Abstract
Background
The movement profile of older adults with compromised function is unknown, as is the relationship between these profiles and the development of major mobility disability (MMD)—a critical clinical outcome. We first describe the dimensions of movement in older adults with compromised function and then examine whether these dimensions predict the onset of MMD.
Methods
Older adults at risk for MMD (N = 1,022, mean age = 78.7 years) were randomized to receive a structured physical activity intervention or health education control. We assessed MMD in 6-month intervals (average follow-up of 2.2 years until incident MMD), with activity assessed at baseline, 6-, 12- and 24-month follow-up via accelerometry.
Results
A principal components analysis of 11 accelerometer-derived metrics yielded three components representing lifestyle movement (LM), extended bouts of moderate-to-vigorous physical activity (MVPA), and stationary body posture. LM accounted for the greatest proportion of variance in movement (53%). Within health education, both baseline LM (HR = 0.74; 95% CI 0.62 to 0.88) and moderate-to-vigorous physical activity (HR = 0.69; 95% CI 0.54 to 0.87) were associated with MMD, whereas only LM was associated with MMD within physical activity (HR = 0.74; 95% CI 0.61 to 0.89). There were similar nonlinear relationships present for LM in both physical activity and health education (p < .04), whereby risk for MMD was lower among individuals with higher levels of LM.
Conclusions
Both LM and moderate-to-vigorous physical activity should be central in treatment regimens for older adults at risk for MMD.
Trial Registration
clinicaltrials.gov Identifier NCT01072500
Keywords: Exercise, Accelerometry, Physical activity, Disability, Sedentary
Disability in the United States due to mobility limitations (ie, self-reported difficulty walking or climbing stairs) represents a major public health challenge, particularly among older adults for whom the prevalence rate in 2016 was 22.6% (1). Magnifying this problem are two trends. First, recent projections indicate by 2035, older adults will begin to outnumber children, and this disparity will escalate to 14.8 million by 2060 (2). Second, the rate of disability is increasing among middle-aged adults because of obesity (3,4). Disability confers enormous psychosocial and economic costs and leads to higher rates of morbidity and mortality (5–7). Leveraging accelerometry data collected during the LIFE Study (8), this investigation provides a detailed and interpretable characterization of patterns of movement among older adults with mobility limitations with the goal of examining how different movement profiles affect the risk for developing major mobility disability (MMD). We chose an analytical approach that we felt would optimize the utility of our findings for the development of future movement-related interventions.
There is considerable evidence that inactivity is a major risk factor for the incidence of MMD during aging (9,10). The LIFE Study aimed to determine if structured physical activity (PA) could prevent persons at high risk for MMD from transitioning to a state of MMD (11). The results were encouraging in that over an average of 2.6 years of follow-up, PA significantly reduced both incident and persistent MMD as compared to health education (HE). However, intriguing and unanswered questions remain. For example, how much lifestyle-related movement (variability and amount of non-exercise PA, excluding extended bouts of moderate-to-vigorous PA [MVPA]) do older adults with limitations in mobility engage in? Does lifestyle movement (LM), alongside time spent standing still rather than sitting or lying, reduce one’s risk for MMD, and do these dimensions of movement respond to a structured PA intervention? There is a growing body of evidence demonstrating the negative impact of sustained sedentary behavior (SB; low energy expenditure [≤1.5 metabolic equivalents] and a seated or laying body position) on health (12) and physical functioning (13,14). Consistent with a broader conceptualization of movement behavior, researchers are now arguing against the use of single metrics (eg, minutes of MVPA) in accelerometry analyses in favor of analytic approaches capturing multiple facets of movement and their relationship to health outcomes (15).
The first aim of this study is to characterize the major dimensions of movement for older adults with limitations in mobility, and the extent to which these dimensions change in response to an exercise intervention. We hypothesized that participation in MVPA would increase in response to a structured PA intervention, but that other dimensions of movement would be unaffected. Second, by examining data on participants in the HE group, we aimed to evaluate whether there were prospective relationships between baseline scores on each movement dimension and the development of MMD during the normal course of aging. Given that LM is replaced by time spent sitting as individuals age and should account for the majority of time spent in PA (16), we hypothesized that low levels of LM and more time spent in SB would predict the development of MMD independent of MVPA. The study design also enabled us to examine these relationships among persons engaged in structured PA.
Methods
Trial Design and Participants
The methods for the LIFE Study have been published in detail elsewhere (8,11). Briefly, LIFE was an eight-center, single blind, randomized clinical trial conducted between February 2010 and December 2013. Mass mailing was the primary means of recruitment. Eligible participants were 70–89 years of age; low-active; at high risk for MMD; able to walk 400 m in less than 15 minutes without help; had no major cognitive impairment; and able to safely participate in the intervention as assessed via medical history, physical examination, and resting electrocardiograph. For details on inclusion and exclusion, see Fielding and colleagues (8).
Randomization
The LIFE Study involved randomization with stratification by field center and sex to either PA or a HE control group. Both groups received a face-to-face orientation appointment with a trained health educator to review details of their intervention assignment, discuss expectations, and answer any outstanding questions.
Interventions
The multicomponent PA intervention focused primarily on walking, with a goal of 150 min/wk of MVPA, plus 10 minutes of balance and stretching and 10 minutes of primarily lower-body strengthening activity. Participants attended center-based sessions twice weekly and aimed to achieve an additional 3–4 PA sessions each week for the duration of the study. Participants began activities at a light intensity and progressed over the first 2–3 weeks of training to a rating of perceived exertion (17) of 13/20 while walking (somewhat hard), and 15–16 during strength training (hard). The HE program covered topics related to healthy aging excluding exercise-related topics. The program also included 5–10 minutes of gentle upper-extremity stretching exercises. Participants attended these sessions weekly for the initial 26 weeks of the study and monthly thereafter.
Measures
Major mobility disability
MMD was a dichotomous variable defined as the inability to complete a 400 m walk test within 15 minutes. This test was completed at baseline and at 6-month follow-up intervals (18).
Actigraphy
We instructed participants to wear an ActiGraph GT3X accelerometer (ActiGraph, Pensacola, FL) on the right hip for seven consecutive days. We examined accelerometer data collected at baseline, 6-, 12-, and 24-month visits. Data were processed using ActiLife (v4.4.1). Wear time was calculated using the Choi and colleagues (19) algorithm, classifying non-wear as 90-minute periods of zero counts per minute (CPM) with a 1-minute spike tolerance. Manual data cleaning procedures were used to leverage the device’s inclinometer, enabling us to distinguish SB from non-SB, including standing still. We removed days averaging more than 10 lying-to-standing transitions/hour, and followed this with a sensitivity analysis implementing a more stringent cut point more than 2 lying-to-standing transitions per hour (see Supplementary Material, “Accelerometer Processing” section for rationale and description). Individuals with less than 3 valid days of accelerometer data were removed from analyses, resulting in a total of 1,022 individuals at baseline (835.2 min/d on 7.5 days on average), and 1,053 individuals at month 6 (825.1 min/d on 6.6 days on average); 690 of these had valid data at both baseline and month 6. Investigation of participant characteristics revealed no meaningful differences between those with and without valid accelerometer data. We computed 14 metrics, including average hourly postural shifts (ie, number of transitions from SB [laying or sitting body posture, ≤100 CPM] to non-SB), counts/hour, minute/hour of SB, light-intensity physical activity (LPA), and MVPA (20); average number of 10 min bouts/d of MVPA; variance in transitions, counts, LPA, and MVPA; and variance in consecutive hourly differences in LPA and MVPA where lower variability represented greater consistency in movement. See Supplementary Material, “Accelerometer Processing” for detailed description of all accelerometer calculations.
Analyses
At baseline, 65% of participants had less than 1 min/h of MVPA, and 95% had less than 3 min/h, resulting in little variability among participants. Consequently, we dropped MVPA minute/hour and associated variances from further consideration. With the remaining 11 metrics, we conducted a principal components analysis (PCA) with varimax rotation, selecting components with eigenvalues more than 1.0 for further analyses. For the selected principal components, we calculated scores at baseline, 6-, 12-, and 24-months using the baseline PCA coefficients, allowing for an examination of the shift in the distribution of these scores within intervention groups using mixed effects models and kernel density plots.
Next, we explored associations between baseline PCA scores and future risk of MMD using Cox proportional hazards regression, stratifying baseline hazard on sex. Within intervention groups, we initially fit a model that contained all three continuous component scores as predictors, and subsequently added selected baseline covariates including the following: age, body mass index, race/ethnicity, short physical performance battery (21) score (<8, ≥8), and history of high blood pressure, diabetes, cardiovascular disease, arthritis, lung disease, and fractures. In the models containing covariates, we explored for nonlinearity in the relationship between component scores and MMD over the continuous range of each component by adding terms for third-degree B-spline curves, allowing for fitting of smoothed nonlinear relationships. We tested for statistical significance of these nonlinear components using likelihood ratio tests. We fit all models within each intervention group to allow for separate examination of the relationship between movement and MMD both in normal aging, and following a structured PA regimen. Finally, recognizing concerns over validity of the inclinometer signal (22), we recomputed all metrics except standing still minute/hour without the use of the inclinometer as follows: less than 100 CPM was classified as SB (23), LPA was classified as 100–1040 CPM, at least 1041 CPM defined MVPA (20), and moving from SB to LPA or MVPA defined a transition. As results did not differ meaningfully except that standing still was excluded, we elected to present these analyses in the Supplementary Tables 2 and 4, Figures 2 and 4), and continue with inclinometer-calculated variables to retain the standing still metric.
Results
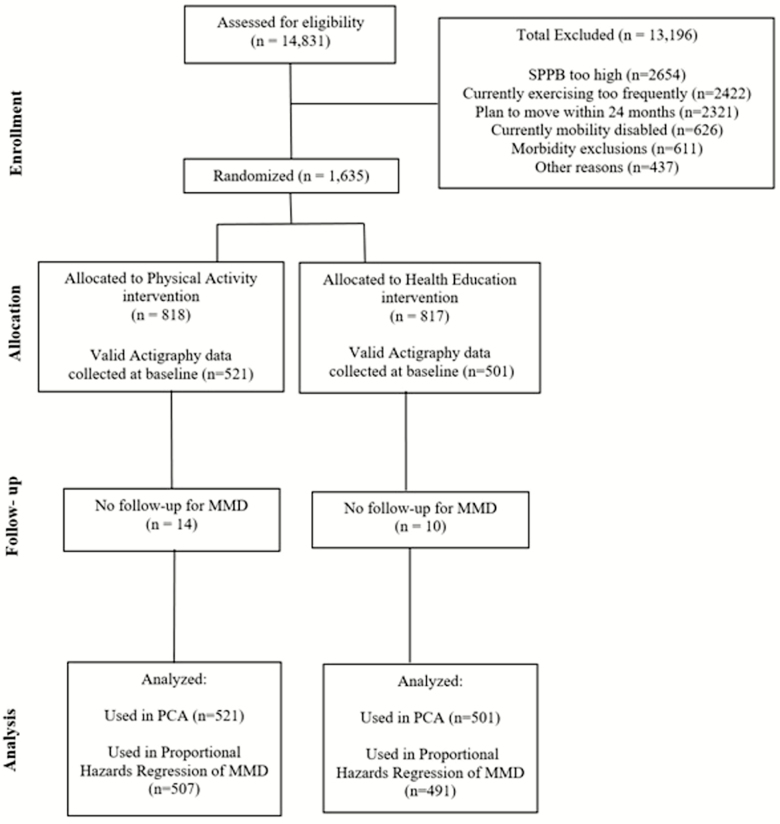
Of the 14,831 individuals assessed for eligibility, we randomized 1,635 to PA or HE. Valid baseline accelerometer data were available from 1,022 individuals (835.2 min/d on 7.5 days on average; Figure 1). Average participant age was 78.7 years, 67% were women, and 24% came from minority racial groups (Table 1) with an average follow-up time for MMD (time until censoring or an event) of 2.2 years. The group had compromised physical function with an average short physical performance battery score of 7.4±1.6 and an average 400 m walk time of 506.3±110.5 seconds. They were also characterized by considerable comorbidity.
Figure 1.
Study flow
Table 1.
Baseline Characteristics
| Overall (N = 1,022) | |
|---|---|
| Age (y, SD) | 78.6, 5.3 |
| Sex | |
| Female (n, %) | 685, 67 |
| Race/ethnicity | |
| White (n, %) | 777, 76 |
| African American/black (n, %) | 147, 18 |
| Hispanic (n, %) | 23, 3 |
| Other (n, %) | 25, 3 |
| Education | |
| ≥College (n, %) | 551, 67 |
| SPPB (mean, SD) | 7.4 (1.6) |
| 400 m walk time (s, SD) | 506.3 (110.5) |
| 400 m walk speed (m/s, SD) | 0.83 (.16) |
| Body mass index (kg/m2, SD) | 30.4, 6.1 |
| Diabetes (n, %) | 192, 23 |
| Heart failure/congestive heart failure (n, %) | 23, 3 |
| Myocardial infarction (n, %) | 49, 6 |
| Stroke (n, %) | 50, 6 |
Characterizing Movement in Older Adults With Limitations in Mobility
Preliminary screening resulted in a final set of 11 variables, with a PCA yielding three independent dimensions with eigenvalues more than 1.0, capturing 84.2% of the variance in these items. The first component represented LM, capturing amount and variability in movement and transitions excluding sustained bouts of MVPA, and explaining 53% of total variance. The remaining components represent sustained bouts of MVPA (hereafter referred to as MVPA; accounting for 17.4% of variance), and body posture, which contrasted time spent standing versus sitting or lying down and accounted for 13.8% of variance explained. Table 2 provides a description of these variables with their component loadings; higher scores represent greater volumes of LM, MVPA, and standing versus sitting/lying. Similar initial and second components were obtained when variables were processed without the inclinometer and using a transitions cut point of 2 (see Supplementary Tables 2 and 3). Likewise, analyses yielded similar results when we conducted the PCA separately for women and men (see Supplementary Tables 4 and 5).
Table 2.
Varimax-Rotated Component Loadings at Baseline
| Metric | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| Sedentary time: minutes/hour sitting or lying down calculated for each day and then averaged over a week | −0.48584 | −0.25608 | −0.82564 |
| Standing still time: minutes/hour calculated for each day and then averaged over a week | −0.06086 | −0.09255 | 0.98313 |
| Light activity time: minutes/hour spent in light activity (time between sedentary and MVPA levels) calculated for each day and averaged over a week | 0.89898 | 0.30534 | 0.09983 |
| Variability in light activity level: Square root of variance of minutes/hour of in light activity (time between sedentary and MVPA levels) calculated for each day and then averaged over a week | 0.89515 | 0.23585 | −0.01156 |
| Variability in light activity differences: Square root of variance of consecutive differences in minutes per hour of light activity (time between sedentary and MVPA levels) calculated for each day and then averaged over a week | 0.88116 | 0.22712 | −0.02199 |
| Transitions: Average transitions/hour calculated for each day and averaged over a week | 0.84115 | 0.13589 | 0.21232 |
| Variability in transitions: Square root of variance of transitions/hour calculated for each day and then averaged over the days in the week | 0.76491 | −0.05186 | 0.12755 |
| Activity level: Average counts/hour calculated for each day and averaged over a week | 0.55403 | 0.75303 | 0.00288 |
| Variability in activity level: Square root of variance of counts/hour calculated for each day and then averaged over a week | 0.22280 | 0.91332 | 0.01719 |
| Bouts of standing and moving: Average number of 10 min bouts standing and moving/day | 0.51480 | 0.73402 | −0.01113 |
| Bouts of MVPA: Average number of 10 min bouts of MVPA/day | −0.13094 | 0.87569 | 0.06764 |
| Eigenvalue | 5.8 | 1.9 | 1.5 |
| Proportion variation explained | 53.0% | 17.5% | 13.8% |
Note: MVPA = moderate-to-vigorous physical activity.
Effect of Interventions on Change in Scores Over 6 Months
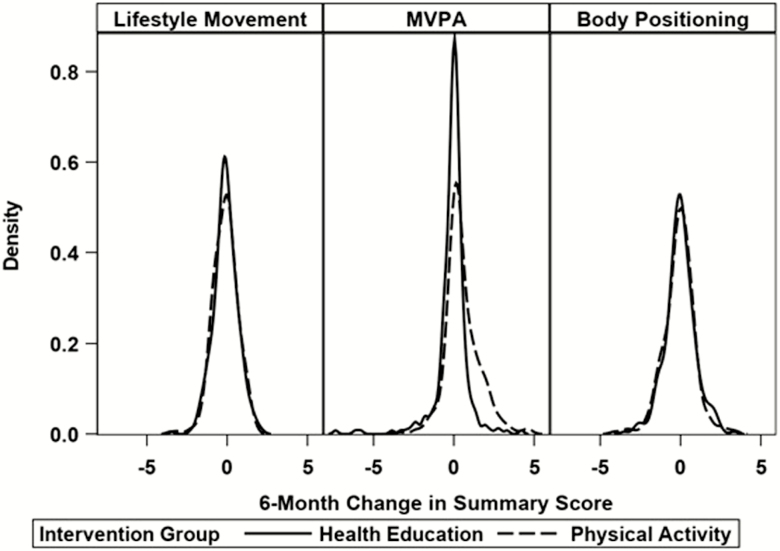
Over the course of 24 months, LM scores displayed slight, nonsignificant decreases in HE (0.29; 95% CI −0.35 to −0.22), and PA (0.26; 95% CI −0.32 to −0.20). By contrast, MVPA decreased in the HE group by 0.02 standard deviations (95% CI −0.09 to 0.06), and increased in the PA group by 0.43 (95% CI 0.36 to 0.50; group difference p < .001). Finally, body posture scores were unchanged with nonsignificant decreases in both HE (0.07; 95% CI −0.15 to −0.01) and PA (0.11; 95% CI −0.17 to −0.04). Supplementary Figure 1 shows the adjusted means from the mixed models across all follow-up assessments and Figure 2 shows the kernel density plots of the distributions of 6-month change by intervention group (the time interval of greatest change) and illustrates the shift in the distribution of MVPA within the PA group. Supplementary Figure 2 demonstrates adjusted means using variables calculated without the inclinometer, and Supplementary Figures 3 and 4 show the kernel density plots of the distributions of 6-month change by intervention group, calculated without the inclinometer and using a transitions cut point of 2, respectively.
Figure 2.
Density plots of distribution of 6-month change by intervention group
Linear Relationships Between Baseline Movement Component Scores and MMD
Table 3 provides estimates of the unadjusted and adjusted hazard ratios relating component summary scores to incident MMD. After adjustment for covariates, baseline LM was related to the incidence of MMD, irrespective of whether participants were randomized to PA (HR = 0.74; 95% CI 0.61 to 0.89) or HE (HR = 0.74; 95% CI 0.62 to 0.88). The MVPA component was significantly associated with MMD after adjustment for baseline covariates within HE (HR = 0.69; 95% CI 0.54 to 0.87), but not within PA (HR = 0.82; 95% CI 0.63 to 1.05). In addition, across the first 6 months of the study, there was a low but significant pattern for increases in MVPA to be related to decreases in LM for both PA and HE (Spearman rs = −0.27, p < .0001). There was no significant effect for the dimension that captured body positioning. Supplementary Tables 6 and 7 provide similar results for the sensitivity analyses when not using the inclinometer and a transitions cut point of 2.
Table 3.
Hazard Ratios Relating Baseline Principal Component Scores to MMD Within Intervention Groups
| Models | Predictor | PA HR (95% CI) |
HE HR (95% CI) |
|---|---|---|---|
| Unadjusted | Lifestyle movement | 0.67 (0.56 to 0.81) | 0.68 (0.58 to 0.80) |
| Sustained MVPA | 0.73 (0.57 to 0.94) | 0.62 (0.49 to 0.78) | |
| Body positioning | 0.89 (0.75 to 1.06) | 0.94 (0.81 to 1.10) | |
| Adjusted* | Lifestyle movement | 0.74 (0.61 to 0.89) | 0.74 (0.62 to 0.88) |
| Sustained MVPA | 0.82 (0.63 to 1.05) | 0.69 (0.54 to 0.87) | |
| Body positioning | 0.94 (0.79 to 1.13) | 0.99 (0.84 to 1.15) |
Notes: All models stratified the baseline hazard on sex.
*Adjusted for baseline age, BMI, race/ethnicity, SPPB, history of high blood pressure, CVD, arthritis, lung disease, history of fracture, and diabetes. BMI = body mass index; CI = confidence interval; CVD = cardiovascular disease; HE = health education; HR = hazard ratio; PA condition = physical activity condition; MMD = major mobility disability; MVPA = moderate-to-vigorous physical activity; SPPB = short physical performance battery.
Nonlinear Relationships Between Baseline Movement Component Scores and MMD
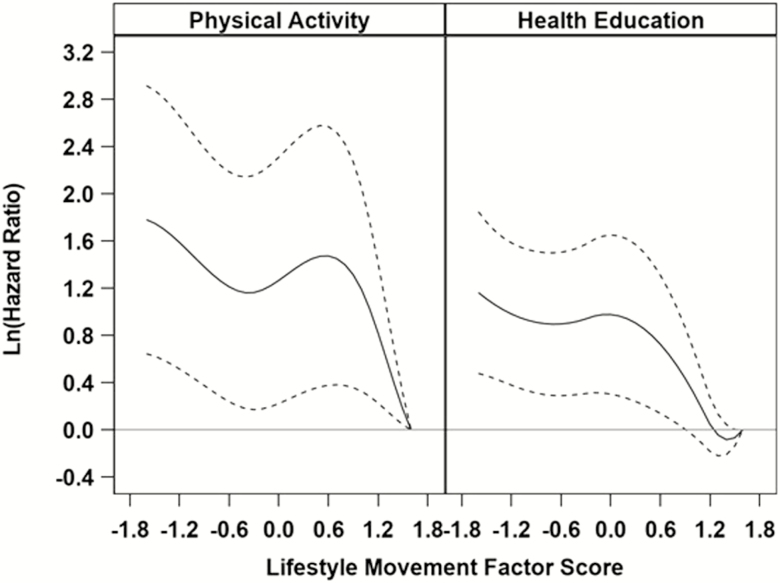
B-spline models identified the presence of nonlinear relationships between LM scores and MMD within the PA and HE groups (PA, p = .02; HE, p = .04), but not for the MVPA or body positioning components (ps > .05 for nonlinear terms). Figure 3 provides a plot of log-transformed HRs in the PA and HE groups relative to a person within each group with a baseline LM component score equal to 1.6 (reference level), after adjusting for the baseline covariates. It is clear that within both conditions, risk of MMD decreases with increasing scores on LM. Within both HE and PA, risk for developing MMD is similar for individuals with LM scores less than 1.0. As scores rise above 1.0, the HRs quickly approach 0 on the log scale (1.0 after taking the exponent). A similar result was obtained from a sensitivity analysis when not using the inclinometer (see Supplementary Figure 5) and when using the transitions cut point of 2.0 (see Supplementary Figure 6); however, the relationship in HE was found to be linear for this subset of the full data set.
Figure 3.
Nonlinear relationship between long-transformed hazard ratios and lifestyle movement scores. Log-transformed hazard ratios relative to a value of 1.6 within each group; there is a significant nonlinear component (p = .02 for PA, p = .04 for HE). Models are stratified the baseline hazard on sex and adjusted for baseline age, BMI, race/ethnicity, SPPB, history of high blood pressure and fracture, CVD, arthritis, lung disease, and diabetes. BMI = body mass index; CVD = cardiovascular disease; HE = health education; PA = physical activity; SPPB = short physical performance battery.
Discussion
The purpose of this study was to provide an interpretable, multifaceted characterization of dimensions of movement in older adults with limitations in mobility, to evaluate whether a structured PA intervention influenced scores on these dimensions, and to determine whether baseline movement scores predicted MMD. This is a timely contribution, given the developing interest in moving beyond single-metric assessments of movement behavior (15,24). The analysis of accelerometry metrics led to the identification of three dimensions: LM, MVPA, and stationary body positioning. LM, which captured amount and variability in SB to non-SB transitions and movement excluding sustained bouts of MVPA, dominated the analysis, explaining 53% of the variance in the movement-related metrics. Consistent with our hypothesis, the PA intervention in the LIFE Study (11) was selective in its effect on movement patterns; affecting change only in MVPA. This result is important given accumulating evidence on the health benefits of reducing sedentary time (16,25), highlighting the importance of interventions specifically designed to replace sitting with LM (26,27). Such programs are more likely to be readily adopted by this group, as our previous work suggests that older adults with limitations in mobility are better equipped to engage in activity prescriptions that are lower in intensity and distributed over time as opposed to high intensity, single bouts of MVPA (28,29).
Particularly interesting were relationships between baseline movement scores and the development of MMD (24). Within the HE group, a condition that reflects the effects of normal aging, baseline levels of LM and MVPA exhibited moderate and consistent prospective relationships with the onset of MMD. Under an assumption of linearity and within covariate-adjusted results, a 1SD difference in LM corresponded to a 26% reduction in risk for MMD, and an equivalent difference in MVPA scores corresponded to a 31% reduction in risk; findings that are strengthened by the orthogonality of the component scores. Within the PA group, a 1SD difference in LM was associated with a 26% reduction in MMD, whereas an equivalent difference in MVPA was associated with a reduction of 18%. Although it is beyond the scope of this article to disentangle the causal pathway leading to the disparity in risk reduction between HE and PA for MVPA (ie, 31% vs 18%), this difference is likely due to participation in MVPA among those in the PA intervention. Indeed, the main outcome from LIFE Study reported a significant reduction in MMD among participants in PA versus HE (11). We would also emphasize to readers that analyses allowing for nonlinear effects revealed that the effect of baseline LM on MMD was due largely to those individuals with low-to-moderate scores on this dimension of movement. It is worth noting that contrary to our hypothesis, time spent standing still when contrasted with SB was unrelated to the incidence of MMD. This finding should be viewed as preliminary, as the variable capturing standing still is reliant on the ActiGraph’s inclinometer function, the validity of which has been questioned (22). Still this finding is of interest and merits additional work using devices better equipped at capturing body posture, as a common SB intervention technique is to replace sitting with standing still (30). With regard to MMD as well as several cardiometabolic outcomes (30), encouraging light movement and increasing postural shifts by prescribing frequent, short bouts of standing is likely to confer greater benefit than sustained standing.
Collectively, these findings underscore the need for clinicians and researchers to consider both LM and MVPA (31) in the promotion of healthy aging as opposed to a singular emphasis on exercise behavior, a recommendation that aligns well with previous findings from the LIFE Study demonstrating total daily activity energy expenditure is predictive of mortality (32). In addition, SB peaks among those aged 70-85 years (33), placing these older adults at increased risk for morbidity, and accelerated rates of MMD (16,34,35), and such negative health effects tend to persist when controlling for MVPA (36,37). Importantly, the LIFE PA intervention had little effect on SB (38) and increases in sitting typically replace LM, not MVPA, with some studies reporting a nearly perfect inverse correlation between LM and SB (16). Duvivier and colleagues (39) conducted a pooled analysis of three studies including adults and older adults wherein they compared 14 h/d of sitting, replacing 5–6 h/d with light-intensity walking and standing still, or 1 h/d of moderate-to-vigorous cycling. The authors noted that exercise participation alone improved circulating markers of endothelial dysfunction, while LM improved metabolic function. The authors concluded that both types of movement are necessary, especially for cardiometabolic health.
Strengths and Limitations
This study has a number of important strengths. The LIFE Study recruited a large sample of older adults from multiple sites, was a randomized clinical trial, tracked PA behavior using accelerometry, and followed the development of incident MMD for an average of 2.2 years. It also adds to an exciting and growing body of evidence using multiple analytic techniques and a combination of accelerometer features to study movement and healthy aging (24). Where our analyses prioritized an easily interpreted solution in to guide future intervention development, other research teams are using sophisticated methods such as machine learning to enhance our ability to predict mobility disability (24).
Limitations include the fact that the sample was predominantly white and had a higher percentage of participants with advanced education. Although we controlled for potential confounders separately within intervention groups, unidentified baseline confounders may have affected our findings. Moreover, we investigated changes within a control condition as a proxy for normal aging. Additional replication in an observational study is warranted, as it is possible that the healthy aging educational content had a small effect on lifestyle behaviors among those in the HE condition. In addition, the use of a hip-worn ActiGraph inclinometer required additional post-processing and necessitated the exclusion of days with excessive noise in the signal. Future replication of this research would benefit from the use of sensors worn at multiple locations (eg, thigh, lower back, hip, shoe) to best differentiate between postures, and to better detect very short movement bouts. Finally, our analyses do not address how an intervention targeting LM or body positioning may affect future incidence of MMD, as our results show that the LIFE intervention did not affect these dimensions of movement. Whereas there exists the possibility that LM may be a marker of poor health, the inclusion of age, functional health and comorbid conditions into the model had little effect on attenuating the effect of baseline LM on MMD.
Conclusions
This study adds to the rapidly growing body of research interested in better understanding the relationship between movement and health in older adults (15,24) by characterizing the dimensions of movement among older adults who were at risk for MMD. We found that LM best captured patterns of activity for this at-risk population of older adults. Irrespective of intervention assignment, we observed significant relationships between lower baseline LM scores and incident MMD across 2.2 years of follow-up. The results align with recent federal guidelines (40) and support the notion that both LM and MVPA should be placed center stage in the development of treatment regimens for older adults at risk for MMD, and in the promotion of movement medicine within health care.
Funding
This work is supported by the National Institutes of Health and National Institute on Aging (UO1 AG22376) and the National Heart, Lung and Blood Institute (3U01AG022376-05A2S).
Conflict of interest statement
None reported.
Supplementary Material
Acknowledgments
For a full list of the LIFE Study investigators, see Supplementary Material “Research Investigators for the LIFE Study.”
References
- 1. Kraus L. 2016 Disability Statistics Annual Report Durham, NH: University of New Hampshire. https://disabilitycompendium.org/sites/default/files/user-uploads/2016_AnnualReport.pdf. Accessed June 4, 2018.
- 2. US Census Bureau. An Aging Nation: Projected Number of Children and Older Adults https://www.census.gov/library/visualizations/2018/comm/historic-first.html. Accessed June 4, 2018.
- 3. Rejeski WJ, Ip EH, Bertoni AG, et al. ; Look AHEAD Research Group. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366:1209–1217. doi: 10.1056/NEJMoa1110294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev. 2010;11:671–685. doi: 10.1111/j.1467-789X.2009.00679.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 6. Shumway-Cook A, Ciol MA, Yorkston KM, Hoffman JM, Chan L. Mobility limitations in the Medicare population: prevalence and sociodemographic and clinical correlates. J Am Geriatr Soc. 2005;53:1217–1221. doi: 10.1111/j.1532-5415.2005.53372.x [DOI] [PubMed] [Google Scholar]
- 7. Cesari M, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, et al. Evidence for the domains supporting the construct of intrinsic capacity. J Gerontol A Biol Sci Med Sci. 2018;73:1653–1660. doi: 10.1093/gerona/gly011 [DOI] [PubMed] [Google Scholar]
- 8. Fielding RA, Rejeski WJ, Blair S, et al. ; LIFE Research Group. The lifestyle interventions and independence for elders study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–1237. doi: 10.1093/gerona/glr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. LaCroix AZ, Guralnik JM, Berkman LF, Wallace RB, Satterfield S. Maintaining mobility in late life. II. Smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. 1993;137:858–869. doi:10.1093/oxfordjournals.aje.a116747 [DOI] [PubMed] [Google Scholar]
- 10. Miller ME, Rejeski WJ, Reboussin BA, Ten Have TR, Ettinger WH. Physical activity, functional limitations, and disability in older adults. J Am Geriatr Soc. 2000;48:1264–1272. doi:10.1111/j.1532-5415.2000.tb02600.x [DOI] [PubMed] [Google Scholar]
- 11. Pahor M, Guralnik JM, Ambrosius WT, et al. ; LIFE study investigators. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manini TM, Lamonte MJ, Seguin RA, et al. Modifying effect of obesity on the association between sitting and incident diabetes in post-menopausal women. Obesity (Silver Spring). 2014;22:1133–1141. doi: 10.1002/oby.20620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santos DA, Silva AM, Baptista F, et al. Sedentary behavior and physical activity are independently related to functional fitness in older adults. Exp Gerontol. 2012;47:908–912. doi: 10.1016/j.exger.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 14. Seguin R, Lamonte M, Tinker L, et al. Sedentary behavior and physical function decline in older women: findings from the women’s health initiative. J Aging Res. 2012;2012:271589. doi: 10.1155/2012/271589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiroma EJ, Schrack JA, Harris TB. Accelerating accelerometer research in aging. J Gerontol A Biol Sci Med Sci. 2018;73:619–621. doi: 10.1093/gerona/gly033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dunstan DW, Howard B, Healy GN, Owen N. Too much sitting—a health hazard. Diabetes Res Clin Pract. 2012;97:368–376. doi: 10.1016/j.diabres.2012.05.020 [DOI] [PubMed] [Google Scholar]
- 17. Borg GA. Perceived exertion: a note on “history” and methods. Med Sci Sports. 1973;5:90–93. doi:10.1249/00005768-197300520-00017 [PubMed] [Google Scholar]
- 18. Manini TM, Beavers DP, Pahor M, et al. Effect of physical activity on self-reported disability in older adults: results from the LIFE study. J Am Geriatr Soc. 2017;65(5):980–988. doi: 10.1111/jgs.14742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi L, Ward SC, Schnelle JF, Buchowski MS. Assessment of wear/nonwear time classification algorithms for triaxial accelerometer. Med Sci Sports Exerc. 2012;44:2009–2016. doi: 10.1249/MSS.0b013e318258cb36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Copeland JL, Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Act. 2009;17:17–30. doi: 10.1123/japa.17.1.17 [DOI] [PubMed] [Google Scholar]
- 21. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 22. An HS, Kim Y, Lee JM. Accuracy of inclinometer functions of the activPAL and ActiGraph GT3X+: a focus on physical activity. Gait Posture. 2017;51:174–180. doi: 10.1016/j.gaitpost.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mankowski RT, Aubertin-Leheudre M, Beavers DP, et al. ; LIFE Research Group. Sedentary time is associated with the metabolic syndrome in older adults with mobility limitations—the LIFE study. Exp Gerontol. 2015;70:32–36. doi: 10.1016/j.exger.2015.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kheirkhahan M, Tudor-Locke C, Axtell R, et al. Actigraphy features for predicting mobility disability in older adults. Physiol Meas. 2016;37:1813–1833. doi: 10.1088/0967-3334/37/10/1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38:105–113. doi: 10.1097/JES.0b013e3181e373a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nicklas BJ, Gaukstern JE, Beavers KM, Newman JC, Leng X, Rejeski WJ. Self-monitoring of spontaneous physical activity and sedentary behavior to prevent weight regain in older adults. Obesity (Silver Spring). 2014;22:1406–1412. doi: 10.1002/oby.20732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clemson L, Fiatarone Singh MA, Bundy A, et al. Integration of balance and strength training into daily life activity to reduce rate of falls in older people (the LiFE study): randomised parallel trial. BMJ. 2012;345:e4547. doi: 10.1136/bmj.e4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rejeski WJ, Axtell R, Fielding R, et al. ; LIFE Study Investigator Group. Promoting physical activity for elders with compromised function: the Lifestyle Interventions and Independence for Elders (LIFE) study physical activity intervention. Clin Interv Aging. 2013;8:1119–1131. doi: 10.2147/CIA.S49737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rejeski WJ, Marsh AP, Brubaker PH, et al. Analysis and interpretation of accelerometry data in older adults: the LIFE Study. J Gerontol A Biol Sci Med Sci. 2016;71:521–528. doi: 10.1093/gerona/glv204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benatti FB, Ried-Larsen M. The effects of breaking up prolonged sitting time: a review of experimental studies. Med Sci Sports Exerc. 2015;47:2053–2061. doi: 10.1249/MSS.0000000000000654 [DOI] [PubMed] [Google Scholar]
- 31. Rillamas-Sun E, LaMonte MJ, Evenson KR, et al. The influence of physical activity and sedentary behavior on living to age 85 years without disease and disability in older women. J Gerontol A Biol Sci Med Sci. 2018;73:1525–1531. doi: 10.1093/gerona/glx222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manini TM, Everhart JE, Patel KV, et al. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296:171–179. doi: 10.1001/jama.296.2.171 [DOI] [PubMed] [Google Scholar]
- 33. Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–881. doi: 10.1093/aje/kwm390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Westerterp KR. Physical activity as determinant of daily energy expenditure. Physiol Behav. 2008;93:1039–1043. doi:10.1016/j.physbeh. 2008.01.021 [DOI] [PubMed] [Google Scholar]
- 35. Buman MP, Hekler EB, Haskell WL, et al. Objective light-intensity physical activity associations with rated health in older adults. Am J Epidemiol. 2010;172:1155–1165. doi: 10.1093/aje/kwq249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35:976–983. doi: 10.2337/dc11-1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gennuso KP, Gangnon RE, Matthews CE, Thraen-Borowski KM, Colbert LH. Sedentary behavior, physical activity, and markers of health in older adults. Med Sci Sports Exerc. 2013;45:1493–1500. doi: 10.1249/MSS.0b013e318288a1e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wanigatunga AA, Ambrosius WT, Rejeski WJ, et al. Association between structured physical activity and sedentary time in older adults. JAMA. 2017;318:297–299. doi: 10.1001/jama.2017.7203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duvivier BMFM, Bolijn JE, Koster A, Schalkwijk CG, Savelberg HHCM, Schaper NC. Reducing sitting time versus adding exercise: differential effects on biomarkers of endothelial dysfunction and metabolic risk. Sci Rep. 2018;8:8657. doi: 10.1038/s41598-018-26616-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Report. Washington, DC: US Department of Health and Human Services; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.