2) and Noninstillers (n = 37) | |||
| Body system classification of AE and SAE | |||
| GU Infectious | 3 SAEs, 0 AE | 4 SAEs, 0 AE | 1 SAE, 0 AE |
| GU Other | 1 SAEs, 0 AE | 0 SAE, 0 AE | 0 SAE, 0 AE |
| GI | 0 SAE, 0AE | 0 SAE, 0 AE | 1 SAE, 0 AE |
| Other | 4 SAE, 0 AE | 2 SAE, 0 AE | 0 SAE, 0 AE |
| Total | 8 SAE, 0 AE | 6 SAE, 0 AE | 2 SAE, 0 AE |
AE, adverse event; SAE, serious adverse event.
Relatedness of AEs+SAEs to the intervention
Of the 13 AEs, 6 (46.2%) were experienced by adults that had instilled LGG® at any time prior to the AE; all 6 of these AEs were rated as moderate in their severity [per US Food and Drug Administration (FDA) definition],26 and included irritation after instilling (1), UTI (1), emotional discomfort due to a UTI (1), UTI and kidney infection (2), and migraine (1). Two of these AEs, irritation instilling LGG® (1) and UTI/kidney infection (1) were considered by the participants to have been ‘caused by LGG®’ and a third (migraine) was considered by the participant as ‘maybe being caused by LGG®’.
Of the 46 SAEs, 22 (47.8%) occurred during phase II (baseline). The remainder (24 or 52.2%) occurred during the ensuing 12 months (phases II and III combined, at any time after LGG® could have been instilled). Of all SAEs, 23.9% (11) occurred at some point after the participant indicated they had used LGG®. Of these 11 SAEs, 4 were considered by the DSMB to be ‘unlikely to be related’ to the study drug, and included bladder infection (1), prostate infection (1), severe kidney infection (1), and coughing and urinary symptoms (1), while 7/11 were definitely unrelated to the study drug. None were determined by the DSMB to be ‘definitely’, ‘probably’, or ‘possibly’ related to the study drug.
Variability of S/AEs by study phase
For the 27 participants who entered the intervention phase but never instilled, an average of 0.51 AEs+SAEs occurred in phase I (baseline) and 0.074 occurred on average during the remaining 12 months of the study. They experienced an average of 0.30 GU AEs (GU+GU Infections) in phase I and no GU AEs in phases II or III.
For the 64 who instilled at least once, during the baseline phase they experienced an average of 0.36 AEs+SAEs, including an average of 0.16 GU AEs. Over the next 12 months these 64 ‘Instillers’ experienced an average of 0.28 AEs+SAEs, including an average of 0.16 GU AEs. The paired t tests comparing experience (AE+SAE or GU AE) in the baseline 6 months with the 12 months of phases II and III were not statistically significant for total AE count [t(63) = 0.76] or for those AEs designated GU or GU infectious [t(63) = 0; both p > 0.40].
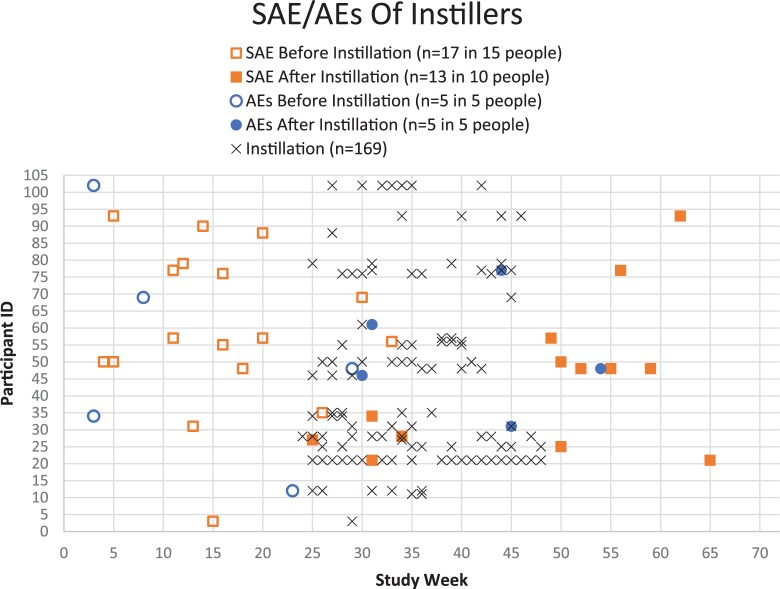
Occurrences of GU AEs+SAEs decreased over the 18 months of the study, but for ‘Instillers’ the correlation between total AEs during the final 12 months and total doses instilled was significant (r = 0.291, p = 0.02). One individual instilled 41 times, while the next highest dose count was 14. Without this outlier, the correlation coefficient dropped (r = 0.255) and the association marginally failed to reach significance (p = 0.055). With and without this extreme dose value, the association between dose and total AE count was not strong (squaring the correlation coefficients shows these share 6.5–8.5% of variance). In summary, ‘Instillers’ had the same rate of GU AEs+SAEs in the first 6 months as in the last 12 months, but their overall AE+SAE rate decreased slightly from 0.36 (first 6 months) to 0.28 (last 12 months) events per person, on average. Figure 3 shows the distribution of doses according to the total number of AEs and SAEs experienced over the 18-month study for those who did and did not instill (zero doses included). The participant instilling 41 times experienced two AEs; both occurred after at least one instillation.
Figure 3.
Timeline of S/AEs among Instillers.
AE, adverse event; SAE, serious adverse event.
Rate of S/AEs by exposure to intervention
We further described all AEs+SAEs by person-time in the study to better summarize participant experience (total unique AE+SAE events divided by the total number of weeks participants filled in the survey, multiplied by 52 weeks). The events per person-year were computed separately for Instillers and Noninstillers.
For Instillers, pre-installation rate was based on the number of weeks from consent to the week prior to first instillation, while for Noninstillers it was the number of weeks they participated each period. Instillers experienced 0.084 events/person-year prior to any installation. During the installation phase (defined per individual at first instillation and ending after their 48th week in the study), Instillers experienced 0.43 events/person-year. During postinstallation (defined per individual from the week after their last instillation through their 72nd week of the study), the rate of AE+SAE was 0.178 events/person-year. For Noninstillers, the event rates were 1.35, 0.906, and 0.206 events/person-year in the first, second, and third phases, respectively. Since the groups are not comparable, no inferences were planned or done.
Tolerability
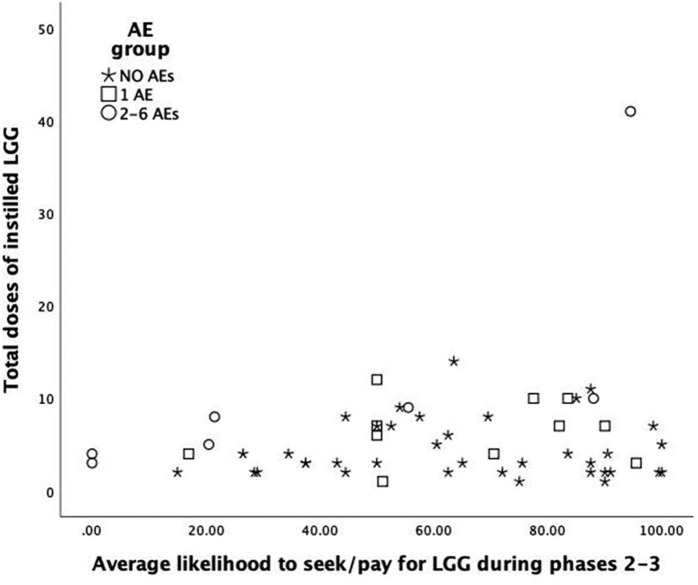
Of the 64 Instillers, only 55 provided tolerability ratings for these analyses; and for these raters, total AE+SAE experience was classified as none (n = 37; 67.3%), one (n = 11; 20.0%) or 2–6 (n = 7; 12.7%) AEs+SAEs. The average ratings of likelihood to seek and pay for the intervention, rated at the ends of the phase II (intervention) and phase III (washout) did not differ significantly across the AE+SAE experience groups [F(2, 47) = 2.4, p = 0.07]. However, the average tolerability ratings of those with the greatest numbers of AEs+SAEs (2–6) were more than 20 points below the averages of the other two AE+SAE count groups, which were nearly identical (0 AEs+SAEs = 63.9; 1 AE+SAE = 64.1). Figure 4 shows that the two lowest likelihood ratings, as well as one of the highest, were recorded for those with 2–6 total AEs+SAEs over the 18-month study. The next lowest, and four highest, were among those with 0 AEs+SAEs. Figure 4 suggests that total AEs+SAEs was unrelated to the total number of doses of LGG® instilled. The distributions of ratings for likelihood of seeking/paying for the intervention (x axis) were very wide for all AEs+SAEs experience groups, including those with no AEs+SAEs (stars in Figure 4).
Figure 4.
Likelihood to seek and pay for intervention by AE and dose.
AE, adverse event.
Discussion
Safety and tolerability
In this first-in-human study, we demonstrate that one or two doses of intravesical LGG® self-instilled within a 22–30 h period in response to urine that is more cloudy or foul-smelling, is safe and well tolerated among adults and children with NLUTD who manage their bladders with IC. The study also provides preliminary demonstration that the SMP-Pro, which directs people with NLUTD when to or not to instill LGG®, is useable by patients. Because we learned that assessment of symptoms must be done in real time with the SMP-Pro to determine more precisely whether there are difficulties in the SMP-Pro for its use as a self-management tool, further studies are needed to generate estimates of correct/incorrect implementation of the protocol itself. Our conclusions are supported by the following:
AEs+SAEs occurred throughout the study at a rate that did not associate with LGG® use;
AEs+SAEs that occurred in Instillers varied across and within people;
rates of AEs+SAEs were highest in phase I (baseline) and subsequently decreased;
in general, the rate of AEs+SAEs did not increase with exposure to LGG®;
there were one, two, and five GU infections (the most likely to be related to the intervention) AEs+SAEs that occurred ⩽1 week,1–2 weeks, or >2 weeks, respectively, following instillation;
there were only three each GU infectious AEs+SAEs that occurred after 1 of the 357 total instillations (1.7%);
of 357 total instillations, only 1 (0.28%) resulted in irritation and withdrawal from the study; and
SAEs that occurred after LGG® were determined by an independent DSMB as unlikely to be associated with the intervention.
Specifically, the data suggest that the rate of AE+SAEs was greatest prior to any instillation of LGG® for both children and adults. Moreover, Noninstillers tended to average more events than those who instilled. It was unexpected that there were so many more SAEs than AEs; this exemplifies the natural history of secondary conditions occurring among people with NLUTD due to SCI/D, SB, and MS, suggesting that future studies must consider AEs and SAEs in analysis plans and study designs. In sum, our results suggest, and we cautiously conclude, that the intervention is safe and well tolerated.
It is generally difficult to define ‘tolerability’. ICH E9 defines tolerability as ‘the degree to which overt AEs can be tolerated by the subject’.26 In that sense, tolerability has been measured by observing rates of death, treatment discontinuations or dose interruptions/reductions, use of supportive therapies, hospital admissions, etc. Our definition of tolerability is consistent with the ICH E9 perspective, but is also representative of the nonrandomized design of this study and maintains our focus on the patient experience. While there was a trend for those with the greatest number of AEs+SAEs to rate their likelihood to seek out and pay for this intervention as less likely, variability in these likelihood ratings was very high. The conclusion that AE+SAE experience and willingness to seek out and pay for the intervention are independent is not due to sample size, but might be due to variability among participants.
The evidence on probiotics
Probiotics are defined as ‘a preparation of, or a product containing viable, defined microorganisms in sufficient numbers, which alter the microflora (by implantation or colonization) in a compartment of the host and by that exert beneficial health effects in the host’.27 Probiotics are increasingly being studied as to their potential health benefits when used in food or medicinally, and include LGG®, Lactobacillus reuteri, Lactobacillus bifidobacterial, strains of Lactobacillus casei, Lactobacillus acidophilus, Escherichia coli strains, selected enterococci Saccharomyces boulardii (yeast), and others.28
While there has been relatively little study of probiotics targeting the GU tract, as early as 1999, a relationship between UTIs and absence or lack of Lactobacillus was suggested.29 Potential mechanisms of probiotic organisms in the GU tract include establishing a barrier against ascension and colonization by reducing uropathogen adherence and growth, as well as effecting immune function. However, continued mechanistic work remains to be done as to how probiotics exert their effects.
Low virulence E. coli for UTI prevention
In a 2017 Cochrane review of ‘probiotics’ for preventing UTI among people with NLUTD, three studies with 110 subjects were identified.25–27 All of these involved intravesical instillation of low virulence E. coli. AE reporting was limited to two of the three studies and included autonomic dysreflexia (one case) and UTI in participants colonized with the E. coli intervention agent. Comparatively, our study had a similar number of participants (n = 103) as the three reviewed studies combined; however, we studied a different intravesical-delivered probiotic in a more homogenous population (the Cochrane studies included people with any type of NLUTD using any bladder management strategy). Thus, direct comparisons between the Cochrane reviewed studies cannot be made with ours due to the significant differences in the interventions, study populations, and outcomes measures.
Oral probiotics for UTI prevention
The only other comparable completed trial is the ProSCIUTTU trial, a randomized double-blind factorial-design placebo-controlled trial of 24 weeks of oral L. reuteri GR-1 or LGG® + Bifidobacterium BB, or both, to prevent UTI among people with SCI. In this trial, there were 207 participants (SCI with NLUTD who utilized any bladder management) stratified across four groups.30 AEs included bowel accidents and increased frequency of bowel movements, UTI, abdominal cramps, blocked urinary catheter, and rash. Among 207 participants, there were 15 AEs occurring during the 24 weeks; during our 24-week baseline/observation period we observed 26 AEs+SAEs, nearly double that in the ProSCIUTTU trial,30 among fewer participants. We suspect the differences are due to our use of a very structured and liberal definition of AE/SAE to best capture safety. Further, we selectively recruited participants at risk for GU infection AEs+SAEs based on their history of at least two UTIs in the past year, so we would anticipate a high, or higher, number of AE+SAEs. Lastly, it is not surprising that the ProSCIUTTU trial also reported more gastrointestinal AEs as the probiotic was taken orally. In contrast, we observed few GI events (three SAEs, two occurring pre-instillation).
Conclusion
Two doses of intravesical LGG® self-administered within a 22–30 h period in response to cloudier or more bad-smelling, stronger, fouler or more pungent than normal urine, and in the absence of symptoms suggestive of infection, is safe and well tolerated among people with NLUTD who manage their bladders with IC. We hypothesize that these symptoms represent a potentially pre-infectious, transitional, state of bladder inflammation that may be amenable to bacterial interference. We do not propose treatment of UTI with probiotics at this time. Research and clinical care promoting probiotics needs to consider the evidence base as it specifically relates to strain of probiotic, route of administration, dosing frequency and duration, patient population, and outcome measure(s).
Acknowledgments
We would like to acknowledge i-Health, a division of DSM Nutritional Products, who supplied the LGG® used in this study.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by PCORI (grant number AD-1310-08215 to SLG).
Conflict of interest statement: SLG holds a use patent for intravesical Lactobacillus.
ORCID iD: Suzanne L. Groah  https://orcid.org/0000-0003-1213-1959
https://orcid.org/0000-0003-1213-1959
Contributor Information
Suzanne L. Groah, MedStar National Rehabilitation Hospital, 102 Irving St, NW, Washington, DC 20010, USA; Department of Rehabilitation Medicine, Georgetown University Medical Center, Washington, DC, USA.
Amanda K. Rounds, MedStar National Rehabilitation Hospital, Washington, DC, USA MedStar Health Research Health Institute, Hyattsville, MD, USA.
Inger H. Ljungberg, MedStar National Rehabilitation Hospital, Washington, DC, USA MedStar Health Research Health Institute, Hyattsville, MD, USA.
Bruce M. Sprague, Division of Urology, Children’s National Health System, Washington, DC, USA
Jamie K. Frost, Collaborative for Research on Outcomes and Metrics and Departments of Neurology and Biostatistics, Bioinformatics & Biomathematics, Georgetown University Medical Center, Washington, DC, USA
Rochelle E. Tractenberg, Collaborative for Research on Outcomes and Metrics and Departments of Neurology and Biostatistics, Bioinformatics & Biomathematics, Georgetown University Medical Center, Washington, DC, USA
References
- 1. Cardenas DD, Moore KN, Dannels-McClure A, et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized, multicenter trial. PM R 2011; 3: 408–417. [DOI] [PubMed] [Google Scholar]
- 2. Haisma JA, van der Woude LH, Stam HJ, et al. Complications following spinal cord injury: occurrence and risk factors in a longitudinal study during and after inpatient rehabilitation. J Rehabil Med 2007; 39: 393–398. [DOI] [PubMed] [Google Scholar]
- 3. Werhagen L, Gabrielsson H, Westgren N, et al. Medical complication in adults with spina bifida. Clin Neurol Neurosurg 2013; 115: 1226–1229. [DOI] [PubMed] [Google Scholar]
- 4. Consortium for Spinal Cord Medicine. Bladder management for adults with spinal cord injury: a clinical practice guideline for health-care providers. J Spinal Cord Med. 2006; 29: 527–573. [PMC free article] [PubMed] [Google Scholar]
- 5. Cardenas DD, Hoffman JM, Kirshblum S, et al. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil 2004; 85: 1757–1763. [DOI] [PubMed] [Google Scholar]
- 6. DeJong G, Tian W, Hsieh CH, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil 2013; 94: S87–S97. [DOI] [PubMed] [Google Scholar]
- 7. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International clinical practice guidelines from the infectious diseases society of America. Clin Infect Dis 2010; 50: 625–663. [DOI] [PubMed] [Google Scholar]
- 8. Madden-Fuentes RJ, McNamara ER, Lloyd JC, et al. Variation in definitions of urinary tract infections in spina bifida patients: a systematic review. Pediatrics 2013; 132: 132–139. [DOI] [PubMed] [Google Scholar]
- 9. The prevention and management of urinary tract infections among people with spinal cord injuries. National institute on disability and rehabilitation research consensus statement. January 27-29, 1992. J Am Paraplegia Soc 1992; 15: 194–207. [DOI] [PubMed] [Google Scholar]
- 10. Vaidyanathan S, Soni BM, Dundas S, et al. Urethral cytology in spinal cord injury patients performing intermittent catheterisation. Paraplegia 1994; 32: 493–500. [DOI] [PubMed] [Google Scholar]
- 11. Schlager TA, Grady R, Mills SE, et al. Bladder epithelium is abnormal in patients with neurogenic bladder due to myelomeningocele. Spinal Cord 2004; 42: 163–168. [DOI] [PubMed] [Google Scholar]
- 12. Groah S, Perez-Losada M, Caldovic L, et al. MP20–08 pyuria and asymptomatic bacteriuria is associated with novel and specific urine microbiomes. J Urol 2015; 193: e226. [Google Scholar]
- 13. Jayawardena V, Midha M. Significance of bacteriuria in neurogenic bladder. J Spinal Cord Med 2004; 27: 102–105. [DOI] [PubMed] [Google Scholar]
- 14. Naber KG, Bergman B, Bishop MC, et al. EAU guidelines for the management of urinary and male genital tract infections. Urinary tract infection (UTI) working group of the health care office (HCO) of the European association of urology (EAU). Eur Urol 2001; 40: 576–588. [DOI] [PubMed] [Google Scholar]
- 15. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the infectious diseases society of America and the European society for microbiology and infectious diseases. Clin Infect Dis 2011; 52: e103–e120. [DOI] [PubMed] [Google Scholar]
- 16. Pannek J. Treatment of urinary tract infection in persons with spinal cord injury: guidelines, evidence, and clinical practice. J Spinal Cord Med 2011; 34: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tractenberg RE, Garver A, Ljungberg IH, et al. Maintaining primacy of the patient perspective in the development of patient-centered patient reported outcomes. PLoS One 2017; 12: e0171114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tractenberg RE, Groah SL, Rounds AK, et al. Preliminary validation of the urinary symptom questionnaire for individuals with neuropathic bladder using intermittent catheterization (USQNB-IC): a patient-centered patient reported outcome. PLoS One 2018; 13: e0197568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fouts DE, Pieper R, Szpakowski S, et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med 2012; 10: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Groah SL. Neurogenic bladder and the urine microbiome: the good, the bad and the ugly. Presented at the American Spinal Injury Association Annual Meeting, April 2017, Albuquerque, NM. [Google Scholar]
- 21. Forster CS, Hseieh M, Perez-Losada M, et al. A single intravesical instillation of lactobacillus rhamnosus GG is safe in children and adults with neuropathic bladder: a phase Ia clinical trial. J Spinal Cord Med 2019; 17: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the infectious diseases society of America. Clin Infect Dis 2010; 50: 625–663. [DOI] [PubMed] [Google Scholar]
- 23. CONSORT Group. Consort transparent reporting of trials, http://www.consort-statement.org/ (accessed 19 March 2019).
- 24. US Food & Drug Administration. Safety Reporting Requirements for INDs (Investigational New Drug Applications). Safety Reporting Requirements for INDs (Investigational New Drug Applications) and BA/BE (Bioavailability/Bioequivalence) Studies, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/safety-reporting-requirements-inds-investigational-new-drug-applications-and-babe. (2012, accessed 23 September 2019)
- 25. US Food and Drug Administration. CFR- code of federal regulations Title 21. US Department of Health and Human Services, Office of Disease Prevention and Health, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=312.32. (accessed 5 June 2019). [Google Scholar]
- 26. European Medicines Agency. ICH Topic E9 Statistical Principles for Clinical Trials, https://www.ich.org/products/guidelines/efficacy/efficacy-single/article/statistical-principles-for-clinical-trials.html (1998, accessed 5 June 2019).
- 27. Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics – approaching a definition. Am J Clin Nutr 2001; 73: 361s–364s. [DOI] [PubMed] [Google Scholar]
- 28. de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. In: Stahl U, Donalies UEB, Nevoigt E. (eds) Food Biotechnology. Vol 111 Berlin: Springer, 2008, pp. 1–66. [DOI] [PubMed] [Google Scholar]
- 29. Reid G. Potential preventive strategies and therapies in urinary tract infection. World J Urol 1999; 17: 359–363. [DOI] [PubMed] [Google Scholar]
- 30. Lee BB, Toh SL, Ryan S, et al. Probiotics [LGG-BB12 or RC14-GR1] versus placebo as prophylaxis for urinary tract infection in persons with spinal cord injury [ProSCIUTTU]: a study protocol for a randomised controlled trial. BMC Urol 2016; 16: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]