Abstract
Systemic inflammation is associated with increasing age. Yet, there are limited data about the association between age and systemic inflammation within older adults, and whether older age is also associated with cellular and nuclear signaling markers of inflammation. In community-dwelling older adults (N = 262, 60–88 years), systemic levels of C-reactive protein, interleukin-6, and soluble tumor necrosis factor receptor II; levels of toll-like receptor-4–stimulated monocytic production of interleukin-6 and tumor necrosis factor α; and resting nuclear levels of activated nuclear factor kappa B and signal transducer and activator of transcription (STAT1, STAT3, STAT5) were evaluated. Adjusting for demographic and clinical factors, multivariate linear regression tested the association between age and each inflammatory marker. Age was positively associated with increased levels of interleukin-6 and soluble tumor necrosis factor receptor II (p’s < .05) and with increases in STAT1, STAT3, and STAT5 activation (p’s < .05). However, no relationship was found between age and C-reactive protein, toll-like receptor-4–stimulated interleukin-6/tumor necrosis factor alpha α production, or nuclear factor kappa B. Within a community-dwelling sample of older adults, older age is associated with increases in STAT activation, along with increases of systemic inflammatory cytokines. In older adults, heterogeneity in age-related increases in inflammatory disease risk may be related to individual variability in inflammation.
Keywords: Inflammation, STAT signaling, NF-κB, C-reactive protein, Proinflammatory cytokines
Inflammation represents a key hallmark of biological aging, which has the potential to provide a mechanistic understanding of why older adults show increased chronic disease risk (1–3). “Usual” aging is associated with rising systemic markers of inflammation or “inflammaging” (1–4), and the importance of inflammation to geriatrics research is further emphasized by the designation of inflammation as one of the seven “pillars of aging” on which research recommendations were focused by the Trans-NIH GeroScience Interest Group (1). Not only does systemic inflammation increase with age, but such increases are prognostic for numerous age-related disorders and mortality, including cardiovascular disease (5), diabetes mellitus, and several cancers (6,7); indeed, inflammation is thought to be linked to 20% of all cancer deaths worldwide (8–10).
The vast majority of studies in humans have examined differences in systemic inflammation between groups of younger and older adults, and/or the association of age across the life span with markers of systemic inflammation such as circulating levels of C-reactive protein (CRP), interleukin (IL)-6 (11), and soluble tumor necrosis factor receptor II (sTNF-RII) (12). However, within older adults, there is marked variability in age-related increases in inflammatory disease risk when compared with adults overall (2), and it is not known whether older adults show individual variability in systemic markers of inflammation in relation to age. Prior research has interrogated this question in regard to adaptive immune responses and found that lymphoproliferative responses to IL-2 decrease across broad age range. Furthermore, adults older than 60 years of age show marked individual variability with about one third showing an attenuated adaptive immune response when compared with adults (13).
Few studies have evaluated the associations between age and cellular sources of systemic inflammation, although monocytes, which make up about 5% of circulating leukocytes, are a major contributor to proinflammatory cytokine production in peripheral blood. Furthermore, the nuclear factor kappa B (NF-κB) transcription control signaling pathway plays a key role in the cellular expression of proinflammatory genes, and animal models have implicated NF-κB as the molecular culprit of inflammaging (14). Yet, there is limited research on the relationship between age and activation of NF-κB in human adults and especially on individual variability of NF-κB in older adults. Finally, no study, to the best of our knowledge in humans, has systematically examined whether age influences the evolutionary conserved, signal transducer and activator of transcription (STAT) family proteins, nor examined the relationship of these STAT family proteins to other markers of systemic and cellular inflammation in older adults. STAT family proteins transduce signals through the cytoplasm and function as transcription factors in the nucleus by regulating cytokine-inducible gene transcription (15). STAT family proteins comprise seven members: STAT1, STAT2, STAT3, STAT4, STAT5 (which comprises the closely related protein members STAT5a and STAT5b), and STAT6. Inducers of STAT transcription include various inflammatory cytokines, such that IL-6 serves as an inducer of STAT3; interferon γ (IFNγ) as an inducer of STAT1; and IL-2 as an inducer of STAT5 (15). Together, the NF-κB and STAT family proteins are pivotal regulators in inflammatory signaling, which have been described as drivers of age-associated transcriptional changes (16).
This study addresses several gaps in inflammaging research, by examining individual variability in markers of inflammation in older adults and interrogating the upstream cellular and transcriptional sources of systemic inflammation with measurement of toll-like receptor-4 (TLR-4)–stimulated monocytic production of IL-6 and TNFα, and nuclear levels of transcriptional pathways including NF-κB and three STAT family proteins (ie, STAT1, STAT3, and STAT5) in peripheral blood mononuclear cells (PMBC), as well as monocyte and lymphocyte subsets.
Method
Participants
This cross-sectional study recruited community-dwelling adults aged 60 years and older, residing in Los Angeles, CA, within 10 miles of the University of California, Los Angeles (UCLA) Westwood campus. The study received oversight and approval from the UCLA Institutional Review Board (IRB#11-000656). As previously described (17), age-targeted sampling methods were used to complete a telephone survey of biobehavioral factors related to social integration in a community-dwelling sample of older adults. From this survey sample of 2,541 adults older 60 years and older, 552 participants who showed varying levels of social integration were invited to undergo an in-person visit with blood sampling; 287 persons agreed to participate. Among the participants who agreed to participate, 23 persons did not meet eligibility criteria and 2 participants withdrew, resulting in a final sample of 262 older adults.
Given the study focus on inflammation, exclusion criteria were implemented to minimize the confounding influence of comorbid conditions or medications on testing the relationship between age and inflammation. Hence, participants (n = 23) who reported the presence of medical conditions or use of medication known to influence inflammation were excluded. Exclusion criteria were morbid obesity (ie, body mass index ≥ 40 kg/m2); current cardiovascular or neurological diseases; pain disorders; inflammatory disorders (ie, rheumatoid arthritis, autoimmune disorders); chronic or acute infectious illnesses; current mental disorders (ie, major depressive disorder, substance use disorder, insomnia disorder); cognitive impairment; and current use of hormone-containing medications (eg, steroids), nonsteroidal anti-inflammatory drugs or analgesics (eg, opioids), immune-modifying drugs that target specific immune response agents (eg, TNF inhibitors), antidepressant and/or antipsychotic medications, and/or tobacco smoking or excessive caffeine consumption (>600 mg/d).
Procedures
During the in-person visit, demographic and clinical characteristics were obtained including body mass index (BMI). Participants also underwent a medical interview regarding past or current history of medical disorders; psychiatric status was assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders. As noted, older adults who reported medical disorders or medications with known effects on inflammation were excluded; the presence of other comorbidities was evaluated using the Chronic Disease Score (CDS), which tallies the use of prescription medications, ranging from 0 (ie, medication free) to 29 (ie, polypharmacy); the CDS correlates strongly with physician ratings of severity of medical illness (18).
Blood samples were obtained by a study phlebotomist via venipuncture between 8 am and 10 am of the in-person visit to minimize influences of circadian factors. EDTA whole blood samples were chilled immediately after collection, then centrifuged, and plasma stored at −80°C until assayed for CRP, IL-6, and sTNF-RII. Heparinized whole blood was collected at room temperature and processed within 4 hours for evaluation of TLR-4-stimulated monocytic production of IL-6 and TNFα, and peripheral blood mononuclear cells (PBMCs) were purified for evaluation of nuclear levels of NF-κB, STAT1, STAT3, and STAT5.
Assays of Inflammatory Markers
Systemic inflammation was evaluated by circulating levels of CRP, IL-6, and sTNF-RII. CRP was quantified by high-sensitivity ELISA (Immundiagnostik, ALPCO Immunoassays, Salem, NH) according to the manufacturer’s protocol, but with an extended standard curve to a lower limit of detection of 0.2 mg/L. IL-6 and sTNF-RII were quantified by high- and regular-sensitivity ELISAs, respectively, according to the manufacturer’s protocols (R&D Systems, Minneapolis, MN). All samples were run in duplicate, with intra-assay and interassay precision of all immunoassays less than or equal to 9%.
Cellular inflammation was evaluated by TLR-4-stimulated monocytic production of IL-6 and TNFα as assayed by fluorescent antibody staining and flow cytometry as previously described (19). Briefly, heparinized whole blood was treated with brefeldin A with and without lipopolysaccharide and incubated for 4 hours with mixing at 37°C. Fixed and permeabilized white cells were stained for cell surface CD14 and intracellular IL-6 and TNFα. CD14+ events were acquired by three-color flow cytometry to determine the net percentages of TLR-4-stimulated production of IL-6 and/or TNFα in three different monocyte subsets: monocytes producing IL-6 only, monocytes producing TNFα only, and monocytes coproducing both IL-6 and TNFα simultaneously.
Nuclear inflammatory signaling via transcription factors was evaluated by spontaneous levels of activated NF-κB, STAT1, STAT3, and STAT5, as assayed by flow cytometry in total PBMC, as well as in monocyte and lymphocyte subpopulations as previously described (20). Briefly, purified PBMC from heparinized whole blood were fixed, then treated with 90% methanol to permeabilize the nuclear membrane. Intranuclear levels of activated (phosphorylated) NF-κB (p65), STAT1 (pTyr-701), STAT3 (pTyr-705), and STAT5 (pTyr-694) were determined by single-color flow cytometry using phycoerythrin-labeled antibodies specific for the phosphorylated forms of each transcription factor. Cell Quest software was used to gate on total PBMC, monocytes only, or lymphocytes only; because methanol fixation necessary to permeabilize the nuclear membrane rendered cell surface molecules unrecognizable for routine phenotyping, monocytes and lymphocytes were gated based on forward versus side scatter. A histogram analysis plot was then used to determine the nuclear levels of each activated transcription factor within each cell population based on the mean fluorescence intensity (MFI).
Statistical Analysis
All analyses were conducted using SPSS version 24.0 (IBM Inc., Armonk, NY) and GraphPad Prism version 7 (GraphPad Software, La Jolla, CA). Non-normally distributed data (ie, CRP, IL-6, and sTNF-RII) were natural log-transformed. To evaluate whether age was associated with markers of systemic (ie, CRP, IL-6, and sTNF-RII), cellular (ie, TLR-4-stimulated monocytic production of IL-6 and/or TNFα), or nuclear (ie, spontaneous levels of NF-κB, STAT1, STAT3, and STAT5 in total PBMC, monocytes only, and lymphocytes only) inflammation, multivariate linear regression modeling was used, adjusting for demographic (sex, race, and education) and clinical factors (BMI and CDS), which have been previously found to be related to inflammation (21). We created 3 different models: Model 1 was the unadjusted model (age only); Model 2 included demographic variables (Model 1 + sex, race, and education); and Model 3 additionally included clinical measures (Model 2 + BMI and CDS). Strength of associations was indexed by the unstandardized regression coefficient (B), indicating the average change in the dependent variable associated with a 1-unit change in the independent variable. To test whether the variability of inflammatory markers differed across the age range, we used a post hoc Glesjer test of heteroscedasticity (22). In addition, we tested whether mean levels of systemic, cellular, or nuclear inflammatory markers differed between males and females by using a post hoc independent t-test. Furthermore, bivariate Pearson correlations were performed to examine the relationships between components of systemic, cellular, and nuclear inflammatory domains. Statistical significance was set at an α-level of .05.
Results
Sample Characteristics
The mean age of the sample was 71.9 ± 7.9 years (range 60–88 years), with 50.8% females, 13% non-Whites, and a mean BMI of 26.3 ± 4.2 kg/m2. For an overview of the sample’s demographic and clinical characteristics, see Table 1. This healthy community-dwelling older adult sample had a median CRP concentration of 1.0 mg/L with an interquartile range of 0.5–2.8 mg/L, a median IL-6 concentration of 1.6 pg/mL with an interquartile range of 1.3–2.9 pg/mL, and a median sTNF-RII concentration of 2.6 ng/mL with an interquartile range of 2.2–3.1 ng/mL. Hence, the sample’s systemic inflammatory characteristics were normal to low compared with other adult population-based studies (23–25), consistent with the exclusion criteria to exclude those with an ongoing inflammatory disorder. For an overview of the sample’s systemic, cellular, and nuclear inflammatory characteristics, see Table 2.
Table 1.
Demographic and Clinical Characteristics of the Older Adult Sample (N = 262)
| Variable | Mean (SD) |
| Age, mean years (± SD) | 71.9 (7.9) |
| Sex, female n (%) | 133 (50.8) |
| Race, n (%) | |
| White | 228 (87.0) |
| African American | 20 (7.6) |
| Other | 14 (5.4) |
| Education, mean years (± SD) | 16.4 (2.8) |
| Employed, n (%) | 174 (66.4) |
| Marital status, n (%) | |
| Never been married | 36 (13.7) |
| Married | 115 (43.9) |
| Separated | 3 (1.1) |
| Widowed | 40 (15.3) |
| Divorced | 65 (24.9) |
| Other | 3 (1.1) |
| Body mass index, mean kg/m2 (± SD) | 26.3 (4.2) |
| Chronic Disease Score, mean (± SD) | 2.0 (2.2) |
Table 2.
Levels of Inflammatory Markers in the Older Adult Sample
| Inflammatory Marker | Median (IQR) | Mean (± SD) |
|---|---|---|
| Systemic inflammation (n = 173)a | ||
| CRP, mg/L | 1.0 (0.5–2.8) | |
| IL-6, pg/mL | 1.6 (1.3–2.9) | |
| sTNF-RII, ng/mL | 2.6 (2.2–3.1) | |
| Cellular inflammation (n = 168)b | ||
| Monocytes producing IL-6 only, % | 25.1 (16.0) | |
| Monocytes producing TNFα only, % | 37.3 (14.6) | |
| Monocytes coproducing IL-6 + TNFα, % | 16.7 (11.3) | |
| Nuclear signaling (n = 123)c | ||
| NF-κB, MFI | ||
| PBMC | 27.4 (14.5) | |
| Monocyte | 42.9 (35.6) | |
| Lymphocyte | 21.7 (8.5) | |
| STAT1, MFI | ||
| PBMC | 36.0 (8.9) | |
| Monocyte | 65.4 (12.1) | |
| Lymphocyte | 24.5 (7.7) | |
| STAT3, MFI | ||
| PBMC | 26.2 (11.1) | |
| Monocyte | 44.7 (15.8) | |
| Lymphocyte | 18.7 (9.4) | |
| STAT5, MFI | ||
| PBMC | 29.2 (6.4) | |
| Monocyte | 48.2 (10.9) | |
| Lymphocyte | 21.8 (5.8) |
Notes: CRP = C-reactive protein; IL-6 = interleukin-6; IQR = interquartile range; NF-κB = nuclear factor kappa B; MFI = mean fluorescence intensity; TNFα = tumor necrosis factor alpha; sTNF-RII = soluble tumor necrosis factor receptor II; STAT = signal transducer and activator of transcription; PBMC = peripheral blood mononuclear cells.
aSystemic (circulating) marker data were natural log-transformed for analyses, but nontransformed median and IQR are shown for ease of interpretation. bNet percentages of toll-like receptor-4 (TLR-4)-stimulated monocytes producing IL-6 only, producing TNFα only, and coproducing both IL-6 and TNFα simultaneously. cSpontaneous intranuclear levels of activated NF-κB, STAT1, STAT3, and STAT5 were assayed in total PBMC, as well as monocyte and lymphocyte subsets.
Associations of Age With Systemic, Cellular, and Nuclear Inflammatory Markers
Adjusted multivariate linear regression modeling was used to examine whether age in this sample of older adults (60–88 years) was associated with systemic, cellular, and nuclear inflammatory markers.
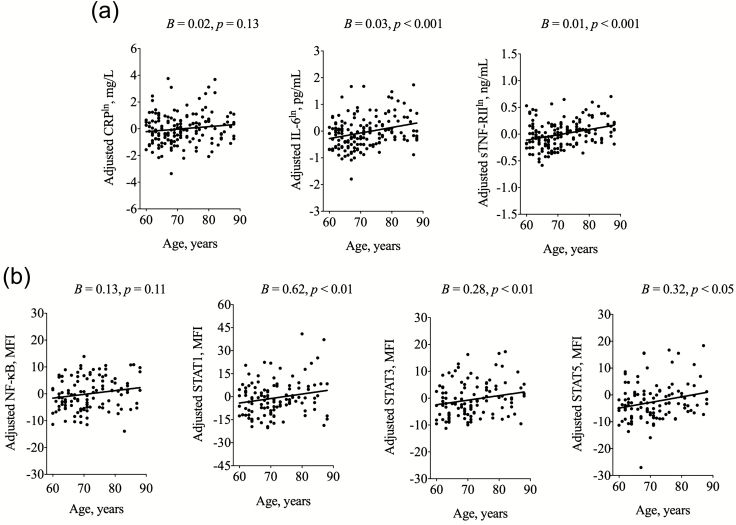
Within the domain of systemic inflammation, age was positively associated with systemic (circulating) levels of IL-6 (B = 0.03, p < .001) and sTNF-RII (B = 0.01, p < .001), but not with levels of CRP (Figure 1a and Table 3). As an example, for every year of chronological age, there is on average an increase of 0.1 pg/mL of IL-6. Consistent with other reports (26,27), BMI was associated with levels of CRP (B = 0.11, p < .001) and IL-6 (B = 0.05, p < .01), but there was no age × BMI interaction in this older adult sample. A post hoc independent t-test showed no sex differences in levels of systemic inflammatory markers. Moreover, associations between age and systemic inflammation did not differ between males and females (ie, no age × sex interactions). In addition, non-African American race was associated with higher systemic levels of CRP (B = 1.50, p < .001), IL-6 (B = 0.68, p < .05), and sTNF-RII (B = 0.27, p < .01). However, there were no age × race interactions.
Figure 1.
(a) Associations of age with systemic markers of inflammation. CRP = C-reactive protein; IL-6 = interleukin-6; sTNF-RII = soluble tumor necrosis factor receptor II. Shown are scatterplots and linear prediction lines for associations of age with markers of systemic inflammation. All scatterplots are adjusted for sex, race, education, body mass index, and Chronic Disease Score; unstandardized regression coefficients (B) and p values from multivariate linear regression modeling are also shown. Residualized levels (ie, error between predicted and observed value) of CRP, IL-6, and sTNF-RII are plotted on the y-axis on a natural log (ln) scale; range of y-axis varies due to differing circulating concentrations in mg/L, pg/mL, and ng/mL, respectively. (b) Associations of age with nuclear inflammatory markers. NF-κB = nuclear factor kappa B; STAT = signal transducer and activator of transcription; MFI = mean fluorescence intensity. Shown are scatterplots and linear prediction lines for associations of age with nuclear inflammatory signaling in unstimulated total peripheral blood mononuclear cells (PBMCs). All scatterplots are adjusted for sex, race, education, body mass index, and Chronic Disease Score; unstandardized regression coefficients (B) and p values from multivariate linear regression modeling are also shown. Residualized levels (ie, error between predicted and observed value) of NF-κB, STAT1, STAT3, and STAT5 are plotted on the y-axis.
Table 3.
Age and Systemic Markers of Inflammation
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| B | 95% CI | B | 95% CI | B | 95% CI | |
| CRPln | ||||||
| Age (y) | 0.01 | −0.01; 0.04 | 0.02 | −0.01; 0.04 | 0.02 | −0.01; 0.04 |
| Sex, female | 0.02 | −0.37; 0.40 | 0.19 | −0.19; 0.56 | ||
| Race, Black | 0.45 | −0.23; 1.13 | 0.17 | −0.50; 0.84 | ||
| Race, other | 1.43** | 0.54; 2.31 | 1.50*** | 0.68; 2.33 | ||
| Education (y) | 0.01 | −0.07; 0.07 | 0.01 | −0.05; 0.08 | ||
| Body mass index (kg/m2) | 0.11*** | 0.06; 0.15 | ||||
| Chronic Disease Score | −0.03 | −0.12; 0.06 | ||||
| IL-6ln | ||||||
| Age (y) | 0.03*** | 0.01; 0.04 | 0.03*** | 0.02; 0.04 | 0.03*** | 0.02; 0.05 |
| Sex, female | −0.10 | −0.34; 0.14 | −0.03 | −0.27; 0.21 | ||
| Race, Black | 0.18 | −0.24; 0.60 | 0.05 | −0.37; 0.48 | ||
| Race, other | 0.65* | 0.11; 1.19 | 0.68* | 0.15; 1.20 | ||
| Education (y) | 0.03 | −0.02; 0.07 | 0.03 | −0.01; 0.07 | ||
| Body mass index (kg/m2) | 0.05** | 0.02; 0.07 | ||||
| Chronic Disease Score | −0.01 | −0.07; 0.05 | ||||
| sTNF-RIIln | ||||||
| Age (y) | 0.01*** | 0.01; 0.02 | 0.01*** | 0.01; 0.02 | 0.01*** | 0.01; 0.02 |
| Sex, female | −0.01 | −0.08; 0.07 | 0.03 | −0.05; 0.11 | ||
| Race, Black | 0.04 | −0.10; 0.17 | −0.02 | −0.16; 0.12 | ||
| Race, other | 0.27** | 0.09; 0.44 | 0.27** | 0.10; 0.45 | ||
| Education (y) | −0.01 | −0.02; 0.01 | −0.01 | −0.03; 0.002 | ||
| Body mass index (kg/m2) | 0.01 | −0.002; 0.02 | ||||
| Chronic Disease Score | 0.02 | −0.002; 0.04 |
Notes: CRP = C-reactive protein; IL-6 = interleukin-6; sTNF-RII = soluble tumor necrosis factor receptor II. Unstandardized regression coefficients (B) with 95% confidence intervals (CI) indicating associations with circulating markers of systemic inflammation. Model 1: unadjusted model (age only); Model 2: Model 1 + adjusted for sex, race and education; Model 3: Model 2 + adjusted for comorbidity as measured by the body mass index and Chronic Disease Score.
*p < .05, **p < .01, ***p < .001.
Within the domain of cellular inflammation, age was not associated with TLR-4-stimulated monocytic production of IL-6 only, TNFα only, or coproduction of IL-6 and TNFα (all p > .05, data not shown). In addition, a post hoc independent t-test showed no sex differences in the percentage of TLR-4-stimulated monocytic production of IL-6 and/or TNFα.
Within the domain of nuclear inflammatory signaling, age was not associated with levels of activated NF-κB, but was positively associated with levels of each of the activated STAT family proteins in the total PBMC population, including STAT1 (B = 0.62, p < .01), STAT3 (B = 0.28, p < .01), and STAT5 (B = 0.32, p < .05; Figure 1b and Table 4); in addition, a post hoc Glesjer test of heteroscedasticity revealed that older age was associated with greater variability in nuclear levels of activated STAT1 (p < .05), but not STAT3 or STAT5.
Table 4.
Age and Nuclear Inflammatory Signaling
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| B | 95% CI | B | 95% CI | B | 95% CI | |
| NF-κB | ||||||
| Age (y) | 0.09 | −0.06; 0.23 | 0.09 | −0.06; 0.23 | 0.13 | −0.03; 0.28 |
| Sex, female | 1.74 | −0.55; 4.03 | 1.08 | −1.34; 3.50 | ||
| Race, Black | 3.15 | −1.17; 7.47 | 3.94 | −0.47; 8.35 | ||
| Race, other | 0.25 | −4.55; 5.05 | 0.09 | −4.70; 4.88 | ||
| Education (y) | 0.01 | −0.44; 0.45 | 0.03 | −0.42; 0.48 | ||
| Body mass index (kg/m2) | −0.12 | −0.37; 0.14 | ||||
| Chronic Disease Score | −0.4 | −0.98; 0.19 | ||||
| STAT1 | ||||||
| Age (y) | 0.52** | 0.18; 0.86 | 0.55** | 0.23; 0.88 | 0.62** | 0.26; 0.98 |
| Sex, female | −6.18* | −11.34; −1.01 | −6.27* | −11.74; −0.80 | ||
| Race, Black | 6.41 | −3.3; 16.15 | 6.09 | −3.89; 16.08 | ||
| Race, other | 8.94 | −1.89; 19.77 | 9.24 | −1.60; 20.09 | ||
| Education (y) | 0.62 | −0.38; 1.63 | 0.72 | −0.29; 1.74 | ||
| Body mass index (kg/m2) | 0.34 | −0.23; 0.91 | ||||
| Chronic Disease Score | −0.56 | −1.87; 0.76 | ||||
| STAT3 | ||||||
| Age (y) | 0.23* | 0.02; 0.43 | 0.23* | 0.04; 0.42 | 0.28** | 0.07; 0.49 |
| Sex, female | 0.23 | −2.78; 3.24 | −0.26 | −3.45; 2.94 | ||
| Race, Black | 4.90 | −0.78; 10.57 | 5.33 | −0.50; 11.16 | ||
| Race, other | 15.74*** | 9.42; 22.05 | 15.73*** | 9.39; 22.07 | ||
| Education (y) | 0.26 | −0.33; 0.85 | 0.31 | −0.28; 0.91 | ||
| Body mass index (kg/m2) | 0.04 | −0.29; 0.37 | ||||
| Chronic Disease Score | −0.47 | −1.24; 0.30 | ||||
| STAT5 | ||||||
| Age (y) | 0.29* | 0.03; 0.54 | 0.30* | 0.05; 0.54 | 0.32* | 0.05; 0.59 |
| Sex, female | 0.25 | −3.66; 4.15 | −0.29 | −4.43; 3.85 | ||
| Race, Black | 6.69 | −0.63; 14.01 | 7.41 | −0.15; 15.0 | ||
| Race, other | 15.33*** | 7.20; 23.45 | 15.15*** | 6.96; 23.34 | ||
| Education | −0.09 | −0.85; 0.68 | −0.08 | −0.86; 0.71 | ||
| Body mass index (kg/m2) | −0.14 | −0.57; 0.29 | ||||
| Chronic Disease Score | −0.27 | −1.28; 0.75 |
Notes: CI = confidence interval; NF-κB = nuclear factor kappa B; STAT = signal transducer and activator of transcription. Unstandardized regression coefficients (B) with 95% CI indicating associations with spontaneous nuclear inflammatory signaling in total peripheral blood mononuclear cells (PBMC). Model 1: unadjusted model (age only); Model 2: Model 1 + adjusted for sex, race and education; Model 3: Model 2 + adjusted for comorbidity as measured by the body mass index and Chronic Disease Score.
*p < .05, **p < .01, ***p < .001.
A post hoc independent t-test showed that males had higher mean levels of activated STAT1 in the total PMBC population (males: 38.6 ± 17.2 MFI vs. females: 33.4 ± 10.6 MFI, p < .05) and in the lymphocyte subpopulation (males: 26.2 ± 9.1 MFI vs. females: 22.7 ± 7.7 MFI, p < .05). Accordingly, female sex was associated with lower nuclear levels of activated STAT1 in the total PBMC population (B = −6.27, p < .05). Moreover, we found a trend of females having slightly higher mean levels of activated NF-κB in the total PMBC population (males: 26.3 ± 10.7 MFI vs. females: 28.4 ± 8.8 MFI, p < .07) and in the lymphocyte subpopulation (males: 20.7 ± 5.1 MFI vs. females: 22.7 ± 6.3 MFI, p < .06). However, overall associations between age and levels of nuclear inflammatory markers did not differ between males and females (ie, no age × sex interactions). In addition, non-African American race was associated and with higher PBMC-derived levels of activated STAT3 (B = 15.72, p < .001) and STAT5 (B = 15.15, p < .001), but not with NF-κB or STAT1. There were no age × race interactions. Within the monocyte and lymphocyte subpopulations, similar results were found; age was associated with STAT1 (B = 0.94, p < .05), STAT3 (B = 0.39, p < .05), and STAT5 (B = 0.45, p < .05) in monocytes (Supplementary Table 1a) and with STAT1 (B = 0.35, p < .01) and STAT3 (B = 0.19, p < .05) in lymphocytes (Supplementary Table 1b), but not with NF-κB in either monocytes or lymphocytes.
Relationships Between the Inflammatory Markers
Within each of the inflammatory domains, significant correlations were observed between inflammatory markers (ie, correlations within systemic inflammatory markers, within cellular inflammatory markers, and within nuclear inflammatory markers). Across inflammatory domains, systemic inflammation correlated with nuclear inflammatory signaling in PBMCs, such that CRP (r = .28, p < .01) and IL-6 (r = .22, p < .05) correlated with STAT3, and sTNF-RII correlated with STAT1 (r = .25, p < .01), STAT3 (r = .32, p < .01), and STAT5 (r = .25, p < .01). Moreover, NF-κB in PBMCs correlated with cellular inflammation, as indexed by TLR-4-stimulated monocytic production of IL-6 (r = .31, p < .05) and IL-6/TNFα coproduction (r = .23, p < .05). However, neither systemic inflammatory markers nor STAT family proteins correlated with TLR-4-stimulated monocytic production of IL-6 and TNFα. Relationships of monocyte and lymphocyte subpopulations with systemic and cellular inflammatory markers were similar to the ones between PBMC and systemic and cellular inflammatory markers. For an overview of the correlational relationships between systemic, cellular, and nuclear inflammatory markers, see Supplementary Table 2.
Discussion
The present study sought to examine the relationship between age and multiple domains of inflammation-associated immune biomarkers in a large sample of community-dwelling older adults. Biomarkers of interest comprised circulating inflammatory markers (ie, systemic levels of CRP, IL-6, sTNF-RII); percentage of TLR-4-stimulated IL-6/TNFα-production measured in CD14+ monocytes; and nuclear inflammatory markers measured in the total PMBC population, as well as lymphocyte and monocyte subpopulations (ie, nuclear levels of activated NF-κB and STAT family proteins). In line with our hypothesis, increasing age more than 60 years was associated with higher nuclear levels of activated STAT1, STAT3, and STAT5, along with higher systemic levels of IL-6 and sTNF-RII. However, within this older adult population, age was not significantly related to systemic CRP, TLR-4-stimulated monocytic production of IL-6 and TNFα, or nuclear levels of activated NF-κB.
What might be the implications of the association between age and inflammation over time? In adults older than 65 years, epidemiological studies have found that the threshold value of IL-6 (ie, 3.19 pg/mL or greater) predicts a 2-fold greater risk of death when compared with levels of IL-6 in the lowest quartile (10). Applying this threshold of mortality risk to our sample and the estimated increase of IL-6 (ie, 0.1 pg/mL per year), the average aged older adult who is at or above the median of IL-6 (ie, 1.6 pg/mL) will be at or above the line of mortality risk within 15 years.
Although several lines of independent research suggest that older age is associated with higher levels of circulating markers of inflammation (11,12), research on age-related changes in STAT signaling is surprisingly sparse. Indeed, there are only limited data on increases in basal STAT3 and STAT5 levels from a very small number of older adult participants (22), and the present study is the first to show in a relatively large sample of older adults that increasingly older age is related to higher spontaneous levels of STAT signaling. Our human observations are consistent with age-related changes in transcriptional activity of STAT3 (28) and STAT5 (29) in murine models; however, these animal studies compared younger animals with older animals, rather than looking at a continuous age range. Hence, our data are novel in demonstrating that even in older adults, older age is associated with progressively higher levels of STAT signaling over a continuous age range of 60–88 years.
It is well-documented that STAT3 is linked to IL-6 signaling (30–32), STAT1 to IFN signaling (33–35), and STAT5 to IL-2 signaling (36–38). Moreover, both enhancement and blunting of STAT signaling pathways have been associated with dysfunctional immunity. For instance, STAT3 gain-of-function mutations have been linked to autoimmunity, whereas STAT3 loss-of-function mutations associated with immunodeficiency (39); other lines of research have indicated that both gain-of-function mutations and loss-of-function mutations of STAT1 play a role in mycobacterial and viral diseases and that deficiency of STAT5 contributes to some autoimmune diseases such as arthritis (15). Hence, it is possible that our findings might have clinical implications for late-life disease risk.
In contrast to STAT signaling, spontaneous nuclear levels of activated NF-κB were not associated with older age between 60 and 88 years, even though NF-κB has been characterized as a molecular culprit of inflammaging (14) and a stimulus of a proinflammatory molecular profile found in aging cells (ie, senescence-associated secretory phenotype) (40). Interestingly, previous work has demonstrated that immune cells from older adults show less pronounced NF-kB activation (41) and p65/RelA induction (42) in response to experimentally induced immune cell activation when compared with immune cells from younger or middle-aged adults. In turn, other work has shown that naive immune cells from older adults show upregulated gene expression regulated by NF-kB when compared with naive immune cells from younger adults (43). Together, these observations suggest that the NF-kB transcriptional control pathway is an important contributor to the coexistence of blunted immune responses and heightened chronic inflammation found in older adults (44,45).
In sum, our data support findings from an array of previous transcriptome profiling studies that have also linked older age to increased levels of proinflammatory gene expression (particularly through NF-kB and STAT pathways; eg, (46–51)).
Similar to NF-κB, TLR-4-stimulated IL-6/TNFα-production measured in CD14+ monocytes was not associated with age, which indeed might be due to the age restriction of our sample (aged 60–88 years). However, the absence of an association between age and cellular inflammation might be due to a defect in the ability of older adults to respond to TLR-4 stimulation. In adults, we have found that sleep deprivation induces a robust activation of TLR-4-stimulated monocytic IL-6/TNFα production. In contrast, in older adults, sleep deprivation fails to induce an increase in TLR-4-stimulated monocytic IL-6/TNFα-production, indicating that older adults show blunted response to TLR-4 stimulation (52).
Whereas older age was associated with higher systemic levels of IL-6 and sTNF-RII, replicating previous findings (53–56), older age was not associated with levels of CRP. Given that previous research has shown increases of CRP with advancing age (54,56), the lack of association between age and CRP might be due to our sample characteristics, that is, we included relatively healthy older adults with a restricted age range (60–88 years), who as a group showed CRP levels in the low-risk range. Nevertheless, within this older population, BMI was a significant correlate of systemic inflammation, consistent with previous work (26,27), which supports evidence that inflammation plays an important role in age-associated obesity and diabetes mellitus.
Prior research has found that levels of markers of systemic inflammation are higher in females when compared with males, although sample populations have primarily included only adults (21). Interestingly, in this older adult sample, sex differences in CRP, IL-6, and sTNF-RII were not found, indicating that differences in reproductive hormones between adult versus older adult females may contribute to varying levels of systemic inflammation. Nevertheless, older adult females showed lower levels of STAT1 and possibly higher NF-κB when compared with older adult males. Moreover, non-African American race was also a strong predictor of a variety of inflammatory markers in our sample, which might be due to sample characteristics with 92.4 non-African American (1.5% Asian, 0.8% Native Hawaiian/Pacific Islander; 87% White; 2.3% other) and 7.6% African American. Although the existing literature is somewhat contradictory (21), the majority of research demonstrated that the African American population has higher levels of CRP, IL-6, and TNFα when compared with the white population. This study is novel in also showing that race is associated with activation of STAT3 and STAT5 family proteins.
Consistent with prior evidence and with known relationships between cytokines and STAT signaling (57–59), analyses across inflammatory domains showed that systemic inflammation correlated with STAT signaling. For example, sTNF-RII, which is cleaved from the surface of cells following exposure to TNFα, correlated with STAT1, STAT3, and STAT5, underlining that TNFα induces phosphorylation of latent cytosolic STAT3 and STAT5b (60). In contrast, the lack of correlation between sTNF-RII and NF-κB might be related to evidence that sTNF-RII, despite its high TRAF2 binding capability, is considered a “poor” activator of the NF-κB signaling pathway compared with other TNF superfamily receptors (61).
Finally, cellular inflammation as evaluated by TLR-4-stimulated monocytic production of IL-6 and TNFα was not correlated with systemic inflammation. The lack of correlation was not surprising because systemic inflammation and cellular inflammation (as evaluated here) are distinct inflammatory domains: systemic inflammatory markers indicate a naturally occurring state of in vivo inflammation, whereas TLR-4-stimulated production of proinflammatory cytokines serves to evaluate in vitro the immune system’s innate capacity to respond to a microbial stimulus.
Strengths and Limitations
Our study shows several strengths and brings various aspects of novelty to gerontology research. First, this study is novel because no prior research has ever systematically examined the effect of age beginning at age 60 and going up to the late 80s on transcription factors of the STAT protein family. Second, because prior human research has examined age-related increases in proinflammatory cytokines primarily by looking at T cells as a source of cytokine production, this study is innovative in examining TLR-4-stimulated production of proinflammatory cytokines in monocytes, which are a major cellular source of proinflammatory cytokine production. Third, no prior research has ever systematically explored the correlational relationships within a comprehensive panel of inflammatory domains (ie, systemic, cellular, and nuclear domains) in a large and healthy sample of older adults. However, we recognize some limitations of our study. Systemic IFN and IL-2 could not be evaluated in this study, as they typically circulate at levels below detection by immunoassays. This constrained us from evaluating systemic and nuclear relationships of the IFN/STAT1 and the IL-2/STAT5 signaling pathways. Moreover, other ligands that activate STAT proteins, molecular cross-talk and antagonism between STAT proteins, and inhibitory mechanisms that negatively downregulate STAT protein signaling were not accounted for and should be considered when interpreting our results. Furthermore, even after excluding participants for a variety of medical conditions and controlling for medication use (ie, CDS), a residual probability of confounding by morbidity remained (ie, confounding by undiagnosed medical illnesses that were not captured during the screening interview). Finally, the focus of our sample for this multidomain study of inflammation was on adults of 60 years and older, and future studies could evaluate STAT signaling pathways and cellular monocytic responsiveness to lipopolysaccharide in a broader age range including younger and middle-aged adults.
Conclusion
Healthy aging beyond age 60 is associated with increasing systemic inflammation and nuclear inflammatory signaling of STAT family proteins. Moreover, such age-related increases in systemic inflammation appear to be tracked more strongly to increased activation of nuclear STAT signaling than to the NF-κB pathway. Our findings suggest that levels of activated nuclear STAT proteins could serve as a useful new biomarker of aging, which might be related the risk of chronic age-related diseases.
Funding
This work was supported by the Max Kade Foundation (D.P.), the National Institutes of Health (R01AG034588 to M.R.I.), and Norman Cousins Center for Psychoneuroimmunology.
Conflict of Interest
None reported.
Supplementary Material
References
- 1. Kennedy BK, Berger SL, Brunet A, et al. . Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl. 1):S4–S9. doi: 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 3. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Franceschi C, Bonafè M, Valensin S, et al. . Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 5. Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.CIR.0000055014.62083.05 [DOI] [PubMed] [Google Scholar]
- 6. Vasto S, Carruba G, Lio D, et al. . Inflammation, ageing and cancer. Mech Ageing Dev. 2009;130:40–45. doi: 10.1016/j.mad.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 7. Pierce BL, Ballard-Barbash R, Bernstein L, et al. . Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 9. Volpato S, Guralnik JM, Ferrucci L, et al. . Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women’s health and aging study. Circulation. 2001;103:947–953. doi: 10.1161/01.CIR.103.7.947 [DOI] [PubMed] [Google Scholar]
- 10. Harris TB, Ferrucci L, Tracy RP, et al. . Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/S0002-9343(99)00066-2 [DOI] [PubMed] [Google Scholar]
- 11. Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245 [DOI] [PubMed] [Google Scholar]
- 12. Gerli R, Monti D, Bistoni O, et al. . Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121:37–46. doi: 10.1016/S0047-6374(00)00195-0 [DOI] [PubMed] [Google Scholar]
- 13. Murasko DM, Nelson BJ, Matour D, Goonewardene IM, Kaye D. Heterogeneity of changes in lymphoproliferative ability with increasing age. Exp Gerontol. 1991;26:269–279. [DOI] [PubMed] [Google Scholar]
- 14. Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflammaging. Ageing Res Rev. 2008;7:83–105. doi: 10.1016/j.arr.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 15. O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Brown ZK, Van Nostrand EL, Higgins JP, Kim SK. The inflammatory transcription factors NFkappaB, STAT1 and STAT3 drive age-associated transcriptional changes in the human kidney. PLoS Genet. 2015;11:e1005734. doi: 10.1371/journal.pgen.1005734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cho JH, Olmstead R, Choi H, Carrillo C, Seeman TE, Irwin MR. Associations of objective versus subjective social isolation with sleep disturbance, depression, and fatigue in community-dwelling older adults. Aging & Ment Health. 2018:1–9. doi: 10.1080/13607863.2018.1481928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-G [DOI] [PubMed] [Google Scholar]
- 19. Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756 [DOI] [PubMed] [Google Scholar]
- 20. Irwin MR, Witarama T, Caudill M, Olmstead R, Breen EC. Sleep loss activates cellular inflammation and signal transducer and activator of transcription (STAT) family proteins in humans. Brain Behav Immun. 2015;47:86–92. doi: 10.1016/j.bbi.2014.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O’Connor MF, Bower JE, Cho HJ, et al. . To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glejser H. A new test for heteroskedasticity. J Am Stat Assoc. 1969;64:315–323. [Google Scholar]
- 23. Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Engl J Med. 2005;352:1611–1613. doi: 10.1056/NEJM200504143521525 [DOI] [PubMed] [Google Scholar]
- 24. Cho HJ, Kivimäki M, Bower JE, Irwin MR. Association of C-reactive protein and interleukin-6 with new-onset fatigue in the Whitehall II prospective cohort study. Psychol Med. 2013;43:1773–1783. doi: 10.1017/S0033291712002437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marcos-Pérez D, Sánchez-Flores M, Maseda A, et al. . Frailty in older adults is associated with plasma concentrations of inflammatory mediators but not with lymphocyte subpopulations. Front Immunol. 2018;9:1056. doi: 10.3389/fimmu.2018.01056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131 [DOI] [PubMed] [Google Scholar]
- 27. Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. J Parenter Enteral Nutr. 2004;28:410–415. doi: 10.1177/0148607104028006410 [DOI] [PubMed] [Google Scholar]
- 28. Chazaud B, Mouchiroud G. Inflamm-aging: STAT3 signaling pushes muscle stem cells off balance. Cell Stem Cell. 2014;15:401–402. doi: 10.1016/j.stem.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 29. Albright JW, Bream JH, Bere EW, Young HA, Winkler-Pickett R, Ortaldo JR. Aging of innate immunity: functional comparisons of NK/LAK cells obtained from bulk cultures of young and aged mouse spleen cells in high concentrations of interleukin-2. Exp Gerontology. 2004;39:73–82. doi: 10.1016/j.exger.2003.09.017 [DOI] [PubMed] [Google Scholar]
- 30. McLoughlin RM, Jenkins BJ, Grail D, et al. . IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci USA. 2005;102:9589–9594. doi: 10.1073/pnas.0501794102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fielding CA, McLoughlin RM, McLeod L, et al. . IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181:2189–2195. doi: 10.4049/jimmunol.181.3.2189 [DOI] [PubMed] [Google Scholar]
- 32. Takeda K, Clausen BE, Kaisho T, et al. . Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/S1074-7613(00)80005-9 [DOI] [PubMed] [Google Scholar]
- 33. Majoros A, Platanitis E, Szappanos D, et al. . Response to interferons and antibacterial innate immunity in the absence of tyrosine-phosphorylated STAT1. EMBO Rep. 2016;17:367–382. doi: 10.15252/embr.201540726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/S0092-8674(00)81289-1 [DOI] [PubMed] [Google Scholar]
- 35. Shuai K, Liao J, Song MM. Enhancement of antiproliferative activity of gamma interferon by the specific inhibition of tyrosine dephosphorylation of Stat1. Mol Cell Biol. 1996;16:4932–4941. doi: 10.1128/mcb.16.9.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin JX, Leonard WJ. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 2000;19:2566–2576. doi: 10.1038/sj.onc.1203523 [DOI] [PubMed] [Google Scholar]
- 37. Imada K, Bloom ET, Nakajima H, et al. . Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med. 1998;188:2067–2074. doi: 10.1084/jem.188.11.2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakajima H, Liu XW, Wynshaw-Boris A, et al. . An indirect effect of Stat5a in IL-2-induced proliferation: a critical role for Stat5a in IL-2-mediated IL-2 receptor alpha chain induction. Immunity. 1997;7:691–701. doi: 10.1016/S1074-7613(00)80389-1 [DOI] [PubMed] [Google Scholar]
- 39. Vogel TP, Milner JD, Cooper MA. The Ying and Yang of STAT3 in human disease. J Clin Immunol. 2015;35:615–623. doi: 10.1007/s10875-015-0187-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal. 2012;24:835–845. doi: 10.1016/j.cellsig.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 41. Gupta S, Bi R, Kim C, Chiplunkar S, Yel L, Gollapudi S. Role of NF-kappaB signaling pathway in increased tumor necrosis factor-alpha-induced apoptosis of lymphocytes in aged humans. Cell Death Differ. 2005;12:177–183. doi: 10.1038/sj.cdd.4401557 [DOI] [PubMed] [Google Scholar]
- 42. Bektas A, Zhang Y, Wood WH 3rd, et al. . Age-associated alterations in inducible gene transcription in human CD4+ T lymphocytes. Aging (Albany, NY). 2013;5:18–36. doi: 10.18632/aging.100522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bektas A, Zhang Y, Lehmann E, et al. . Age-associated changes in basal NF-κB function in human CD4+ T lymphocytes via dysregulation of PI3 kinase. Aging (Albany, NY). 2014;6:957–974. doi: 10.18632/aging.100705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bektas A, Schurman SH, Sen R, Ferrucci L. Human T cell immunosenescence and inflammation in aging. J Leukoc Biol. 2017;102:977–988. doi: 10.1189/jlb.3RI0716-335R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bektas A, Schurman SH, Sen R, Ferrucci L. Aging, inflammation and the environment. Exp Gerontol. 2018;105:10–18. doi: 10.1016/j.exger.2017.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harries LW, Hernandez D, Henley W, et al. . Human aging is characterized by focused changes in gene expression and deregulation of alternative splicing. Aging Cell. 2011;10:868–878. doi: 10.1111/j.1474-9726.2011.00726.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Holly AC, Melzer D, Pilling LC, et al. . Towards a gene expression biomarker set for human biological age. Aging Cell. 2013;12:324–326. doi: 10.1111/acel.12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jylhävä J, Raitanen J, Marttila S, Hervonen A, Jylhä M, Hurme M. Identification of a prognostic signature for old-age mortality by integrating genome-wide transcriptomic data with the conventional predictors: the Vitality 90+ Study. BMC Med Genomics. 2014;7:54. doi: 10.1186/1755-8794-7-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reynolds LM, Ding J, Taylor JR, et al. . Transcriptomic profiles of aging in purified human immune cells. BMC Genomics. 2015;16:333. doi: 10.1186/s12864-015-1522-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peters MJ, Joehanes R, Pilling LC, et al. ; NABEC/UKBEC Consortium The transcriptional landscape of age in human peripheral blood. Nat Commun. 2015;6:8570. doi: 10.1038/ncomms9570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Magalhães JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carroll JE, Carrillo C, Olmstead R, et al. . Sleep deprivation and divergent toll-like receptor-4 activation of cellular inflammation in aging. Sleep. 2015;38:205–211. doi: 10.5665/sleep.4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Giuliani N, Sansoni P, Girasole G, et al. . Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp Gerontol. 2001;36:547–557. [DOI] [PubMed] [Google Scholar]
- 54. Paik JK, Chae JS, Kang R, Kwon N, Lee SH, Lee JH. Effect of age on atherogenicity of LDL and inflammatory markers in healthy women. Nutr Metab Cardiovasc Dis. 2013;23:967–972. doi: 10.1016/j.numecd.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 55. Hager K, Machein U, Krieger S, Platt D, Seefried G, Bauer J. Interleukin-6 and selected plasma proteins in healthy persons of different ages. Neurobiol Aging. 1994;15:771–772. doi: 10.1016/0197-4580(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 56. Ferrucci L, Corsi A, Lauretani F, et al. . The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anderson AE, Pratt AG, Sedhom MA, et al. . IL-6-driven STAT signalling in circulating CD4+ lymphocytes is a marker for early anticitrullinated peptide antibody-negative rheumatoid arthritis. Ann Rheum Dis. 2016;75:466–473. doi: 10.1136/annrheumdis-2014-205850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Isomäki P, Junttila I, Vidqvist KL, Korpela M, Silvennoinen O. The activity of JAK-STAT pathways in rheumatoid arthritis: constitutive activation of STAT3 correlates with interleukin 6 levels. Rheumatology (Oxford). 2015;54:1103–1113. doi: 10.1093/rheumatology/keu430 [DOI] [PubMed] [Google Scholar]
- 59. Zhang D, Sun M, Samols D, Kushner I. STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J Biol Chem. 1996;271:9503–9509. doi: 10.1074/jbc.271.16.9503 [DOI] [PubMed] [Google Scholar]
- 60. Miscia S, Marchisio M, Grilli A, et al. . Tumor necrosis factor alpha (TNF-alpha) activates Jak1/Stat3-Stat5B signaling through TNFR-1 in human B cells. Cell Growth Differ. 2002;13:13–18. [PubMed] [Google Scholar]
- 61. Grech AP, Gardam S, Chan T, et al. . Tumor necrosis factor receptor 2 (TNFR2) signaling is negatively regulated by a novel, carboxyl-terminal TNFR-associated factor 2 (TRAF2)-binding site. J Biol Chem. 2005;280:31572–31581. doi: 10.1074/jbc.M504849200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.