Abstract
Visceral adipose tissue (VAT) inflammation plays a central role in longevity and multiple age-related disorders. Cellular senescence (SEN) is a fundamental aging mechanism that contributes to age-related chronic inflammation and organ dysfunction, including VAT. Recent studies using heterochronic parabiosis models strongly suggested that circulating factors in young plasma alter the aging phenotypes of old animals. Our study investigated if young plasma rescued SEN phenotypes in the VAT of aging mice. With heterochronic parabiosis model using young (3 months) and old (18 months) mice, we found significant reduction in the levels of pro-inflammatory cytokines and altered adipokine profile that are protective of SEN in the VAT of old mice. These data are indicative of protection from SEN of aging VAT by young blood circulation. Old parabionts also exhibited diminished expression of cyclin-dependent kinase inhibitors (CDKi) genes p16 (Cdkn2a) and p21 (Cdkn1a/Cip1) in the VAT. In addition, when exposed to young serum condition in an ex vivo culture system, aging adipose tissue–derived stromovascular fraction cells produced significantly lower amounts of pro-inflammatory cytokines (MCP-1 and IL-6) compared to old condition. Expressions of p16 and p21 genes were also diminished in the old stromovascular fraction cells under young serum condition. Finally, in 3T3-preadipocytes culture system, we found reduced pro-inflammatory cytokines (Mcp-1 and Il-6) and diminished expression of cyclin-dependent kinase inhibitor genes in the presence of young serum compared to old serum. In summary, this study demonstrates that young milieu is capable of protecting aging adipose tissue from SEN and thereby inflammation.
Keywords: Aging, Adipose tissue, Senescence, Inflammation, Plasma factors
Adipose tissue is at the crossroad of longevity and age-associated diseases (1). Not only adipose tissue distributions change with age, but inflammation is primed at the adipose tissue prior to other organs during aging and onset of obesity (2). Moreover, interventions that delay or limit fat tissue turnover, redistribution, or dysfunction in experimental animals enhance life span (3–10). We recently demonstrated that elevated endoplasmic reticulum stress response promotes white adipose tissue inflammation (11) and diminished autophagy activity induces endoplasmic reticulum stress in aging adipose tissue (12). Understanding the molecular mechanism of adipose tissue inflammation and its reversal is crucial for therapeutic strategies for aging disorders.
Cellular senescence (SEN) is a fundamental aging mechanism that contributes to age-related organ dysfunction and chronic inflammation. The phenomenon of SEN is characterized by the upregulation of cyclin-dependent kinase inhibitor genes: p16 (Cdkn2a) and p21 (Cdkn1a/Cip1), along with display of senescence-associated secretary phenotypes. Recent evidences suggest that accumulation of senescent cell is a root cause of adipose tissue dysfunction and inflammation in aging (13). Manipulations that extend longevity also diminishes senescent cell burden in the aging adipose tissue (14). Our analyses on gonadal fat of aging male C57/Bl6 mice have demonstrated that TLR4-deficient mice were protected from senescence and inflammation (15). We further demonstrated that reduced adipose tissue inflammation in old TLR4-deficient mice was linked to reduced endoplasmic reticulum stress, augmented autophagy activity, and diminished senescence (15).
Heterochronic parabiosis experiments, in which young and old mice are surgically joined together for shared circulation, suggested that plasma factors can rejuvenate aging phenotypes (16,17). Recent research on parabiosis model also demonstrated that young plasma rescues old animals from age-associated anomalies in skeletal muscle (16,18), brain (19–21), and heart (22) and in immune (23) and kidney function (24). Recently, several tentative serum factors have been identified that regulate aging and SEN (22) including growth differentiation factor 11 (GDF11) (18,25) and eotaxin-1 (26,27) (CCL11). However, the exact mechanisms linking these factors to the aging phenotypes are often controversial and poorly defined. In this study, we embarked on to test whether young cellular milieu reverses senescence phenomenon and inflammation in the visceral adipose tissue (VAT) of old mice.
Experimental Methods
Mice
C57BL/6 young (4–6 months) and old (18–22 months) male mice were obtained from the Jackson Laboratory. Mice were maintained in a pathogen-free environment provided by the Unit for Laboratory Animal Medicine at the University of Michigan (Ann Arbor, MI) until they were used. All the experimental research in this study was approved by the University of Michigan University Committee on Use and Care of Animals.
Parabiosis Procedure
In general, mice that have same genetic background and similar body weight and size are selected to ensure harmonious cohabitation. In this study, however, different weight and size between young and old mice were inevitable. We used C57/BL6 male mice for our parabiosis studies. We surgically joined the circulation of young (Y-4 months) and old (O-18 months) mice for 4 weeks using the following procedure that was adopted from published methods (28).
The paired two animals were anesthetized using an injectable anesthetic (sodium pentobarbital, 40–60 mg/kg, ip), and the opposite side of each animal (right side of the left animal vs left side of the right animal) was shaved and cleaned with at least three iodine scrubs alternated with warmed saline, sterile water, or alcohol. As suggested by Institutional Animal Care and Use Committee (IACUC) reviewers that this was a type III surgery, carprofen (5 mg/kg, sc) was given preemptively and 48 hours postoperatively for each animal. An ophthalmic ointment was applied to prevent desiccation or corneal injury during the procedure.
A longitudinal skin incision from 0.5 cm above the elbow all the way to 0.5 cm below the knee joint was made using sharp scissors on the prepared side of each animal. The skin along the incision was gently separated from subcutaneous fascia using forceps. The right elbow and knee of the animal on the left were joined to the left elbow and knee of the animal on the right respectively using 2-0/3-0 silk sutures. The incision was closed with simple interrupted sutures on both ventral and dorsal sides. To prevent dehydration, 0.5 mL of warm saline was administered subcutaneously to each animal.
Animals were returned to their home cage with food pellets on the floor. In general, animals were able to adjust to the parabiotic existence after recovered from anesthesia, moving to eat and drink together. Skin sutures were removed under isoflurane (2% via a vaporizer) anesthesia 7 days after surgery. Blood chimerism usually occurs around 2 weeks following the surgery. Parabiotic state was maintained for 4 weeks. The mortality rate was 7%, 1 of total 14 pairs.
At the end of the experiment, the mice were killed to separate the pairs to collect plasma, VAT, liver, heart, brain, and muscle. The parabiosis experiment was repeated twice for a total of three experiments.
Isolation of Serum
Following CO2 euthanasia, blood samples were collected from young (4 month) or old (20 month) mice through retro-orbital plexus. Collected blood was kept on ice for 30-minute clotting and then centrifuged at 2,000 rpm for 15 minutes. The serum portion of blood was then collected and separated into aliquots for storage at −80°C until further usage.
Isolation of Adipose Tissue
Careful inspection was done to exclude aged animals with cancer or lymphoma. Gonadal fat pads from mice were excised under sterile condition. Fat tissue was fractionated into adipocyte and stromovascular fraction (SVF) portions, as described earlier (11).
Cell Culture and Treatment
3T3-preadipocytes (ATCC) or adipose tissue–derived old SVFs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) media containing 10% heat-inactivated fetal bovine serum (FBS) for 18 hours of incubation in a 37°C/5% CO2 incubator. Cells were then plated in 6-well dishes and cultured in presence of young or old plasma or fetal bovine serum (10% vol) for 3 days. Cells were then harvested for total RNA and protein lysates for analyses. Expression of mRNA was analyzed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and protein amounts by western blotting.
Cytokine Assays
Pro-inflammatory cytokines (MCP-1 and IL-6) in the adipose tissue lysates were analyzed by commercial standardized murine ELISA kits (R&D Systems).
Adipokine Array
The relative expressions of 38 mouse adipokines (ADKs) were analyzed by proteome profiler adipokine array kits (R&D Systems).
qRT-PCR
Total RNA was isolated by standard protocol for real-time quantitative PCR analysis. Relative expression of mRNA was calculated by 2^(−ddCt) method using Ct values of respective genes as published earlier (11,12). The primers used for respective genes are the following:
p16 (forward: 5′-CATGTTGTTGAGGCTAGAGAGG-3′; reverse: 5′-CACCGTAGTTGAGCAGAAGAG), p21 (forward: 5′-AAGTGTGCCGTTGTCTCTTC; reverse: 5′-AGTCAAAGTTCCACCGTTCTC),
Il-6 (forward: 5′-CTTCCATCCAGTTGCCTTCT; reverse: 5′-CTCCGACTTGTGAAGTGGTATAG),
Tnf-α (5′-TTGTCTACTCCCAGGTTCTCT; reverse: 5′-GAGGTTGACTTTCTCCTGGTATG-3′),
Mcp-1 (5′-CTCACCTGCTGCTACTCATTC-3′; reverse: 5′-ACTACAGCTTCTTTGGGACAC-3′)
Western Blotting
Protein expression was analyzed using standard western blotting techniques (29). The primary antibodies for p16 (ab108349) and p21 (ab109199) and α-tubulin (ab7291) were obtained from Abcam, and antibodies for stat3 (12640S), p-stat3 (94994) were obtained from Cell Signaling Technology.
Statistical Analysis
Statistical significance of the differences between means was tested using Student’s t test for two groups. For multiple comparison, Tukey’s post hoc test preceded by an analysis of variance was applied. GraphPad Prism7 software was used for all statistical analyses. Statistical significances were expressed as p < .05 (*), p < .01 (**), and p < .001 (***) in the figures.
Results
Increased Body Weight and VAT Mass in Young but No Change in Old Mice due to Heterochronic Parabiosis
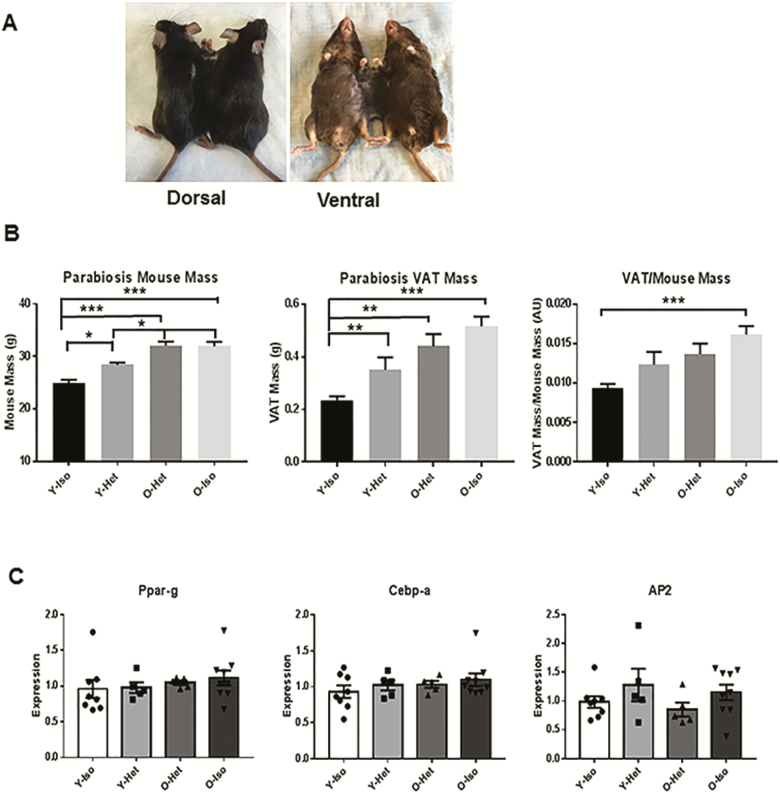
To test the role of young milieu on old VAT, we performed parabiosis experiments by surgically joining young and old (heterochronic), young and young (Y-isochronic), or old and old (O-isochronic) mice. Following 4 weeks of shared circulation, the mice were killed and we analyzed for total body weight and VAT weight of the parabionts. Compared to the Y-Iso group, we found significant differences in the body weight of Y-Het, O-Het, and O-Iso pairs (Figure 1B, first panel). Similarly, VAT weight was significantly lower in Y-Iso compared to the rest of the group (Figure 1B, middle panel). However, no significant difference was observed between O-Het and O-Iso groups, suggesting that young environment does not alter adiposity in the old mouse. On the other hand, differences in the body weight and VAT mass were found between Y-Iso and Y-Het groups, indicating that old plasma had an adipogenic effect on young VAT. These data also indicate that although young environment has no effects on adiposity or body weight, old plasma was capable of increasing VAT mass and body weight of young mice. We also observed significant difference in the VAT:body weight ratio between Y-Iso and O-Iso groups, and Y-Iso and Y-Het groups (Figure 1B, third panel). However, no significant difference was observed between O-Het or O-Iso groups. To address the adipogenic effect of old environment on the young VAT, we analyzed mRNA expression of known adipogenic genes (Ppar-g, Cebp-a, and Ap2) in all the groups, but did not find any significant differences in either of the groups (Figure 1C).
Figure 1.
Increased body weight (BW) and visceral adipose tissue (VAT) mass in young but no change in old mice because of heterochronic parabiosis. (A) Photographs of heterochronic pairs both dorsal and ventral views. (B) Graphical representation of body weight and VAT weight and the ratio of BW:VAT of young (4 month) isochronic (Y-Iso), young heterchronic (Y-Het), old (20 month) heterochronic (O-Het), and old isochronic (O-Iso) pairs (n = 12 each) following 4 weeks of parabiosis. Statistical significance was determined by Tukey’s multiple comparison test followed by analysis of variance and indicated as *p < .05, **p < .001, ***p < .0001. (C) Relative mRNA abundance of Ppar-g, Cebp-a, and Ap2 in the parabiosis groups were also plotted in the graph after normalization (n is represented by no. of dots) with Gapdh expression. Statistical significance was determined by paired t test.
Reduced Adipose Tissue Inflammation in the Old Mice Following Heterochronic Parabiosis
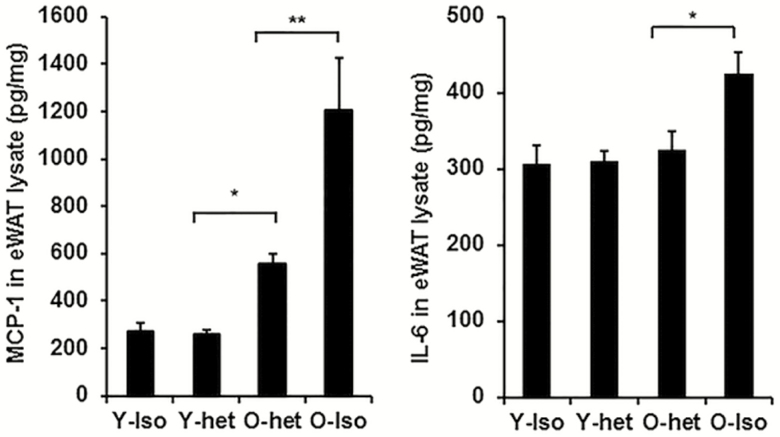
We then analyzed the levels of pro-inflammatory cytokines (MCP-1 and IL-6) in the VAT lysates (Figure 2). We found no difference in the levels of MCP-1 and IL-6 in Y-Iso and Y-Het groups. Notably, there were significant differences in the MCP-1 but not IL-6 levels between Y-Het and O-het groups, suggesting intrinsic inflammatory status in aging VAT. Importantly, both IL-6 and MCP-1 were significantly diminished in the O-Het compared to the O-Iso mice (Figure 2). Our data, therefore, suggest that sharing young environment reduces old VAT inflammation without affecting adiposity or body weight.
Figure 2.
Young plasma diminishes pro-inflammatory and senescence phenotypes in old adipose tissue in heterochronic parabiosis system. Mouse (C57/Bl6: male) adipose tissue samples were collected from either isochronic young (Y-iso) or old (O-iso), and heterochronic young (Y-het) or old (O-het) groups following 4 weeks of parabiosis. (A) Levels of MCP-1 and (B) IL-6 in the adipose tissue lysate as quantified by ELISA. Statistical significance was determined by paired t test (n = 6 for each group) and expressed as * p < .05 or **p < .001.
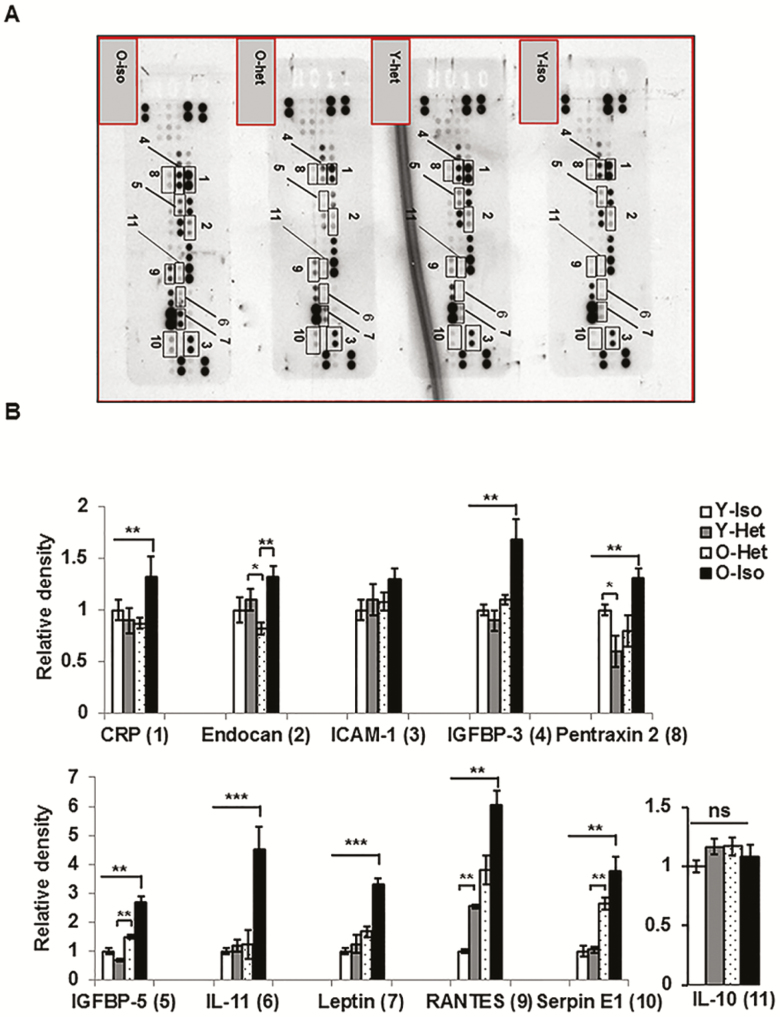
Diminished Abundance of SEN-Promoting ADKs in the Old Adipose Tissue Following Heterochronic Parabiosis
We performed adipokine array on VAT lysates from the parabionts and analyzed the relative abundance of 38 different ADKs. Array data on adipose tissue lysates revealed that the protein levels of 10 ADKs (Figure 3A and B) were significantly diminished in O-Het compared to O-Iso parabionts. These ADKs include CRP, endocan, intercellular adhesion molecule 1 (ICAM1), insulin-like growth factor binding protein 3 (IGFBP-3), insulin-like growth factor binding protein 5 (IGFBP-5), IL-11, leptin, pentraxin 2, regulated on activation normal T cell expressed and secreted (RANTES), and serpin E1/plasminogen activator inhibitor 1 (PAI-1). Importantly, the majority of the ADKs are tightly linked to SEN (30–35). Except for IGFBP-3, IGFBP-5, and RANTES, no significant differences are observed in the ADK profile between Y-Het and Y-Iso parabionts. These results strongly indicate that young plasma factors are able to preserve young ADK milieu in aging VAT. As IL-10 overexpression has been shown to decrease with aging and has a protective role in inflammation in skeletal muscle (36), we wanted to examine whether IL-10 has any role in reversing VAT inflammation. In our analyses of IL-10 from the adipokine array, we did not find any significant differences between O-Het or O-Iso parabionts (Figure 3), negating the role of IL-10 in the suppression of VAT inflammation in old. To further address the mechanism of reduced inflammation in the O-het, we also analyzed the STAT3 phosphorylation, and suppressor of cytokine signaling (SOCS) family of proteins in the VAT lysate of the parabiosis pairs. We observed reduced p-STAT levels in the O-Het compared to O-Iso, Y-Iso, or Y-het groups; however, these differences were not statistically significant (Supplementary Figure 1). Interestingly, we observed elevated SOCS2 and SOCS3 levels in the O-Het compared to O-Iso, but not significantly different from Y-Iso or Y-het groups (Supplementary Figure 2). This may explain the reduced inflammation in the O-Het compared to O-Iso groups, yet does not hold true for Y-Iso or Y-Het groups. Nevertheless, the difference might reflect the reduced inflammation in the O-Het group compared to the rest of the parabiosis pairs.
Figure 3.
Diminished abundance of cellular senescence–promoting adipokines (ADKs) protein in old adipose tissue following heterochronic parabiosis. (A) Representative image of three independent ADK arrays performed on visceral adipose tissue lysates from the parabionts. Proteins of interest are labeled with rectangles and adjacent numbers. The expression of each ADK band is normalized with internal reference spots and is plotted in (B). Statistical significances were determined by paired t test (n = 6 for each group) and expressed as *p < .05, **p < .001, ***p < .0001.
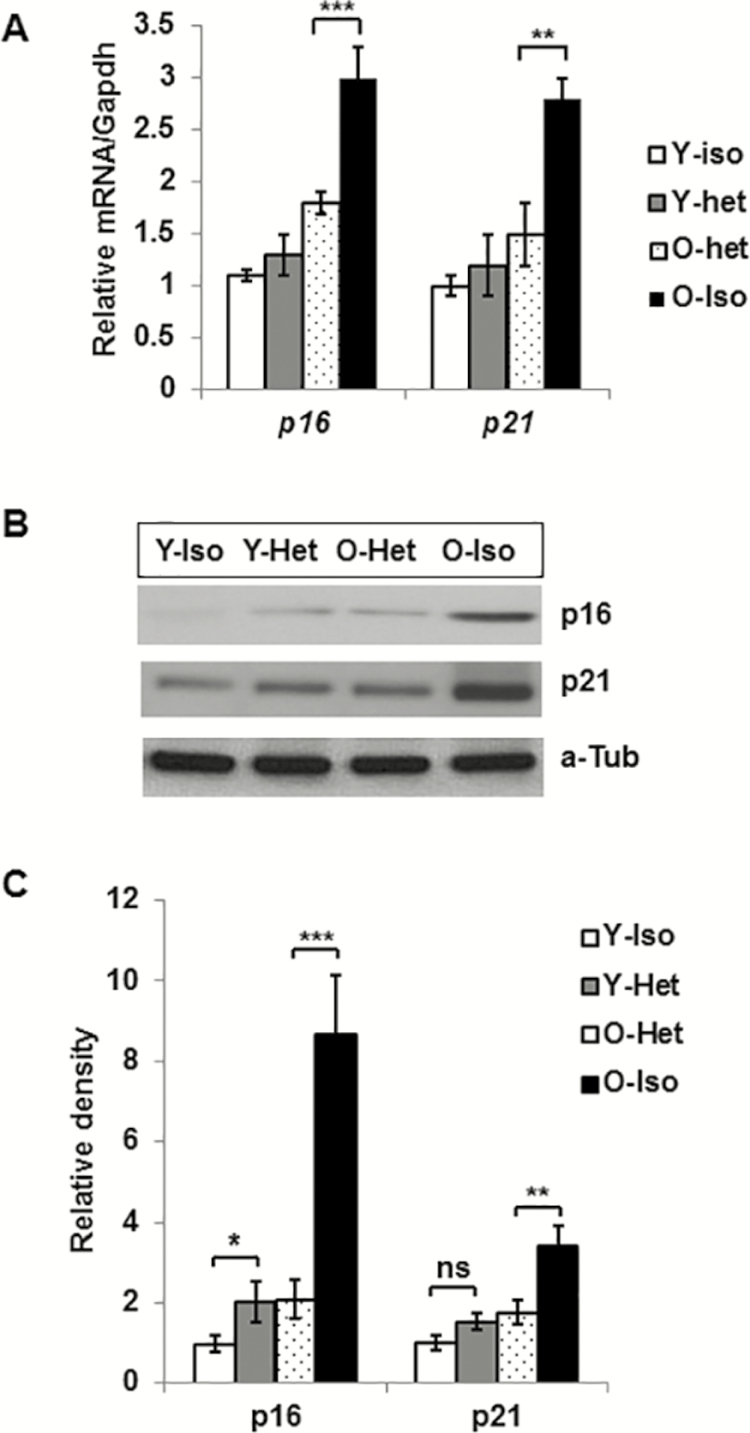
Reduced p16 and p21 Expression in Old VAT in Heterochronic Parabiosis
mRNA and protein expression of SEN-associated genes, p16 and p21, is significantly diminished in the VAT of O-Het compared to O-Iso parabionts (Figure 4). This supports the notion that young circulatory milieu not only reduces the expression of pro-inflammatory cytokines or SEN-promoting ADKs, but also limits the expression of genes directly associated with SEN induction.
Figure 4.
Reduced levels of p16 and p21 gene products in aging visceral adipose tissue following heterochronic parabiosis. (A) Graphical presentation of relative mRNA of p16 and p21 in different parabionts (n = 6 for each group). (B) Representative image of western blot analysis of p16 and p21 protein expression. (C) The relative protein abundance of p16 and p21 were expressed (n = 4) after the density was normalized with the respective α-Tubulin. Statistical significance was determined by paired t test and expressed as *p < .05, **p < .001.
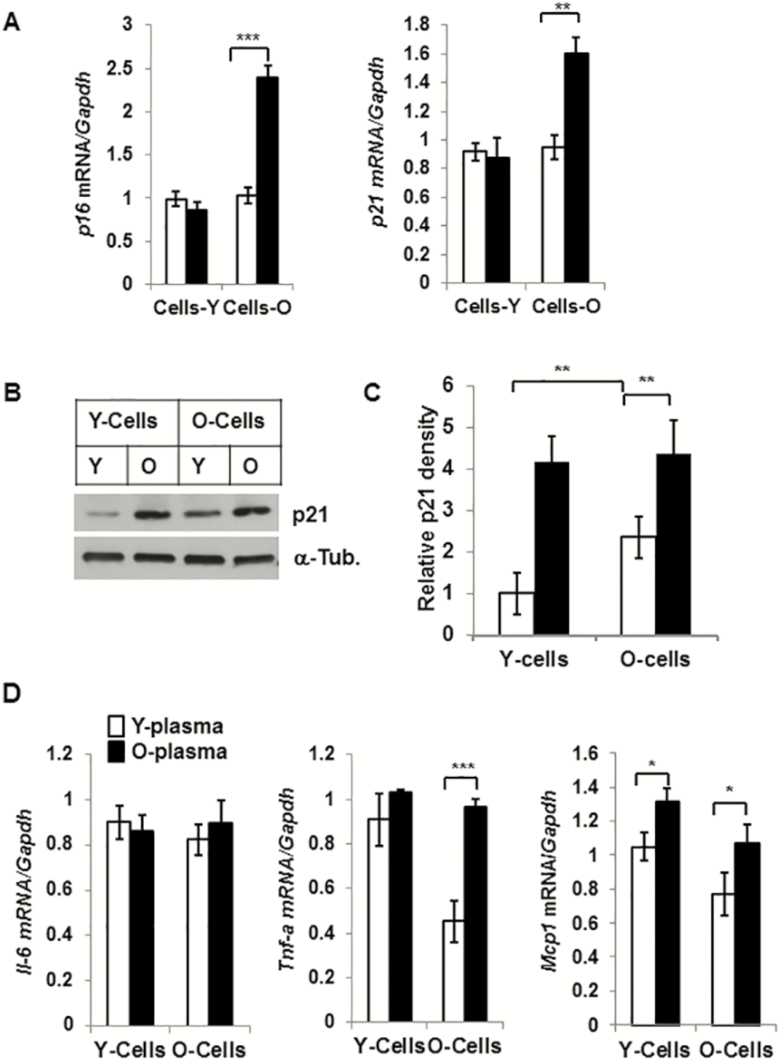
Reduced Expression of Senescence-Associated and Pro-inflammatory Genes in Old SVFs Cultured With Young Serum
Epididymal adipose tissue–derived SVFs from young (4 month) and old (20 month) mice were cultured in presence of young or old serum (10% vol/vol) for 3 days, then harvested for total RNA and protein lysates. The levels of p16 and p21 were significantly reduced, both in mRNA (Figure 5A) and in protein content (Figure 5B and C), compared to exposure to old serum condition. In addition, mRNA of Tnf-α and Mcp-1 but not Il-6 in the old SVFs was significantly diminished in the presence of young serum (Figure 5D). Taken together, these data further support that young serum is capable of reducing aging SVFs from SEN and pro-inflammatory phenotype.
Figure 5.
Reduced expression of senescence-associated and pro-inflammatory genes in old stromovascular fractions (SVFs) cultured with young plasma. Epididymal adipose tissue–derived SVFs from young (4 months) and old (20 months) were cultured in presence of young or old serum (10%) for 3 days and were harvested for total RNA and protein lysates. mRNA expression of p16 and p21 was analyzed by quantitative reverse transcription-polymerase chain reaction and plotted in (A) and relative protein abundance (n = 3) was measured after the density was normalized with the respective α-Tubulin band (B and C). Relative mRNA abundance of Il-6, Tnf-α and Mcp-1 was also plotted in the graph (D). The significance levels *p < .05 or **p < .01 or ***p < .001 were determined by Student’s t test using means and standard error of the mean of three independent (n = 3) experiments.
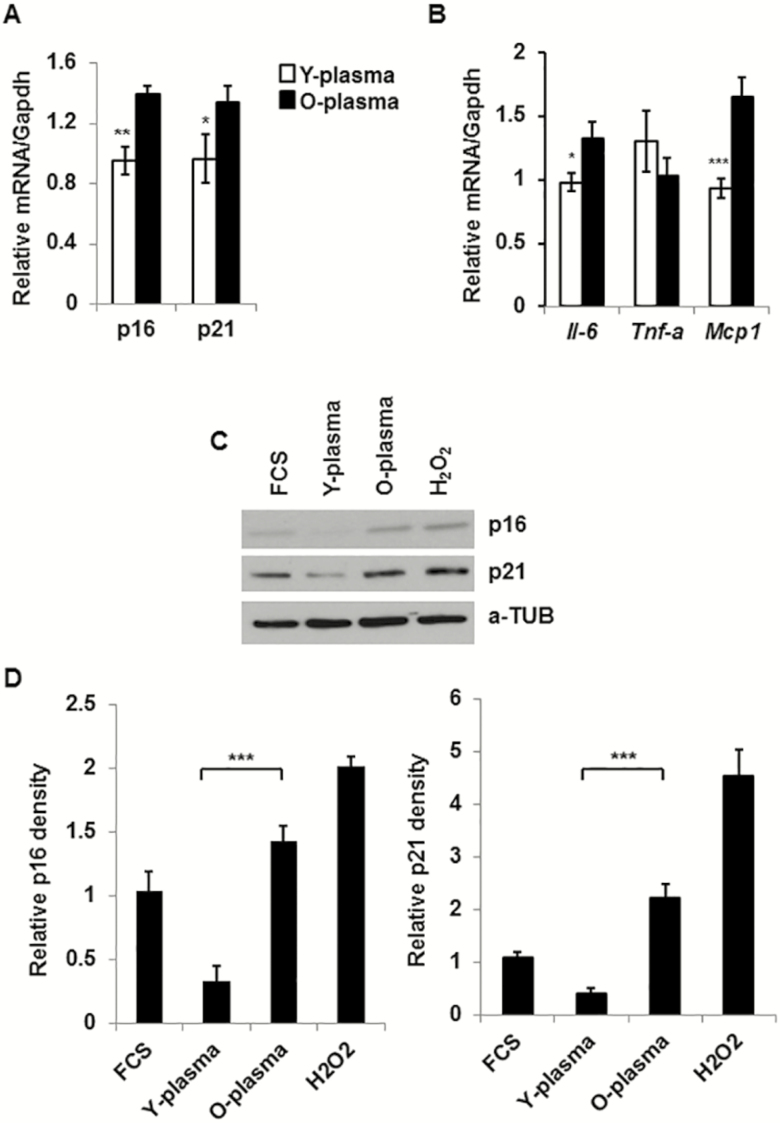
Expression of CDKs and Pro-inflammatory Cytokine Genes Are Diminished Both in 3T3-Preadipocytes and in Old SVFs Cultured With Young Serum
In 3T3-preadipocytes culture system, expressions of both p16 and p21 gene products were significantly reduced both in mRNA (Figure 6A) and in protein levels (Figure 6C) in the presence of young compared to old serum. Cells were also treated with H2O2, as a known inducer of senescence that served as a positive control in our experiment. Cells were also cultured in the presence of 10% fetal bovine serum, as an additional control for serum in the analysis. Expression of pro-inflammatory cytokines IL-6 and Mcp-1 but not Tnf-α mRNA was also reduced by the young compared to old serum condition (Figure 6B).
Figure 6.
Reduced expression of pro-inflammatory and cellular senescence genes in 3T3-preadipocytes and in primary stromovascular fractions (SVFs), cultured in the presence of young plasma. 3T3-preadipocytes or adipose tissue–derived old SVFs were cultured in the presence of young or old plasma (10%) for 3 days. Cells were harvested for total RNA and protein lysates. Expression of mRNA was analyzed by quantitative reverse transcription-polymerase chain reaction and protein analysis by western blotting. (A) Relative mRNA expressions of p16 (Cdkn2a) and p21 (Cdkn1a/Cip1) were evaluated after normalization with Gapdh (n = 4). (B) Relative expression of Il-6, Tnf-α, and Mcp-1 was expressed following normalization with Gapdh expression (n = 4). (C) Representative image of protein expressions of p16 and p21 in 3T3-preadipocyte lysates under different conditions. The relative protein abundance was shown in graph (D) after the density was normalized with the respective α-Tubulin band (n = 3). The significance levels *p < .05 or **p < .01 or ***p <.001 were determined by Student’s t test using means and standard error of the mean of three independent experiments.
Discussion
A fundamental aging mechanism that likely contributes to chronic diseases and age-related dysfunction is SEN (37). SEN is a terminal arrest of proliferation when cells are subjected to various cellular stresses including telomere dysfunction, DNA damage, and genotoxic and oxidative stress (38–40). Senescent cells have been demonstrated to disrupt tissue structure and function through the secretion of pro-inflammatory cytokines and chemokines and proteases, a feature known as senescence-associated secretory phenotype (41). The senescence-associated secretory phenotype is believed to serve as a link between senescent cell accumulation and local and systemic dysfunction and diseases. Consistence with a role for SEN in causing age-related organ dysfunction, clearing senescence cells by drug-inducible suicide gene enhances health span and delays multiple age-related phenotypes in genetically modified progeroid mice (42). Thus, interventions that reduce the burden of senescent cells could ameliorate age-related disabilities and chronic diseases (43,44). Targeting senescent cells in aging adipose tissue for therapeutic intervention is supported by evidence of reduced SEN burden in long-lived mouse models (14), and also by evidence of improved health span by targeting SEN cells using drugs (senolytics) in mouse models (42,44,45). The use of senolytics is potentially promising, but long-term use of these drugs may not be suitable because of their off-target and adverse side effects (46,47).
Recent heterochronic parabiosis studies strongly suggest that circulating factors in the young plasma alter aging phenotypes in multiple organs (16,18,48,49). However, the specific plasma factor and underlying mechanisms have not been elucidated. This study demonstrates for the first time that young blood circulation reverses the senescence characteristics as well as pro-inflammatory phenotypes of old adipose tissue. This phenomenon was supported by the heterochronic parabiosis experiments (Figures 2–4), ex vivo treatment of SVFs with serum from young and old mice (Figure 5), and 3T3-preadipocyte culture system (Figure 6).
By pairing young with old mice in heterochronic parabiosis, we demonstrated reduced expression of both p16 and p21 gene products in old VAT, overexpression of which are considered to be characteristic of SEN. In addition, profiles of 10 ADKs of O-het were also reduced compared with the O-Iso and without significant effects on the Y-het groups. These ADKs are CRP, endocan, ICAM1, IGFBP-3, IGFBP-5, IL-11, leptin, pentraxin 2, RANTES, and serpin E1/PAI-1. Of note, the majority of these ADKs are either cause or consequence of SEN (30–35). In order to rule out the possibility of IL-10-mediated suppression of inflammation in O-Het parabionts, we analyzed the levels of IL-10, using the ADK array data and found no significant differences among all four groups. To further address the mechanism of reduced inflammation in the O-het, we also analyzed the STAT3 phosphorylation and SOCS family of proteins as downstream target of cytokine signaling in the VAT lysate of the parabiosis pairs. We observed reduced p-STAT3 signal; however, these differences were not statistically significant (Supplementary Figure 1). Interestingly, we found significantly elevated SOCS2 and SOCS3 levels in the O-Het compared to O-Iso groups, but not in the Y-Iso or Y-het groups (Supplementary Figure 2). This difference in SOCS2 and SOCS3 levels explains the reduced inflammation in the O-Het compared to O-Iso groups, but does not address an equation in the context of Y-Iso or Y-Het groups. We reconcile that the young cells have their own intrinsic mechanism of suppression of cytokine signaling networks that differ from aging cells. Nevertheless, the shuttle difference in p-STAT3 and SOCS levels in the O-Het might reflect the reduced inflammation in the O-Het group.
Therefore, aging environment provides a cellular context that is conducive to senescence in old VAT, but which can still be reversed in the presence of young environment. This observation was further supported by the follow-up experiments using ex vivo culture of SVFs and in vitro culture of 3T3-preadipocytes. Our observation is in agreement with the recent observations that serum from calorie-restricted animal delays senescence and extends life span of normal human fibroblast (50), and young plasma reverses age-dependent senescence in the hepatic tissue (51). More importantly, it should be noted that because heterochronic parabiosis in reality leads to a “dilution effect” for many shared factors, that effects could conceivably be due to absolute less exposure to geronic factors in the old blood by dilution with young blood, which may contain much lower levels of these factors, such as cytokines.
We provide evidence that like many other organs, senescence in the VAT of old mice was also diminished by sharing circulation with young. However, the mechanism of this reversal needs further investigation.
Our previous works have demonstrated impaired autophagy in aging VAT as one of the causes of adipose tissue inflammation. It is possible that the beneficial effect of young circulating factors could be mediated via the restoration of autophagy-mediated protein degradation. In support of this, it was recently demonstrated that young plasma restores autophagy in rat liver (51). We cannot rule out the possibility that the circulatory factor(s) may induce epigenetic alterations in the aging VAT. Recent reports also suggested plasma factors can alter nuclear concentration of DNA methyltransferases (52) and histone deacetyletranferases, including silent mating type information regulation 2 homolog 1 (50).
Funding
This project has been funded partly by National Institutes of Health grants AG020628, AG028268, HL58984 and University of Michigan Research Career Development core (RCDC) and Claude D. Pepper Older American Independence Center (OAIC) grant (AG024824 to A.K.G.). This work has also been supported partially by grants DK020572 (MDRC), DK089503 (MNORC), and 1U2CDK110678-01 (UM-MMPC).
Conflict of interest statement
None declared.
Supplementary Material
References
- 1. Cartwright MJ, Schlauch K, Lenburg ME, et al. Aging, depot origin, and preadipocyte gene expression. J Gerontol A Biol Sci Med Sci. 2010;65:242–251. doi: 10.1093/gerona/glp213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van der Heijden RA, Sheedfar F, Morrison MC, et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging (Albany NY). 2015;7:256–268. doi: 10.18632/aging.100738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barzilai N, Gupta G. Revisiting the role of fat mass in the life extension induced by caloric restriction. J Gerontol A Biol Sci Med Sci. 1999;54:B89–B96; discussion B97. [DOI] [PubMed] [Google Scholar]
- 4. Masoro EJ. Caloric restriction and aging: controversial issues. J Gerontol A Biol Sci Med Sci. 2006;61:14–19. [DOI] [PubMed] [Google Scholar]
- 5. Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223299/5606/572 [DOI] [PubMed] [Google Scholar]
- 6. Selman C, Tullet JM, Wieser D, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang GR, Chiu YS, Wu YY, et al. Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J Pharmacol Sci. 2009;109:496–503. doi:JST.JSTAGE/jphs/08215FP [DOI] [PubMed] [Google Scholar]
- 8. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muzumdar R, Allison DB, Huffman DM, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menon V, Zhi X, Hossain T, et al. The contribution of visceral fat to improved insulin signaling in Ames dwarf mice. Aging Cell. 2014;13:497–506. doi: 10.1111/acel.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghosh AK, Garg SK, Mau T, O’Brien M, Liu J, Yung R. Elevated endoplasmic reticulum stress response contributes to adipose tissue inflammation in aging. J Gerontol A Biol Sci Med Sci. 2015;70:1320–1329. doi: 10.1093/gerona/glu186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghosh AK, Mau T, O’Brien M, Garg S, Yung R. Impaired autophagy activity is linked to elevated ER-stress and inflammation in aging adipose tissue. Aging (Albany NY). 2016;8:2525–2537. doi: 10.18632/aging.101083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stout MB, Tchkonia T, Pirtskhalava T, et al. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY). 2014;6:575–586. doi: 10.18632/aging.100681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghosh AK, O’Brien M, Mau T, Yung R. Toll-like receptor 4 (TLR4) deficient mice are protected from adipose tissue inflammation in aging. Aging (Albany NY). 2017;9:1971–1982. doi: 10.18632/aging.101288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mccay CM, Pope F, Lunsford W, Sperling G, Sambhavaphol P. Parabiosis between old and young rats. Gerontologia. 1957;1:7–17. [DOI] [PubMed] [Google Scholar]
- 18. Sinha M, Jang YC, Oh J, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith LK, He Y, Park JS, et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21:932–937. doi: 10.1038/nm.3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bouchard J, Villeda SA. Aging and brain rejuvenation as systemic events. J Neurochem. 2015;132:5–19. doi: 10.1111/jnc.12969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. doi: 10.1038/nm.3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzalez-Valdes I, Hidalgo I, Bujarrabal A, et al. Bmi1 limits dilated cardiomyopathy and heart failure by inhibiting cardiac senescence. Nat Commun. 2015; 6:6473. doi: 10.1038/ncomms7473 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Kim MJ, Miller CM, Shadrach JL, Wagers AJ, Serwold T. Young, proliferative thymic epithelial cells engraft and function in aging thymuses. J Immunol. 2015;194:4784–4795. doi: 10.4049/jimmunol.1403158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang Q, Ning Y, Liu D, et al. A young blood environment decreases aging of senile mice kidneys. J Gerontol A Biol Sci Med Sci. 2018;73:421–428. doi: 10.1093/gerona/glx183 [DOI] [PubMed] [Google Scholar]
- 25. Egerman MA, Cadena SM, Gilbert JA, et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22:164–174. doi: 10.1016/j.cmet.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Panizzutti B, Gubert C, Schuh AL, et al. Increased serum levels of eotaxin/CCL11 in late-stage patients with bipolar disorder: an accelerated aging biomarker? J Affect Disord. 2015;182:64–69. doi: 10.1016/j.jad.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 27. Hoefer J, Luger M, Dal-Pont C, Culig Z, Schennach H, Jochberger S. The “aging factor” eotaxin-1 (CCL11) is detectable in transfusion blood products and increases with the donor’s age. Front Aging Neurosci. 2017;9:402. doi: 10.3389/fnagi.2017.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamran P, Sereti KI, Zhao P, Ali SR, Weissman IL, Ardehali R. Parabiosis in mice: a detailed protocol. J Vis Exp. 2013;80. doi: 10.3791/50556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghosh AK, Brindisi M, Yen YC, et al. Structure-based design, synthesis and biological evaluation of novel β-secretase inhibitors containing a pyrazole or thiazole moiety as the P3 ligand. Bioorg Med Chem Lett. 2015;25:668–672. doi: 10.1016/j.bmcl.2014.11.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim KS, Kim MS, Seu YB, Chung HY, Kim JH, Kim JR. Regulation of replicative senescence by insulin-like growth factor-binding protein 3 in human umbilical vein endothelial cells. Aging Cell. 2007;6:535–545. doi: 10.1111/j.1474-9726.2007.00315.x [DOI] [PubMed] [Google Scholar]
- 31. Kim KS, Seu YB, Baek SH, et al. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol Biol Cell. 2007;18:4543–4552. doi: 10.1091/mbc.e07-03-0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pascal T, Debacq-Chainiaux F, Boilan E, Ninane N, Raes M, Toussaint O. Heme oxygenase-1 and interleukin-11 are overexpressed in stress-induced premature senescence of human WI-38 fibroblasts induced by tert-butylhydroperoxide and ethanol. Biogerontology. 2007;8:409–422. doi: 10.1007/s10522-007-9084-8 [DOI] [PubMed] [Google Scholar]
- 33. Gorgoulis VG, Pratsinis H, Zacharatos P, et al. p53-dependent ICAM-1 overexpression in senescent human cells identified in atherosclerotic lesions. Lab Invest. 2005;85:502–511. doi: 10.1038/labinvest.3700241 [DOI] [PubMed] [Google Scholar]
- 34. Zhao X, Dong Y, Zhang J, et al. Leptin changes differentiation fate and induces senescence in chondrogenic progenitor cells. Cell Death Dis. 2016;7:e2188. doi: 10.1038/cddis.2016.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dimri GP, Itahana K, Acosta M, Campisi J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol Cell Biol. 2000;20:273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dagdeviren S, Jung DY, Friedline RH, et al. IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. FASEB J. 2017;31:701–710. doi: 10.1096/fj.201600832R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kirkland JL, Tchkonia T. Clinical strategies and animal models for developing senolytic agents. Exp Gerontol. 2015;68:19–25. doi: 10.1016/j.exger.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gewirtz DA. Autophagy and senescence: a partnership in search of definition. Autophagy. 2013;9:808–812. doi: 10.4161/auto.23922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mech Ageing Dev. 2008;129:467–474. doi: 10.1016/j.mad.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kang C, Elledge SJ. How autophagy both activates and inhibits cellular senescence. Autophagy. 2016;12:898–899. doi: 10.1080/15548627.2015.1121361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- 42. Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Breccia M, Molica M, Alimena G. How tyrosine kinase inhibitors impair metabolism and endocrine system function: a systematic updated review. Leuk Res. 2014;38:1392–1398. doi: 10.1016/j.leukres.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 47. Demaria M, Ohtani N, Youssef SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260 [DOI] [PubMed] [Google Scholar]
- 49. Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Cabo R, Liu L, Ali A, et al. Serum from calorie-restricted animals delays senescence and extends the lifespan of normal human fibroblasts in vitro. Aging (Albany NY). 2015;7:152–166. doi: 10.18632/aging.100719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu A, Guo E, Yang J, et al. Young plasma reverses age-dependent alterations in hepatic function through the restoration of autophagy. Aging Cell. 2018;17:1–13. doi: 10.1111/acel.12708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Horsburgh S, Todryk S, Toms C, Moran CN, Ansley L. Exercise-conditioned plasma attenuates nuclear concentrations of DNA methyltransferase 3B in human peripheral blood mononuclear cells. Physiol Rep. 2015;3:1–10. doi: 10.14814/phy2.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.