Abstract
Syndiotactic polystyrene (SPS) physical gels containing a large amount of the ionic liquid 1-butylpyridinium bromide ([C4py]Br) were systematically prepared and their physical properties were examined in detail. The gels had stable forms for a long time, having storage elastic modulus values of normal gels. They showed nearly the same values of the electrical conductivity (∼7 mS/cm) as those of the mixed solutions of [C4py]Br, suggesting that the distribution of [C4py]Br was uniform in these gels and that the charge transportation in these SPS gels was not interrupted by a three-dimensional network of SPS fibrils consisting of the SPS δ crystalline phases.
Introduction
Syndiotactic polystyrene (SPS), which is a useful heat-resistant engineering plastic material with high workability, was first synthesized in 1986 by using a metallocene catalyst.1 SPS is known to have complex polymorphic behavior2−4 and forms physical gels when dissolved in some organic compounds.5 Thermoreversible gels of SPS have been extensively studied so far since Kobayashi et al.6 Finally, SPS gels are established to have a three-dimensional network of fibrils consisting of the δ crystalline phases such as a δ clathrate phase7,8 or a δ intercalate phase.9−11 If all of the solvent molecules are evacuated from SPS gels, the X-ray diffraction peaks of the SPS samples are identical to those of SPS δ empty crystalline phase,12 which is monoclinic (space group P21/a; a = 1.74 nm; b = 1.18 nm; c = 0.78 nm; γ = 117°) and exhibits per unit cell 2 identical cavities centered on the center of symmetry and bounded by 10 phenyl rings.12,13 Previously, we examined where more than 60 solvent compounds could form SPS gels.14−16 Finally, it was concluded that the formation of SPS thermoreversible gels was strongly dependent on the molecular volume of the solvent and compatibility between SPS and the solvent and that there is a “gelation range” of solvent molecules as for molecular volume size and solubility parameter.
An ionic liquid (IL) is defined as a salt whose melting temperature is, at most, 100 °C. Because it has unique properties, such as nonflammability, negligible volatility, wide electrochemical window, and high ionic conductivity, the application of ILs has become a topic of advanced research.17−21 If SPS gels containing a large fraction of ILs can be prepared, they would be a useful soft material for batteries and sensors.
The first trial in obtaining SPS gels containing ILs was carried out using partially sulfonated SPS.22 The gels could include 30 wt % of 1-ethyl-3-methylimidazolium dicyanamide, an IL, but this did not show any electric conductivity. In 2017, Jana et al. reported that the membranes made of SPS aerogel with a thickness of ∼100 μm were swollen with 1-n-butyl-methylpyrrolidinium bis(trifluoromethane sulfonyl)imide, an IL, and that the ionic conductivity of the membrane was found to be 6.33 × 10–4 S/cm.23
In the present paper, we show our method of preparing SPS gels having a large amount of IL that are perfectly in gel form and electrically conductive.
Results and Discussion
Preparation of SPS Gels Containing ILs
We have already examined the structure and properties of SPS/pyridine (Py) gels.16 Taking into account good compatibility between SPS and Py, we tried to produce SPS gels containing ILs having a pyridinium ring. The thermogravimetry (TG) plots showed that the temperatures where the thermal decomposition process of 1-butyl-4-methylpyridinium tetrafluoroborate and 1-butylpyridinium bis(trifluoromethanesulfonyl)imide occurred happened to overlap with the temperatures where SPS decomposition occurred, which started above 320 °C. However, the thermal decomposition of 1-butylpyridinium bromide ([C4py]Br) started above 200 °C and ended below 300 °C. Therefore, we realized that we could determine the fractions of SPS, [C4py]Br, and solvent in SPS gels from the TG plots of the gels. Thus, we chose [C4py]Br for the purpose of producing SPS gels containing a plenty of IL. [C4py]Br whose melting point was 95 °C can easily be liquefied by its exposure to the open air for long time periods because it is hygroscopic. However, the addition of 1 wt % water into [C4py]Br was found to give the stable liquid state. Thus, we used a mixture of [C4py]Br with 1 wt % water. Hereafter, we call this solution to be ILBr.
Before finding the better preparation conditions of SPS gels containing ILBr, we tried the following experiments step-by-step. First, we tried to directly dissolve SPS pellets in ILBr at high temperatures, but this ended in failure. Thus, we chose some solvents able to form SPS physical gels and tried to mix ILBr in them. The solvents used included chloroform, Py, toluene, tetrahydrofuran (THF), piperidine, and pyrrolidine. ILBr was insoluble with toluene, THF, and piperidine even at high temperatures. Only less than 10 wt % of ILBr was found to be soluble in these. Although ILBr was slightly soluble in chloroform, SPS did not dissolve in a mixture of ILBr and chloroform. Finally, ILBr was found to be mixed at any ratio with Py and pyrrolidine at room temperature.24 Then, we dissolved SPS in Py and pyrrolidine-containing ILBr at high temperatures and successfully obtained SPS gels by cooling at room temperature. For example, 5 wt % SPS gels were formed with Py with 10 wt % of ILBr or with pyrrolidine with 20 wt % of ILBr.
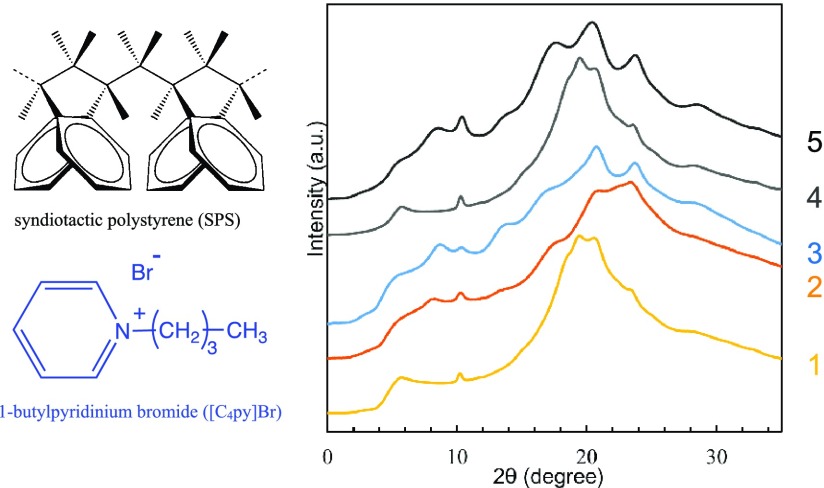
Now that SPS gels containing an IL were formed, their structures were examined by the wide-angle X-ray diffraction (WAXD) measurements (Figure 1). The fibrils of SPS/Py gels were reported to be of SPS/Py δ intercalate phase10,11 [the typical reflection peaks are at 2θ = 5.7° (010) and 10.3° (200)] when the gels were wet;16 however, by drying SPS/Py gels at 40 °C for 24 h under vacuum, they changed to SPS δ empty phase [2θ = 8.6° (010) and 10.4° (2̅10)]12 because of the desorption of Py molecules.
Figure 1.
WAXD profiles of SPS/Py/[C4py]Br gels [Py: [C4py]Br = 9:1 (weight ratio)] (1–3) and SPS/Py gels (4, 5). SPS/Py/[C4py]Br gels; (1) wet gel, (2) just after drying under vacuum, (3) dried powder after washing with methanol. SPS/Py gels; (4) wet gel, (5) powder gel after drying under vacuum.
The wet gels made of 5 wt % SPS with Py containing 10 wt % of [C4py]Br showed the same WAXD profiles as 5 wt % SPS/Py wet gels, meaning that the fibrils forming a three-dimensional network were the SPS δ intercalate phase. We dried this SPS/Py/[C4py]Br gel at 40 °C for 24 h under vacuum and found a reflection peak at 2θ = 8.3° corresponding to the 010 with the SPS δ monoclinic phase having guest molecules.7,8 In the case of SPS/Py gel, all of the guest Py molecules were desorbed after drying under vacuum and the crystalline phase changed to SPS δ empty phase. Therefore, there is a possibility that [C4py]Br can be a guest molecule inside the cavity of the SPS nanoporous δ phase because the cation part of [C4py]Br is 9.59 Å × 4.99 Å × 1.82 Å (87.1 Å3)25 and the cavity volume of SPS δ empty phase is 120–160 Å3. When the SPS/Py/[C4py]Br gels were dried under vacuum, [C4py]Br remained as the liquid state without being evaporated. Therefore, we washed out [C4py]Br with methanol from the dried gels. Along with washing, the reflection peak at 2θ = 8.3 turned out to be shifted to 8.7°. In fact, there are two nanoporous-crystalline δ phases, the monoclinic one with typical 2θ = 8.3°–8.4° as well as a triclinic one with 2θ = 8.7°.26 Thus, the change of the WAXD profiles before and after extraction with methanol could be the transformation from the nanoporous-crystalline monoclinic δ phase toward the triclinic one. In any way, the fibrils forming a three-dimensional network in SPS/Py/[C4py]Br gel was established to be of the SPS nanoporous-crystalline δ phases. Figure S1 shows the scanning electron microscopy (SEM) pictures of SPS fibrils.
So far, it can be concluded that (1) the shape of SPS gels containing [C4py]Br is kept to be stable due to the three-dimensional network of SPS fibrils whose structure is of SPS/Py δ intercalate phase and (2) the fibril structure is not destroyed even by evaporation of Py under vacuum and by direct surrounding of ILBr because the structure of SPS fibrils is maintained by the formation of SPS δ clathrate phase with either Py or [C4py]Br as a guest. Thus, we can expect that the contact of [C4py]Br with SPS/Py gels would increase the fraction of [C4py]Br in SPS gels without getting collapsed.
Therefore, we carried out the following experiments. First, we prepared 1.2 g SPS gels with mixed solvents of Py and ILBr, with a weight ratio of Py/ILBr = 9:1 in screw tubes. Second, we mounted 0.6 g of ILBr or Py solutions of ILBr as shown in Figure S2, with different ratios on the SPS/Py/ILBr gels and left them at 25 °C for 1 week. The concentration of SPS was of 5 wt %.
The SPS gels kept the same shapes as those in the initial stage even after the mount of ILBr for 1 week. The WAXD charts of all SPS gels after mounting ILBr or ILBr solutions show that the fibril structure of the gels was still made with SPS δ intercalate crystalline phase. We measured TG/differential thermal analysis (DTA) of the SPS gel part (Figure S2). In these wet gels, each fraction of SPS, Py, and [C4py]Br was calculated because the plateau regions were observed.27 The fractions of [C4py]Br against all solvents in the SPS gels were 16, 21, and 46 wt %, respectively, when the mounting solvents were 20, 50, and 100 wt % of ILBr. These values were found to be nearly the same of the fractions of [C4py]Br in all of the mixed solvents (13, 24, and 41%) calculated when all [C4py]Br molecules were uniformly distributed in the SPS gel and the liquid that was mounted on the gel. Although the contact area of the interface between the gel and liquid is not wide, all of the solvent molecules appear to be mixed and reach the equilibrium state, where each solvent is uniformly distributed. This means that the interface of SPS/Py gels does not interrupt [C4py]Br from entering the SPS gel part and that the gel network composed of the SPS δ crystalline phase is so stable that the gel shape should be maintained. Thus, we understand that the immersion of SPS/Py gels in ILBr would be a good way of increasing the fraction of [C4py]Br in the gels.
Finally, we have established the preparation way of SPS gels where the weight ratio of [C4py]Br is 80 wt % or more. The preparation way can be summarized as follows: first, SPS was dissolved in Py at high temperatures such as 140 °C and cooled at room temperature. The gel samples for the measurements of viscoelasticity and electrical conductivity were prepared by casting the Py solutions of SPS into each regular mold after cooling the solution around 120 °C. After keeping the gels at room temperature for 1 day, the gels were immersed in ILBr or Py solutions of ILBr. We obtained SPS gels containing various concentrations of [C4py]Br by changing the immersion time or the composition of ILBr in the liquid that SPS gels were immersed in. The fraction of [C4py]Br in an SPS gel was measured with TG/DTA. Though SPS/Py gels were slightly opaque at 5 wt % SPS, the color of the gels turned brown after immersion in ILBr.
Viscoelastic Properties of SPS/Py/[C4py]Br Gels
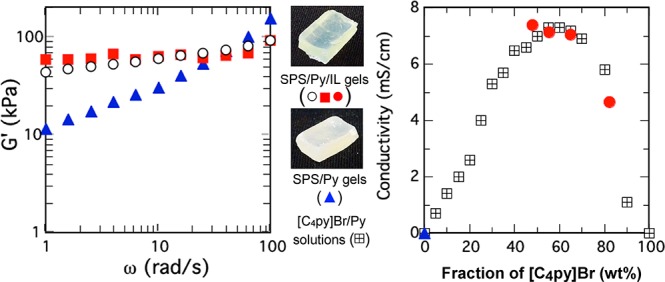
Now that the SPS gels with high fractions of an IL compound were prepared with ease, we studied their physical properties, such as viscoelasticity and electrical conductivity. To evaluate the viscoelastic properties of the SPS/Py/[C4py]Br gels, dynamic frequency rheological measurements were carried out using a rheometer. Figure 2 shows the frequency dependence of the storage elastic modulus, G′, for SPS/Py/[C4py]Br and SPS/Py. Each gel was 5 wt % of SPS. The results are summarized as follows: (i) The G′ value of each gel increased with an increase of frequency, but the frequency dependence of the SPS gels containing [C4py]Br was less than that of the SPS/Py gel without [C4py]Br. (ii) The reproducibility was so high that the inside of the gels was assumed to be uniform (the G′ values of SPS/Py/[C4py]Br gels containing 35 and 41 wt % of [C4py]Br were nearly the same at each frequency as shown in Figure 2). (iii) The G′ values of SPS gels containing ∼40 wt % [C4py]Br were found to be tens of thousands Pascal, indicating that the SPS/Py/[C4py]Br gels had enough mechanical strength to keep stable shapes. Thus, our SPS/Py/[C4py]Br gels are expected to utilize as good soft materials. For example, the values are similar to the ionogel prepared with gelator and poly(methyl methacrylate).28 Thus, it is ascertained that we have succeeded in preparing stable gels with a large amount of IL only by using inherent SPS, namely, without being chemically modified and without adding any reagents such as gelators.
Figure 2.
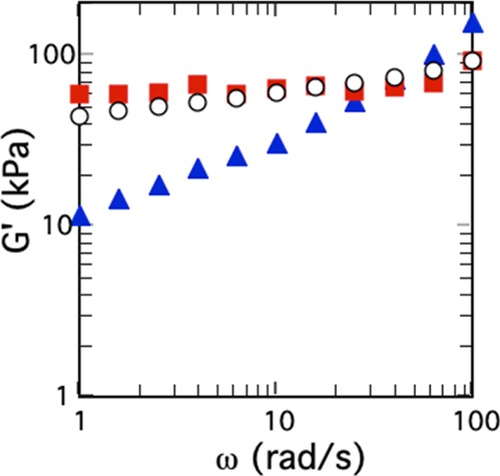
Viscoelastic properties of SPS/Py/[C4py]Br [the fractions of [C4py]Br were 35 (black circle) and 41 wt % (red square) and SPS/Py gels (blue triangle)] at 25 °C: the SPS concentrations of all of the gels were 5 wt %.
Guenet et al. observed that the loss elastic modulus, G″, became larger than G′ at much lower frequencies, when fibril–fibril interaction in a gel was not strong enough so that there was a phenomenon of junction modification by chain slipping under stress.29,30 We expected that the large fraction of ILs might weaken the interaction between SPS fibrils. However, the frequency dependence of G″ (Figure S3) did not show the change Guenet et al. observed. The junction of fibril networks in SPS/Py/[C4py]Br gel was found to be unchanged by the addition of a large amount of [C4py]Br.
Electrical Conductivity of SPS/Py/[C4py]Br Gels
Last, we examined the electrical conductivity of SPS/Py/[C4py]Br gels by changing the fraction of [C4py]Br and SPS. We used a low resistivity meter. To keep the same conditions for the measurements of conductivity, all of the gels were prepared in the same template made of polytetrafluoroethylene (PTFE). We obtained each fraction of SPS, Py, and [C4py]Br in a gel from the TG chart of the SPS/Py/[C4py]Br gel (Figure 3). The fractions of an SPS gel were expressed to be as (w, x, y; z) where w, x, and y are the wt % of [C4py]Br, Py, and SPS in a gel, and z is the fraction (wt %) of [C4py]Br in the total solvents, that is, [C4py]Br and Py. For example, the fractions calculated from line 3 of Figure 3 are (79.2, 17.3, 3.46; 82.0), where the fraction of [C4py]Br in the solvents of the gel, that is, z, was obtained by 79.2/(79.2 + 17.3) × 100 (=82.0 wt %).
Figure 3.
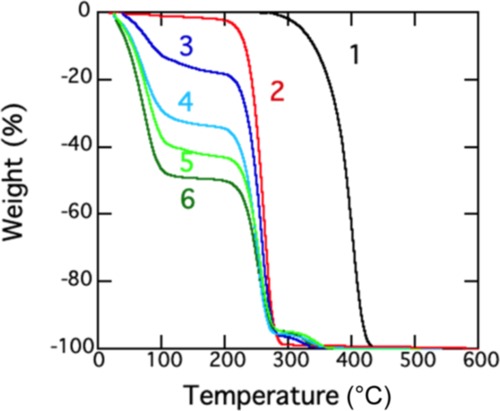
TG traces of SPS/Py/[C4py]Br gels at a scan rate of 10 °C/min: (1) SPS pellet, (2) ILBr, and (3–6) SPS/Py/[C4py]Br gels (5 wt % of SPS) with different fractions of [C4py]Br (see the definition of (w, x, y; z) in the text). (3) (79.2, 17.3, 3.46; 82.0), (4) (62.1, 33.6, 4.21; 64.9), (5) (52.6, 42.3, 5.03; 55.4), (6) (45.6, 49.5, 4.94; 47.9).
In Figure 4, the electrical conductivity of SPS/Py/[C4py]Br gel (SPS, 5 wt %) was plotted as a red circle against the fraction of [C4py]Br in all of the solvents inside the gel. Needless to say, the electrical conductivity of SPS/Py gel was not measurable due to its high resistivity. However, all SPS gels containing [C4py]Br were found to be electrically conductive, as shown in Figure 4. The highest value of conductivity observed in Figure 4 was 7.4 mS/cm for SPS/Py/[C4py]Br where [C4py]Br was 48 wt % in the gel. The conductivity was around 7 mS/cm at ∼40 wt % of [C4py]Br in the solvents of the SPS gel, which is nearly the same as the SPS gel whose G′ value was tens of thousands Pascal (Figure 2). Thus, our SPS/Py/[C4py]Br gels are concluded to be not only stable but also conductive.
Figure 4.
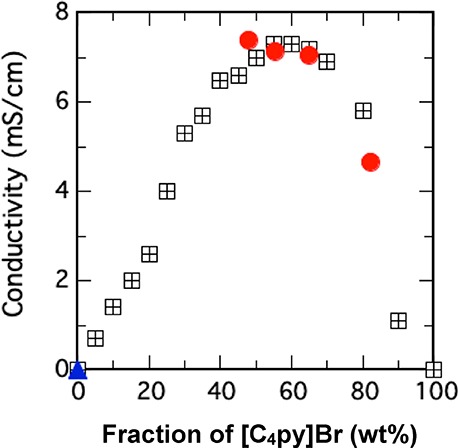
Electrical conductivity of SPS/Py/[C4py]Br gels (red circle) and SPS/Py gels (blue triangle), solutions of Py and [C4py]Br (black square) at a fraction value of [C4py]Br/([C4py]Br + Py).
Figure 4 demonstrates that the SPS gel containing 79 wt % [C4py]Br had a lower conductivity than the gel containing a lower fraction of [C4py]Br. As a matter of fact, the conductivity of [C4py]Br without Py was 3 μS/cm.31 To obtain the electrical conductivity of the mixed solutions between Py and [C4py]Br,32 we used an electrode as the conductivity sensor (black square of Figure 4). As it is already known,17−21 the conductivity of the solution with ILs tends to increase compared with that of the inherent IL itself. In the case of [C4py]Br and Py, the mixture of 60 wt % [C4py]Br turned out to show the highest conductivity (∼7 mS/cm). The measurement methods were different between the SPS gels and the mixed solvents, but the values of electrical conductivity appear to be nearly the same. Note that the conductivity values of the SPS gels and the mixed solvents were nearly the same at the same composition of [C4py]Br. The conductivity values of SPS/Py/[C4py]Br gels whose fractions of [C4py]Br in the solvents, that is, [C4py]Br and Py, were 45–65 wt % were found to be around 7 mS/cm, which was almost identical to that of the solutions.
In addition, we measured the conductivity of SPS/Py/[C4py]Br with 9.1 wt % of SPS ([C4py]Br 60%, Py 31%) [[C4py]Br/([C4py]Br + Py) = 66%] to be 7.1 mS/cm, which was identical to that of the 5 wt % SPS gel. From the standpoint of conductivity, it follows from these results that (1) the distribution of the IL ([C4py]Br) is uniform in the SPS/Py/[C4py]Br gels and (2) the charge transportation in these SPS gels is not interrupted by the three-dimensional network of SPS fibrils. SPS is a typical electrically insulating material, but the existence of SPS fibrils in the gels does not prevent the solvent molecules from diffusing in the area where solvent molecules gather together. This result is consistent with our studies on the molecular motion in SPS gels using fluorescent probe molecules.33
Moreover, we kept this gel sample (SPS 9.1 wt %) for half year in a screw tube. Syneresis of Py did not occur and the size did not change, while a little syneresis was observed for an SPS/Py gel with 10 wt % SPS.
In conclusion, we prepared SPS gels containing high concentrations of [C4py]Br by the immersion of SPS/Py gels in [C4py]Br. Some ionogels reported are so stiff that the G′ values were over 100 kPa, but our gels have relatively low G′ values. Moreover, the electrical conductivity was as high as 7 mS/cm. Thus, we can conclude that we developed an efficient soft material with a stable shape and high conductivity.
Experimental Section
Materials
SPS was kindly supplied by Idemitsu Kosan Co., Ltd. It is 98% syndiotactic with an Mw of 152 000, and an Mw/Mn of 1.9. SPS was dissolved with a solvent in a screw tube by heating the mixture at high temperature after influx of dry nitrogen gas for 1 min. The solvents used included chloroform, Py, toluene, THF (Wako Co.), piperidine, and pyrrolidine (Sigma-Aldrich). The ILs used were 1-butylpyridinium bromide ([C4py]Br) (Sigma-Aldrich, Tokyo Kasei), 1-butyl-4-methylpyridinium tetrafluoroborate (Sigma-Aldrich), and 1-butylpyridinium bis(trifluoromethanesulfonyl)imide (Tokyo Kasei). All of the ILs and solvents were used without purification. The heating temperature of a sample was sometimes higher than the boiling temperature of the solvent by 10–20 °C. In the case of SPS/Py gels and SPS/pyrrolidine gels with 5–10 wt % SPS, the heating temperatures were 130–140 and 120–130 °C, respectively. After SPS was perfectly dissolved, the solutions were well stirred by using a vortex mixer, heated again for 2 min, and finally cooled at room temperature (around 25 °C) for 1 day.
Measurements
WAXD patterns were measured at room temperature using a Rigaku MicroMax 7 HFM with a beam of Cu Kα radiation (λ = 0.15418 nm) that was finely focused by a confocal mirror and collimator. SPS wet gels and dried powders measured for the WAXD were filled in a capillary made of soda lime glass with a diameter of 1.0 mm (Hilgenberg GmbH). The diffraction patterns were detected by a Rigaku R-Axis IV++ and recorded on an imaging plate. The data were analyzed by using FIT2D program. Diffraction intensities at constant 2θ shown in a diffraction pattern were obtained by the integration of all diffraction intensities around the beam.
The TG/DTA data of SPS gels, which were filled in platinum pans, were obtained using a Rigaku Thermo Plus EVO2 TG/DTA 81205Z instrument in air while increasing the temperature from 15 to 700 °C at a rate of 10 °C/min. Gels for the dynamic frequency rheological measurements were prepared in a PTFE bottle whose inner diameter was 25 mm. SPS/Py solutions with 5 wt % SPS (around 120 °C) were poured to the vessel and cooled at room temperature. The height of the gels was arranged to be 1 cm. The fraction of [C4py]Br in the SPS gels for this purpose was increased by the mount of ILBr, that is, a mixture of [C4py]Br with 1 wt % water, on the gel in the vessel. The measurements were carried out with an ARES G-2 rheometer (TA Instruments) whose stage for samples had a diameter of 2.5 cm.
SEM pictures were obtained using a JEOL JSM-6300 or JEOL JSM-6510LV after drying an SPS/Py gel on a glass plate under vacuum at 40 °C for more than 2 days. Dried SPS samples were stuck on a double-sided tape of carbon and coated with platinum. In the case of SPS/Py/[C4py]Br gels, the same method did not give the clear SEM pictures of SPS networks due to the disturbance of [C4py]Br. Thus, we washed the dried SPS/Py/[C4py]Br gel with methanol and dried the gel powder.
We used a low resistivity meter (Loresta-GX MCP-T700, Mitsubishi Chemical Analytech) with a 4-pin probe (ASP probe). We obtained values of electrical conductivity of our SPS gels by measuring their surface resistivity and by using calculation software of the machine, where we inputted each size of our gels (length, breadth, and height). To keep the same conditions for the measurements of conductivity, all of the gels were prepared in the same template made of PTFE: the sizes were 1 cm × 5 cm × 0.5 cm (depth). Moreover, we obtained the electrical conductivity of the mixed solutions between Py and [C4py]Br, and we used a Conducell 4USF-PG 120 conductivity sensor (Hamilton) connected to a Stratos Eco 2405 Cond converter (Knick).
Acknowledgments
This work was supported by Grant-in-aid for Scientific Research (C) (15K05625 and 18K05233) from the Ministry of Education, Science, Sports, and Culture of Japan. The research was partially carried out at the Research Institute of Green Science and Technology, Shizuoka University. The authors are grateful to Dr. N. Ishihara, Idemitsu Kosan Co., for the supply of SPS.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02310.
SEM pictures of dried SPS/Py gel and dried SPS/Py/ [C4py]Br gel; image of how to mount ILBr or ILBr/Py on an SPS gel and thermogravimetry traces of SPS/Py/[C4py]Br gels; and frequency dependence of the loss elastic modulus (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ishihara N.; Seimiya T.; Kuramoto M.; Uoi M. Crystalline syndiotactic polystyrene. Macromolecules 1986, 19, 2464–2465. 10.1021/ma00163a027. [DOI] [Google Scholar]
- Milano G.; Guerra G. Understanding at molecular level of nanoporous and co-crystalline materials based on syndiotactic polystyrene. Prog. Mater. Sci. 2009, 54, 68–88. 10.1016/j.pmatsci.2008.07.001. [DOI] [Google Scholar]
- Guerra G.; Daniel C.; Rizzo P.; Tarallo O. Advanced materials based on polymer cocrystalline forms. J. Polym. Sci., Part B: Polym. Phys. 2012, 50, 305–322. 10.1002/polb.23035. [DOI] [Google Scholar]
- Gowd E. B.; Tashiro K.; Ramesh C. Structural phase transitions of syndiotactic polystyrene. Prog. Polym. Sci. 2009, 34, 280–315. 10.1016/j.progpolymsci.2008.11.002. [DOI] [Google Scholar]
- Guenet J. M.Polymer–Solvent Molecular Compounds; Elsevier: Amsterdam, 2008. [Google Scholar]
- Kobayashi M.; Nakaoki T.; Ishihara N. Molecular conformation in glasses and gels of syndiotactic and isotactic polystyrenes. Macromolecules 1990, 23, 78–83. 10.1021/ma00203a015. [DOI] [Google Scholar]
- Daniel C.; Guerra G.; Musto P. Clathrate Phase in Syndiotactic Polystyrene Gels. Macromolecules 2002, 35, 2243–2251. 10.1021/ma011531q. [DOI] [Google Scholar]
- van Hooy-Corstjens C. S. J.; Magusin P. C. M. M.; Rastogi S.; Lemstra P. J. A Comparative Study on Gels and Clathrates of Syndiotactic Polystyrene: Solvent Mobility in Polymer–Solvent Compound. Macromolecules 2002, 35, 6630–6637. 10.1021/ma012211+. [DOI] [Google Scholar]
- Daniel C.; Deluca M. D.; Guenet J.-M.; Brûlet A.; Menelle A. Thermoreversible gelation of syndiotactic polystyrene in benzene. Polymer 1996, 37, 1273–1280. 10.1016/0032-3861(96)80854-8. [DOI] [Google Scholar]
- Petraccone V.; Tarallo O.; Venditto V.; Guerra G. An intercalate molecular complex of syndiotactic polystyrene. Macromolecules 2005, 38, 6965–6971. 10.1021/ma051075w. [DOI] [Google Scholar]
- Malik S.; Rochas C.; Guenet J. M. Thermodynamic and structural investigations on the different forms of syndiotactic polystyrene intercalates. Macromolecules 2006, 39, 1000–1007. 10.1021/ma051852s. [DOI] [Google Scholar]
- De Rosa C.; Guerra G.; Petraccone V.; Pirozzi B. Crystal structure of the emptied clathrate form (δe form) of syndiotactic polystyrene. Macromolecules 1997, 30, 4147–4152. 10.1021/ma970061q. [DOI] [Google Scholar]
- Milano G.; Venditto V.; Guerra G.; Cavallo L.; Ciambelli P.; Sannino D. Shape and Volume of Cavities in Thermoplastic Molecular Sieves Based on Syndiotactic Polystyrene. Chem. Mater. 2001, 13, 1506–1511. 10.1021/cm001089a. [DOI] [Google Scholar]
- Itagaki H.; Tokami T.; Mochizuki J. A trial to clarify a cause of forming physical gels: Morphology of syndiotactic polystyrene in n-alkylbenzene. Polymer 2012, 53, 5304–5312. 10.1016/j.polymer.2012.09.025. [DOI] [Google Scholar]
- Mochizuki J.; Tokami T.; Kusuki S.; Sano T.; Itagaki H. Physical gels of syndiotactic polystyrene with fragrant molecules. Macromol. Chem. Phys. 2013, 214, 1912–1920. 10.1002/macp.201300089. [DOI] [Google Scholar]
- Mochizuki J.; Sano T.; Tokami T.; Itagaki H. Decisive properties of solvent able to form gels with syndiotactic polystyrene. Polymer 2015, 67, 118–127. 10.1016/j.polymer.2015.04.042. [DOI] [Google Scholar]
- Welton T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. 10.1021/cr980032t. [DOI] [PubMed] [Google Scholar]
- Wasserscheid P.; Keim W. Ionic Liquids—New “Solutions” for Transition Metal Catalysis. Angew. Chem., Int. Ed. 2000, 39, 3772–3789. . [DOI] [PubMed] [Google Scholar]
- Wilkes J. S. A short history of ionic liquids—from molten salts to neoteric solvents. Green Chem. 2002, 4, 73–80. 10.1039/b110838g. [DOI] [Google Scholar]
- Plechkova N. V.; Seddon K. R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. 10.1039/b006677j. [DOI] [PubMed] [Google Scholar]
- A Ueki T.; Watanabe M. Macromolecules in Ionic Liquids: Progress, Challenges, and Opportunities. Macromolecules 2008, 41, 3739–3749. 10.1021/ma800171k. [DOI] [Google Scholar]; B Watanabe M. Design and materialization of ionic liquids based on an understanding of their fundamental properties. Electrochemistry 2016, 84, 642–653. 10.5796/electrochemistry.84.642. [DOI] [Google Scholar]
- Dembna A.; Venditto V.; Albunia A. R.; Califano R.; Guerra G. Sulfonated Syndiotactic Polystyrene: sorption of ionic liquid in the amorphous phase and of organic guests in the crystalline phase. Polym. Adv. Technol. 2013, 24, 56–61. 10.1002/pat.3049. [DOI] [Google Scholar]
- Raut P.; Liang W.; Chen Y.-M.; Zhu Y.; Jana S. C. Syndiotactic Polystyrene-Based Ionogel Membranes for High Temperature Electrochemical Applications. ACS Appl. Mater. Interfaces 2017, 9, 30933–30942. 10.1021/acsami.7b09155. [DOI] [PubMed] [Google Scholar]
- 1-Butylpyridinium bromide without 1 wt % of water was not so soluble with pyridine and pyrrolidine.
- Reinert L.; Batouche K.; Lévêque J.-M.; Muller F.; Bény J.-M.; Kebabi B.; Duclaux L. Adsorption of imidazolium and pyridinium ionic liquids onto montmorillonite: Characterisation and thermodynamic calculations. Chem. Eng. J. 2012, 209, 13–19. 10.1016/j.cej.2012.07.128. [DOI] [Google Scholar]
- Acocella M. R.; Rizzo P.; Daniel C.; Tarallo O.; Guerra G. Nanoporous triclinic δ modification of syndiotactic polystyrene. Polymer 2015, 63, 230–236. 10.1016/j.polymer.2015.02.058. [DOI] [Google Scholar]
- The fraction of water was not determined by our TG/DTA charts because of the small amount of water added and the strong interaction between pyridine and water.
- Kataoka T.; Ishioka Y.; Mizuhata M.; Minami H.; Maruyama T. Highly Conductive Ionic-Liquid Gels Prepared with Orthogonal Double Networks of a Low-Molecular-Weight Gelator and Cross-Linked Polymer. ACS Appl. Mater. Interfaces 2015, 7, 23346–23352. 10.1021/acsami.5b07981. [DOI] [PubMed] [Google Scholar]
- McKenna G. B.; Guenet J.-M. The effects of solvent type on the concentration dependence of the compression modulus of thermoreversible isotactic polystyrene gels. J. Polym. Sci., Part B: Polym. Phys. 1988, 26, 267–276. 10.1002/polb.1988.090260205. [DOI] [Google Scholar]
- Daniel C.; Dammer C.; Guenet J.-M. On the definition of thermoreversible gels: the case of syndiotactic polystyrene. Polymer 1994, 35, 4243–4246. 10.1016/0032-3861(94)90604-1. [DOI] [Google Scholar]
- To be exact, this conductivity was measured for ILBr, which included 1 wt % of water.
- We prepared the solutions of Py and [C4py]Br by mixing Py and ILBr, which contained 1 wt % of water. DTA measurements at a scan rate of 10 °C/min did not show any peak of water, although the sharp peak due to [C4py]Br appeared around 104 °C. Thus, we judged that water molecules had strong interaction with Py molecules so that their roles were involved in the Py phases.
- Itagaki H.; Mochizuki J. Size and Distribution of Free Volume in Thermoreversible Gels of Syndiotactic Polystyrene. Macromolecules 2005, 38, 9625–9630. 10.1021/ma051743d. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.