Abstract
Purpose
The aim of this retrospective study is to evaluate medium-term results of undersized balloon angioplasty and stenting for symptomatic high-grade (70–99%) stenosis of a major intracranial artery with Enterprise stent.
Methods
This study included 68 consecutive symptomatic (recurrent transient ischemic attack (TIA) or ischemic stroke under dual antiplatelet treatment) patients with high-grade (70–99%) stenosis of a major intracranial artery who were endovascularly treated with undersized balloon angioplasty and Enterprise stent deployment between July 2012 and December 2017. Primary outcomes were any stroke or death within 30 days after procedure. Secondary outcomes were technical success rates, stroke and restenosis during the follow-up period.
Results
A total of 68 lesions in 68 patients (mean age: 62 ± 7 years) were treated with a technical success rate of 99%. The degree of pre-procedural stenosis was 92 ± 6% and dropped to 12 ± 10% after stent deployment. No patient developed any stroke or death during the periprocedural period. Intracranial hemorrhage was observed in 1 (1.5%) patient. In 60 (88%) patients with available imaging follow-up in-stent restenosis was observed in 2 patients. Mean follow-up period was 22 ± 17 months (range 6–72) and none of the patients experienced recurrent TIA or stroke during the follow-up period.
Conclusion
In this retrospective single-center study undersized balloon angioplasty and deployment of a self-expandable stent with relatively low radial force was safe and effective for endovascular treatment of high-grade intracranial arterial stenosis with high technical success rate, low periprocedural complication rates and favorable medium-term follow-up results.
Keywords: Enterprise, intracranial high-grade stenosis, stenting, transluminal angioplasty
Introduction
Intracranial atherosclerotic stenosis (ICAS) is estimated to be the cause of up to 10% of ischemic strokes in the USA.1 Its prevalence is particularly high in Asian, Hispanic and African populations.2,3 Patients with symptomatic ICAS are at high risk of subsequent stroke even with best medical therapy.4,5 Patients with symptomatic high-grade (70–99%) intracranial stenosis have higher risk of recurrent stroke up to 20% within 1 year despite aggressive antithrombotic treatment.6–9 Therefore, intracranial angioplasty and stent placement have become an alternative therapeutic modality for stroke prevention in patients with severe stenosis who fail medical therapy.9–11
So far, two randomized controlled trials (Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) and Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT)) have been implemented to evaluate aggressive medical treatment and endovascular intervention with stenting in patients with ICAS.7,12 Both trials have demonstrated superiority of medical treatment alone over endovascular treatment and thus the future of neuroendovascular treatment of ICAS has been debated.7,12,13 The inferiority of endovascular treatment was mainly driven by periprocedural complications.13 The United States Food and Drug Administration in March 2012, however, announced that the endovascular treatment with the Wingspan stent (Stryker Neurovascular, Kalamazoo, Michigan, USA) remains a treatment option for patients with recurrent stroke despite medical management.14
The Wingspan stent system, which was the device exclusively used in SAMMPRIS has been criticized for its rigidity and open-cell design with high radial force.15–17 Furthermore, deployment of the Wingspan into tortuous, long vascular segments and arterial bifurcations has been particularly associated with higher rates of complications because of its rigidity.15,16 The Enterprise stent (Codman Neurovascular, Raynham, Massachusetts, USA), which was originally designed for neck remodeling in the treatment of intracranial aneurysms, features a closed-cell design, special carrier system and lower radial force and has been suggested as an effective alternative for stenting high degree symptomatic ICAS.17 Another major issue of percutaneous transluminal angioplasty and stenting (PTAS) for ICAS is high rates of in-stent restenosis (ISR). Reported ISR rates with Enterprise is lower when compared with Wingspan series.17 In this retrospective case series, we report medium-term follow-up results from patients who, at our department, underwent undersized balloon angioplasty and stenting for high-grade (70–99%) stenosis of a major intracranial artery with Enterprise stent system.
Materials and methods
Patient population and lesion characteristics
Informed consent was obtained from all participants and our local institutional ethics review board approved this study. Between July 2012 and December 2017, 124 patients with recurrent transient ischemic attack (TIA) or ischemic stroke under dual antithrombotic treatment, who demonstrated high-grade intracranial stenosis at computed tomography angiography (CTA) or magnetic resonance angiography (MRA), were referred to our clinic for diagnostic cerebral angiography. Among them, cerebral angiography confirmed high-grade stenosis in 96 patients. We could not obtain written informed consent from 6 patients. Patients with non-atherosclerotic stenosis (n = 7), vascular malformation (n = 2) and occlusive lesions (n = 13) were excluded. This study included 68 patients with high-grade (70–99%) stenosis of a major intracranial artery (intracranial internal carotid artery (ICA), middle cerebral artery (MCA), intracranial vertebral artery or basilar artery). TIA and ischemic stroke was confirmed by clinical evaluation and magnetic resonance imaging (MRI). Digital subtraction angiography (DSA) was used to quantitate the severity of the stenosis as defined by the Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) study criterion. Collected demographic data of the patients included age, comorbidities, medical therapy for prevention of stroke and stroke history.
MRI and diagnostic DSA were performed before PTAS. Morphology of the targeted segment of the stenotic artery and stenosis degree were measured by using DSA and three dimensional reconstruction images. Transluminal angioplasty and stenting procedures were delayed at least two weeks from the latest ischemic event onset, except for two patients. We recorded the locations, length of the stenotic segment, reference artery diameter and degree of stenosis.
Procedures
All of the patients were under dual antithrombotic treatment with aspirin and clopidogrel. For the patients with a suspicion of irregular drug taking the loading dose of aspirin (300 mg) and clopidogrel (75 mg) was given at least three consecutive days prior to intervention. We are routinely performing response testing for clopidogrel. All of the procedures were performed under general anesthesia, using a biplane angiography system (Allura FD 20x20, Philips Healthcare, The Netherlands). Arterial access was obtained via femoral artery introducers. A 6 F guiding catheter was placed at the common carotid artery or subclavian artery according to localization of the target vessel. Following femoral puncture 5000 units of intravenous heparin was given and during the procedure 1000 units of intravenous heparin per hour was proceeded to maintain the double-activated clotting time. After placement of the guiding catheter, a rotational angiography and 3D reconstruction images were obtained to better measure the parent artery diameter and length of the stenosis. Under a roadmap guidance the stenosis was bypassed with a Transend floppy microwire, followed by an undersized predilatation with a balloon catheter (Gateway, Stryker). The balloon catheter was chosen according to diameter of the parent artery, as the diameter of the balloon catheter at its nominal pressure would not exceed 80% of the diameter of the parent artery. After withdrawal of the balloon catheter, the delivery microcatheter was advanced over an exchange microwire. After placement of the delivery microcatheter Enterprise stents were deployed covering the targeted stenosis. The stent was chosen as the diameter of the stent would be at least 1 mm wider than that of the target vessel, and the exceeding length of the stent would be at least 5 mm longer than that of the target lesion. A post-stenting angiography was performed immediately after stent deployment and 20 minutes later an additional run was performed to rule out thrombus formation within the stent or distal thromboembolism. During the procedures, we kept the blood pressure of the patients under 120–140 mmHg to avoid reperfusion or hyperperfusion injury. Patients were observed in an intensive care unit and neurological examination was performed at regular intervals. Systolic blood pressure was strictly controlled at <140 mmHg during intensive care unit follow-up.
The immediate post-interventional and follow-up degree of any residual stenosis was recorded. Treatment of 100 mg aspirin daily for life and 75 mg clopidogrel daily for 6 months was recommended after discharge.
Clinical and angiographic follow-up
The primary outcome parameters were occurrence of an acute infarct or TIA in treated territory or death within 30 days after endovascular treatment as defined by the guidelines of the SAMMPRIS trial. The secondary outcomes were technical success, occurrence of >50% ISR confirmed by DSA or CTA, and occurrence of recurrent stroke or TIA in treated territory during the follow-up period. Technical success was defined as deployment of the stent accurately covering the lesion resulting in a residual stenosis less than 50%. A TIA was defined as neurological worsening with complete recovery within 24 hours. A stroke was defined as a sudden-onset neurological deficit that persisted for at least 24 hours. We defined symptomatic brain hemorrhage as parenchymal, subarachnoid, or intraventricular hemorrhage detected by computed tomography or MRI, associated with new neurological signs or symptoms lasting 24 hours including seizures.
Follow-up DSA was scheduled at 3 and 6 months, and thereafter follow-up was yearly by DSA, CTA or MRA.
Statistical analysis
The statistical methods were predominantly descriptive. Continuous data were presented as means ( ± standard deviation), and categoric data were presented as percentages.
Results
Patients and lesion characteristics
This study included 68 patients (12 women, and 56 men) with a mean age of 62 ± 7 years (range 46–82). A total of 68 lesions in 68 patients were endovascularly treated. All patients presented with an ischemic stroke (61 patients, 90%) or TIA (7 patients, 10%). Procedures were delayed at least two weeks, except for two patients whose procedures were performed 9 and 11 days from the qualifying event. The average time interval between the procedure and the event was 18 ± 9 days (range 9–36).
Angioplasty and stent placement were performed in the following locations: 21 in the intracranial ICA, 8 in M1 segment of the MCA, 19 in the basilar artery and 20 in the intradural vertebral artery. Demographic and lesion characteristics of the patients are summarized in Table 1.
Table 1.
Demographic and lesion characteristics of the patients.
| Characteristics | Results |
|---|---|
| Number of patients | 68 |
| Age (years) | 62 ± 7 (46–82) |
| Clinical presentation | |
| Stroke | 61 (90%) |
| Transient ischemic attack | 7 (10%) |
| Gender | |
| Male | 56 (82%) |
| Female | 12 (18%) |
| Number of lesions | 68 |
| Location of lesions | |
| Internal carotid artery | 21 |
| Middle cerebral artery | 8 |
| Basilar artery | 19 |
| Vertebral artery | 20 |
Procedural results
All procedures were a combination of angioplasty followed by stent placement performed with the patient under general anesthesia. The overall technical success rate was 99%. The mean severity of pre-procedural stenosis was 92 ± 6% and mean degree of residual vascular stenosis after stent deployment was determined as 12 ± 10%. Mean reference artery diameter was 2.7 ± 0.9 mm (range 1.66–3.82). Gateway percutaneous transluminal angioplasty (PTA) balloon catheters were used for balloon angioplasty. Mean length of the balloon catheters was 16.6 ± 3 mm (10–20 mm) and average stent length was 25 ± 6 mm (14–37 mm).
Stent sizes were 4 × 23 mm for 10 lesions, 4 × 30 mm for 19 lesions, 4 × 40 mm for 3 lesions, 4.5 × 22 mm for 20 lesions, 4.5 × 28 mm for 8 lesions, 4.5 × 30 mm for 3 lesions, and 4.5 × 37 mm for 4 lesions.
In one patient with basilar artery stenosis, we performed angioplasty with Gateway PTA balloon catheter; however, we failed to achieve sufficient dilatation and we could not perform stent deployment.
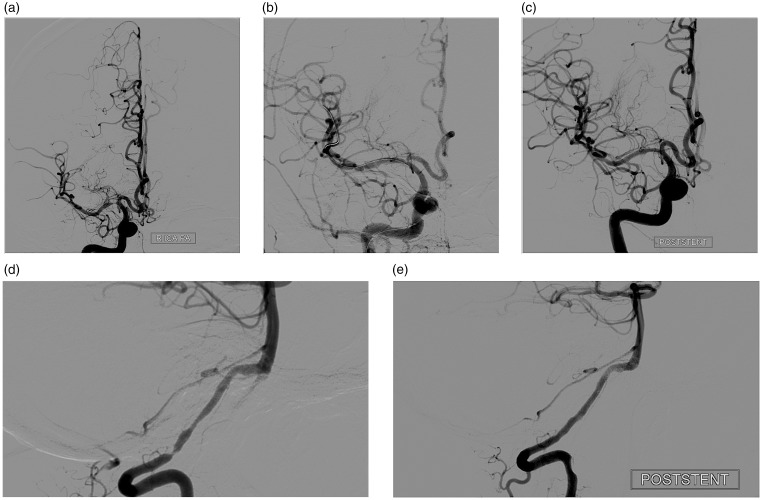
Procedure-related complications were encountered in 1 (1.5%) patient. The patient presented with a recurrent stroke due to high-grade stenosis at V4 segment of the right vertebral artery and also had occlusion at the right ICA and left vertebral artery (Figure 2). In the procedure of this patient, a subarachnoid hemorrhage was observed as a procedural complication. It was clinically relevant; however, the patient recovered completely without neurological deficit. No patient developed a stroke or TIA in treated territory or death within 30 days after endovascular treatment. Stent migration occurred in one patient during the procedure. The patient had a stenosis at M1 segment of the MCA; a second stent was deployed after migration of the first stent and no further complication occurred.
Figure 2.
A 65-year-old male patient was referred with a recurrent stroke due to right vertebral artery stenosis and had also occlusion at right internal carotid artery and left vertebral artery (a and b). After undersized balloon angioplasty with Gateway an abnormal location of the tip of the microwire in anterior inferior cerebellar artery (AICA) was detected (c and d). An Enterprise stent was deployed and after deployment digital subtraction angiography (DSA) demonstrates near 10% residual stenosis (e). After deployment of the stent, a selective angiography of the AICA was performed due to suspicion of a microwire perforation and DSA demonstrates extravasation from a small branch of the AICA (f). Coil embolization was performed and on the completion of DSA total embolization of the extravasation and almost no residual stenosis at vertebral artery was observed (g).
Follow-up results
The National Institutes of Health Stroke Scale (NIHSS) scores of the patients were evaluated by neurologists at discharge, and the NIHSS scores of the patients were between 0 and 6. There was imaging follow-up in 60 (88%) patients. For the other 8 patients only clinical follow-up could be possible: at 1 and 2 months for 3 patients and at 1, 2 and 3 months for 5 patients. Follow-up DSA was available at the 12th month of the procedure for 47 patients and at the 6th month of the procedure for 13 patients. Mean follow-up period was 22 ± 17 months (range 6–72). ISR was observed in 2 patients (3%), defined as an in-stent stenotic lesion of more than 50% on the follow-up DSA series. One patient had occluded the Enterprise stent at the 12-month follow-up DSA, and one patient developed 50–60% ISR at follow-up DSA. These two patients were both under antiplatelet drug therapy like all other patients. No patient experienced recurrent TIA or stroke during the follow-up period. Procedural and follow-up results are summarized in Table 2.
Table 2.
Procedural and follow-up results.
| Technical success rate (%) | 99 |
| Mean pre-procedural stenosis degree (%) | 92 ± 6 |
| Mean post-procedural stenosis degree (%) | 12 ± 10 |
| Mean reference artery diameter (mm) | 2.7 ± 0.9 (1.66–3.82) |
| Mean balloon catheter length (mm) | 16.7 ± 3 (10–20) |
| Mean stent length (mm) | 25 ± 6 (14–37) |
| Mean follow-up period (months) | 22 ± 17 (6–72) |
| In-stent restenosis (n (%)) | 2 (2.94) |
Discussion
In this study, we report our single-center PTAS experience of high-grade symptomatic ICAS with Enterprise and our medium-term follow-up results. We achieved a high technical success rate (99%) with no 30 day stroke or death, and favorable follow-up results.
The best treatment option for the symptomatic ICAS still remains controversial.17,18 The SAMMPRIS trial is a cornerstone in the treatment of symptomatic ICAS followed by the VISSIT study. These two randomized controlled trials evaluated the safety and efficacy of PTAS for treatment of ICAS. Both trials included patients with TIA or stroke within 30 days prior to enrollment that was attributed to severe (70–99%) intracranial stenosis.13,14 They compared aggressive medical treatment represented by dual antiplatelet medication alone with a combination of PTAS and aggressive medical treatment. Both of the trials were prematurely terminated due to a significantly higher 1-month stroke and/or death rates (SAMMPRIS 15%; VISSIT 24%) in patients randomized to intracranial stent placement.13 Furthermore, long-term results of the both studies also failed to show benefit from stenting. Best medical therapy was found to be superior when compared with PTAS.
Many factors have been pointed out to be related to the high periprocedural complication rates in SAMMPRIS trial.19–22 Technical aspects of the procedure especially with Wingspan stent system which was exclusively used in SAMMPRIS were criticized.4,17,19 Technical failures of the Wingspan stent system have been reported as delivery, deployment and retrieval failures. These technical failures were related to the tortuousness of the cerebral vasculature, the open-cell design of the Wingspan stent system that stimulates neointimal hyperplasia; and the high radial force of the stent system.16,19,21
Recently, a multicenter prospective trial of stent placement in patients with high-grade intracranial stenosis from China enrolled 100 patients.14 The results of this multicenter study demonstrated that, modifications in patient selection and procedural aspects can reduce the 1 month stroke and/or death rate following intracranial stent placement.14 Additionally, the Chinese stenting registry reported better outcomes when compared with SAMMPRIS and VISSIT trials. In the Chinese registry the primary outcome of any stroke, TIA, or death within 30 days was 4.3%. Reasons for the superior results of this study may be due to factors that differ from SAMMPRIS and VISSIT, such as more rigorous patient selection criteria, freedom of the interventionalists to select devices, and delay in the stenting procedure for a recommended time of at least 3 weeks after the last event.13,23
In our series we did not observe any stroke, TIA or death during the periprocedural period. Intracranial hemorrhage was observed in one patient due to a microwire perforation of a small branch of anterior inferior cerebellar artery. The patient developed a subarachnoid hemorrhage in the prepontine and cerebellopontine cisterns and the 4th ventricle. During the procedure the microwire perforation was noticed and the ruptured artery was subsequently embolized with detachable metallic coils. The patient woke without any neurological deficit.
Our overall procedure-related complication rate was 1.5% which is comparable to reported complication rates. In the study of Lee et al., 24 symptomatic patients with 30 intracranial arterial stenotic lesions refractory to medical therapy were treated with Enterprise stenting followed by undersized angioplasty, and periprocedural complication rate was 10% per lesion.19 Feng et al. reported three complications (6.8%) during the periprocedural period in 42 patients with Enterprise stent.17 Combined neurological morbidity and mortality rate at 30 days was 2 patients (0.9%) in the study of Vajda et al.21 In these retrospective single-center studies, stenting of the symptomatic ICAS with Enterprise stent appeared reasonably safe and effective as in our study. In the other studies, using Wingspan stent for the treatment of ICAS, reported 1-month complication rates ranged from 6 to 21%.24–26
Technical success rates for PTAS with Enterprise has been reported very high as Vajda et al., Feng et al. and Lee et al. all reported 100% technical success rates with the Enterprise stent system.17,19,21 We think that the high technical success rates and low periprocedural complication rates with Enterprise stent, that was originally developed for neck remodeling of the wide-neck intracranial aneurysms, may be attributable to its design. The stent delivery system of the Enterprise has a good accessibility with its flexible tip. Deployment of the stent is also possible in tortuous vascular segments and stenosis involving arterial bifurcation. Furthermore, its design allows redeployment and recapturing of the stent if it deploys less than 70% of its length. Stent migration following deployment of Enterprise has been reported as a critical drawback in some previous studies, demonstrated in one of the cases in this study. We had to deploy another stent covering the stenosis after migration with no further complication.
High rates of ISR has been a major issue for PTAS of ICAS. One well-known mechanism for ISR is stimulation of the intimal hyperplasia by the radial force of the stent. In the study of Krischek et al. radial force of the Enterprise stent was found to be lower when compared with Wingspan stent.27 Therefore, Enterprise stent has been used with the expectation of lower rates of ISR compared to Wingspan stent. We observed ISR in 2 patients (3%) during a mean follow-up period of 22 ± 17 months in this study. It is similar to the results in the studies of Lee et al. and Feng et al. with Enterprise stents.17,19 The ISR rates of Wingspan procedures have been reported to range from 7.5 to 42.8% with a mean follow-up period of 6–13 months.17,28 Many factors other than stent radial force such as stent thrombogenicity, vascular intimal injury by balloon dilatation without stenting, lesion length, lesion at anterior circulation and residual stenosis have been reported to be related with ISR.26,27 Lower ISR with Enterprise than Wingspan may be thus attributed to the closed-cell design of the Enterprise stent, with lower radial force compared with the Wingspan.
The best treatment option for the symptomatic ICAS still remains controversial. Although the randomized trials showed better short-term and long-term results with the best medical treatment, SAMMPRIS reported a recurrent stroke rate of 12.2% at 1 year despite best medical therapy. PTAS may be a treatment option for prevention of recurrent stroke due to high-grade ICAS.
This study has several limitations; first is the retrospective design. Second, as this study reports a single-center experience, the sample size is relatively small. However, to the best of our knowledge, this single-center experience of PTAS for high-grade symptomatic ICAS includes the largest sample size treated with Enterprise stent placement with medium-term follow-up results in the literature. And third, another limitation of this study is the composition of the targeted lesions. Our series included 8 MCA lesions out of 68 lesions; on the other hand, almost half of the targeted lesions in the SAMMPRIS trial, 38% of the targeted lesions in the study of Gao et al.14 and more than half of the targeted lesions in the study of Feng et al.17 were located at MCA. This may have influence on the comparison of the results. Prospective, multicenter, randomized controlled trials with long-term follow-up and larger sample size are needed to determine the safety and efficacy of Enterprise stent system for the treatment of symptomatic ICAS.
Conclusion
Undersized balloon angioplasty and deployment of a self-expandable stent with relatively low radial force is relatively safe and effective for endovascular treatment of symptomatic high-grade intracranial arterial stenosis with high technical success rate, low periprocedural complication rates and favorable medium-term follow-up results.
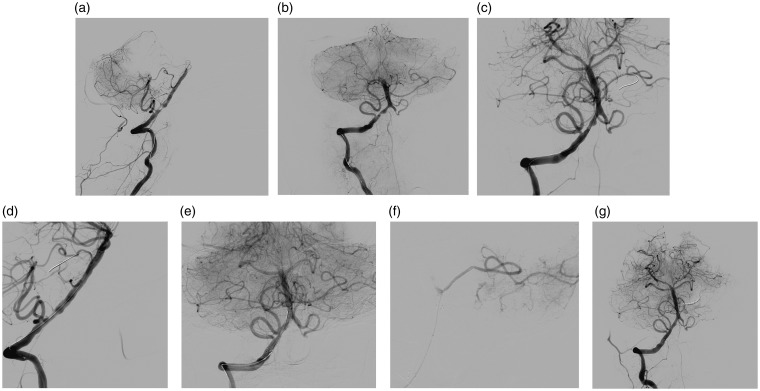
Figure 1.
A 54-year-old male patient with high-grade stenosis at right middle cerebral artery (MCA) and left vertebral artery was referred after a recurrent stroke. He was symptomatic (had a stroke) because of the stenosis at the M1 segment of right MCA. Digital subtraction angiography (DSA) demonstrated a severe stenosis at M1 segment of right MCA (a). Percutaneous transluminal angioplasty was performed with Gateway (b) and an Enterprise stent was deployed. DSA after percutaneous transluminal angioplasty and stenting (PTAS) revealed minimal residual stenosis (<10%) (c). Selective left vertebral DSA of the same patient also demonstrated a severe stenosis at V4 segment of left vertebral artery (d). Because of the vertebrobasilar insufficiency symptoms of the patient, PTAS was performed (but this lesion was not included by the study). Final angiography after PTAS with Enterprise revealed minimal residual stenosis (e).
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Kim GE, Yoon W, Kim SK, et al. Incidence and clinical significance of acute reocclusion after emergent angioplasty or stenting for underlying intracranial stenosis in patients with acute stroke. AJNR Am J Neurodadiol 2016; 37: 1690–1695. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorelick PB, Wong KS, Bae HJ, et al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008; 39: 2396–2399. [DOI] [PubMed] [Google Scholar]
- 3.Feldmann E, Daneault N, Kwan E, et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology 1990; 40: 1541–1545. [DOI] [PubMed] [Google Scholar]
- 4.Möhlenbruch MA, Pfaff J, Herweh C, et al. One-pass endovascular treatment of intracranial atherosclerotic stenosis with a novel PTA balloon and self-expanding microstent. Neuroradiology 2016; 58: 893–899. [DOI] [PubMed] [Google Scholar]
- 5.Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis and treatment. Lancet Neurol 2013; 12: 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011; 365: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014; 383: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006; 113: 555–563. [DOI] [PubMed] [Google Scholar]
- 9.Park S, Kim JH, Kwak JK, et al. Intracranial stenting for severe symptomatic stenosis: self-expandable versus balloon-expandable stents. Interv Neuroradiol 2013; 19: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.In HS, Lee HY, Park JY, et al. Intracranial stenting in patients with atherosclerotic stenosis associated with various aneurysms in the same diseased arterial segment. AJNR Am J Neuroradiol 2010; 31: 1895–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu SC, Cheng HK, Cheng, et al. Angioplasty and stenting for intracranial atherosclerotic stenosis: position statement of the Hong Kong Society of Interventional and Therapeutic Neuroradiology. Hong Kong Med J 2013; 19: 69–73. . [PubMed] [Google Scholar]
- 12.Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 2015; 313: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 13.Wabnitz A, Chimowitz M. Angioplasty, stenting and other potential treatments of atherosclerotic stenosis of the intracranial arteries: past, present and future. J Stroke 2017; 19: 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao P, Wang D, Zhao Z, et al. Multicenter prospective trial of stent placement in patients with symptomatic high-grade intracranial stenosis. AJNR Am J Neuroradiol 2016; 37: 1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimoto M, Shobayashi Y, Takemoto K, et al. Structural analysis for Wingspan stent in a perforator model. Interv Neuroradiol 2013; 19: 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao LB, Park S, Lee D, et al. Mechanism of procedural failure related to wingspan. Neurointervention 2012; 7: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Z, Duan G, Zhang P, et al. Enterprise stent for the treatment of symptomatic intracranial atherosclerotic stenosis: an initial experience of 44 patients. BMC Neurol 2015; 15: 187 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Huang Q, Zhang Y, et al. Wingspan stents for the treatment of symptomatic atherosclerotic stenosis in small intracranial vessels: safety and efficacy evaluation. AJNR Am J Neuroradiol 2012; 33: 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KY, Chen DY, Hsu HL, et al. Undersized angioplasty and stenting of symptomatic intracranial tight stenosis with Enterprise: Evaluation of clinical and vascular outcome. Interv Neuroradiol 2016; 22: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qureshi AI, Al-Senani FM, Husain S, et al. Intracranial angioplasty and stent placement after stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis (SAMMPRIS) trial: present state and future considerations. J Neuroimaging 2012; 22: 1–13. [DOI] [PubMed] [Google Scholar]
- 21.Vajda Z, Schmid E, Guthe T, et al. The modified Bose method for the endovascular treatment of intracranial atherosclerotic arterial stenoses using the Enterprise stent. Neurosurgery 2012; 70: 91–101. [DOI] [PubMed] [Google Scholar]
- 22.Hoak DA, Lutsep HL. Management of Symptomatic Intracranial Stenosis. Curr Cardiol Rep 2016; 18: 83. [DOI] [PubMed] [Google Scholar]
- 23.Fiorella D, Levy EI, Turk AS, et al. US multicenter experience with the Wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke 2007; 38: 881–887. . [DOI] [PubMed] [Google Scholar]
- 24.Bose A, Hartmann M, Henkes H, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke 2007; 38: 1531–1537. [DOI] [PubMed] [Google Scholar]
- 25.Zaidat OO, Klucznik R, Alexander MJ, et al. The NIH registry on use of the Wingspan stent for symptomatic 70–99% intracranial arterial stenosis. Neurology 2008; 70: 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding D, Starke RM, Crowley RW, et al. Role of stenting for intracranial atherosclerosis in the post-SAMMPRIS era. Biomed Res Int 2013; 2013: 304320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krischek O, Miloslavski E, Fischer S, et al. A comparison of functional and physical properties of self-expanding intracranial stents [Neuroform3, Wingspan, Solitaire, Leo+, Enterprise]. Minim Invasive Neurosurg 2011; 54: 21–28. [DOI] [PubMed] [Google Scholar]
- 28.Levy EI, Turk AS, Albuquerque FC, et al. Wingspan in-stent restenosis and thrombosis: incidence, clinical presentation, and management. Neurosurgery 2007; 61: 644–650. [DOI] [PubMed] [Google Scholar]