Abstract
Background: We estimated the prevalence and incidence of amyloid-β deposition (A), small-vessel disease (V), and neurodegeneration (N) biomarker positivity in community-dwelling cognitively normal individuals (CN). We determined the longitudinal association between the respective biomarker indices with progression to all-cause mild cognitive impairment (MCI) and its amnestic and nonamnestic subtypes.
Methods: CN participants, recruited by advertising, underwent brain [C-11]Pittsburgh Compound-B (PiB)-positron emission tomography (PET), magnetic resonance imaging, and [F-18]fluoro-2-deoxy-glucose (FDG)-PET, and were designated as having high or low amyloid-β (A+/A−), greater or lower white matter hyperintensities burden (V+/V−) and diminished or normal cortical glucose metabolism (N+/N−). MCI was adjudicated using clinical assessments. We examined the association between A, V, and N biomarker positivity at study baseline and endpoint, with progression to MCI using linear regression, Cox proportional hazards and Kaplan–Meier analyses adjusted for age and APOE-ε4 carrier status.
Results: In 98 CN individuals (average age 74 years, 65% female), A+, V+, and N+ prevalence was 26%, 33%, and 8%, respectively. At study endpoint (median: 5.5 years), an A+, but not a V+ or N+ scan, was associated with higher odds of all-cause MCI (Chi-square = 3.9, p = .048, odds ratio, 95% confidence interval = 2.6 [1.01–6.8]). Baseline A+, V+, or N+ were not associated with all-cause MCI, however, baseline A+ (p = .018) and A+N+ (p = .049), and endpoint A+N+ (p = .025) were associated with time to progression to amnestic, not nonamnestic, MCI.
Conclusion: Longitudinal assessments clarify the association between amyloid-β and progression to all-cause MCI in CN individuals. The association between biomarker positivity indices of amyloid-β and neurodegeneration, and amnestic MCI reflects the underlying pathology involved in the progression to prodromal Alzheimer’s disease.
Keywords: Alzheimer’s, Biomarkers, Mild cognitive impairment
Cognitively normal (CN) individuals with in vivo evidence of Alzheimer’s disease (AD) pathology in the brain are considered to have preclinical AD (1), a target population for AD-modifying drug trials (2). While this definition of preclinical AD incorporates biomarkers of amyloid-β deposition and neurodegeneration, it largely ignores small-vessel disease, which was noted to coexist with AD pathology since the first case report described by Alois Alzheimer (3). As amyloid-β deposition, small-vessel disease, and neurodegeneration are common age-related changes in the brain that influence cognitive outcomes, it is essential to characterize the three processes simultaneously.
Amyloid-beta (Aβ) is deposited in the brain several years prior to the clinical onset of AD and is associated with progression to mild cognitive impairment (MCI), a transient preclinical phase of AD (4). In CN older adults, elevated levels of Aβ in the brain are present in 21%–25% of individuals at age 65 years and in 30%–65% at 85 years (5,6). White matter hyperintensities (WMH), markers of small-vessel disease on MRI, have been reported to occur in over 90% of older adults with and without dementia (7) and in CN older adults, and are associated with progression to MCI (8). Synaptic dysfunction, assessed by decreased [F-18]fluoro-2-deoxy-glucose-PET (FDG)-PET uptake in the brain is a marker of neurodegeneration (9) reported in 29% of CN older adults (10) and is also associated with progression to MCI (9). Estimates of Aβ and/or neurodegeneration positivity have largely focused on dementia-free samples (summarized by Jansen and colleagues (11)), which could include those with MCI (10,12,13). Therefore, the estimates derived from such mixed samples may not be generalizable to a CN sample to estimate burden of preclinical AD. In addition, estimates of AD-biomarker positivity in CN samples have not examined markers of coexisting small-vessel disease (13–15). Therefore, to enable sample-size calculations for clinical trials targeting preclinical AD, it is imperative to study AD biomarkers in CN individuals and examine small-vessel disease biomarkers due to its high prevalence in older populations (7).
We previously reported on the association between Aβ, small vessel disease and neurodegeneration positivity determined at study baseline with clinical outcomes 2 years later in dementia-free adults with a mean age of 85.5 years (12). Individuals with normal levels of Aβ, lower burden of WMH and absence of hypometabolism can accrue changes in these biomarkers with advancing age—the rate of conversion from Aβ-negative to Aβ-positive status was estimated at 3.1% per year in a CN sample (14). Therefore, the relationships between biomarker status and clinical outcome may differ when biomarker positivity is assessed at baseline as opposed to a later point in the follow-up. Here, we examined cognitive outcomes of biomarker positivity for Aβ, small-vessel disease, and neurodegeneration in a carefully screened sample of CN older adults.
The aim of the study was to estimate the prevalence and incidence of biomarker positivity for Aβ deposition determined using Pittsburgh Compound-B (PiB)-PET, small-vessel disease quantified using WMH severity and neurodegeneration assessed by hypometabolism using FDG-PET. We then determined the relationship between the respective biomarker positivity status at baseline and at last follow-up, with progression to MCI in CN older adults.
Methods
Participants
Participants (n = 98) comprised community-dwelling older adults aged 65–90 years (mean 73.7 ± 5.6 years) screened for major psychiatric or neurological disorders, depression, use of medications affecting cognition, and any preclusions for brain MRI as reported previously (16). They were recruited from advertisements in the community, from a cohort of control volunteers at the University of Pittsburgh Alzheimer’s Disease Research Center (ADRC), and from the University of Pittsburgh Pepper Registry, which is a registry of studies on mobility, balance, and aging. Inclusion criteria were age >60 years, a Mini-Mental State Examination score >27 and cognitively normal based detailed cognitive assessment, and a Clinical Dementia Rating scale score = 0 (17). The cognitive assessments included tests of memory (Word List Learning from the Consortium to Establish a Registry in Alzheimer’s Disease battery, Logical Memory Story A from the Wechsler Memory Scale—Revised, modified Rey Osterrieth (R–O) figure recalls), visuospatial construction (modified block design subtest from the Wechsler Adult Intelligence Scale—Revised (WAIS-R), copying of the R–O figure), language (semantic and letter fluency, Boston Naming Test), and attention and executive functions (Trail Making Test A & B, Digit Symbol Substitution Test, and digit spans forward and backward from the WAIS-R) (16). Participants were followed over the subsequent 1–10 years with clinical assessments performed annually and brain imaging performed every 1–2 years during the study period. Participants with any psychiatric, brain injury, neurological, and unstable medical conditions were excluded. We also excluded those with incomplete data for this analysis.
Outcomes
Participants were classified as cognitively normal or MCI at every annual clinical assessment. Adjudication of MCI was performed by three investigators (W.E.K., B.E.S., and H.J.A.) utilizing questionnaires ascertaining participants’ subjective cognitive complaints and neuropsychological test scores, with the following criteria: (i) concern regarding change in cognition; (ii) impairments in one or more cognitive domains; and (iii) preservation of independence in functional abilities (18). We also categorized MCI as amnestic MCI (aMCI, predominant memory impairment) and nonamnestic MCI (naMCI, predominantly nonmemory impairments).
Imaging
PiB-PET methodology followed a standard procedure (19) that involved injection of [11C]PiB (15 mCi) followed by a 30-minute PiB-PET study (6 × 300-second frames) acquisition 50–70 minutes postinjection. [18F]FDG was administered (7 mCi over 20 seconds) intravenously after PiB injection. FDG-PET images were acquired over 25 minutes in the 35–60 minutes postinjection interval. A structural T1-weighted MPRAGE MR image was acquired for each subject, using the Siemens 3T MR scanner. Details of image registration, regions of interest delineation, and measures of PiB retention and FDG uptake were described previously (20).
Aβ positivity was determined on PiB-PET using the sparse k-mean clustering (SKM) (20). SKM regional cutoffs were obtained for cortical regions with a predilection for Aβ deposition (anterior cingulate, frontal, lateral temporal, parietal and precuneus cortices, and anterior ventral striatum). Individuals with regional PiB binding that exceeded this predetermined cutoff were designated Aβ positive (A+) and those not meeting this cutoff were designated as normal Aβ (A−) (20).
Neurodegeneration was quantified using FDG-PET. Decreased FDG uptake in the posterior cortices (parietal, precuneus, occipital, and inferior temporal) was used to designate neurodegeneration positivity (N+) at a cutoff of 1.025 SUVR (21).
MRI was performed on a 1.5 T at the start of study, and upgraded to a 3.0 T scanner (GE Medical Systems, Milwaukee, WI) during the study period. WMH, surrogate markers or small-vessel disease burden, were assessed on T2-FLAIR sequences on both 1.5 T (TR = 9,004 msec, TE = 172.5 msec [effective]; TI = 2,200 msec, number of excitations = 1, interleaved acquisition over 24 slices, 4-mm slice thickness, 1 mm gap) and 3T (TR = 9,160 msec, TE = 90 msec [effective]; TI = 2,500 msec, number of excitations = 1, interleaved acquisition over 48 slices, 3-mm slice thickness with no gap). WMH volume was rendered on these FLAIR sequences, however, we did not combine the two volume measures due to differences in MRI acquisition and the magnets used. Instead, we rated severity of WMH using the Cardiovascular Health Study (CHS) WMH visual scale (22). Blinded to demographic, clinical, or other imaging data, raters (W.E.K., H.J.A., A.D.C., and N.K.N.) scored WMH on MRIs on the 10-point scale (Grade 0 to Grade 9) (23) and agreements on ratings were recorded on a consensus basis. Participants were designated as having a high small-vessel disease burden (V+) using a cutoff score of 3 or more, which corresponds to beginning confluence of WMH linked to cognitive impairment (23,24).
Statistical Methods
Descriptive statistics for baseline characteristics were assessed as means and standard deviations (SD) for continuous variables and % (n) for categorical variables. Two sample t test were used to test differences between the continuous variables and Chi-squares test (or Fisher’s as appropriate) for the categorical variables.
Prevalence of biomarker positivity was measured by dividing the number of respective positive individuals at baseline by the population at risk. Incident biomarker positivity and incident MCI was measured by the number of new biomarker positive individuals and new MCI cases divided by the respective number at-risk (biomarker negative individuals, and all CN participants) at baseline. Person-years was the total number of years that each individual was under observation or until an MCI/biomarker outcome was reached and measured by multiplying the number of years (from baseline to first incident case or, to final follow-up in nonincident cases) by the number of individuals.
To evaluate the association between final clinical status and each biomarker at baseline and at last follow-up, linear logistic models were used; models were both unadjusted and adjusted for age and APOE-ε4 carrier status. We also performed an exploratory analysis to examine the relationship between presence of one or multiple positive biomarkers and time to progression to MCI using Cox proportional hazards models. Kaplan–Meier plots were used to graphically demonstrate these relationships.
Results
Baseline Characteristics
In this sample of 98 CN individuals, mean age 73.7 years (± 5.6 years), 65% were female, 21% were APOE-ε4 carriers, and 87% were Caucasian. The follow-up assessments for imaging and clinical outcomes were performed over an average of 3.5 and 4.3 years, respectively (range: 1–10 years).
Table 1 shows the baseline characteristics of the group based on biomarker and final clinical status. Biomarker positive individuals were generally older than biomarker negative individuals and these differences in age were statistically significant for the A+ versus A− but not the V+ versus V−, or N+ versus N− groups. Proportion of APOE-ε4 carriers were significantly greater in the A+ group compared with the A− group but no significant differences were noted in the V and N biomarker groups. Racial differences were also significant for the A+ versus A− groups. At study baseline, individuals who later progressed to MCI were older than those who remained CN through the study duration but these differences were not statistically significant.
Table 1.
Participant Characteristics at Study Baseline
| Baseline Aβ Status | Baseline Small-vessel Disease Status | Baseline Neurodegeneration Status | Clinical Status at Endpoint | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Characteristics | A+ (n = 25) |
A− (n = 73) |
Statistic, df | V+ (n = 32) |
V− (n = 66) |
Statistic, df | N+ (n = 8) |
N− (n = 90) |
Statistic, df | MCI (n = 24) |
CN (n = 74) |
Statistic, df |
| Age at Baseline mean (SD), years | 76.0 (5.8) | 72.9 (5.4) | t = −2.3, df = 38.9* | 75.4 (6.1) | 72.9 (5.2) | t = −1.9, df = 53.6 | 71.6 (4.1) | 73.9 (5.7) | t = 1.4, df = 9.6 | 75.7 (6) | 73.1 (5.4) | t = −1.9, df = 36.1 |
| Female sex No. (%) |
13 (52%) | 51 (69.8%) | χ2 = 1.9, df = 1 | 22 (68.8%) | 42 (63.6%) | χ 2 = 0.07, df = 1 | 5 (62.5%) | 59 (69.5%) | a | 13 (54.1%) | 51 (68.9%) | χ 2 = 1.2, df = 1 |
| Caucasian race No. (%) |
22 (88%) | 63 (86.3) | a,* | 28 (87.5%) | 57 (86.3%) | a | 8 (100%) | 77 (85.5) | a | 22 (91.6%) | 63 (85.1%) | a |
| Education mean (SD), years |
14.9 (2.9) | 15.1 (2.5) | t = 0.4, df = 37 | 15.3 (2.8) | 14.9 (2.5) | t = −0.7, df = 55.9 | 15.1 (2.4) | 15.1 (2.6) | t = −0.1, df = 8.6 | 14.5 (2.5) | 15.2 (2.6) | t = 1.2, df = 40.6 |
| APOE-ε4 carrierb No. (%) |
10 (0.1) | 11 (0.1) | χ 2 = 5.0, df = 1* | 7 (0.1) | 14 (0.2) | χ 2 = 0, df = 1 | 1 (0.01) | 20 (0.2) | a | 7 (0.1) | 14 (0.2) | χ 2 = 0.3, df = 1 |
| MMSE mean (SD) |
28.4 (1.7) | 28.6 (1.4) | t = 0.5, df = 36 | 28.6 (1.5) | 28.6 (1.5) | t = −0.1, df = 60.3 | 29.4 (0.9) | 28.5 (1.5) | t = −2.4, df = 10.9* | 28.4 (1.3) | 28.6 (1.6) | t = 0.6, df = 44.9 |
Note: Welch Two Sample t test.
χ2 test with Yates’ continuity correction.
MMSE = Mini-Mental Status Examination.
aFisher’s Exact Test used instead of χ 2 test with Yates’ continuity correction. bAPOE-ε4: apolipoprotein- ε4 allele (data missing for 8 subjects, n = 92).
*p < .05.
The differences in PiB-PET SUVR, WMH scale scores and FDG-PET SUVR for the respective A, V, and N groups is shown in Supplementary Table 1. The N− group had a significantly higher WMH scale score than N+ groups. Otherwise, there were no significant differences between the biomarker status groups, beyond each respective biomarker defining group membership.
Prevalence and Incidence of Biomarker Positivity and MCI
Table 2 shows the prevalent and incident biomarker positivity for the whole sample further subgrouped by tertiles of age. The prevalence estimates of Aβ, small-vessel disease, and neurodegeneration positive biomarkers were 26%, 33%, and 8%, respectively. In at-risk CN individuals, the overall incidence rate of Aβ positivity was estimated at 6 per 100 person-years, and that of small-vessel disease and neurodegeneration positivity was estimated at 2 per 100 person-years each. Incidence rates of positivity indices for Aβ deposition, but not small-vessel disease or neurodegeneration, increased with age.
Table 2.
Prevalence, Incidence, and Incidence Rates of Respective Biomarker Positivity
| Aβ biomarker positivity (A+) | Small-vessel Disease biomarker positivity (V+) | Neurodegeneration biomarker positivity (N+) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence | Incidencea | Person-years | Incidence Rate (per 100 person-years) | Prevalence | Incidencea | Person-years | Incidence rate (per 100 person-years) | Prevalence | Incidencea | Person-years | Incidence rate (per 100 person-years) | |
| Whole group | 25/98 (26%) | 15/54 (28%) | 245 | 6 | 32/98 (33%) | 6/46 (13%) | 244 | 2 | 8/98 (8%) | 8/69 (12%) | 332 | 2 |
| 60–69 y | 5/29 (17%) | 2/16 (13%) | 69 | 3 | 6/29 (21%) | 0/15 (0) | 81 | 0 | 3/29 (10%) | 5/18 (3%) | 82 | 6 |
| 70–76 years | 6/36 (17%) | 7/23 (30%) | 108 | 6 | 12/36 (33%) | 4/17 (24%) | 102 | 4 | 3/36 (8%) | 1/29 (3%) | 153 | 1 |
| 77–90 years | 14/3 (42%) | 6/15 (40%) | 68 | 9 | 14/33 (42%) | 2/14 (14%) | 62 | 3 | 2/33 (6%) | 2/22 (9%) | 98 | 2 |
Note: Aβ = Amyloid-β; WMH = White matter hyperintensities.
aThe denominator for incidence is based on the number of individuals who were both: (i) biomarker negative at baseline and (ii) had at least one imaging follow-up for that biomarker.
All participants were CN at study entry; therefore, we ascertained only incident MCI in this sample. Incident rate of MCI were estimated at 6 cases per 100 person-years for the whole sample (60–69 years: 5 cases per 100 person-years; 70–76 years: 4 cases per 100 person-years; and 77–90 years: 11 cases per 100 person-years).
Association Between Biomarker Positivity and Progression to MCI
During a median of 5.5 years (interquartile range: 4.2) 24 of the 98 baseline CN individuals progressed to MCI. At baseline, there were no significant differences in the number of A+/A−, V+/V−, or N+/N− individuals who later progressed to MCI compared with those who remained CN (Supplementary Table 2).
Table 3 shows the proportions of A, V, and N biomarker positive and negative proportions in the CN and MCI groups. At last follow-up assessment, the proportion of A+ MCI individuals exceeded that of A− MCI individuals, and proportion of A− CN individuals exceeded A+ CN individuals (Chi-square = 3.9, p = .048, odds ratio [OR] [95% confidence interval {CI} = 2.6 [1.0–6.8], Table 3). Of the 15 incident A+ cases, 6 progressed to MCI. There were no significant differences in the last follow-up assessment in the numbers of those with V+ versus V− or, with N+ versus N− in those with MCI compared to CN individuals (Table 3).
Table 3.
Biomarker Positivity and Clinical Status at Last Follow-Up
| Clinical Status | Aβ | Small-vessel Disease | Neurodegeneration | |||
|---|---|---|---|---|---|---|
| A+ | A− | V+ | V− | N+ | N− | |
| MCI = 24 | 14 | 10 | 9 | 15 | 5 | 19 |
| CN = 74 | 26 | 48 | 27 | 47 | 10 | 64 |
| Pearson χ 2 | 3.9 | 0.01 | 0.7 | |||
| Odds Ratio (95% CI) | 2.6 (1.01, 6.8)* | 1 (0.4, 2.7) | 1.7 (0.5, 5.4) | |||
| Odds Ratio Adjusted for Age and APOE-ε4 (95% CI) | 1.8 (0.6, 5.1) | 0.7 (0.2, 2.1) | 2.3 (0.6, 8.7) | |||
Note: A = Amyloid-β biomarker status; APOE-ε4 = Apolipoprotein-ε4 carrier status; N = Neurodegeneration biomarker status; CN = Cognitively normal older individuals; MCI = Mild cognitive impairment; V = Small-vessel disease biomarker status.
*p < .05.
Supplementary Table 3 depicts the beta-coefficient, p value, hazard ratio, and 95% confidence interval for univariate and multivariate model revealing a lack of association between baseline and last observation of A, V, and N positivity and progression to MCI. Supplementary Figures 1–3 depicts the Kaplan–Meier plots demonstrating the lack of relationship between baseline and endpoint A, V, and N biomarker positivity and MCI outcomes, respectively.
We found that the presence of all three positive biomarkers combined was significantly associated with progression to MCI (p = .0005); however, these subsamples were unbalanced (n = 5 [A+, V+, and N+] compared with n = 30 [A−, V−, and N−]). Any combination of two positive biomarkers versus no positive biomarkers was not significantly associated with progression to MCI. No other inflection point along the continuous distribution of each biomarker better predicted conversion from normal to MCI.
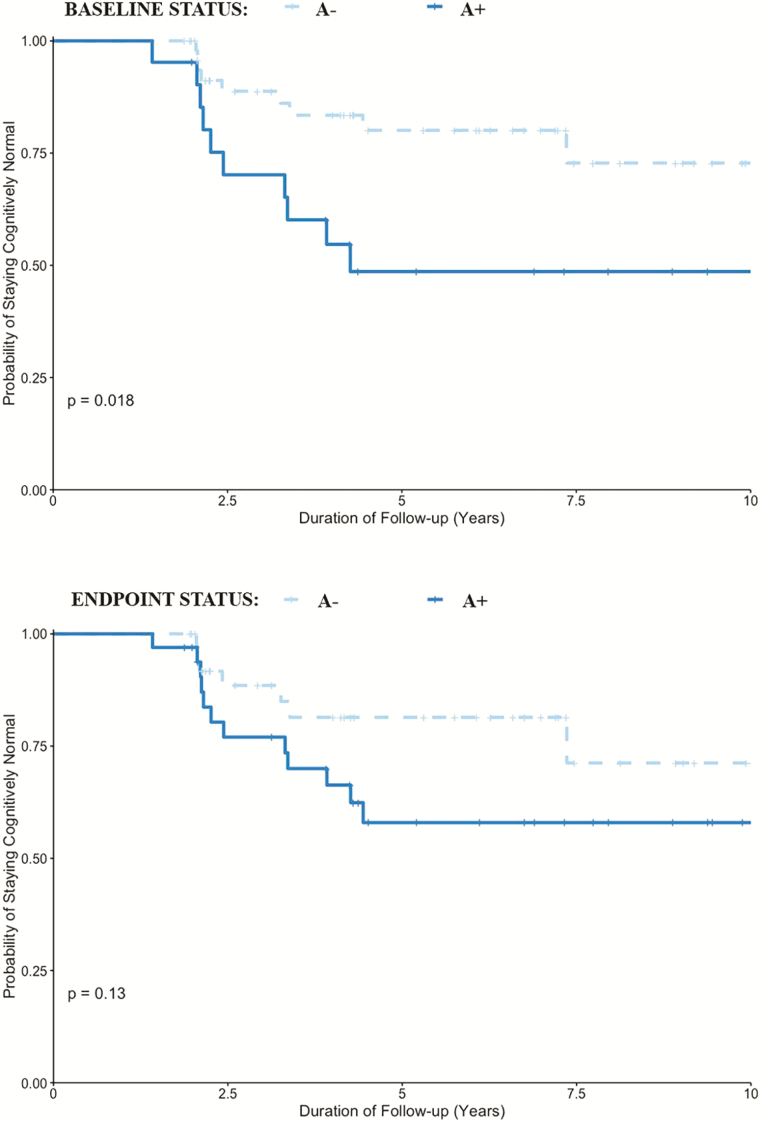
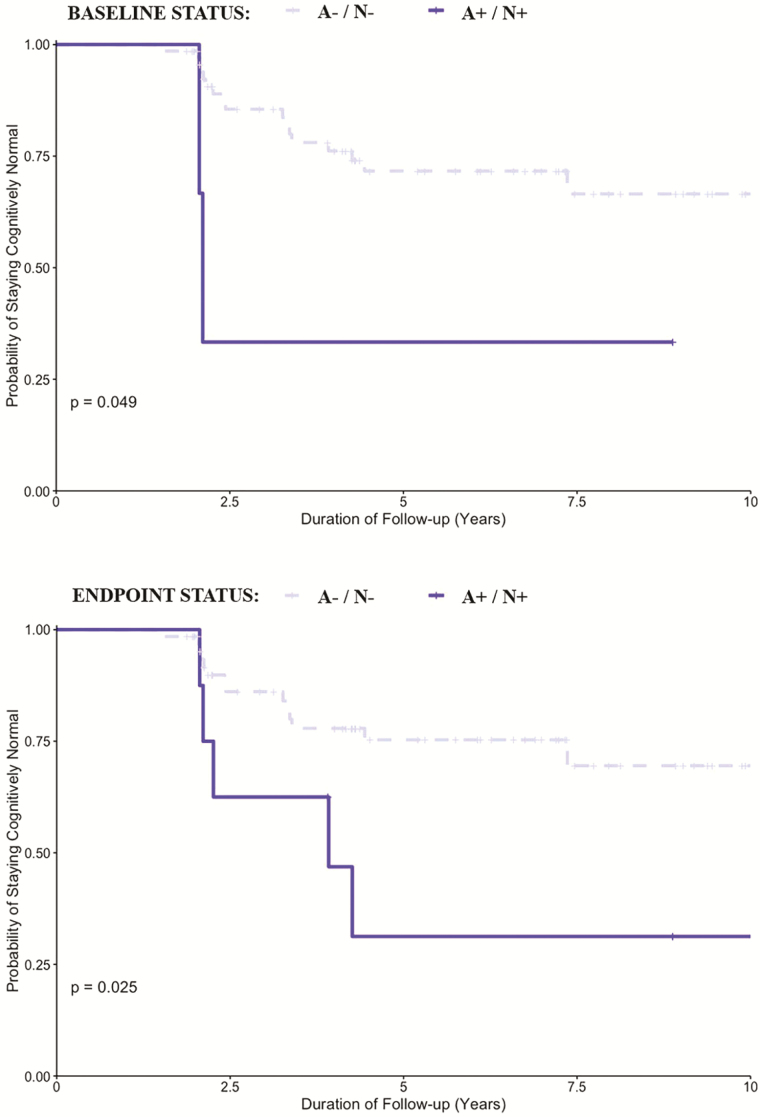
At last follow-up assessment, 18 of the 24 MCI individuals were aMCI, and 6 were naMCI. Baseline A+, but not a V+ scan, was associated with higher odds of progression to aMCI (Chi-square = 5.9, p = .035, OR [95% CI] = 3.6 [1.2–10.6]), not naMCI (p = .6). In addition, time to progression to aMCI, not naMCI, was significantly associated with A+ biomarker status at study baseline (p = .018), not endpoint (p = .13, Figure 1). A combined biomarker positivity of A+ and N+ scan at study endpoint (Chi-square = 10.3, p = .007, OR [95% CI] = 9.1 [1.9–42.9] but not at baseline (Chi-square = 4.3, p = .097, OR [95% CI] = 9.1 [0.78–106.9]), was associated with higher odds of aMCI when compared with staying cognitively normal. Time to progression to aMCI, not naMCI, was associated with combined biomarker positivity of A+ and N+ at study baseline (p = .049), and particularly at study endpoint (p = .025, Figure 2). Of the 6 of 15 incident A+ individuals who progressed to MCI, 3 were aMCI and 3 were naMCI, and of these, none of the aMCI were V+ or N+, whereas 1 individual (77–90 years age tertile) in the naMCI group was both V+ and N+. V+ alone, or in combination with the other two biomarkers, had no significant association with aMCI or naMCI. Proportions of incident aMCI and naMCI increased with age (Supplementary Table 4).
Figure 1.
Kaplan–Meier plots demonstrating the relationship between brain amyloid-β (A) biomarkers assessed at study baseline (top section) and endpoint (bottom section) with time (years) to progression to amnestic mild cognitive impairment in cognitively normal individuals.
Figure 2.
Kaplan–Meier plots demonstrating the relationship between biomarker positivity for combined biomarker positivity of amyloid-β and neurodegeneration (A/N) at study baseline (top section) and at endpoint (bottom section) with time (years) to progression to amnestic mild cognitive impairment in cognitively normal individuals.
Discussion
Aβ burden increased with age in our sample of CN older adults such that prevalence of amyloid positivity doubled, and the incidence rates of amyloid positivity tripled from the lowest age tertile (60–69 years) to the highest age tertile (77–90 years). Also, the prevalence and incidence of positivity indices of small-vessel disease but not neurodegeneration increased with age. These estimates are amongst a few that look at prevalence and incidence rates of A, V, and N biomarker positivity in a single CN older adult sample.
The prevalence estimates of amyloid positivity of 26% in our sample falls within the range expected in dementia-free older adults (25), and are in keeping with findings from the Mayo Clinic Study of Aging (15), Australian Imaging, Biomakers Lifestyle study (4,26), and others (14,27). However, our estimate of prevalent A+ status was lower than the 45% estimated in Alzheimer’s Disease Neuroimaging Initiative recruited largely from academic memory clinics (28). The differences in the prevalence across samples are likely due to varying cutoffs for amyloid positivity and populations differences, besides others.
Our estimated V+ prevalence of 33% is the same as that estimated in the CHS cohort (mean age 70 years) (29), and is similar to the 36% reported in an Asian cohort of older adults (30). Our estimate of the prevalence of N+ status was lower than the 25%–29% estimated previously (10,31). Lower estimates than those previously reported may be explained by the fact that 74 participants remained CN at last follow-up, which in some cases was up to 10 years from baseline assessment, accounting for greater number of person-years, therefore, decreasing our overall estimates of incidence rates.
Several studies have shown that Aβ positivity in dementia-free older adults is related to cognitive decline and progression to MCI (13–15,32). We did not find a significant association between baseline A+ status with progression to MCI. A proportion of those with A− status at baseline progressed to A+ over the study period thereby increasing the odds of MCI with A+ status at last follow-up. That is, the baseline A− group harbored several individuals at a subthreshold level of amyloid positivity who would soon progress to both A+ and MCI (6 of the 15 incident A+ individuals progressed to MCI). This suggests that A− individuals on a trajectory of Aβ deposition can become A+ over a brief period and progress to MCI. The discrepancy between findings from this sample and other larger studies may have several explanations. One may be that our sample was relatively smaller than other studies and not powered to show the cognitive effects of Aβ over the relatively short follow-up time that averaged 4 years. In addition, the study criteria were stringent to ensure that all participants were CN at study entry. Therefore, longer follow-up may be needed to observe the effects of Aβ on cognition in this sample. These findings suggest that cross-sectional analysis may blunt the association between Aβ and progression to MCI, which may be revealed longitudinally. The association between A+ status and incident MCI at last follow-up was not significant when adjusted for age and APOE-e4 carrier status which raises the possibility that Aβ status may have a poor predictive utility for intermediate cognitive outcomes such as all-cause MCI. The finding that an A+ status at baseline was associated with higher odds of aMCI, and time to progression to the aMCI, may reflect the underlying pathological substrate involved in AD. Furthermore, time to progression to aMCI, not naMCI, was significantly associated with the biomarker status combination of A+ and N+ scan at both study timepoints. The stronger relationship between time to progression to aMCI and A+N+ status at study endpoint compared with at study baseline is in keeping with prior research indicating that Aβ deposition is an early event in evolution of AD, and hypometabolism from neuronal dysfunction may follow over a period of time leading to prodromal AD (33–35).
In contrast with studies that found a relationship between small-vessel disease and cognitive decline (24) and neurodegeneration and MCI (36,37), we found no significant independent associations between these two biomarkers and progression to all-cause MCI. WMH and hypometabolism, indices of small-vessel disease, and neurodegeneration, are not specific to AD pathology and have varied etiology whereas Aβ deposition is a core feature of AD pathology resulting in cognitive decline and MCI. Our findings are in keeping with results from Alzheimer’s Disease Neuroimaging Initiative that showed that WMH burden has no independent association with progression to MCI (38). Some suggest that small-vessel disease, but not neurodegenerative markers, predict incident AD (37). The discrepancy between these findings may have to do with burden of vascular disease sampled, thresholds used and variability in cohorts. In addition, markers of V and N are not solely attributable to vascular (39) and neurodegenerative processes, respectively (40). The clinical implications of these findings indicate that FDG-PET and FLAIR MRI may have limited utility in predicting transition to all-cause MCI. The small numbers of naMCI (n = 6) in our sample may explain why we did not find an association between small-vessel disease biomarkers and naMCI.
Aβ, small-vessel disease, and neurodegeneration can interact with one another. Our exploratory analysis suggested that presence of all three states (A+, V+, and N+) were significantly associated with progression to MCI; however, the sample was too small to draw firm conclusions. Yet, it underscores the “healthy” phenotype of our sample compared with another older sample from the same geographic region that had a larger proportion of three-biomarker positivity and a stronger relationship with MCI (12). Aβ deposition and small-vessel disease coexist and influence neurodegeneration (41). Small-vessel disease augments the expression of clinical symptoms in AD (42), and is independently associated with neurodegeneration (43). Longer follow-up period may make these interrelationships between biomarkers and progression to MCI more apparent.
There are some limitations that need to be considered. The burden of small-vessel disease in our sample was likely low based on the study criteria employed; therefore, these findings may not be generalizable to populations with greater vascular disease burden. We used visual rating scale scores to designate small-vessel disease positivity biomarkers because we switched to a updated MRI scanner midway in the study, however, severity of WMH ratings did not change when using the updated scanner. We did not characterize cardiovascular risk and treatments of cardiac risk which can influence small-vessel disease (44). We used established thresholds to define biomarker positivity for the three indices, however, these were not developed or optimized for defining transition from normal to MCI or the two MCI subtypes assessed.
There are several strengths of this study. The recruitment of high-functioning CN sample at baseline allows us to test the conceptual model that biomarker changes may extends 15–20 years prior to MCI (45). This study also shows an association between amyloid-β alone at study baseline, and in combination with neurodegeneration biomarker at study endpoint, and time to progression to aMCI, in keeping with evolution of AD pathology and neuronal dysfunction leading to development of prodromal AD. Based on recent evidence of vascular contribution to AD pathology (46), we include a biomarker for the vascular component that is missing in the proposed conceptual framework for study of AD and related dementias (1). We did not impute missing data or transform data. We utilized PiB-PET and FDG-PET uniformly from initiation of the study providing a rich dataset, with some participants being followed for approximately 10 years, enabling an accurate estimate of incidence rates.
Conclusion
This study estimates prevalence and incidence of biomarker positivity for amyloid, small-vessel disease, and neurodegeneration in a community-dwelling CN sample. Amyloid positivity assessed longitudinally, had greater odds of progression to MCI, whereas small-vessel disease or neurodegeneration assessed cross-sectionally or longitudinally was not associated with progression to all-cause MCI. Biomarker positivity for amyloid-β alone, and in combination with neurodegeneration over the study period, was associated with time to progression to the AD prodrome, aMCI.
Funding
This work was supported by grants K23 AG049945 (PI: N.K.N.) and RF01 AG025516 (PI: W.E.K. and H.J.A.) from the National Institute on Aging.
Conflict of Interest
GE Healthcare holds a license agreement with the University of Pittsburgh. W.E.K. and C.A.M. are coinventors of PiB and, as such, have a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this manuscript. All other authors have no conflicts of interest with this work.
Supplementary Material
References
- 1. Jack CR Jr, Bennett DA, Blennow K, et al. ; Contributors NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sperling RA, Rentz DM, Johnson KA, et al. . The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karran E, De Strooper B. The amyloid cascade hypothesis: are we poised for success or failure? J Neurochem. 2016;139(Suppl 2):237–252. doi: 10.1111/jnc.13632 [DOI] [PubMed] [Google Scholar]
- 4. Villemagne VL, Pike KE, Chételat G, et al. . Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–192. doi: 10.1002/ana.22248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mathis CA, Kuller LH, Klunk WE, et al. . In vivo assessment of amyloid-beta deposition in nondemented very elderly subjects. Ann Neurol. 2013;73:751–761. doi: 10.1002/ana.23797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aizenstein HJ, Nebes RD, Saxton JA, et al. . Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Leeuw FE, de Groot JC, Achten E, et al. . Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boyle PA, Yu L, Fleischman DA, et al. . White matter hyperintensities, incident mild cognitive impairment, and cognitive decline in old age. Ann Clin Transl Neurol. 2016;3:791–800. doi: 10.1002/acn3.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Leon MJ, Convit A, Wolf OT, et al. . Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET). Proc Natl Acad Sci USA. 2001;98:10966–10971. doi: 10.1073/pnas.191044198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landau SM, Mintun MA, Joshi AD, et al. ; Alzheimer’s Disease Neuroimaging Initiative Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jansen WJ, Ossenkoppele R, Knol DL, et al. ; Amyloid Biomarker Study Group Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez OL, Klunk WE, Mathis C, et al. . Amyloid, neurodegeneration, and small vessel disease as predictors of dementia in the oldest-old. Neurology. 2014;83:1804–1811. doi: 10.1212/WNL.0000000000000977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jack CR Jr., Wiste HJ, Weigand SD, et al. . Age-specific and sex-specific prevalence of cerebral beta-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. Lancet Neurol. 2017;16:435–444. doi: 10.1016/S1474-4422(17)30077-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vlassenko AG, Mintun MA, Xiong C, et al. . Amyloid-beta plaque growth in cognitively normal adults: longitudinal [11C]Pittsburgh compound B data. Ann Neurol. 2011;70:857–861. doi: 10.1002/ana.22608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberts RO, Aakre JA, Kremers WK, et al. . Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol. 2018;75:970–979. doi: 10.1001/jamaneurol.2018.0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nebes RD, Snitz BE, Cohen AD, et al. . Cognitive aging in persons with minimal amyloid-β and white matter hyperintensities. Neuropsychologia. 2013;51:2202–2209. doi: 10.1016/j.neuropsychologia.2013.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morris JC, Edland S, Clark C, et al. . The consortium to establish a registry for Alzheimer’s disease (CERAD). Part IV. Rates of cognitive change in the longitudinal assessment of probable Alzheimer’s disease. Neurology. 1993;43:2457–2465. doi: 10.1212/WNL.43.12.2457 [DOI] [PubMed] [Google Scholar]
- 18. Albert MS, DeKosky ST, Dickson D, et al. . The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Price JC, Klunk WE, Lopresti BJ, et al. . Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146 [DOI] [PubMed] [Google Scholar]
- 20. Cohen AD, Mowrey W, Weissfeld LA, et al. . Classification of amyloid-positivity in controls: comparison of visual read and quantitative approaches. Neuroimage. 2013;71:207–215. doi: 10.1016/j.neuroimage.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yau WW, Tudorascu DL, McDade EM, et al. . Longitudinal assessment of neuroimaging and clinical markers in autosomal dominant Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2015;14:804–813. doi: 10.1016/S1474-4422(15)00135-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yue NC, Arnold AM, Longstreth WT Jr., et al. . Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology. 1997;202:33–39. doi: 10.1148/radiology.202.1.8988189 [DOI] [PubMed] [Google Scholar]
- 23. Manolio TA, Kronmal RA, Burke GL, et al. . Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.STR.25.2.318 [DOI] [PubMed] [Google Scholar]
- 24. Ding D, Xiong Y, Zhao Q, et al. . White matter hyperintensity predicts the risk of incident cognitive decline in community dwelling elderly. J Alzheimers Dis. 2018;61:1333–1341. doi: 10.3233/JAD-170876 [DOI] [PubMed] [Google Scholar]
- 25. Chételat G, La Joie R, Villain N, et al. . Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. Neuroimage Clin. 2013;2:356–365. doi: 10.1016/j.nicl.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rowe CC, Ellis KA, Rimajova M, et al. . Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 27. Jack CR Jr., Wiste HJ, Weigand SD, et al. . Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology. 2013;81:1732–1740. doi: 10.1212/01.wnl.0000435556.21319.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS; Alzheimer’s Disease Neuroimaging Initiative Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA. 2017;317:2305–2316. doi: 10.1001/jama.2017.6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Longstreth WT Jr, Manolio TA, Arnold A, et al. . Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.STR.27.8.1274 [DOI] [PubMed] [Google Scholar]
- 30. Hilal S, Mok V, Youn YC, Wong A, Ikram MK, Chen CL. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry. 2017;88:669–674. doi: 10.1136/jnnp-2016-315324 [DOI] [PubMed] [Google Scholar]
- 31. Landau SM, Fero A, Baker SL, Koeppe R, Mintun M, Chen K, et al. . Measurement of longitudinal beta-amyloid change with 18F-florbetapir PET and standardized uptake value ratios. J Nucl Med. 2015;56:567–574. doi: 10.2967/jnumed.114.148981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Villemagne VL, Pike KE, Darby D, Maruff P, Savage G, Ng S, et al. . Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer’s disease. Neuropsychologia. 2008;46:1688–1697. doi: 10.1016/j.neuropsychologia.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 33. Lowe VJ, Kemp BJ, Jack CR Jr., et al. . Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50:878–886. doi: 10.2967/jnumed.108.058529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen AD, Klunk WE. Early detection of Alzheimer’s disease using PiB and FDG PET. Neurobiol Dis. 2014;72 Pt A:117–122. doi: 10.1016/j.nbd.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen AD, Price JC, Weissfeld LA, et al. . Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: an example of brain reserve. J Neurosci. 2009;29:14770–14778. doi: 10.1523/JNEUROSCI.3669-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lowe VJ, Weigand SD, Senjem ML, et al. . Association of hypometabolism and amyloid levels in aging, normal subjects. Neurology. 2014;82:1959–1967. doi: 10.1212/WNL.0000000000000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brickman AM, Provenzano FA, Muraskin J, et al. . Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch Neurol. 2012;69:1621–1627. doi: 10.1001/archneurol.2012.1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lo RY, Jagust WJ, Alzheimer’s Disease Neuroimaging Initiative Vascular burden and Alzheimer disease pathologic progression. Neurology. 2012;79:1349–1355. doi: 10.1212/WNL.0b013e31826c1b9d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wardlaw JM, Allerhand M, Doubal FN, et al. . Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology. 2014;82:1331–1338. doi: 10.1212/WNL.0000000000000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Berti V, Mosconi L, Pupi A. Brain: normal variations and benign findings in fluorodeoxyglucose-PET/computed tomography imaging. PET Clin. 2014;9:129–140. doi: 10.1016/j.cpet.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Villeneuve S, Reed BR, Madison CM, et al. . Vascular risk and Abeta interact to reduce cortical thickness in AD vulnerable brain regions. Neurology. 2014;83:40–47. doi: 10.1212/WNL.0000000000000550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277:813–817. doi: 10.1001/jama.1997.03540340047031 [DOI] [PubMed] [Google Scholar]
- 43. den Heijer T, van der Lijn F, Ikram A, et al. . Vascular risk factors, apolipoprotein E, and hippocampal decline on magnetic resonance imaging over a 10-year follow-up. Alzheimers Dement. 2012;8:417–425. doi: 10.1016/j.jalz.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 44. Dufouil C, Chalmers J, Coskun O, et al. ; PROGRESS MRI Substudy Investigators Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163 [DOI] [PubMed] [Google Scholar]
- 45. Jansen WJ, Ossenkoppele R, Tijms BM, et al. ; Amyloid Biomarker Study Group Association of cerebral amyloid-β aggregation with cognitive functioning in persons without dementia. JAMA Psychiatry. 2018;75:84–95. doi: 10.1001/jamapsychiatry.2017.3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21:1318–1331. doi: 10.1038/s41593-018-0234-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.