Abstract
Background
The accumulation of deficits model for frailty has been used to develop an electronic health record (EHR) frailty index (eFI) that has been incorporated into British guidelines for frailty management. However, there have been limited applications of EHR-based approaches in the United States.
Methods
We constructed an adapted eFI for patients in our Medicare Accountable Care Organization (ACO, N = 12,798) using encounter, diagnosis code, laboratory, medication, and Medicare Annual Wellness Visit (AWV) data from the EHR. We examined the association of the eFI with mortality, health care utilization, and injurious falls.
Results
The overall cohort was 55.7% female, 85.7% white, with a mean age of 74.9 (SD = 7.3) years. In the prior 2 years, 32.1% had AWV data. The eFI could be calculated for 9,013 (70.4%) ACO patients. Of these, 46.5% were classified as prefrail (0.10 < eFI ≤ 0.21) and 40.1% frail (eFI > 0.21). Accounting for age, comorbidity, and prior health care utilization, the eFI independently predicted all-cause mortality, inpatient hospitalizations, emergency department visits, and injurious falls (all p < .001). Having at least one functional deficit captured from the AWV was independently associated with an increased risk of hospitalizations and injurious falls, controlling for other components of the eFI.
Conclusions
Construction of an eFI from the EHR, within the context of a managed care population, is feasible and can help to identify vulnerable older adults. Future work is needed to integrate the eFI with claims-based approaches and test whether it can be used to effectively target interventions tailored to the health needs of frail patients.
Keywords: Frailty, Falls, Health Services, Multimorbidities
Frailty is commonly defined as a biological process resulting in decreased reserve, producing an increased vulnerability to acute stressors (1). Numerous studies have proposed instruments to quantify frailty; either via the frailty phenotype (2), functional measures (3), or frailty indices (FIs) based on the theoretical model of deficit accumulation (4). Regardless of the measurement modality, frailty predicts a myriad of negative health outcomes (5), including disability (6), health care utilization (7), long-term care admission (8), and mortality (1,9). Increasingly, the literature also suggests that frailty itself may be reversible—perhaps more so than disability (10,11). Frailty should be an appealing focus for screening in primary care due to its predictive ability and potential as a therapeutic target for the prevention of falls, disability, and unnecessary or burdensome hospitalizations (12). However, in the United States, frailty assessment has not been broadly implemented; barriers include not only a lack of consensus among prevalent frailty instruments, but time and resource constraints in busy clinicians’ offices (13).
In England, the accumulated-deficit model of frailty has facilitated the development of an electronic health record (EHR) based frailty index (eFI) utilizing primary care data (14). The appeal of the eFI is that its’ automatic calculation could ameliorate clinician time concerns in screening for frailty. In fact, the eFI has already been incorporated into guidelines for frailty management by The British Geriatrics Society and the National Health Service (NHS) (10,14). While the proliferation of EHR systems should permit the adoption of a similar approach in the United States, implementation is more complex outside of unified health systems like the NHS. While there are exceptions such as the Veterans Affairs (VA) system, EHRs in the United States will often reflect an open cohort of patients, one in which patients may enter or exit from observation over time, at least partially driven by utilization of multiple health systems (15). It is unclear to what extent an EHR-based FI could be calculated for a fixed population of older individuals in the United States, for example, patients within a Medicare Accountable Care Organization (ACO). The magnitude of incomplete data also has important implications for frailty screening as part of population health management. While frailty indices derived from administrative claims can alleviate some concerns with incomplete ascertainment (16–18), they would be difficult to use at the point of care given time lags inherent with claims data. Finally, an additional limitation of the NHS eFI and work in the VA (18) is that they primarily incorporate deficits through diagnosis codes. Such an approach may capture advanced disease, but tends toward under-reporting of subclinical contributors to frailty (19); perhaps missing subtler deficits and functional impairments that may still impact risk or may benefit from focused intervention (20). Medicare’s recent implementation of Annual Wellness Visits (AWVs), which require cognitive and functional screening as well as falls risk assessment, provides an opportunity to include more subtle functional status changes in building a FI (21).
The goal of the present work was to adapt the eFI adopted by the NHS to a U.S. health care system, applying it to a population of older adults enrolled in a Medicare ACO. Our primary research question was to estimate the frequency with which individuals in our Medicare ACO would have sufficient data captured in the EHR to estimate a FI. In addition, we assessed the predictive performance of our adapted eFI, including and excluding functional data from Medicare AWVs, with respect to incident outcomes including injurious falls, health care utilization, and all-cause mortality.
Methods
Population
We identified patients in our EHR (Epic, Verona, WI) attributed to our Medicare Shared Savings Plan Accountable Care Organization (MSSP-ACO). Patients included were at least 65 years of age as of July 1, 2016. We used a 2-year lookback period in order to define our adapted eFI, using data from July 1, 2014 to July 1, 2016.
Composition of the Adapted EMR-Based Frailty Index (eFI)
As shown in Supplementary Tables 1A–C, we modeled our adapted eFI after the deficits included in the eFI of Clegg and colleagues. For deficits based on diagnosis codes, we utilized diagnosis codes from all sources (outpatient, inpatient, emergency department, etc.) and employed ICD-9-CM and ICD-10-CM code definitions largely based on previous validated algorithms (22,23) or the Clinical Classification Software for ICD-9-CM (https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp). As our data covers the time period where our health system switched to coding using ICD-10-CM, we mapped deficit definitions based on ICD-9-CM that lacked an analog for ICD-10-CM using the 2017 CMS General Equivalence Mapping Files (https://www.cms.gov/Medicare/Coding/ICD10/2017-ICD-10-CM-and-GEMs.html). For individuals with no observed diagnosis codes during the 2-year look back period, we assumed this implied little to no prior interaction with the health system, and so we set all diagnosis code based deficits to missing for such individuals. Finally, as a measure of multimorbidity, we used the algorithm of Quan and colleagues (22) to estimate the weighted Charlson Comorbidity Index.
We included laboratory measures and vital signs from outpatient encounters in the eFI from the lab-based FI of Howlett and colleagues [(24); Supplementary Table 1B]. If multiple measurements were available during the look-back period, each measurement was individually scored as a deficit, with the individual deficit scores then averaged to obtain an overall deficit score. We also included functional data from the Medicare Annual Wellness Visits (AWVs, https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/AWV_chart_ICN905706.pdf; Supplementary Table 1C). We used the latest AWV for individuals with multiple AWVs during the look-back period. The eFI included 54 total deficits and was calculated as the unweighted sum of the score for each deficit, divided by the total number of nonmissing items. Consistent with recommendations for constructing frailty indices, we required ≥30 nonmissing items (25). Because we had 33 deficits involving diagnosis codes, we did not want an absence of diagnosis codes and other data (for individuals with ≥1 code in the past 2 years) to necessarily imply robust health status. We additionally excluded individuals that did not have at least 9 of the 20 items based on laboratory measurements, smoking status, body mass index, and blood pressure measured in the past 2 years. As a sensitivity analysis, we also considered a different requirement based on having sufficient contact with the health care system in the past 2 years, which we defined as having at least two outpatient encounters with a measured blood pressure. The primary difference with this approach is that implicitly assumes that a lack of diagnosis codes implies robust health status, and it does not require the presence of any of the nondiagnosis code based-items in calculating the eFI (with the exception of blood pressure).
Incident Outcomes
We extracted encounter information on the incidence of emergency department (ED) visits, inpatient hospitalizations, and outpatient encounters in the year following our index date (July 1, 2016) for defining the eFI. Injurious falls were identified based on ICD-10 diagnosis codes (Supplementary Table 1A). We additionally required that diagnosis codes for an injurious fall were linked to an encounter that entailed an ED visit or inpatient encounter. We similarly extracted dates of death from the EHR for the same follow-up time period. Mortality information in our EHR is supplemented through a monthly deterministic linkage (based on name, age, gender, date of birth, and race/ethnicity) to the North Carolina State Center for Health Statistics death index, which captures any individuals that die within North Carolina.
Statistical Analysis
We examined the association between the eFI and all-cause mortality using Cox proportional hazards regression. We examined discriminatory ability by calculating c-statistics (using 10-fold cross-validation) and the estimated explained relative risk measure of Heller (26). We estimated the incidence of health care encounters, emergency department (ED) visits, inpatient hospitalizations, and injurious falls using the mean cumulative count (MCC) estimator (27), which allows for recurrent events and the competing risk of death. Standard errors for the MCC were estimated using bootstrap resampling (N = 1,000). While regression methods have been proposed to handle recurrent events and a competing terminal event (28), we have found that current software implementations are not suited to large data sets. Therefore, to address potential confounding effects with respect to utilization and injurious falls, we reverted to a Cox proportional hazards framework allowing for recurrent events, but not a competing terminal event. In all models, we included age, sex, race/ethnicity, and the Charlson Comorbidity Index as covariates. To attempt to control for informed presence bias, we also adjusted for the number of health care encounters in the past 2 years (29). We adjusted for both the number of past outpatient encounters (<5, 5 to <10, and 10 or more) and the number of past emergency department visits or inpatient hospitalizations (0, 1, or ≥2). All analyses were performed using the R Statistical Computing Environment (R Core Team, Vienna, Austria) or SAS v9.4 (SAS, Cary, NC).
Results
There were 12,798 individuals attributed to our health system’s ACO population as of July 1, 2016. There was sufficient data in the EHR to calculate the eFI in 9,013 (70.4%) individuals (Table 1). The median and maximum eFI scores were 0.19 and 0.61, respectively, with the majority of ACO enrollees categorized as prefrail (0.10 < eFI ≤ 0.21, 46.5%) or frail (eFI > 0.21, 40.1%) (30). We did observe a slightly higher burden of frailty in women, as 40.6% of women were categorized as frail (eFI > 0.21) compared with 39.4% in men (Supplementary Table 2). Conversely, men exhibited a slightly higher burden of comorbidity, with a median Charlson Comorbidity Index of 3 versus a median of 2 in women (p < .001). For ACO patients deemed to have insufficient data to calculate the eFI, 809 (21.3%) had no diagnosis codes recorded in the past 2 years, with the remaining individuals tending only to have data recorded for blood pressure, body mass index, and smoking status, above and beyond at least one diagnosis code (Supplementary Table 3). As expected, these individuals exhibited a lower rate of contact with the health care system (Table 1), as they had a lower number of healthcare encounters in the past 2 years (median of 2 encounters vs 12 encounters for those where eFI could be defined) and were less likely to have completed a Medicare AWV in the past 2 years (8.2% vs 42.1%, p < .001).
Table 1.
Characteristics of Medicare Shared Savings Program Accountable Care Organization Population Stratified by whether or not the Electronic Frailty Index (eFI) could be Calculated From the Electronic Health Record
| Characteristic | Sufficient Data toCalculate eFI N = 9,013 | Insufficient Datato Calculate eFI N = 3,785 | p Value |
|---|---|---|---|
| Age, years, mean ± SD | 75.0 ± 7.3 | 74.9 ± 7.1 | .612 |
| Age, no. (%) | .211 | ||
| 65 to <75 years | 4,895 (54.3) | 2,118 (56.0) | |
| 75 to <85 years | 2,953 (32.8) | 1,205 (31.8) | |
| 85 years or more | 1,165 (12.9) | 462 (12.2) | |
| Female sex, no. (%) | 5,194 (57.6) | 1,930 (51.0) | <.001 |
| Race/ethnicity, no. (%) | <.001 | ||
| White | 7,554 (83.8) | 3,411 (90.1) | |
| Black | 1,178 (13.1) | 259 (6.8) | |
| Hispanic | 124 (1.4) | 62 (1.6) | |
| Other | 153 (1.7) | 49 (1.3) | |
| Unknown | 4 (0.0) | 4 (0.1) | |
| Median household income based on zip code >$53,657, no. (%)a | 2,921 (36.9) | 806 (32.4) | <.001 |
| No. of health care encounters, median (IQR) | 12 (7–19) | 2 (1–4) | <.001 |
| No. of outpatient encounters in past 2 years, no. (%) | <.001 | ||
| <5 | 1,219 (13.5) | 2,917 (77.1) | |
| 5 to <10 | 2,566 (28.5) | 545 (14.4) | |
| 10 or more | 5,228 (58.0) | 323 (8.5) | |
| No. of ED visits or inpatient encounters in past 2 years, no. (%) | <.001 | ||
| 0 | 5,682 (63.0) | 3,199 (84.5) | |
| 1 | 1,602 (17.8) | 362 (9.6) | |
| 2 or more | 1,729 (19.2) | 224 (5.9) | |
| Medicare Annual Wellness Visit in past 2 years, no. (%) | 3,791 (42.1) | 312 (8.2) | <.001 |
| eFI, median (IQR) | 0.19 (0.13–0.26) | — | — |
| eFI, no. (%) | — | ||
| eFI ≤ 0.10 | 1,210 (13.4) | — | |
| 0.10 < eFI ≤ 0.20 | 3,858 (42.8) | — | |
| 0.20<eFI ≤ 0.30 | 2,581 (28.6) | — | |
| 0.30 < eFI ≤ 0.40 | 1,018 (11.3) | — | |
| eFI > 0.40 | 346 (3.8) | — |
Notes: Participants were required to be at least 65 years of age as of July 1, 2014, and to be ≤95 years of age on July 1, 2016. Electronic frailty index (eFI) based on 2-year look back period from July 1, 2014 to July 1, 2016. SD = standard deviation; IQR = interquartile range; ED = emergency department.
aMedian household income = $53,657 in 2014. U.S. Bureau of the Census, Median Household Income in the United States [MEHOINUSA646N], retrieved from FRED, Federal Reserve Bank of St. Louis; https://fred.stlouisfed.org/series/MEHOINUSA646N, February 4, 2018.
Associations with all-cause mortality
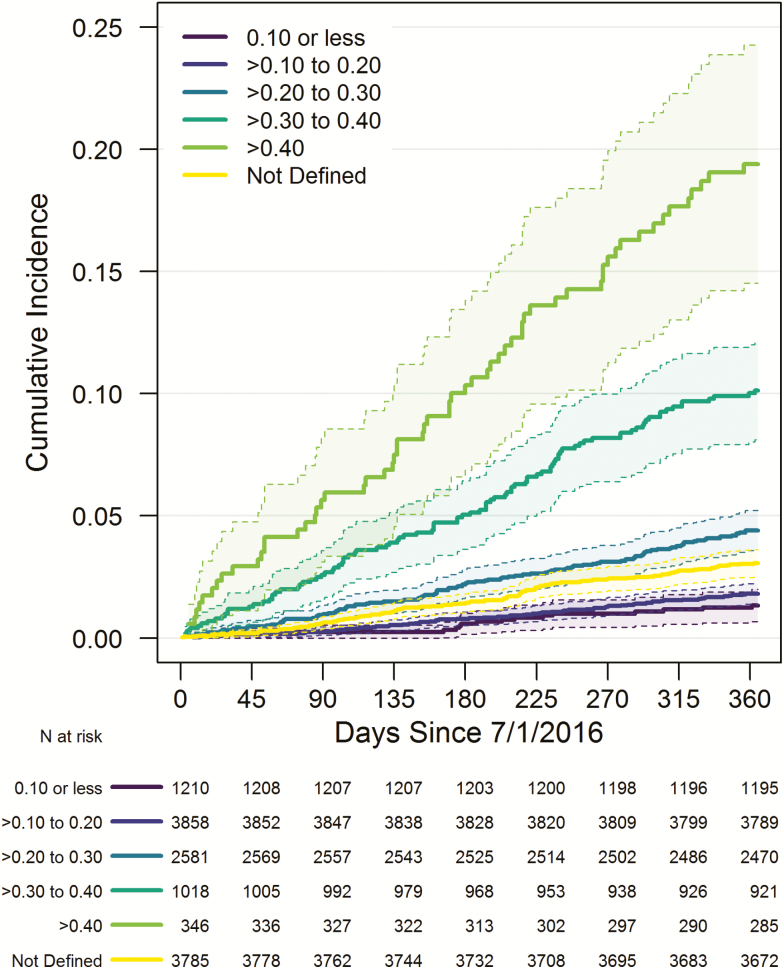
Figure 1 shows Kaplan–Meier estimates of all-cause mortality stratified by eFI score. As expected, we observed increased mortality risk with increasing eFI score. Table 2 examines the association of the eFI with all-cause mortality based on a multivariable Cox regression model. The strongest predictors of mortality were the Charlson Comorbidity Index (estimated explained relative risk (eeRR) = 11.2%) and number of ED or inpatient encounters in the past 2 years (eeRR = 6.2%). However, even when accounting for these factors and age, the eFI was independently associated with mortality (eeRR = 3.5%). Based on 10-fold cross-validation, the c-statistic for a model based only on the eFI was 0.740 and 0.790 for the full multivariable model given in Table 2. Using a threshold of eFI >0.19 (31), the sensitivity for 90-day mortality was 83.1%, with a specificity of 52.3% (Supplementary Table 4). We also observed that ACO patients where the eFI could not be calculated exhibited a higher mortality rate than patients with eFI >0.10 but ≤0.20 (Figure 1). When we investigated what factors might explain this level of mortality risk (data not shown), one of the strongest correlates appeared to be the number of ED or inpatient encounters in the past 2 years (Supplementary Figure 1), recognizing that comorbidity burden and the eFI were not calculated for these individuals.
Figure 1.
Frailty status based on the electronic health record frailty index (eFI) and incidence of all-cause mortality. Shaded areas denote 95% point-wise confidence intervals.
Table 2.
Association Between the Electronic Frailty Index (eFI) and All-Cause Mortality
| Variable | Hazard Ratio (95% CI) | p Value | ExplainedRelative Risk |
|---|---|---|---|
| Age (per 5 year increase) | 1.23 (1.15–1.32) | <.001 | 4.3% |
| Sex (male) | 1.6% | ||
| Female | 0.68 (0.55–0.84) | <.001 | |
| Race (white) | 0.1% | ||
| Nonwhite | 0.85 (0.64–1.14) | .285 | |
| No. of outpatient encounters in past 2 years (<5) | 1.0% | ||
| 5 to <10 | 1.04 (0.64–1.67) | .881 | |
| 10 or more | 0.76 (0.48–1.20) | .240 | |
| No. of ED visits or inpatient encounters in past 2 years (0) | 6.2% | ||
| 1 | 1.69 (1.24–2.32) | .001 | |
| 2 or more | 2.43 (1.79–3.31) | <.001 | |
| Weighted Charlson Comorbidity Index | 1.17 (1.13–1.21) | <.001 | 11.2% |
| eFI (per 0.1 increase) | 1.33 (1.15–1.53) | <.001 | 3.5% |
eFI denotes electronic Frailty Index, CI Confidence Interval, and ED Emergency Department. Hazard Ratios based on multivariable Cox regression model. Explained Relative Risk is the estimated explained relative risk measure of Heller (26).
Associations with incident health care utilization and injurious falls
Supplementary Figure 2 shows unadjusted mean cumulative count (MCC) estimates for incident health care utilization and injurious falls, accounting for recurrent events and the competing risk of death. For all outcomes, we observed increasing event rates with increasing frailty. As an example, the MCC estimate for injurious falls was 24.28 (95% confidence interval [CI]: 18.49 to 30.92) falls per 100 individuals with eFI >0.40 over 365 days, as compared with 0.66 (95% CI: 0.24 to 1.16) falls for individuals with eFI ≤0.10. As with mortality, individuals in the subgroup where the eFI could not be calculated exhibited similar rates of inpatient encounters, ED encounters injurious falls to those individuals categorized as prefrail. Table 3 displays adjusted estimates of the association between the eFI and utilization or falls, adjusting for age, comorbidity, and past health care utilization. The eFI was a strong predictor of all outcomes we examined (p < .001). While comorbidity based on the Charlson Index was also a significant predictor of overall health care utilization and inpatient encounters, it was not a significant predictor of ED visits (hazard ratio [HR] = 1.00, 95% CI: 0.98 to 1.02) or injurious falls (HR = 0.99, 95% CI: 0.95 to 1.04). Finally, we examined discriminatory ability by modeling time to the first event for each outcome except overall healthcare encounters (data not shown). For these models, which do not consider recurrent events, the c-statistics were 0.724 (inpatient), 0.691 (ED), and 0.749 (injurious falls) based on the eFI, and 0.741 (inpatient), 0.739 (ED), 0.791 (injurious falls) based on a full model additionally including age, sex, race/ethnicity, past utilization, and comorbidity.
Table 3.
Association Between the Electronic Frailty Index (eFI), Health Care Utilization, and Injurious Falls
| Variable | Health Care Encounters | Emergency Department Visits | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| No. of outpatient encounters in past 2 years (<5) | ||||
| 5 to <10 | 1.24 (1.16–1.33) | <.001 | 1.17 (0.89–1.53) | .255 |
| 10 or more | 1.97 (1.84–2.12) | <.001 | 1.42 (1.11–1.83) | .006 |
| No. of ED visits or inpatient encounters in past 2 years (0) | ||||
| 1 | 1.07 (1.04–1.11) | <.001 | 1.97 (1.66–2.33) | <.001 |
| 2 or more | 1.15 (1.10–1.20) | <.001 | 3.75 (3.16–4.44) | <.001 |
| Weighted Charlson Comorbidity Index | 1.02 (1.01–1.02) | <.001 | 1.00 (0.98–1.02) | .952 |
| eFI (per 0.1 increase) | 1.20 (1.17–1.22) | <.001 | 1.36 (1.26–1.46) | <.001 |
| Variable | Inpatient Encounters | Injurious Falls | ||
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| No. of outpatient encounters in past 2 years (<5) | ||||
| 5 to <10 | 1.14 (0.85–1.51) | .383 | 1.22 (0.60–2.46) | .583 |
| 10 or more | 1.14 (0.87–1.50) | .352 | 1.04 (0.53–2.05) | .914 |
| No. of ED visits or inpatient encounters in past 2 years (0) | ||||
| 1 | 1.75 (1.48–2.08) | <.001 | 1.99 (1.38–2.86) | <.001 |
| 2 or more | 2.32 (1.95–2.76) | <.001 | 3.16 (2.23–4.50) | <.001 |
| Weighted Charlson Comorbidity Index | 1.05 (1.02–1.07) | <.001 | 0.99 (0.95–1.04) | .749 |
| eFI (per 0.1 increase) | 1.62 (1.50–1.76) | <.001 | 1.66 (1.42–1.93) | <.001 |
Note: Hazard ratios based on multivariable Cox regression model accounting for recurrent events, additionally adjusting for age, sex, and race/ethnicity. CI = confidence interval; ED = emergency department.
Contribution of functional data from Annual Wellness Visits
Table 4 displays the results of sensitivity analyses where we removed the eight AWV functional items from the eFI calculation and modeled those items separately to see if they were an independent predictor of utilization and injurious falls after accounting for age, comorbidity, past health care utilization, and the remaining elements in the eFI. For these analyses, we classified individuals as functionally intact (average AWV item score < 0.125) versus some functional impairment (average AWV item score ≥ 0.125), which mathematically corresponds to having at least one full deficit across the eight functional items. Having some functional deficit was not associated with overall health care utilization, ED encounters, or mortality (HR = 1.34, 95% CI: 0.73 to 2.44). However, having some functional deficit was associated with an increased rate of inpatient encounters (HR = 1.44, 95% CI: 1.13 to 1.84) and injurious falls (HR = 1.85, 95% CI: 1.07 to 3.21).
Table 4.
Incremental Contribution of Medicare Annual Wellness Visit Data to Association Between the eFI, Health Care Utilization, and Injurious Falls
| Healthcare Encounters | Emergency Department Visits | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value |
| eFI (per 0.1 increase)a | 1.24 (1.20–1.28) | <.001 | 1.29 (1.13–1.46) | <.001 |
| ≥1 Deficit from Medicare AWVb | 1.03 (0.98–1.07) | .275 | 1.17 (0.93–1.48) | .172 |
| Variable | Inpatient Encounters | Injurious Falls | ||
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| eFI (per 0.1 increase)a | 1.78 (1.52–2.07) | <.001 | 1.45 (1.11–1.89) | .007 |
| ≥1 Deficit from Medicare AWVb | 1.44 (1.13–1.84) | .003 | 1.85 (1.07–3.21) | .028 |
Note: Analyses based on subgroup of 3,791 individuals that completed a Medicare Annual Wellness Visit (AWV) between July 1, 2014 and July 1, 2016.
aeFI calculated removing eight functional items from the AWV.
bBecause some functional items from the AWV are not scored as yes or no deficits, actual threshold is an average deficit score ≥0.125, which mathematically corresponds to having at least one full deficit among the eight AWV items. Hazard ratios based on multivariable Cox regression model accounting for recurrent events, adjusting for age, sex, race/ethnicity, number of outpatient encounters in the past 2 years, number of emergency department or inpatient encounters in the past 2 years, and the Charlson Comorbidity Index. CI = confidence interval.
Sensitivity Analyses for Data Requirements to Calculate eFI
Finally, we investigated the impact of the requirement of ≥30 nonmissing items and 9 out of 20 items based on laboratory measurements, smoking status, body mass index, and blood pressure measured to calculate the eFI. If we instead require a minimum of two outpatient encounters (with measured blood pressure) in the past 2 years, this reduced the number of ACO patients for which the eFI could not be calculated from 3,785 (29.6%) to 1,967 (15.4%, Supplementary Table 5). The majority of patients that were re-classified from missing to a calculable eFI score had an eFI ≤0.20 (1,594/1,912, 83.4%), thereby reducing the proportion of the population classified as frail (eFI > 0.21) from 40.1% to 35.9%. Despite this shift toward including presumably healthier individuals, the association of the eFI with all-cause mortality did not appreciably change (Supplementary Table 6), with the eeRR being only slightly attenuated from 3.5% to 2.8%.
Discussion
Despite consensus recommendations for the importance of routine screening for frailty in older adults (10,32), widespread implementation in the United States has been slow (12,33). Previous research has demonstrated the potential to integrate the EHR with the deficit accumulation approach to frailty (5,14,18), although this work has been based in health systems with more closed patient populations. Our study demonstrates that it is feasible to adapt an EHR-based FI within the context of a Medicare ACO population, albeit with some important caveats which we will discuss below. Within our Medicare ACO population, the adapted eFI exhibited distributional characteristics consistent with expectation, that is, higher levels of frailty for women compared with men, and a maximal observed value less than 0.7 (34). In addition, the eFI was independently associated with incident injurious falls, inpatient hospitalizations, ED encounters, and all-cause mortality. The eFI demonstrated reasonable discriminative ability (c-statistics > 0.70), comparable to previous studies (5,14).
A concern with risk indices built from the EHR is missing data, loss to follow-up, and other potential sources of bias (35). Our analyses highlight these concerns as we were not able to calculate the eFI for ~15% or ~30% of the adults attributed to our ACO, depending on the data requirement applied in calculating the eFI. Missing data likely partially explains why the prevalence of frailty in our population was considerably higher than that observed in community-dwelling populations (36). Presumably healthier individuals would be more likely to have minimal contact with the health system and thus be excluded in calculating the eFI. While patients with insufficient data to calculate the eFI may generally be healthier, this group is not without risk, as its rate of health care utilization and mortality was similar to patients categorized as prefrail in our primary analyses. However, it is important to recognize that insufficient data to calculate the eFI is not necessarily indicative of a complete lack of data from which to assess risk for future outcomes. For example, laboratory results and vital signs from inpatient hospitalizations and ED visits are excluded in calculating the eFI, so as not to count acute exacerbations as chronic deficits. Our analyses indicated that EHR data from prior ED visits and inpatient hospitalizations could be used to identify a higher risk subgroup among patients with insufficient outpatient data to estimate the eFI. In addition, an immediate future research direction would be to examine the extent to which frailty information derived from administrative claims can complement that derived from the EHR (16,17). At a minimum, one would expect claims data to contribute information about patients that received primary care or outpatient care in different health systems in the past.
We found that the additional functional deficits assessed during the Medicare AWVs were associated with the risk of incident injurious falls and ED visits after controlling for an eFI calculated excluding these functional items. While functional limitations were not associated with other health care utilization or all-cause mortality, statistical power for these analyses was likely limited given that follow-up was limited to 1 year, and analyses were limited to the subgroup of patients with an AWV in the past 2 years. While we believe future work should continue to evaluate the prognostic value of functional information, it is important to acknowledge that the uptake of AWVs is currently low, as only 18.2% of Medicare beneficiaries had an AWV in 2015 (37). However, utilization of the AWVs appears to be higher for ACOs and is increasing over time (37). Within our ACO, the proportion of patients with an AWV was 32.2% and 42.1% in fiscal years 2015 and 2016, respectively.
Several limitations of our results should be considered. We evaluated the eFI within the context of an ACO population within a single health system, and so a future need is examining the eFI’s prognostic performance in additional health care systems, and with respect to a broader spectrum of actionable outcomes, such as the use of the eFI for automated frailty screening in the preoperative setting (38). Second, while the discriminatory ability of the eFI was on par with previous studies (5,14), this level of discrimination may be “poor to adequate” (34), and its accuracy to target frailty interventions in clinical practice remains uncertain. Finally, we were also not able to compare the eFI to a clinically based assessment of frailty, such as the frailty phenotype or the Clinical Frailty Scale (2,39).
Our focus on leveraging the EHR to generate a measure of frailty is similar to using a laboratory test to measure a specific physiologic function, where specificity is lower and once detected, a clinical exam is necessary to determine a more specific cause and treatment. This approach is based on the desire to identify at-risk older adults while simultaneously avoiding excess work for already-busy front line clinicians. The eFI could help to discern which older adults may most benefit from targeted evaluation and interventions that preserve mobility, reduce falls, or avoid high-burden, high-cost hospital-based healthcare. Conversely, the eFI could also be used at the end-of-life to target resources such as palliative care, although a recent study in England illustrated that the NHS eFI was likely not currently accurate enough to actionably predict 3-month mortality at the individual level (31). In the future, testing a tool like the eFI to target population health management strategies for frail and prefrail older adults may help to move toward a function-based, rather than disease-based, model of medical care (40).
Funding
The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (UL1TR001420). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding support was also provided by the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332), the J. Paul Sticht Center for Healthy Aging and Alzheimer’s Disease, and the Center for Health Care Innovation at Wake Forest School of Medicine.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the data extraction and analyses performed by members of the Biomedical Informatics Program within the Clinical and Translational Science Institute at Wake Forest School of Medicine.
Author Contributions
N.M.P., J.D.W., and K.E.C. contributed to study design. N.M.P., K.L., and B.J.W. performed data extraction and statistical analyses. All authors participated in drafting the article, editing, and approved the final version of the article.
Conflict of interest statement
None reported.
References
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi:10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 3. Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–1074. doi: 10.1056/NEJMoa020423 [DOI] [PubMed] [Google Scholar]
- 4. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi:10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 5. Drubbel I, de Wit NJ, Bleijenberg N, Eijkemans RJ, Schuurmans MJ, Numans ME. Prediction of adverse health outcomes in older people using a frailty index based on routine primary care data. J Gerontol A Biol Sci Med Sci. 2013;68:301–308. doi: 10.1093/gerona/gls161 [DOI] [PubMed] [Google Scholar]
- 6. Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304:1919–1928. doi: 10.1001/jama.2010.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roe L, Normand C, Wren MA, Browne J, O’Halloran AM. The impact of frailty on healthcare utilisation in Ireland: evidence from the Irish longitudinal study on ageing. BMC Geriatr. 2017;17:203. doi: 10.1186/s12877-017-0579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campitelli MA, Bronskill SE, Hogan DB, et al. The prevalence and health consequences of frailty in a population-based older home care cohort: a comparison of different measures. BMC Geriatr. 2016;16:133. doi: 10.1186/s12877-016-0309-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. doi: 10.1111/j.1532-5415.2006.00738.x [DOI] [PubMed] [Google Scholar]
- 10. Turner G, Clegg A; British Geriatrics Society; Age UK; Royal College of General Practioners Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing. 2014;43:744–747. doi: 10.1093/ageing/afu138 [DOI] [PubMed] [Google Scholar]
- 11. Puts MTE, Toubasi S, Andrew MK, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017;46:383–392. doi: 10.1093/ageing/afw247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walston J, Buta B, Xue QL. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med. 2018;34:25–38. doi: 10.1016/j.cger.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aguayo GA, Donneau AF, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186:420–434. doi: 10.1093/aje/kwx061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45:353–360. doi: 10.1093/ageing/afw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Casey JA, Schwartz BS, Stewart WF, Adler NE. Using electronic health records for population health research: a review of methods and applications. Annu Rev Public Health. 2016;37:61–81. doi: 10.1146/annurev-publhealth-032315-021353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Segal JB, Chang H-Y, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care. 2017;55:716–722. doi: 10.1097/MLR.0000000000000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Orkaby AR, Nussbaum L, Ho Y-L, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002–2012. J Gerontol A Biol Sci Med Sci. 2018. doi: 10.1093/gerona/gly232. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Callahan KE, Wilson LA, Pavon JM, et al. Internal medicine residents’ ambulatory management of core geriatric conditions. J Grad Med Educ. 2017;9:338–344. doi: 10.4300/JGME-D-16-00428.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weir DR, Wallace RB, Langa KM, et al. Reducing case ascertainment costs in U.S. population studies of Alzheimer’s disease, dementia, and cognitive impairment-Part 1. Alzheimers Dement. 2011;7:94–109. doi: 10.1016/j.jalz.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kainkaryam V. The annual wellness visit shared medical appointment: innovative delivery of preventive care to the elderly. J Ambul Care Manage. 2013;36:335–337. doi: 10.1097/JAC.0b013e3182a3e78b [DOI] [PubMed] [Google Scholar]
- 22. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi:10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 23. Tonelli M, Wiebe N, Fortin M, et al. ; Alberta Kidney Disease Network Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. doi: 10.1186/s12911-015-0155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Howlett SE, Rockwood MR, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 2014;12:171. doi: 10.1186/s12916-014-0171-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heller G. A measure of explained risk in the proportional hazards model. Biostatistics. 2012;13(2):315–325. doi: 10.1093/biostatistics/kxr047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dong H, Robison LL, Leisenring WM, Martin LJ, Armstrong GT, Yasui Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am J Epidemiol. 2015;181:532–540. doi: 10.1093/aje/kwu289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rondeau V, Mazroui Y, Gonzalez JR. frailtypack: an R package for the analysis of correlated survival data with frailty models using penalized likelihood estimation or parametrical estimation. J Stat Softw. 2012;47(4):1–28. doi: 10.18637/jss.v047.i04 [DOI] [Google Scholar]
- 29. Goldstein BA, Bhavsar NA, Phelan M, Pencina MJ. Controlling for informed presence bias due to the number of health encounters in an electronic health record. Am J Epidemiol. 2016;184:847–855. doi: 10.1093/aje/kww112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoover M, Rotermann M, Sanmartin C, Bernier J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep. 2013;24:10–17. [PubMed] [Google Scholar]
- 31. Stow D, Matthews FE, Barclay S, et al. Evaluating frailty scores to predict mortality in older adults using data from population based electronic health records: case control study. Age Ageing. 2018;47(4):564–569. doi: 10.1093/ageing/afy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Santos-Eggimann B, Sirven N. Screening for frailty: older populations and older individuals. Public Health Rev. 2016;37:7. doi: 10.1186/s40985-016-0021-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Drubbel I, Numans ME, Kranenburg G, Bleijenberg N, de Wit NJ, Schuurmans MJ. Screening for frailty in primary care: a systematic review of the psychometric properties of the frailty index in community-dwelling older people. BMC Geriatr. 2014;14:27. doi: 10.1186/1471-2318-14-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goldstein BA, Navar AM, Pencina MJ, Ioannidis JPA. Opportunities and challenges in developing risk prediction models with electronic health records data: a systematic review. J Am Med Inform Assoc. 2017;24(1):198–208. doi: 10.1093/jamia/ocw042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70:1427–1434. doi: 10.1093/gerona/glv133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ganguli I, Souza J, McWilliams JM, Mehrotra A. Practices caring for the underserved are less likely to adopt Medicare’s annual wellness visit. Health Aff (Millwood). 2018;37:283–291. doi: 10.1377/hlthaff.2017.1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg. 2017;152:233–240. doi: 10.1001/jamasurg.2016.4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kritchevsky SB, Williamson J. Putting function first. J Nutr Health Aging. 2014;18:467–468. doi: 10.1007/s12603-014-0456-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.