Abstract
Objectives The aim of this study was to describe the process of regeneration of damaged salivary glands due to ionizing radiations by bone marrow mesenchymal stem cells (BM-MSCs) transplantation that have been given hypoxic preconditioning with 1% O 2 concentration.
Materials and Methods Stem cell culture was performed under normoxic (O 2 : 21%) and hypoxic conditions by incubating the cells for 48 hours in a low oxygen tension chamber consisting of 95% N 2 , 5% CO 2 , and 1% O 2 . Thirty male Wistar rats were divided into four groups: two groups of control and two groups of treatment. A single dose of 15 Gy radiation was provided to the ventral region of the neck in all treatment groups, damaging the salivary glands. BM-MSCs transplantation was performed in the treatment groups for normoxia and hypoxia 24-hour postradiation.
Statistical Analysis Statistical analysis was done using normality test, followed by MANOVA test ( p < 0.05).
Results There was a significant difference in the expression of binding SDF1-CXCR4, Bcl-2 ( p < 0.05) and also the activity of the enzyme α-amylase in all groups of hypoxia.
Conclusion BM-MSCs transplantation with hypoxic precondition increases the expression of binding SDF1-CXCR4, Bcl-2 that contributes to cell migration, cell survival, and cell differentiation.
Keywords: bone marrow mesenchymal stem cells, hypoxic precondition, salivary gland defect, SDF1-CXCR4, Bcl-2, α-amylase
Introduction
Salivary gland is one of the normal tissues frequently affected by the head and neck radiation therapy. Irreversible salivary gland defect frequently occurs due to the radiation exposure. The salivary gland damage results in the decrease of saliva production and in a very severe condition called xerostomia. 1 Following irradiation-induced, irreversible hyposalivation often occur because the stem cell sterilization of the primitive salivary glands. 2 It is required to apply an alternative approach to treat the severe damage of glands and the left tissue. One of the alternative approaches for this purpose is the stem cell therapy.
Some factors that affect the success of stem cell therapy include the following: stem cells strongly attach and survive in the defect area and they can integrate with the surrounding microenvironment. 3 However, considering the low amount of survived cells, which shows the low viability of the transplanted stem cells, the effectiveness of stem cell therapy decreases. The low rate of viability is probably due to the conventional method of culture, which was done under normoxic condition with 21% O 2. It is contrary to the in vitro environment, which is a hypoxic condition with 1 to 7% O 2 , 4 depending on the stem cell location and type. Therefore, it is assumed that the same condition as the micro environment is necessary, to get a desirable result regarding the viability of the transplanted stem cells in the injured tissue and is expected to allow the stem cells proliferation and differentiation into the origin-like cells. 5 In addition to the appropriate microenvironment, there are others factors that also play a role in the success of stem cell therapy, the factors that can induce stem cells to migrate into the defect area. One of the mediators that plays an important role in the migration process into the defect area is stromal derived-cell factor 1 (SDF1) through binding with CXCR4 receptor. 6
The purpose of this study was to explain the mechanism of regeneration of salivary gland defect due to ionizing radiations by BM-MSCs transplantation that has been given hypoxic preconditioning with 1% of O 2 concentration.
Materials and Methods
Ethical Approval
All animal studies were performed via a protocol approved by the Institutional Animal Care and Use Committee of Faculty of Veterinary Medicine, Universitas Airlangga, and complied with the National Research Council's guidelines (366-KE) through ethical seminar.
Salivary Gland Damage in Animal Model
Damage to salivary glands in a healthy male Wistar rat was induced by single dose of 15 Gy radiation in the ventral region of the neck. Rats used in this study were 3 to 4 months old and each with 250 to 300 g weight. Rats were kept in an individual plastic cage in the laboratory for Experimental Animal of Institute of Tropical Disease, Universitas Airlangga with adequate ventilation.
Treatment
This research was a true experimental posttest control group design. The bone marrow mesenchymal stem cells (BM-MSCs) were isolated from the femur of Wistar male rats. Stem cell culture was divided into two conditions: normoxic (O 2 : 21%) and hypoxic conditions for 48 hours of incubation in a low oxygen tension chamber consisting of 95% N 2, 5% CO 2 , and 1% O 2 . Forty male Wistar rats were divided into four groups: two groups of control and two groups with damage, each with 10 replicates. They are as follows:
The negative control group (T0–): rats with normal salivary glands (not irradiated) and without MSCs treatment.
The positive control group (T0+): rats with damaged salivary glands and without MSCs treatment.
The treatment Group 1 (T1): rats with damaged salivary glands, given MSCs transplant under normoxia 24-hour postradiation.
The treatment Group 2 (T2): rats with damaged salivary glands, given MSCs transplant under hypoxia 24-hour postradiation.
The regeneration process of salivary gland damage was determined after 4 weeks post BM-MSCs transplantation by studying the expression of several chemokines and proteins, such as binding of SDF1-CXCR4, Bcl-2 on the tissue by immunohistochemistry methods and the activity of enzyme α-amylase produced by acinar cells through enzyme-linked immunosorbent assay (ELISA) activity as a marker of salivary gland regeneration process.
Statistical Analysis
Data were analyzed statistically with normality test and MANOVA test using Statistical Package for the Social Sciences (SPSS) 17.0 (IBMTM, Chicago, Illinois, United States).
Immunohistochemical Methods for Observation of SDF1-CXCR4 and Bcl-2
Immunohistochemistry (IHC) was performed to determine the expression of SDF1-CXCR4 and Bcl - 2. 7 First, salivary glands were transversely incised from paraffin blocks. Monoclonal antibodies, namely anti-SDF1-CXCR4 and anti-Bcl-2 , were used in this technique. Samples were observed under a light microscope at a magnification of 200× to check SDF1-CXCR4 and Bcl-2 expression. The expression of each variable was described by the number of cells with brown discoloration due to diaminobenzidine (DAB-chromogen) in each incision. 8
Histological Observation of Salivary Glands
Light microscopy examination was performed to study salivary gland histology and regenerate acinar cells. After that, samples were prepared for histology in the following steps: submandibular gland of rats was fixed in 10% formalin buffer, followed by dehydration using a series of alcohol, from 70, 80, 90, to 96% (absolute). Gland tissues of rats were cleared in a xylene solution. The tissues were infiltrated with an embedding agent, the liquid paraffin. Microtome was set with a distance at 4 to 6 µ for sectioning, and the sections were placed on a slide. The embedding process must be reversed to get the paraffin wax out of the tissue and allow water soluble dyes to penetrate the sections. Therefore, the slides are “deparaffinized” by washing them through xylenes to alcohols to water before any staining can be done. The routine H&E staining was performed. After that, Canada balsam was used to mount the stained section and covered with a coverslip. Submandibular gland and acinar cell regenerations are observed and identified based on the histological measures of that of the normal tissue.
Results
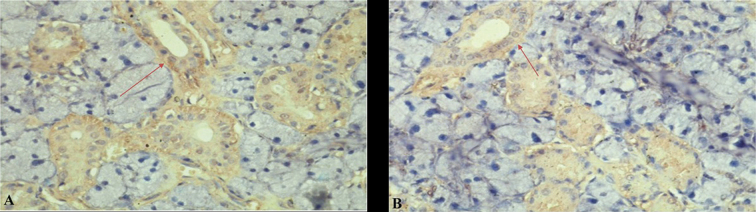
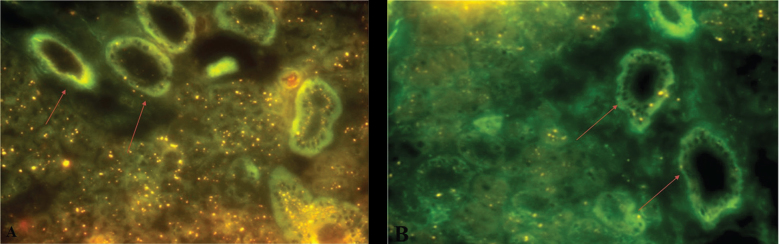
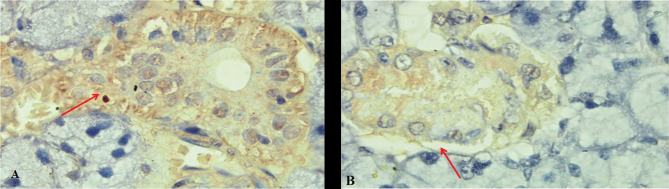
The IHC examination results showed that in the hypoxic group the expression of binding SDF1-CXCR4 and Bcl - 2, and α-amylase enzyme increased significantly than in the normoxia group. The mean of SDF-1 and CXCR4 expression significantly expressed in hypoxic preconditioning group than in the normoxic group. A significant difference ( p < 0.05) between normoxia and hypoxia group with SDF1-CXCR4 binding also showed in MANOVA test ( Table 1 ). It can be seen from comparison of CXCR4 (brown chromogen) expression between treatments that hypoxic group in acute conditions showed mainly CXCR4 expression (that was stronger than the expression in the normoxia group ( Fig. 1 ). It also can be seen from the green color of the microscopic image of salivary gland tissue that occupies most of the ductal basal membrane. The distribution of BM-MSCs, as depicted by labeled PKH 26, shows that green coloration is stronger in hypoxic groups than in the normoxic group ( Fig. 2 ). The mean of Bcl-2 expression was significantly expressed in hypoxic preconditioning group than in the normoxic group; the MANOVA test results indicated a significant difference ( p < 0.05; Table 2 ). It can be seen from a comparison of the expression of Bcl-2 (brown chromogen) between treatments that groups with acute hypoxia showed stronger Bcl - 2 expression compared with the acute normoxia group ( Fig. 3 ). Furthermore, there were significant differences in MANOVA test ( p < 0.05) of α-amylase expression between the treatment groups. The acute hypoxia group showed a significant increase in α-amylase enzyme activity compared with that in the acute normoxia group ( Table 3 ).
Table 1. Average value and standard deviation (SD) expression of SDF1-CXCR4.
| Groups | SDF1-CXCR4 | p- Value | |
|---|---|---|---|
| Mean | SD | ||
| Abbreviation: SD, standard deviation. | |||
| Negative control group (T0–) | 1.640 | 0.555 | 0.000 |
| Positive control group (T0+) | 1.160 | 0.219 | |
| Treatment group 1 (T1) | 2.920 | 0.540 | |
| Treatment group 2 (T2) | 3.720 | 1.825 | |
Fig. 1.
Comparison of CXCR4 (brown chromogen) expression between treatments. In the slide it appears that hypoxic group in acute conditions showed mainly CXCR4 expression ( A ) that was stronger than that in the normoxic group ( B ). Immunohistochemical staining, 400× magnification; Nikon H600L microscope; 300-megapixel camera DS Fi2.
Fig. 2.
Microscopic images of BM-MSCs labeled using PKH 26. The green light in the image shows the distribution of labeled BM-MSCs. Hypoxic condition ( A ) , normoxic condition ( B ).
Table 2. Average values and standard deviations of expression of Bcl-2.
| Groups | Bcl-2 | p- Value | |
|---|---|---|---|
| Mean | SD | ||
| Abbreviation: SD, standard deviation. Note: p < 0.05 is significant. | |||
| Negative control group (T0–) | 23.600 | 5.176 | 0.000 |
| Positive control group (T0+) | 4.000 | 1.870 | |
| Treatment group 1 (T1) | 5.400 | 1.140 | |
| Treatment group 2 (T2) | 11.800 | 1.303 | |
Fig. 3.
Comparison of the expression of Bcl-2 (brown chromogen) between treatments. In the slide, it appears that groups with acute hypoxia ( A ) showed stronger Bcl-2 expression compared with those in the acute normoxic group ( B ) . Immunohistochemical staining, 400× magnification; Nikon H600L microscope; 300-megapixel camera DS Fi2.
Table 3. Average values and standard deviations of α-amylase enzyme expression.
| Groups | α-amylase | p -Value | |
|---|---|---|---|
| Mean | SD | ||
| Abbreviation: SD, standard deviation. Note: p < 0.05 is significant. | |||
| Negative control group (T0–) | 289,259.000 | 18,645.313 | 0.000 |
| Positive control group (T0+) | 95,330.400 | 31,503.973 | |
| Treatment group 1 (T1) | 152,088.200 | 6,434.510 | |
| Treatment group 2 (T2) | 186,118.400 | 5,971.156 | |
Discussion
Stem cell transplantation is one of the promising therapies that is predicted to restore the function of the salivary gland damage by regenerating acinar cells. Stem cell therapy can be successful in addition to the need for adaptive stem cells and when transplanted stem cells are attached and integrated with their niche. Stem cell niches are needed to increase and manage the viability of stem cells transplanted in the damaged area. They have the same microenvironment as the physiological micro-conditions of the original cell and can support stem cells to proliferate and differentiate into the original cell. 4 However, there are problems that still exist in cell-based therapies, namely the delivery cell process or cell migration to the area of injury or what is commonly called homing. It is suspected that several proteins and chemokines play a role in the migration process into the damaged area, such as stromal derived-cell factor 1 (SDF1) through binding with CXCR4 receptor. 1
In the previous study, effect of hypoxic preconditions on the expression of CXCR4 and SDF-1 was studied using IHC. The results showed that 1% hypoxic preconditioning treatment significantly increased CXCR4 and SDF-1 expression, which play an important role in increasing the ability of MSCs to migrate to the damaged area and induce endogenous stem cells to proliferate and differentiate into the desired cells. 9 Thus, it is expected that there will be a process of improving the microenvironment of the resident stem cells, which will eventually lead to the regeneration process. In this study, transplantation of adaptive MSCs was administered by direct injection into salivary glands of Wistar rats damaged by exposure to 15 Gy of single dose ionizing radiations.
The results of this study indicate that BM-MSCs cells that have been given hypoxic preconditioning have better therapeutic ability than those under normoxic conditions so that they can induce cell repair processes. It can be seen from the green color of the microscopic image of salivary gland tissue that occupies most of the ductal basal membrane. This shows the migration of BM-MSCs in the basal membrane of the ducts and acinar cells which are heavily damaged by radiation exposure. The distribution of PKH 26-labeled BM-MSCs shows that green coloration is stronger in hypoxic group than in the normoxic group. The IHC examination results in the hypoxic group showed that the expression of binding SDF1-CXCR4 and Bcl - 2 increased significantly than in the normoxic group.
A recent study states that the activation of SDF-1-CXCR4 bonds in tissues plays a role in the transduction of various signals that can regulate several biological functions, such as cell survival, proliferation, chemotaxis, and cell differentiation, which is in line with result of this study. One of the main functions of SDF1-CXCR4 bond is regulating the trafficking of BM-MSCs cells in during homing in the injured area. 10
Furthermore, the MANOVA test results showed that there were significant differences in the expression of α-amylase between the treatment groups. The hypoxia group showed a significant increase in the activity of α-amylase enzyme compared with that in the acute normoxic group after transplantation. The results of this study indicate the regeneration of the damaged salivary glands. 11
All these have shown the influential effect of low O 2 concentration on MSCs biology and raised serious concern over its therapeutic efficiency and biosafety. 12 Environmental stress to the in vitro cultured MSCs can be caused by higher O 2 concentration. Moreover, in recent years after many transplantation studies, early senescence, longer population doubling time, DNA damage, 13 and poor engraftment have presented clear evidence regarding the negative influence of ambient O 2 concentration on MSCs. 14 A large number of number of studies suggest that hypoxia activates several transcription factors in the nucleus, such as HIF-1α and NFκβ, and these factors interact with paracrine factors such as MEK and PI3K/Akt. 15 All these interactions increase the secretion of several growth factors, such as stromal-derived factor 1 (SDF-1), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF) with increased expression of each receptor such as CXCR4, increased secretion of some anti apoptotic proteins, such as Bcl-2 and Bcl-xL as a survival factor. 16
Conclusion
This study concluded that (1) BM-MSCs transplantation with hypoxic preconditioning of 1% O 2 increases the expression of SDF1-CXCR4 and Bcl-2 that contribute to cell migration and cell survival. (2) BM-MSCs can improve regeneration process of the damaged salivary glands by increasing the activity of the α-amylase as a marker of regeneration process in the salivary gland tissue.
Acknowledgments
The authors would like to thank Universitas Airlangga, Institute of Tropical Disease, Stem Cell Division, Faculty of Dental Medicine and Stem Cell and Tissue Bank Center RSUD Dr. Soetomo, Surabaya, Indonesia.
Footnotes
Conflict of Interest None declared.
References
- 1.Chambers M S, Garden A S, Kies M S, Martin J W. Radiation-induced xerostomia in patients with head and neck cancer: pathogenesis, impact on quality of life, and management. Head Neck. 2004;26(09):796–807. doi: 10.1002/hed.20045. [DOI] [PubMed] [Google Scholar]
- 2.Lombaert I M, Brunsting J F, Wierenga P K. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3(04):e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponnaiyan D, Jegadeesan V. Comparison of phenotype and differentiation marker gene expression profiles in human dental pulp and bone marrow mesenchymal stem cells. Eur J Dent. 2014;8(03):307–313. doi: 10.4103/1305-7456.137631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohni A, Verfaillie C M. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;13:1–10. doi: 10.1155/2013/130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estrada J C, Albo C, Benguría A. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012;19(05):743–755. doi: 10.1038/cdd.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin C Y, Chang F H, Chen C Y. Cell therapy for salivary gland regeneration. J Dent Res. 2011;90(03):341–346. doi: 10.1177/0022034510386374. [DOI] [PubMed] [Google Scholar]
- 7.Rantam F A, Ferdiansyah M, Purwati A. 2nd ed. Surabaya, Indonesia: Airlangga University Press; 2014. Stem Cell Mesenchymal, Hematopoetik dan Model Aplikasi; pp. 145–155. [Google Scholar]
- 8.Crosby K, Simendinger J, Grange C, Ferrante M, Bernier T, Stanen C.Immunohistochemistry protocol for paraffin-embedded tissue section - advertisement. Cell Signal Technol 2016Available at:https://www.jove.com/video/5064/immunohistochemistryprotocol-for-paraffin-embedded-tissue-sections. Accessed on June 15, 2016
- 9.Mulyani S WM, Ernawati D S, Astuti E R, Rantam F A. Hypoxic preconditioning effect on stromal cells derived factor-1 and C-X-C chemokine receptor type 4 expression in Wistar rat's ( Rattus norvegicus ) bone marrow mesenchymal stem cells ( in vitro study) . Vet World. 2018;11(07):965–970. doi: 10.14202/vetworld.2018.965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haque N, Rahman M T, Abu Kasim NH, Alabsi A M. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. ScientificWorldJournal. 2013;2013:632972. doi: 10.1155/2013/632972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim J Y, Ra J C, Shin I S. Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage. PLoS One. 2013;8(08):e71167. doi: 10.1371/journal.pone.0071167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng J, van der Zwaag M, Stokman M A, van Os R, Coppes R P. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol. 2009;92(03):466–471. doi: 10.1016/j.radonc.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Mohamadnejad M, Pournasr B, Bagheri M. Transplantation of allogeneic bone marrow mesenchymal stromal cell-derived hepatocyte-like cells in homozygous familial hypercholesterolemia. Cytotherapy. 2010;12(04):566–568. doi: 10.3109/14653240903511143. [DOI] [PubMed] [Google Scholar]
- 14.Greijer A E, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57(10):1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alcarez T M, Naranjo S S, Jimenez C. Hypoxic induces the activation of the PI3K/Akt cell survival pathway in PC12 cells – protective role in apoptosis. J Biol Chem. 2001;276:22368–22374. doi: 10.1074/jbc.M011688200. [DOI] [PubMed] [Google Scholar]
- 16.Kagami H, Wang S, Hai B. Restoring the function of salivary glands. Oral Dis. 2008;14(01):15–24. doi: 10.1111/j.1601-0825.2006.01339.x. [DOI] [PubMed] [Google Scholar]