Key Points
Question
Does the oral Janus kinase 1 selective inhibitor abrocitinib improve atopic dermatitis signs and symptoms in adults with moderate to severe atopic dermatitis at doses that are well tolerated?
Findings
In this randomized, double-blinded, phase 2b clinical trial including 267 participants, the proportion of patients achieving substantial improvement from baseline was significantly greater for those receiving 200 mg and 100 mg of abrocitinib compared with placebo. Dose-related decreases in platelet count were observed for all doses greater than 10 mg, but platelet values trended upward toward baseline after the maximum decrease at week 4 and despite ongoing treatment with abrocitinib; most adverse events were mild and considered unrelated to treatment.
Meaning
The findings of this study show that 12 weeks of once-daily treatment with 200 mg or 100 mg of oral abrocitinib resulted in significant improvement in the signs and symptoms of atopic dermatitis.
Abstract
Importance
Atopic dermatitis is associated with substantial patient and caregiver burden. Currently available treatments for atopic dermatitis are inadequate or contraindicated for some patients. Abrocitinib (PF-04965842) is an oral Janus kinase 1 selective inhibitor under investigation for the treatment of atopic dermatitis.
Objective
To investigate the efficacy and safety of abrocitinib for patients with moderate to severe atopic dermatitis.
Design, Setting, and Participants
A phase 2b, randomized, double-blinded, placebo-controlled, parallel-group trial was conducted from April 15, 2016, to April 4, 2017, at 58 centers in Australia, Canada, Germany, Hungary, and the United States among 267 patients 18 to 75 years of age with a clinical diagnosis of moderate to severe atopic dermatitis for 1 year or more and inadequate response or contraindication to topical medications for 4 weeks or more within 12 months. Efficacy was assessed in the full analysis set, which was a modified intention-to-treat population that included all patients who received 1 dose or more of the study drug except for 4 patients from 1 site.
Interventions
Participants were randomly assigned 1:1:1:1:1 to receive abrocitinib (200 mg, 100 mg, 30 mg, or 10 mg) or placebo once daily for 12 weeks.
Main Outcomes and Measures
The primary outcome was the proportion of patients achieving an Investigator’s Global Assessment of clear (0) or almost clear (1) with an improvement from baseline of 2 grades or more at week 12. The secondary outcome was the percentage change from baseline in the Eczema Area and Severity Index at week 12.
Results
Of the 267 participants, 144 were women (mean [SD] age, 40.8 [16.1] years). At week 12, 21 of 48 patients receiving 200 mg of abrocitinib (43.8%; P < .001, 2-sided), 16 of 54 patients receiving 100 mg of abrocitinib (29.6%; P < .001), and 3 of 52 patients receiving placebo (5.8%) achieved grades of clear or almost clear on the Investigator’s Global Assessment scale with improvement of 2 grades or more; these rates correspond to maximum effect model-based estimates of 44.5% (95% CI, 26.7%-62.3%) for those receiving 200 mg of abrocitinib, 27.8% (95% CI, 14.8%-40.9%) for those receiving 100 mg of abrocitinib, and 6.3% (95% CI, −0.2% to 12.9%) for those receiving placebo. Reductions in the Eczema Area and Severity Index were 82.6% (90% CI, 72.4%-92.8%; P < .001) for those receiving 200 mg of abrocitinib, 59.0% (90% CI, 48.8%-69.3%; P = .009) for those receiving 100 mg of abrocitinib, and 35.2% (90% CI, 24.4%-46.1%) for those receiving placebo. Adverse events were observed in 184 of 267 patients (68.9%); the most frequently reported adverse events (in ≥3 patients in any group) were dermatitis atopic, upper respiratory tract infection, headache, nausea, and diarrhea. Dose-dependent decreases in platelet count were observed but trended upward toward baseline levels after week 4.
Conclusions and Relevance
Once-daily oral abrocitinib was effective and well tolerated for short-term use in adults with moderate to severe atopic dermatitis. Additional trials are necessary to evaluate long-term efficacy and safety.
Trial Registration
ClinicalTrials.gov identifier: NCT02780167
This randomized clinical trial investigates the efficacy and safety of once-daily oral abrocitinib, a Janus kinase 1 selective inhibitor, for patients with moderate to severe atopic dermatitis.
Introduction
Atopic dermatitis (AD) is a common chronic inflammatory skin disease characterized by intense pruritus and eczematous lesions.1 The burden associated with the disease is substantial, encompassing physical, psychological, social, and economic costs.2 Although emollients and topical anti-inflammatory agents are the cornerstone of AD treatment, they are often insufficient for individuals with moderate to severe disease.3,4 Phototherapy and systemic corticosteroids are treatment options, but the former is limited by accessibility, and the latter is not recommended because of safety concerns.5 Systemic immunosuppressants may be prescribed but are off-label in many countries and are associated with considerable adverse effects that limit treatment duration.3,5 Dupilumab was recently approved in several countries for treatment of moderate to severe AD.6,7 Although this is an important addition to the treatment landscape, some patients do not respond adequately, and others are unwilling or unable to receive subcutaneous injections. Consequently, there remains a need for alternative therapies for AD.
The Janus kinase (JAK) family is a group of cytoplasmic tyrosine kinases that mediate signaling pathways activated by various cytokines.8,9 Relevant to the pathophysiologic characteristics of AD, JAK1 plays a role in type 2 helper T-cell differentiation via interleukin 4 (IL-4) and thymic stromal lymphopoietin signaling (eFigure 1 in Supplement 1).10 Mounting evidence indicates that JAK inhibitors may be effective for the treatment of AD. Positive results have been reported for topical and oral JAK inhibitors for the treatment of moderate to severe AD.11,12,13,14
Abrocitinib (PF-04965842) is an oral JAK1 selective inhibitor that inhibits several key cytokine signaling pathways known to have an important role in the pathophysiologic characteristics of AD, including IL-4, IL-13, IL-31, and interferon γ (eFigure 1 in Supplement 1). The objective of this study was to demonstrate the effectiveness of JAK1 selective inhibition for the management of AD by evaluating the efficacy and safety of abrocitinib (200 mg, 100 mg, 30 mg, and 10 mg) compared with placebo given once daily in adults with moderate to severe AD. Investigator-reported outcomes are reported here; patient-reported outcomes will be included in a future publication.
Methods
Study Design and Participants
This phase 2b, multicenter, randomized, double-blinded, placebo-controlled, parallel-group study was conducted between April 15, 2016, and April 4, 2017, at 58 centers in Australia, Canada, Germany, Hungary, and the United States (NCT02780167) (trial protocol in Supplement 2). A 35-day screening period was followed by a 12-week double-blinded, placebo-controlled treatment period and a 4-week follow-up (eFigure 2 in Supplement 1). The study was conducted in compliance with the Declaration of Helsinki15 and all International Council for Harmonization Good Clinical Practice Guidelines. All local regulatory requirements were followed. This research was approved by institutional review boards or ethics committees at each study site (eTable 1 in Supplement 1). All patients provided written informed consent.
Eligible patients were men or women aged 18 to 75 years with a clinical diagnosis of moderate to severe AD (percentage of affected body surface area [%BSA] ≥10; Investigator’s Global Assessment [IGA] score ≥3; and Eczema Area and Severity Index [EASI] score ≥12) for 1 year or more before day 1 of the study and inadequate response to topical medications (topical corticosteroids or topical calcineurin inhibitors) for 4 weeks or more (based on investigator’s judgment) or inability to receive topical treatment within 12 months before the first dose of study drug because it was medically inadvisable (eg, application to a large %BSA, which is associated with increased risk for systemic absorption and suppression of the hypothalamic-pituitary-adrenal axis, and cutaneous adverse effects such as burning or stinging sensations with topical calcineurin inhibitors or skin atrophy, purpura, telangiectasia, and striae with chronic use of topical corticosteroids). Patients who had used topical corticosteroids or topical calcineurin inhibitors within 1 week of the first dose of study drug were excluded (see eAppendix in Supplement 1 for detailed eligibility criteria). Patients were permitted to use oral antihistamines and nonmedicated emollient (CeraVe lotion [CeraVe]; or Aquaphor [Beiersdorf Inc]) and sunscreen (both provided by the sponsor) during the study. Patients who received prohibited systemic or topical medication for AD before week 12 were discontinued from treatment.
Randomization and Masking
Patients were randomly assigned (1:1:1:1:1) to receive oral abrocitinib (200, 100, 30, or 10 mg) once daily or placebo once daily for 12 weeks. Randomization was via an interactive response technology system. Blinded abrocitinib and placebo tablets were delivered to the study sites in blister packs. Patients, investigators, and sponsors were blinded to study treatment.
Outcome Measures
The primary efficacy end point was the proportion of patients who achieved an IGA of clear (0) or almost clear (1) with an improvement from baseline of 2 grades or more at week 12 (eTable 2 in Supplement 1). The key secondary end point was the percentage change from baseline in EASI at week 12. Additional secondary end points were the proportion of patients who achieved an IGA of clear or almost clear with an improvement of 2 grades or more and percentage change from baseline in EASI at time points other than week 12 and the proportions of patients who achieved 50% or more improvement in EASI (EASI-50), 75% or more improvement in EASI (EASI-75), and 90% or more improvement in EASI (EASI-90); improvement from baseline in pruritus numeric rating scale (NRS) scores; change from baseline in %BSA; and percentage change from baseline in Scoring AD index scores at all time points. Photographs of treatment-eligible AD lesions were obtained for patients at selected study sites at day 1, week 6, and week 12. Photographic services were provided through a central photography vendor (Canfield Scientific Inc), and detailed procedures were provided to ensure consistency. Safety was assessed by monitoring treatment-emergent adverse events (TEAEs), vital signs, and laboratory test results throughout the study.
Statistical Analysis
A sample size of 250 patients was determined to provide approximately 95% power to detect a 33% difference between abrocitinib and placebo with a 1-sided test at a significance level of 0.0125, assuming the placebo response rate was approximately 10%. A conservative approach was adopted by reporting 2-sided P values with a significance level of 0.05. Safety was assessed in the safety analysis set, which included all randomized patients who received 1 dose or more of the study drug. Efficacy was assessed in the full analysis set, which was a modified intention-to-treat population that included all patients who received 1 dose or more of the study drug except for 4 patients from 1 site. Patients from this site were excluded before unmasking the database because of major protocol deviations, which included multiple monitoring findings that revealed lack of principal investigator oversight and study execution issues.
The primary end point was analyzed using simple differences in observed proportions, using normal approximation. Response rates were estimated using a 3-parameter maximum effect model to characterize the dose-response relationship.16 Patients who discontinued treatment or received a prohibited medication for AD before week 12 were considered nonresponders. Although they were encouraged to continue in the follow-up, most withdrew from the trial. For continuous secondary end points, the primary analysis was performed using the mixed-effects model repeated measure, assuming missing at random. Although it is not possible to definitively demonstrate the missing at random assumption, it appeared to be a reasonable approximation based on the pattern of discontinuations and sensitivity analysis. All binary secondary end points were analyzed using a generalized linear mixed model assuming missing at random. The generalized linear mixed model and mixed-effects model repeated measure included time, treatment, and treatment-by-time interaction as fixed effects and baseline value as a covariate. An unstructured variance-covariance matrix was used to model within-patient variability. Missing values were handled by the generalized linear mixed model and mixed-effects model repeated measure without imputation. Because the generalized linear mixed model did not converge for some binary end points, only logistic regression results are reported. Safety data were summarized descriptively. Multiplicity adjustment was not performed. All analyses were performed in SAS, version 9.4 (SAS Institute Inc) and R, version 3.4.4 (R Foundation for Statistical Computing).17
Results
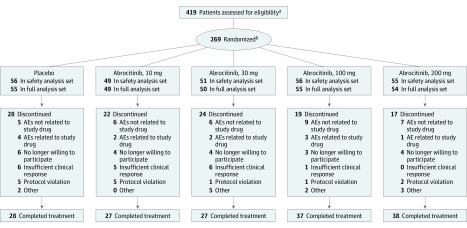
A total of 419 patients were screened, and 267 (safety analysis set) received randomized treatment. Of these, 157 patients (58.8%) completed the study (Figure 1). Discontinuations in the 30-mg abrocitinib, 10-mg abrocitinib, and placebo groups were more frequent than in the 200-mg abrocitinib and 100-mg abrocitinib groups, largely because of insufficient clinical response and use of prohibited medications. The efficacy analysis included 263 patients (full analysis set). Demographics and baseline characteristics were balanced across treatment arms (Table 1).
Figure 1. CONSORT Diagram.
AE indicates adverse event.
aPrimary reasons for screening failure included 30 of 152 patients (19.7%) were not willing or able to comply with study procedures; 19 of 152 patients (12.5%) had evidence of active, latent, or inadequately treated tuberculosis, and 24 of 152 patients (15.8%) had laboratory test result abnormalities.
bTwo randomized patients did not receive study treatment and were, therefore, not included in the safety or full analysis set.
Table 1. Demographic and Baseline Characteristics.
| Characteristic | Placebo (n = 56) | Abrocitinib | |||
|---|---|---|---|---|---|
| 10 mg (n = 49) | 30 mg (n = 51) | 100 mg (n = 56) | 200 mg (n = 55) | ||
| Safety Analysis Set a | |||||
| Age, mean (SD), y | 42.6 (15.1) | 44.3 (15.9) | 37.6 (15.9) | 41.1 (15.6) | 38.7 (17.6) |
| Male sex, No. (%) | 21 (37.5) | 21 (42.9) | 22 (43.1) | 31 (55.4) | 28 (50.9) |
| Race, No. (%) | |||||
| White | 40 (71.4) | 38 (77.6) | 39 (76.5) | 40 (71.4) | 37 (67.3) |
| Black | 10 (17.9) | 5 (10.2) | 4 (7.8) | 7 (12.5) | 13 (23.6) |
| Asian | 4 (7.1) | 5 (10.2) | 5 (9.8) | 8 (14.3) | 5 (9.1) |
| Other | 2 (3.6) | 1 (2.0) | 3 (5.9) | 1 (1.8) | 0 |
| BMI, mean (SD) | 27.1 (5.9) | 28.2 (7.8) | 27.3 (5.6) | 28.0 (6.1) | 28.6 (7.3) |
| Disease duration, median (range), y | 25.6 (1.1-67.1) | 30.2 (1.8-60.6) | 20.5 (1.2-66.6) | 23.8 (1.1-66.7) | 19.6 (1.9-68.8) |
| Full Analysis Set b | (n = 55) | (n = 49) | (n = 50) | (n = 55) | (n = 54) |
| EASI, mean (SD) | 25.4 (12.9) | 28.1 (13.1) | 22.1 (10.7) | 26.7 (11.8) | 24.6 (13.5) |
| %BSA, mean (SD) | 40.1 (22.3) | 44.2 (22.7) | 34.1 (20.8) | 41.9 (22.3) | 38.0 (23.3) |
| IGA grade, No. (%) | |||||
| Moderate (3) | 34 (61.8) | 27 (55.1) | 28 (56.0) | 29 (52.7) | 34 (63.0) |
| Severe (4) | 21 (38.2) | 22 (44.9) | 22 (44.0) | 26 (47.3) | 20 (37.0) |
| SCORAD Index score, mean (SD) | 65.0 (12.1) | 65.3 (13.2) | 62.4 (13.0) | 65.4 (13.7) | 62.7 (13.7) |
| Pruritus NRS score, mean (SD)c | 7.6 (1.8) | 7.6 (1.7) | 7.6 (1.9) | 7.4 (2.2) | 6.9 (2.7) |
Abbreviations: %BSA, percentage of body surface area; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment; NRS, numeric rating scale; SCORAD, Scoring Atopic Dermatitis.
Safety analysis set included all patients who received 1 dose or more of abrocitinib or placebo.
Full analysis set included all patients who received 1 dose or more of abrocitinib or placebo, except for 4 patients who were excluded because of major protocol deviations.
Pruritus NRS data not available for 14 patients (52 patients in placebo group, 45 patients in 10-mg group, 48 patients in 30-mg group, 55 patients in 100-mg group, and 53 patients in 200-mg group).
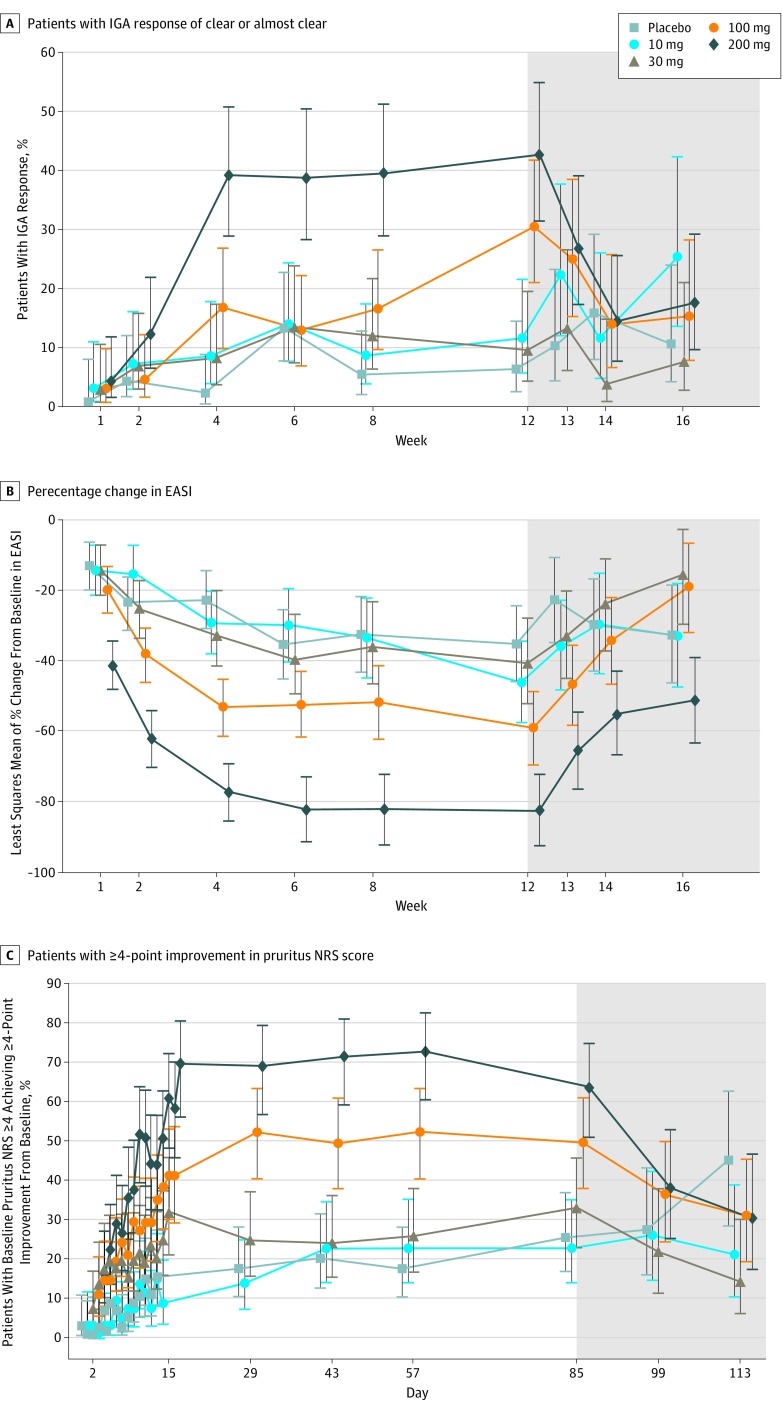
At week 12, 21 of 48 patients receiving 200 mg of abrocitinib (43.8%), 16 of 54 patients receiving 100 mg of abrocitinib (29.6%), 4 of 45 patients receiving 30 mg of abrocitinib (8.9%), 5 of 46 patients receiving 10 mg of abrocitinib (10.9%), and 3 of 52 patients receiving placebo (5.8%) achieved grades of clear or almost clear on the IGA scale with improvement of 2 grades or more from baseline (Table 2 and eFigure 3 in Supplement 1). Significantly greater proportions of patients achieved grades of clear or almost clear on the IGA scale with improvement of 2 grades or more in both the 200-mg and the 100-mg dose groups, compared with placebo. The maximum effect model fit the observed data well (eFigure 3 in Supplement 1), corresponding to rates of 44.5% (95% CI, 26.7%-62.3%) for those receiving 200 mg of abrocitinib, 27.8% (95% CI, 14.8%-40.9%) for those receiving 100 mg of abrocitinib, and 6.3% (95% CI, −0.2% to 12.9%) for those receiving placebo. Reductions in the EASI were 82.6% (90% CI, 72.4%-92.8%) for those receiving 200 mg of abrocitinib, 59.0% (90% CI, 48.8%-69.3%) for those receiving 100 mg of abrocitinib, and 35.2% (90% CI, 24.4%-46.1%) for those receiving placebo. The maximum effect model-estimated placebo-adjusted responses were 38.2% in the 200-mg dose group and 21.5% in the 100-mg dose group, which are close to observed percentages. The proportion of patients who achieved grades of clear or almost clear on the IGA scale with improvement of 2 grades or more in the 30-mg and 10-mg groups were not significantly different from placebo. A dose-dependent response relationship was observed (eFigure 3 in Supplement 1). The proportion of patients achieving grades of clear or almost clear on the IGA scale with improvement of 2 grades or more for the 200-mg group plateaued between weeks 4 and 6 and was maintained through week 12, whereas the proportion for the 100-mg group increased through week 12 (Figure 2A).
Table 2. Efficacy End-Point Summary (Week 12)a.
| Measure | Placebo (n = 52)b | Abrocitinib | |||
|---|---|---|---|---|---|
| 10 mg (n = 46)b | 30 mg (n = 45)a | 100 mg (n = 54)b | 200 mg (n = 48)b | ||
| IGAc | |||||
| Observed response, No. (%) [95% CI] | 3 (5.8) [0.0 to 12.1] | 5 (10.9) [1.9 to 19.9] | 4 (8.9) [0.6 to 17.2] | 16 (29.6) [17.5 to 41.8] | 21 (43.8) [29.7 to 57.8] |
| P value | NA | .36 | .56 | <.001 | <.001 |
| Emax estimated response, % (95% CI)d | 6.3 (–0.2 to 12.9) | 8.2 (2.2 to 14.1) | 12.3 (4.9 to 19.7) | 27.8 (14.8 to 40.9) | 44.5 (26.7 to 62.3) |
| EASI | |||||
| LSM of percentage change from baseline, % (90% CI)e | –35.2 (–46.1 to –24.4) | –31.1 (–42.8 to –19.4) | –40.7 (–52.0 to –29.5) | –59.0 (–69.3 to –48.8) | –82.6 (–92.8 to –72.4) |
| P value | NA | .67 | .56 | .009 | <.001 |
| EASI-50 responders, No. (%) | 14 (26.9) | 12 (26.1) | 15 (33.3) | 30 (55.6) | 38 (79.2) |
| Odds ratio (90% CI)f | NA | 0.96 (0.45 to 2.03) | 1.36 (0.65 to 2.82) | 3.28 (1.66 to 6.48) | 9.67 (4.47 to 20.93) |
| EASI-75 responders, No. (%) | 8 (15.4) | 8 (17.4) | 6 (13.3) | 22 (40.7) | 31 (64.6) |
| Odds ratio (90% CI)f | NA | 1.26 (0.52 to 3.07) | 0.80 (0.31 to 2.06) | 3.86 (1.77 to 8.41) | 9.51 (4.26 to 21.19) |
| EASI-90 responders, No. (%) | 5 (9.6) | 5 (10.9) | 0 | 14 (25.9) | 21 (52.1) |
| Odds ratio (90% CI)f | NA | 1.21 (0.42 to 3.49) | 0.09 (0.01 to 1.07) | 3.18 (1.29 to 7.86) | 9.26 (3.82 to 22.46) |
| Pruritus NRS scoreg | |||||
| ≥4-Point improvement, No. (%) | 13 (25.5) | 10 (22.7) | 15 (33.3) | 25 (50.0) | 28 (63.6) |
| Odds ratio (90% CI)f | NA | 0.86 (0.39 to 1.90) | 1.44 (0.68 to 3.03) | 2.84 (1.40 to 5.76) | 5.11 (2.43 to 10.77) |
| %BSA | |||||
| LSM change from baseline, % (95% CI)e | –13.7 (–18.8 to –8.5) | –7.4 (–13.0 to –1.9) | –12.7 (–18.1 to –7.3) | –20.2 (–25.1 to –15.2) | –28.6 (–33.5 to –23.7) |
| P value | NA | .18 | .83 | .13 | <.001 |
| SCORAD Index score | |||||
| LSM of percentage change from baseline, % (95% CI)e | –29.0 (–36.6 to –21.3) | –26.7 (–35.0 to –18.4) | –30.1 (–38.1 to –22.1) | –49.2 (–56.4 to –42.0) | –69.7 (–76.9 to –62.5) |
| P value | NA | .74 | .87 | .002 | <.001 |
Abbreviations: %BSA, percentage of body surface area; EASI, Eczema Area and Severity Index; EASI-50, 50% or more improvement in EASI; EASI-75, 75% or more improvement in EASI; EASI-90, 90% or more improvement in EASI; Emax, maximum effect; IGA, Investigator’s Global Assessment; LSM, least squares mean; NA, not applicable; NRS, numeric rating scale; SCORAD, Scoring Atopic Dermatitis.
Baseline was defined as the last measurement before first dosing.
Week 12 visits for 18 patients were mapped outside the window (3 patients in placebo group, 3 patients in 10-mg group, 5 patients in 30-mg group, 1 patient in 100-mg group, and 6 patients in 200-mg group).
IGA response was defined as IGA of clear (0) or almost clear (1) with improvement of 2 grades or more from baseline.
Emax model, log [π/(1 − π)] = E0 + Emax × dose/(ED50 + dose), was prespecified as primary analysis for the primary end point in the statistical analysis plan. For discontinued patients, any missing value for all subsequent visits until week 12 was imputed using the nonresponder imputation approach.
Mixed-effects model repeated measure contained fixed factors of treatment, week, treatment by week interaction, and baseline value. The P value was 2-sided. Missing data were not imputed.
Logistic regression model was used including treatment as a main effect and baseline score as a covariate. For discontinued patients, any missing value for all subsequent visits until week 12 was imputed using the nonresponder imputation approach.
Pruritus NRS score was analyzed for patients with baseline NRS score of 4 or higher (51 patients in placebo group, 44 patients in 10-mg group, 45 patients in 30-mg group, 50 patients in 100-mg group, and 44 patients in 200-mg group).
Figure 2. Secondary Efficacy End Points.
A, Proportion of patients who achieved Investigator’s Global Assessment (IGA) of clear or almost clear with 2-grade or more improvement from baseline over time. A logistic regression model was used, including treatment as a main effect, baseline IGA as a covariate. B, Percentage change from baseline in Eczema Area and Severity Index (EASI) over time. C, Proportion of patients with baseline pruritus numeric rating scale (NRS) score of 4 or higher, achieving 4-point or more improvement from baseline over time. Mixed-effects model repeated measure was used and contained fixed factors of treatment, week, treatment by week interaction, baseline value, and unstructured covariance matrix. Error bars denote 90% confidence interval. Missing data were not imputed. Baseline was defined as the last measurement before first dosing. Shaded areas represent the follow-up period when patients were no longer receiving the drug.
At week 12, significantly greater percentage reductions of EASI were observed in both the 200-mg (least squares mean [LSM] difference from placebo, –47.4%; P < .001) and 100-mg (LSM difference from placebo, –23.8%; P = .009) groups compared with placebo, while percentage reductions in the 30-mg and 10-mg groups were not significant (Table 2). Decreases from baseline in EASI for the 200-mg and 100-mg groups plateaued by weeks 4 to 6 and were maintained through week 12 (Figure 2B). Significant differences from placebo in percentage change in EASI were observed as early as week 1 (first postbaseline assessment) in the 200-mg group (LSM difference from placebo, –28.3%; P < .001), and at week 2 in the 100-mg group (–14.9%; P = .03).
At week 12, the proportions of patients who achieved an EASI-75 response was greater in the 200-mg (31 of 48 [estimated response based on logistic regression modeling using nonresponse imputation with treatment as a main effect and baseline score as a covariate, 63.7%; observed response, 64.6%]; P < .001) and 100-mg (22 of 54 [estimated response based on logistic regression modeling using nonresponse imputation with treatment as a main effect and baseline score as a covariate, 41.6%; observed response, 40.7%]; P = .004) groups than in the placebo group (8 of 52 [estimated response based on logistic regression modeling using nonresponse imputation with treatment as a main effect and baseline score as a covariate, 15.6%; observed response, 15.4%]) (Table 2). Similarly, EASI-50 and EASI-90 responses were significantly greater than placebo for both the 200-mg and the 100-mg groups.
At week 12, significant reductions in pruritus NRS scores were observed in the 200-mg (LSM difference from placebo, –25.4%; P = .003) and 100-mg (−20.7%; P = .02) groups compared with placebo. In the subgroup of patients with a baseline pruritus NRS score of 4 or more, 63.6% (28 of 44) in the 200-mg group and 50.0% (25 of 50) in the 100-mg group achieved an improvement of 4 points or more at week 12, compared with 25.5% (13 of 51) who received placebo (Figure 2C). Significant differences from placebo were observed as early as day 2 in the 200-mg group (odds ratio, 6.09; 90% CI, 1.35-27.59; P = .049) and day 5 in the 100-mg group (odds ratio, 8.15; 90% CI, 1.84-36.06; P = .02) (eFigure 4 in Supplement 1). Reductions in pruritus NRS scores plateaued by week 2 in the 200-mg group and week 4 in the 100-mg group and were maintained through week 12 in both groups.
Decreases from baseline in %BSA were observed in all treatment groups, with the greatest reduction observed in the 200-mg group. Significant reductions in %BSA were observed as early as week 1 (first postbaseline assessment) in the 200-mg group (LSM difference from placebo, –10.8%; P < .001). This reduction was maintained through week 12 (–14.9%; P < .001) (Table 2). Patients in the 100-mg group had a significant reduction in %BSA compared with placebo at week 4 (–11.2%; P < .001) and week 8 (–8.63%; P = .04). Decreases from baseline in Scoring AD index scores were observed in all groups, with the highest percentage reduction observed in the 200-mg group (Table 2). These results are all supported by photographic evidence (eFigure 5 in Supplement 1).
A total of 184 of 267 patients (68.9%) experienced 402 TEAEs that were mostly mild (Table 3). Of these, 125 events reported by 64 of 267 patients (24.0%) were considered related to treatment. The most frequently reported of these TEAEs (≥3 patients in any treatment group) included diarrhea, nausea, viral upper respiratory tract infection, headache, and dermatitis atopic; among these, only gastrointestinal disorders were reported substantially more often for patients receiving abrocitinib than those receiving placebo. Serious TEAEs were reported by 9 of 267 patients (3.4%) (eTable 3 in Supplement 1). There were no deaths in the study. Two patients experienced serious adverse events that were considered related to treatment; 1 patient in the 200-mg group developed pneumonia during follow-up after initiation of cyclosporine, which was continued, and the patient recovered with antibiotic treatment; and 1 patient in the 100-mg group developed eczema herpeticum during the treatment period, abrocitinib was permanently discontinued, and the patient recovered with antiviral treatment. There were 2 cases of treatment-emergent herpes zoster (1 patient in the 10-mg group [unrelated to treatment, mild], and 1 patient in the 30-mg group [treatment-related, moderate]) and 2 cases of treatment-emergent herpes simplex (1 patient in the placebo group [treatment-related, moderate], and 1 patient in the 100-mg group [treatment-related, moderate]); none required hospitalization or parenteral antimicrobials (the frequency of these events did not reach the threshold used in Table 3 for reporting most frequently reported TEAEs and are, therefore, not listed in Table 3). A total of 44 of 267 patients (16.5%) discontinued treatment because of TEAEs, of which the most common were (worsening of) dermatitis atopic (20 of 267 [7.5%]), eczema (6 of 267 [2.2%]), and abdominal pain (2 of 267 [0.7%]).
Table 3. Adverse Event Summary.
| Adverse Event | Placebo (n = 56) | Abrocitinib | |||
|---|---|---|---|---|---|
| 10 mg (n = 49) | 30 mg (n = 51) | 100 mg (n = 56) | 200 mg (n = 55) | ||
| Treatment-emergent adverse events, No. (%)a | |||||
| Gastrointestinal disorders | 4 (7.1) | 4 (8.2) | 5 (9.8) | 6 (10.7) | 12 (21.8) |
| Diarrhea | 1 (1.8) | 3 (6.1) | 1 (2.0) | 1 (1.8) | 5 (9.1) |
| Nausea | 1 (1.8) | 3 (6.1) | 3 (5.9) | 1 (1.8) | 8 (14.5) |
| Infections and infestations | 13 (23.2) | 23 (46.9) | 19 (37.3) | 24 (42.9) | 23 (41.8) |
| Upper respiratory tract infection | 5 (8.9) | 3 (6.1) | 5 (9.8) | 3 (5.4) | 5 (9.1) |
| Viral upper respiratory tract infection | 5 (8.9) | 5 (10.2) | 6 (11.8) | 10 (17.9) | 7 (12.7) |
| Nervous system disorders | 4 (7.1) | 2 (4.1) | 8 (15.7) | 7 (12.5) | 7 (12.7) |
| Dizziness | 0 | 0 | 1 (2.0) | 0 | 3 (5.5) |
| Headache | 2 (3.6) | 2 (4.1) | 5 (9.8) | 5 (8.9) | 4 (7.3) |
| Skin and subcutaneous disorders | 11 (19.6) | 10 (20.4) | 10 (19.6) | 17 (30.4) | 10 (18.2) |
| Dermatitis atopic | 7 (12.5) | 8 (16.3) | 9 (17.6) | 7 (12.5) | 7 (12.7) |
| Dermatitis contact | 0 | 0 | 0 | 3 (5.4) | 0 |
| Serious adverse events, No. (%)b | |||||
| Asthma condition aggravated | 0 | 1 (2.0) | 0 | 0 | 0 |
| Asthma | 0 | 0 | 0 | 1 (1.8) | 0 |
| Dermatitis condition aggravated | 1 (1.8) | 0 | 0 | 1 (1.8) | 0 |
| Dermatitis atopic | 1 (1.8) | 0 | 0 | 0 | 0 |
| Eczema herpeticum | 0 | 0 | 0 | 1 (1.8)c | 0 |
| Malignant melanoma | 0 | 1 (2.0) | 0 | 0 | 0 |
| Pneumonia | 0 | 0 | 0 | 0 | 1 (1.8)c |
| Pulmonary embolism | 0 | 0 | 0 | 0 | 1 (1.8) |
Reported for 3 or more patients in any treatment group.
Based on all patients.
Considered related to treatment.
Changes in platelet count were observed for abrocitinib doses of more than 10 mg, with an apparent dose-response (eFigure 6 in Supplement 1). Maximum decreases in platelet count were observed at week 4 in the 200-mg and 100-mg groups (maximum mean change, –29.8% in the 200-mg group and –11.4% in the 100-mg group). Thereafter, platelet count trended upward toward baseline levels by week 12 with ongoing abrocitinib treatment. Despite the decreases, only 2 of 266 patients (0.8%) had platelet counts less than 100 × 103/μL (to convert to ×109/L, multiply by 1.0) (1 patient receiving placebo [normal at screening, but 66 × 103/μL at baseline], and 1 patient receiving 200 mg [261 × 103/μL at baseline]). The patient in the placebo group received blinded study drug at baseline based on screening values, which was considered a protocol deviation. The patient in the 200-mg group had a TEAE of moderate thrombocytopenia during a regularly scheduled visit at day 28 with a platelet count of 36 × 103/μL. Treatment was continued until day 40, and the patient was withdrawn from the study on day 43 based on repeat laboratory testing results that confirmed thrombocytopenia. The patient’s platelet count increased to 61 × 103/μL on day 43 and was considered resolved by day 125 when the count was 267 × 103/μL. The patient also experienced leukopenia and neutropenia on day 14 (considered resolved on days 28 [leukopenia] and 125 [neutropenia]). Reductions in platelet counts were not associated with bleeding or other clinically relevant events. No other clinically meaningful treatment-related trends in clinical laboratory test result abnormalities were observed, including serum lipid and transaminase levels. One patient in the 200-mg group reported a pulmonary embolism, which was not considered related to treatment. The patient had been traveling a long distance by car, and baseline laboratory values were within normal limits.
Discussion
The results of this phase 2b trial show that 200 mg and 100 mg of once-daily oral abrocitinib significantly improved signs and symptoms of AD, with rapid onset of action for disease severity and itch. The proportion of patients who achieved an IGA of clear or almost clear with improvement of 2 grades or more from baseline peaked by weeks 4 to 6 for the 200-mg group but continued to improve through week 12 in the 100-mg group. The reduction in EASI differed significantly from placebo by week 1 for the 200-mg group and by week 2 for the 100-mg group and peaked by weeks 4 to 6 for both groups. The reduction in pruritus NRS scores separated significantly from placebo 2 days after the initiation of treatment for the 200-mg group and peaked by weeks 2 to 4 for both the 200-mg and 100-mg groups. The proportion of patients with an IGA of clear or almost clear with improvement or 2 grades or more observed in this study for the highest dose of abrocitinib was 44.5% (95% CI, 26.7%-62.3%) (vs 6.3% [95% CI, –0.2% to 12.9%] for placebo), which is comparable with the approximate 40% of patients who achieved the end point with dupilumab (vs 8%-10% for placebo) after 16 weeks of treatment.18 However, direct comparisons between the 2 studies are not possible as the inclusion criteria (EASI≥12 in our study [mean at baseline, 25.38], and EASI≥16 in the dupilumab study [median at baseline, 28.6-31.8],18 although IGA grade and %BSA criteria were the same) and length of treatment (12 weeks in this study, and 16 weeks in the dupilumab study18) were different.
Abrocitinib was well tolerated in this 12-week study. The incidence of TEAEs was higher in patients who received abrocitinib at doses of 30 mg or more but did not seem to increase with dose. Gastrointestinal disorders were the only treatment-related adverse events to occur substantially more often in patients who received abrocitinib than those given placebo, but these events were mostly mild. In this short-term study, gastrointestinal events did not appear to result in issues with adherence or withdrawal, but will be monitored in future studies. The potential risk for thromboembolic events will also be monitored in future trials.
The most pronounced hematologic abnormalities that occurred were reduced platelet counts in the 200-mg and 100-mg groups. Maximum reductions were seen at week 4 and moved toward normalization with continued abrocitinib treatment. The decrease in platelet count was not considered clinically relevant in most patients, except for 1 patient in the 200-mg group who experienced leukopenia, neutropenia, and thrombocytopenia, all of which were moderate and considered related to treatment. Thrombocytopenia is generally attributed to JAK2 inhibition19; however, additional studies are necessary to investigate the potential association between JAK1 inhibition and thrombocytopenia.
Limitations
The limitations of this study are like those of other phase 2b trials, including a relatively small sample size and short duration of treatment that limits evaluation of safety. The high discontinuation rate was largely attributable to insufficient clinical response and to protocol-mandated discontinuations (use of any medication for AD [including topical rescue medications]); there were higher rates of these events in the 30-mg, 10-mg, and placebo groups. However, use of rescue medication and discontinuation rates were consistent with similar studies.20 The lack of an open-label extension may also have contributed to the high discontinuation rate.
Conclusions
Because of the limitations of current treatment options, there is an unmet need for novel treatments for moderate to severe AD. In this 12-week, phase 2b study, the 200-mg and 100-mg once-daily doses of the JAK1 inhibitor abrocitinib demonstrated effectiveness and acceptable safety in the treatment of moderate to severe AD, suggesting that JAK1 inhibition alone may be sufficient to produce a clinical effect. The efficacy and safety of 200 mg and 100 mg of abrocitinib are being further evaluated in phase 3 trials.
eFigure 1. Average Daily Inhibiting Concentration for Specified Percentage Effect With Key Cytokines in Atopic Dermatitis
eFigure 2. Study Design
eFigure 3. Proportion of Patients Achieving Investigator’s Global Assessment Clear or Almost Clear With ≥2-Grade Improvement from Baseline at Week 12 (Emax Fitted Curve With Standard Error)
eFigure 4. Proportion of Patients With Baseline Pruritus NRS Score of ≥4 Achieving ≥4-Point Improvement From Baseline Over the First 2 Weeks
eFigure 5. Photographic Evidence
eFigure 6. Mean Absolute Value Versus Time for Platelets
eAppendix. Inclusion and Exclusion Criteria
eTable 1. Investigators
eTable 2. Investigator’s Global Assessment (IGA)
eTable 3. Summary of Serious Adverse Events
Trial Protocol
Data Sharing Statement
References
- 1.Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338-351. doi: 10.1016/j.jaad.2013.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192-199. doi: 10.1111/j.1525-1470.2005.22303.x [DOI] [PubMed] [Google Scholar]
- 3.Boguniewicz M, Alexis AF, Beck LA, et al. Expert perspectives on management of moderate-to-severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol Pract. 2017;5(6):1519-1531. doi: 10.1016/j.jaip.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 4.Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis, section 2: management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116-132. doi: 10.1016/j.jaad.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidbury R, Davis DM, Cohen DE, et al. ; American Academy of Dermatology . Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327-349. doi: 10.1016/j.jaad.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupixent [summary of product characteristics]. Paris, France: Sanofi-Aventis Groupe; 2017. [Google Scholar]
- 7.Dupixent [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals Inc; 2018.
- 8.Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273-287. doi: 10.1111/j.1600-065X.2008.00754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol. 2016;51(3):263-292. doi: 10.1007/s12016-015-8488-5 [DOI] [PubMed] [Google Scholar]
- 10.Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2(3):e24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bissonnette R, Papp KA, Poulin Y, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol. 2016;175(5):902-911. doi: 10.1111/bjd.14871 [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa H, Nemoto O, Igarashi A, Nagata T. Efficacy and safety of topical JTE-052, a Janus kinase inhibitor, in Japanese adult patients with moderate-to-severe atopic dermatitis: a phase II, multicentre, randomized, vehicle-controlled clinical study. Br J Dermatol. 2018;178(2):424-432. doi: 10.1111/bjd.16014 [DOI] [PubMed] [Google Scholar]
- 13.Guttman-Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80(4):913-921. doi: 10.1016/j.jaad.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 14.Guttman-Yassky E, Silverberg JI, Thaçi D, Hong C-H, Mohamed M-E, Othman AA. Primary results from a phase 2b, randomized, placebo-controlled trial of upadacitinib for patients with atopic dermatitis. 76th American Academy of Dermatology Annual Meeting; February 16-20, 2018; San Diego, California. [Google Scholar]
- 15.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Kallen A. Computational Pharmacokinetics. Boca Raton, FL: CRC; 2007. doi: 10.1201/9781420060669 [DOI] [Google Scholar]
- 17.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 18.Simpson EL, Bieber T, Guttman-Yassky E, et al. ; SOLO 1 and SOLO 2 Investigators . Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335-2348. doi: 10.1056/NEJMoa1610020 [DOI] [PubMed] [Google Scholar]
- 19.O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72(suppl 2):ii111-ii115. doi: 10.1136/annrheumdis-2012-202576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130-139. doi: 10.1056/NEJMoa1314768 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Average Daily Inhibiting Concentration for Specified Percentage Effect With Key Cytokines in Atopic Dermatitis
eFigure 2. Study Design
eFigure 3. Proportion of Patients Achieving Investigator’s Global Assessment Clear or Almost Clear With ≥2-Grade Improvement from Baseline at Week 12 (Emax Fitted Curve With Standard Error)
eFigure 4. Proportion of Patients With Baseline Pruritus NRS Score of ≥4 Achieving ≥4-Point Improvement From Baseline Over the First 2 Weeks
eFigure 5. Photographic Evidence
eFigure 6. Mean Absolute Value Versus Time for Platelets
eAppendix. Inclusion and Exclusion Criteria
eTable 1. Investigators
eTable 2. Investigator’s Global Assessment (IGA)
eTable 3. Summary of Serious Adverse Events
Trial Protocol
Data Sharing Statement