Key Points
Question
What is the prognosis in early breast cancer associated with a high 21-gene recurrence score when treated with adjuvant chemotherapy plus endocrine therapy?
Findings
In this secondary analysis of a randomized clinical trial, among 1389 women with early breast cancer and a high score of 26 to 100 by 21-gene assay who received adjuvant chemotherapy, the estimated proportion free from distant recurrence at 5 years was 93%.
Meaning
In women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer, and a high 21-gene recurrence score, a higher proportion were free from distant recurrence when treated with chemoendocrine therapy than expected with endocrine therapy alone.
This secondary anaysis of the TAILORx randomized clinical trial describes clinical outcomes for women with a high 21-gene recurrence score who received adjuvant chemotherapy plus endocrine therapy, a population expected to have a high distant recurrence rate with endocrine therapy alone.
Abstract
Importance
A high 21-gene recurrence score (RS) by breast cancer assay is prognostic for distant recurrence of early breast cancer after local therapy and endocrine therapy alone, and for chemotherapy benefit.
Objective
To describe clinical outcomes for women with a high RS who received adjuvant chemotherapy plus endocrine therapy in the TAILORx trial, a population expected to have a high distant recurrence rate with endocrine therapy alone.
Design, Setting, and Participants
In this secondary analysis of data from a multicenter randomized clinical trial, 1389 women with hormone receptor–positive, ERBB2-negative, axillary node-negative breast cancer, and a high RS of 26 to 100 were prospectively assigned to receive adjuvant chemotherapy in addition to endocrine therapy. The analysis was conducted on May 12, 2019.
Interventions
The adjuvant chemotherapy regimen was selected by the treating physician.
Main Outcomes and Measures
Freedom from recurrence of breast cancer at a distant site, and freedom from recurrence, second primary cancer, and death (also known as invasive disease-free survival [IDFS]).
Results
Among the 9719 eligible women, with a mean age of 56 years (range 23-75 years), 1389 (14%) had a recurrence score of 26 to 100, of whom 598 (42%) had an RS of 26 to 30 and 791 (58%) had an RS of 31 to 100. The most common chemotherapy regimens included docetaxel/cyclophosphamide in 589 (42%), an anthracycline without a taxane in 334 (24%), an anthracycline and taxane in 244 (18%), cyclophosphamide/methotrexate/5-fluorouracil in 52 (4%), other regimens in 81 (6%), and no chemotherapy in 89 (6%). At 5 years, the estimated rate of freedom from recurrence of breast cancer at a distant site was 93.0% (standard error [SE], 0.8%), freedom of recurrence of breast cancer at a distant and/or local regional site 91.0% (SE, 0.8%), IDFS 87.6% (SE, 1.0%), and overall survival 95.9% (SE, 0.6%).
Conclusions and Relevance
The estimated rate of freedom from recurrence of breast cancer at a distant site in women with an RS of 26 to 100 treated largely with taxane and/or anthracycline-containing adjuvant chemotherapy regimens plus endocrine therapy in the prospective TAILORx trial was 93% at 5 years, an outcome better than expected with endocrine therapy alone in this population.
Trial Registration
ClinicalTrials.gov identifier: NCT00310180
Introduction
The 21-gene recurrence score (RS) assay provides prognostic information for distant recurrence in hormone–receptor-positive, ERBB2-negative early breast cancer that is independent of clinicopathologic features,1 and is also predictive of chemotherapy benefit when the RS is high.2,3 Prediction of chemotherapy benefit was initially established in the prospective-retrospective validation study including primary tumor samples from 651 patients enrolled in the B20 trial with hormone receptor–positive, axillary node-negative breast cancer randomized to tamoxifen or chemotherapy plus tamoxifen, whether high RS was defined as 31 to 100 irrespective of ERBB2 RNA expression (n = 651),2 or 26 to 100 in tumor samples that had a low ERBB2 RNA expression score (n = 569).4 For the 122 (21%) patients with a high RS of 26 to 100 in the ERBB2-negative cohort of the B20 validation cohort randomized to tamoxifen or tamoxifen plus an adjuvant chemotherapy regimen (including methotrexate and 5-fluorouracil with or without cyclophosphamide), 10-year distant recurrence-free survival rates were 62% (95% CI, 48%-81%) with tamoxifen alone, and 88% (95% CI, 81%-95%) with chemotherapy plus tamoxifen; there was a statistically significant interaction between chemotherapy treatment and high RS (likelihood ratio test on interaction, P = .01), indicating that high RS was not only prognostic for a high distant recurrence rate, but also predictive of chemotherapy benefit.4 Distant recurrence-free survival rates were similar with endocrine therapy alone in other cohorts, including the prospective-retrospective validation study using primary tumor samples derived from 594 patients with hormone receptor–positive, ERBB2-negative axillary node-negative breast cancer enrolled in the B14 trial who received adjuvant tamoxifen without chemotherapy; 157 (26%) with a high RS of 26 to 100 had an estimated 10-year distant recurrence-free survival rate of 70% (standard error [SE], 3.9%).1
The Trial Assigning Individualized Options for Treatment (TAILORx) was a prospective trial designed to address gaps in our knowledge about the application of the 21-gene RS in hormone receptor–positive, ERBB2-negative, axillary node-negative breast cancer.5 The TAILORx trial results demonstrated that endocrine therapy alone was noninferior to adjuvant chemotherapy plus endocrine therapy in the overall population with an RS of 11 to 25, the primary trial end point, with some chemotherapy benefit noted for trial participants who were aged 50 years or younger with an RS of 16 to 25 in exploratory analysis.6 The trial also demonstrated a low distant recurrence rate of 1% at 5 years and 3% at 9 years with endocrine therapy alone if the RS was 0 to 10 irrespective of age,6,7 and that integration of clinical features with RS provided additional prognostic information for recurrence but not prediction of chemotherapy benefit.8 In this report we provide a descriptive analysis of clinical outcomes, characteristics, and adjuvant chemotherapy regimens for patients with an RS of 26 to 100 assigned to receive chemotherapy, including regimens largely including taxanes and/or anthracyclines, plus endocrine therapy.6,8
Methods
Study Protocol
The trial protocol is available in Supplement 1. This was a prospective clinical trial sponsored by the National Cancer Institute (NCI) that was coordinated by the Eastern Cooperative Oncology Group (ECOG) and subsequently ECOG-ACRIN Cancer Research Group and approved by the National Cancer Institute Central institutional review board or local institutional review board. Women were required to provide written informed consent, including willingness to have treatment assigned or randomized based on the RS results. All women had an Oncotype DX RS assay performed in a central laboratory (Genomic Health, Inc).1 Additional details regarding the study protocol have been previously reported.6,7,8 Clinicians were able to select 1 of several commonly used chemotherapy regimens (eTable 1 in Supplement 2). Participants with a high RS of 26 to 100 and assigned to chemotherapy (arm D) were enrolled in a voluntary prospective registry; as previously described,6 sufficient baseline and follow-up information was available in 1389 of 1737 patients (80%) with a high RS for inclusion in the analysis.
Study End Points
The standardized definitions for efficacy end points (STEEP) criteria were used for end point definitions.9 End points included (1) invasive disease-free survival (IDFS), defined as time from registration to first event, where the first event is ipsilateral breast tumor recurrence, local recurrence, regional recurrence, distant recurrence, contralateral second primary invasive cancer, second primary nonbreast invasive cancer (excluding nonmelanoma skin cancers), or death without evidence of recurrence; (2) distant recurrence-free interval, defined as time from registration to date of distant recurrence of breast cancer, or of death with distant recurrence, if death is the first manifestation of distant recurrence (referred to here as freedom recurrence of breast cancer at a distant site); (3) relapse-free interval, defined as date from registration to first recurrence of breast cancer (ipsilateral breast, local-regional, or distant), or to the date of death with recurrence, if death is the first manifestation of recurrence (referred to here as freedom from recurrence of breast cancer at a distant and/or local-regional site); and (4) overall survival (OS), defined as date from registration to death from any cause.
Statistical Analysis of Clinical Outcomes
The overall sample size was driven by the need to include a sufficient number of patients with an RS of 11 to 25 to test noninferiority of endocrine therapy alone, the experimental arm, to chemotherapy plus endocrine therapy, the standard arm, as previously described.6 The final analysis took place on March 2, 2018, at which time the prespecified number of events required for full information (n = 835) had occurred in the randomized group with an RS of 11 to 25. At the time of the analysis, among 1389 patients with an RS of 26 to 100 included in the analysis, there were 80 distant recurrence events and 134 IDFS events by 5 years and 101 events and 189 IDFS events by 9 years (eTable 2 in Supplement 2). The median follow-up at the time of analysis in the high-risk arm was 61 months (range, 0.1-132 months). There was a larger dropout rate in the high-risk arm (arm D) than the other arms (arms A, B, C) (eFigure in Supplement 2) because of the voluntary nature of the high-risk registry, and because follow-up for the high-risk arm was requested for up to 5 years. Five-year event rates are shown because most distant recurrence events occurred within 5 years (80/101 [80%]), and because follow-up information beyond 5 years was limited for the reasons described. Event-free rates were estimated using the Kaplan-Meier method, with confidence intervals computed using the log-log transform and Greenwood’s variance. Analyses were conducted using R statistical software (version 3.2.3, R Foundation) on May 12, 2019.
Statistical Methods for Projection of Clinical Outcomes With Endocrine Therapy Alone
The expected rate of distant recurrence through 5 and 9 years of patients with RS of 26 to 100 (referred to here as arm D of TAILORx) if treated with endocrine therapy alone was estimated by combining patient-specific distant recurrence risk information from TAILORx arm D with patient-specific chemotherapy benefit information from ERBB2-negative cohort derived from the NSABP B20 trial.4 For each individual patient in TAILORx arm D, the log cumulative hazard of distant recurrence was estimated from a Cox model with effects for RS as a continuous covariate and age category (≤50 vs >50 years). The patient-specific log hazard ratio for chemotherapy effect was estimated from NSABP study B20 patients who were ERBB2-negative and hormone receptor–positive (by reverse transcription polymerase chain reaction) using a Cox model with effects for RS as a continuous covariate, age category (≤50 vs >50 years), randomized chemotherapy use, the 2-way interactions of RS with chemotherapy and age category with chemotherapy, and the 3-way interaction of RS with age category with chemotherapy. Combining these 2 estimates and transforming to the risk scale yielded a patient-specific distant recurrence risk estimate for the scenario in which the patients did not receive chemotherapy. The individual risk estimates were averaged across the TAILORx arm D population, with SEs computed using the Δ method.
Results
Patient and Tumor Characteristics by Adjuvant Chemotherapy Regimen
A total of 10 253 eligible women were registered between April 7, 2006, and October 6, 2010. Among the 9719 eligible participants with follow-up information included in the main analysis set, 1389 (14%) had a recurrence score of 26 to 100, of whom 598 (42%) had an RS of 26 to 30, and 791 (58%) had an RS of 31 to 100. The characteristics of the high-RS group are shown in Table 1. The most common chemotherapy regimens included docetaxel/cyclophosphamide in 589 (42%), an anthracycline without a taxane in 334 (24%), an anthracycline and taxane in 244 (18%), cyclophosphamide/methotrexate/5-fluorouracil (CMF) in 52 (4%), other regimens in 81 (6%), and no chemotherapy in 89 (6%). Patients treated with CMF tended to be older (median age, 61.5 years), and those treated with anthracyclines and taxanes tended to be younger (median age, 53.5 years), compared with those treated with taxane and cyclophosphamide or an anthracycline without a taxane (median age, 57 years). Of 89 patients who received no adjuvant chemotherapy, and were thus nonadherent with assigned treatment, a higher proportion had an RS of 26 to 30 (52 of 89 [58%]) than those who received chemotherapy (546 of 1300 [42%]), whereas other characteristics, such as age, tumor size, and grade were largely similar.
Table 1. Characteristics of Patients in the Recurrence Score of 26 to 100 Cohort Assigned to Chemotherapy Stratified by Chemotherapy Regimen in the Intention-to-Treat Population.
| Study Arm | No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Total | Taxane and Cyclophosphamide | Anthracycline Without Taxane | Anthracycline Plus Taxane | CMF | Other | No Chemotherapy | |
| No. | 1389 | 589 | 334 | 244 | 52 | 81 | 89 |
| Age, y | |||||||
| Median (range) | 56 (23-75) | 57 (23-74) | 57 (31-75) | 53.5 (30-75) | 61.5 (40-75) | 54 (36-75) | 56 (36-75) |
| IQR | (49-63) | (50-63) | (50-63) | (46-60) | (53-67.5) | (49-62) | (50-63) |
| Mean (SD) | 55.8 (9.4) | 56.2 (9.3) | 56.3 (8.8) | 53.3 (9.8) | 60.0 (9.7) | 55.4 (8.9) | 55.9 (9.4) |
| ≤40 | 79 (6) | 33 (6) | 13 (4) | 26 (11) | 1 (2) | 1 (1) | 5 (6) |
| 41-50 | 330 (24) | 127 (22) | 76 (23) | 65 (27) | 10 (19) | 29 (36) | 23 (26) |
| 51-60 | 512 (37) | 217 (37) | 137 (41) | 92 (38) | 12 (23) | 21 (26) | 33 (37) |
| 61-70 | 395 (28) | 185 (31) | 93 (28) | 52 (21) | 20 (38) | 26 (32) | 19 (21) |
| 71-75 | 73 (5) | 27 (5) | 15 (4) | 9 (4) | 9 (17) | 4 (5) | 9 (10) |
| Premenopausal | 407 (29) | 158 (27) | 91 (27) | 99 (41) | 10 (19) | 25 (31) | 24 (27) |
| Postmenopausal | 982 (71) | 431 (73) | 243 (73) | 145 (59) | 42 (81) | 56 (69) | 65 (73) |
| Tumor size, cm | |||||||
| Median (range) | 1.7 (0.2-21.4) | 1.7 (0.5-8.4) | 1.6 (0.2-21.4) | 1.95 (0.6-7.0) | 1.5 (0.6-3.7) | 1.6 (0.5-3.8) | 1.7 (0.6-3.8) |
| IQR | (1.3-2.3) | (1.3-2.3) | (1.2-2.2) | (1.4-2.5) | (1.1-3.7) | (0.5-2.1) | (1.3-2.2) |
| Mean (SD) | 1.88 (0.99) | 1.8 (0.8) | 1.9 (1.4) | 2.1 (1.0) | 1.6 (0.7) | 1.8 (0.8) | 1.8 (0.7) |
| Distribution | |||||||
| ≤1.0 | 188 (14) | 73 (12) | 51 (15) | 31 (13) | 11 (21) | 14 (17) | 8 (9) |
| 1.1-2.0 | 741 (53) | 324 (55) | 180 (54) | 109 (45) | 30 (58) | 44 (54) | 54 (61) |
| 2.1-3.0 | 348 (25) | 153 (26) | 75 (22) | 74 (30) | 9 (17) | 15 (19) | 22 (25) |
| 3.1-4.0 | 91 (7) | 33 (6) | 22 (7) | 21 (9) | 2 (4) | 8 (10) | 5 (6) |
| ≥4.1 | 20 (1) | 5 (1) | 6 (2) | 9 (4) | 0 | 0 | 0 |
| Unknown | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Histologic grade | |||||||
| Low | 89 (7) | 34 (6) | 22 (7) | 17 (7) | 1 (2) | 5 (6) | 10 (11) |
| Intermediate | 590 (43) | 269 (46) | 146 (45) | 90 (38) | 26 (55) | 26 (33) | 33 (38) |
| High | 681 (50) | 277 (48) | 160 (49) | 132 (55) | 20 (43) | 48 (61) | 44 (51) |
| Unknown | 29 | 9 | 6 | 5 | 5 | 2 | 2 |
| ER expression | |||||||
| Negative | 40 (3) | 17 (3) | 10 (3) | 10 (4) | 1 (2) | 2 (2) | 0 |
| Positive | 1349 (97) | 572 (97) | 324 (97) | 234 (96) | 51 (98) | 79 (98) | 89 (100) |
| PgR expression | |||||||
| Negative | 405 (30) | 177 (32) | 75 (23) | 81 (33) | 15 (29) | 30 (38) | 27 (30) |
| Positive | 948 (70) | 381 (68) | 257 (77) | 161 (67) | 37 (71) | 50 (62) | 62 (70) |
| Unknown | 36 | 31 | 2 | 2 | 0 | 1 | 0 |
| Clinical risk | |||||||
| Low | 589 (43) | 256 (44) | 149 (45) | 90 (38) | 26 (55) | 31 (39) | 37 (43) |
| High | 770 (57) | 323 (56) | 179 (55) | 149 (62) | 21 (45) | 48 (61) | 50 (57) |
| Unknown | 30 | 10 | 6 | 5 | 5 | 2 | 2 |
| Primary surgery | |||||||
| Breast conservation | 1021 (74) | 442 (75) | 249 (75) | 174 (71) | 38 (73) | 61 (75) | 57 (64) |
| Mastectomy | 368 (26) | 147 (25) | 85 (25) | 70 (29) | 14 (27) | 20 (25) | 32 (36) |
| Postoperative radiation therapy | |||||||
| Yes | 1300 (94) | 387 (66) | 213 (64) | 157 (64) | 31 (60) | 40 (49) | 28 (31) |
| No/unknown | 89 (6.4) | 202 (34) | 121 (36) | 87 (36) | 21 (40) | 41 (51) | 61 (69) |
| Recurrence score | |||||||
| Median (range) | 32 (26-87) | 32 (26-81) | 32 (26-72) | 36 (26-87) | 30 (26-64) | 32 (26-64) | 29 (26-77) |
| IQR | (28-40) | (28-39) | (28-37) | (30-48) | (28-35) | (28-40) | (27-35) |
| Mean (SD) | 35.2 (9.8) | 34.6 (9.4) | 33.9 (8.2) | 39.3 (11.8) | 32.7 (7.7) | 35.4 (9.4) | 33.5 (10.3) |
| 26-30 | 598 (43) | 267 (45) | 147 (44) | 70 (29) | 30 (58) | 32 (40) | 52 (58) |
| 31-35 | 315 (23) | 133 (23) | 86 (26) | 49 (20) | 10 (19) | 21 (26) | 16 (18) |
| 36-40 | 158 (11) | 59 (10) | 44 (13) | 35 (14) | 6 (12) | 8 (10) | 6 (7) |
| 41-50 | 202 (15) | 91 (15) | 40 (12) | 48 (20) | 4 (8) | 12 (15) | 7 (8) |
| 51-100 | 116 (8) | 39 (7) | 17 (5) | 42 (17) | 2 (4) | 8 (10) | 8 (9) |
| Endocrine Therapy | |||||||
| Premenopausal | |||||||
| AI | 41 (10) | 13 (8) | 7 (8) | 13 (13) | 0 | 5 (20) | 3 (12) |
| OFS | 21 (5) | 4 (3) | 7 (8) | 8 (8) | 0 | 1 (4) | 1 (4) |
| OFS and AI | 31 (8) | 13 (8) | 6 (7) | 10 (10) | 0 | 1 (4) | 1 (4) |
| Tam | 177 (43) | 77 (49) | 35 (38) | 39 (39) | 5 (50) | 10 (40) | 11 (46) |
| Tam and AI | 117 (29) | 48 (30) | 33 (36) | 21 (21) | 4 (40) | 7 (28) | 4 (17) |
| Other | 1 (<1) | 0 | 1 (1) | 0 | 0 | 0 | 0 |
| None reported | 19 (5) | 3 (2) | 2 (2) | 8 (8) | 1 (10) | 1 (4) | 4 (17) |
| Postmenopausal | |||||||
| AI | 695 (71) | 320 (74) | 165 (68) | 99 (68) | 30 (71) | 38 (68) | 43 (66) |
| Other | 256 (26) | 105 (24) | 73 (30) | 39 (27) | 10 (24) | 12 (21) | 17 (26) |
| None reported | 31 (3) | 6 (1) | 5 (2) | 7 (5) | 2 (5) | 6 (11) | 5 (8) |
Abbreviations: AI, aromatase inhibitor; ER, estrogen receptor; IQR, interquartile range; OFS, ovarian function suppression; PgR, progesterone receptor; tam, tamoxifen.
Clinical Outcomes in the Overall Population and by Chemotherapy Regimen
In the overall population assigned to chemotherapy, as summarized in Table 2, the estimated 5-year rates of freedom from recurrence of breast cancer at a distant site were 93.0% (SE, 0.8%), freedom of recurrence of breast cancer at a distant and/or local regional site 91.0% (SE, 0.8%), IDFS 87.6% (SE, 1.0%), and OS 95.9% (SE, 0.6%).
Table 2. Kaplan-Meier Estimates of Clinical Outcomes at 5 Years for Patients With a Recurrence Score of 26 to 100 Assigned to Chemotherapy Stratified by Adjuvant Chemotherapy Regimen in the Intention-to-Treat Populationa.
| Variable | Mean (SE) [95% CI] | ||||||
|---|---|---|---|---|---|---|---|
| All Patients | Taxane and Cyclophosphamide | Anthracycline Without Taxane | Anthracycline and Taxane | CMF | Other | No Chemotherapy | |
| No. | 1389 | 589 | 334 | 244 | 52 | 81 | 89 |
| Invasive disease-free survival | 87.6 (1.0) [85.5-89.4] | 88.1 (1.5) [84.8-90.8] | 87.4 (2.0) [83-90.8] | 88.6 (2.3) [83.2-92.3] | 84 (5.6) [69.3-92.1] | 91.3 (3.4) [81.5-96] | 79.7 (4.9) [67.9-87.5] |
| Freedom from recurrence of breast cancer at a distant site | 93.0 (0.8) [91.4-94.4] | 92.7 (1.2) [90-94.7] | 92.3 (1.6) [88.5-94.9] | 95.1 (1.5) [91-97.3] | 88.5 (4.8) [74.6-95.1] | 95.5 (2.5) [86.8-98.5] | 92.8 (3.1) [83.6-97] |
| Freedom from recurrence of breast cancer at a distant or local-regional site | 91.0 (0.8) [89.1-92.5] | 91.0 (1.3) [88-93.2] | 90.1 (1.8) [86-93] | 93.6 (1.7) [89.1-96.2] | 88.7 (4.8) [74.9-95.1] | 95.5 (2.5) [86.8-98.5] | 84 (4.5) [72.8-90.8] |
| Overall survival | 95.9 (0.6) [94.6-96.9] | 95.8 (0.9) [93.6-97.2] | 96.7 (1.0) [93.9-98.2] | 97.2 (1.1) [93.8-98.7] | 90.1 (4.7) [75.8-96.2] | 97.2 (2.0) [89.2-99.3] | 90.7 (3.7) [80.3-95.7] |
Abbreviation: CMF, cyclophosphamide/methotrexate/5-fluorouracil.
Five-year Kaplan-Meier estimates (percent), standard error, and 95% confidence intervals shown.
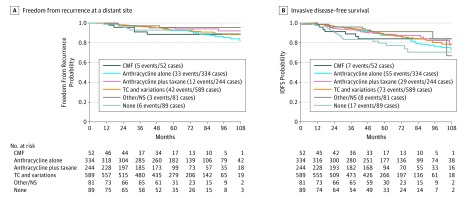
Clinical outcomes stratified by chemotherapy regimens are shown in the Figure, and further described in Table 2 as 5-year Kaplan-Meier estimates. Five-year rates of freedom from recurrence of breast cancer at a distant site ranged from 92.3% (SE, 1.6%) to 95.5% (SE, 2.5%) for all chemotherapy regimens with the exception of CMF, which was associated with an 88.5% (SE, 4.8%) rate; those who received no chemotherapy had similar rates (92.6% SE, 3.1%) compared with those who did receive chemotherapy. Five-year rates of IDFS ranged from 84.0% (SE, 5.6%) to 91.3% (SE, 3.4%) for those who received chemotherapy, but were lower for those who received no chemotherapy (79.7%; SE, 4.9%). Nine-year event rates are shown in eTable 3 in Supplement 2, but should be interpreted with caution because of limited follow-up beyond 5 years, and potential for bias in reporting of clinical events; for the overall population, the estimated 9-year rates of freedom from recurrence of breast cancer at a distant site was 86.8% (SE, 1.7%),
Figure. Kaplan-Meier Curves for Freedom From Recurrence of Breast Cancer at a Distant Site and Invasive Disease-Free Survival (IDFS).
CMF Indicates cyclophosphamide/methotrexate/5-fluorouracil; NS, not specified; TC, taxane and cyclophosphamide.
Cox models for comparison of any chemotherapy regimen vs none with adjustment for tumor size (>2 vs ≤2 cm), age (>65 vs 51-65 vs ≤50 years), grade, and RS revealed estimated hazard ratios of 0.74 (95% CI, 0.32-1.69) for freedom from recurrence of breast cancer at a distant site and 0.48 (95% CI, 0.29-0.80) for IDFS, suggesting inferior outcomes for those with a high RS of 26 to 100 who did not receive chemotherapy as assigned.
If the analysis was restricted to the 1300 patients who received adjuvant chemotherapy, the rate of freedom from recurrence of breast cancer at a distant site at 5 and 9 years was 93.0% (SE, 0.8%) and 86.8% (SE, 1.7%), respectively. For the 546 patients (42%) with an RS of 26 to 30, the rates were 94.6% (SE, 1.1%) and 88.5% (SE, 2.5%) at 5 and 9 years, respectively. For the 754 patients (58%) who had an RS of 31 to 100, the rates were 91.9 (SE, 1.1%) and 85.5% (SE, 2.5%) at 5 and 9 years, respectively. Invasive disease-free survival rates at 5 and 9 years were 88.1% (SE, 1.0%) and 76.2% (SE, 2.3%) in the overall population, 90.5% (SE, 1.4%) and 78.0% (SE, 3.4%) in the RS 26 to 30 group, and 86.3% (SE, 1.4%) and 74.8% (SE, 3.1%) in the RS 31 to 100 group.
Estimation of Expected Outcomes If Treated With Endocrine Therapy Alone
The expected rate of distant recurrence in this cohort if treated with endocrine therapy alone was estimated by combining patient-specific distant recurrence risk information with patient-specific chemotherapy benefit information from the ERBB2-negative cohort of NSABP B20.4 Using this estimation method, the expected rates of freedom from distant recurrence without adjuvant chemotherapy in this cohort were 78.8% (SE, 14.0%) at 5 years and 65.4% (SE, 10.4%) at 9 years. For those with an RS of 26 to 30, the expected rates at 5 and 9 years were 89.6% (SE, 9.2%) and 80.6% (SE, 7.0%), and for those with an RS of 31 to 100 were 70.7% (SE, 17.9%) and 54% (SE, 13.8%), respectively.
Discussion
In this secondary analysis of a prospective clinical trial including 1389 women with hormone-receptor-positive, ERBB2-negative, axillary node-negative breast cancer, and a high 21-gene RS of 26 to 100 assigned to receive adjuvant chemotherapy, the estimated 5-year rate of freedom from recurrence of breast cancer at a distant site were 93.0% (SE, 0.8%) and 86.8% (SE, 1.7%), respectively. These findings were highly consistent with a comparable population with ERBB2-negative disease in the B20 trial treated with methotrexate and 5-fluorouracil with or without cyclophosphamide (88% at 10 years),4 and substantially better than similar patients treated with tamoxifen alone in the prospective validation studies of the 21-gene assay in the B20 cohort (62% at 10 years)4 and B14 cohort (70% at 10 years).1 Outcomes were also similar in patients enrolled in TAILORx trial with an RS of 0 to 107 and 11 to 256 treated with endocrine therapy alone compared with the B20 cohort,5 supporting the use of outcomes from the B20 trial in planning TAILORx. In addition, the projected rates of freedom from recurrence of breast cancer at a distant site in this population if treated with endocrine therapy alone was estimated to be 78.8% (SE, 14.0%) at 5 years and 65.4% (SE, 10.4%) at 9 years when simulating outcomes based on the treatment effect of chemotherapy noted in the ERBB2-negative cohort of the B20 trial,4 providing additional evidence that the outcomes when treated with chemoendocrine therapy were better than expected with endocrine therapy alone in this population.
For IDFS, an end point that also includes local-regional recurrences, contralateral breast cancer, other second primary cancers, and deaths from other causes, the estimated 5- and 9-year IDFS rates were 87.6% (SE, 1.0%) and 75.7% (SE, 2.2%). Taken together with information regarding distant recurrence rates, these findings indicate that about one-half of all events in this population included events other than distant recurrence.
Strengths and Limitations
Strengths of this analysis include the prospective nature of the trial and treatment assignment; the large sample size of the high-RS cohort assigned to chemotherapy (n = 1389) relative to the prior B20 validation study (n = 122)4; the high compliance rate to assigned chemotherapy (94%); the use of systemic adjuvant therapies reflecting current clinical practice,10 including aromatase inhibitors rather than tamoxifen in postmenopausal women, and use of second- and third-generation chemotherapy regimens, including anthracyclines and/or taxanes, in most patients.10 Limitations include the lack of randomization to endocrine therapy alone to prospectively confirm an interaction between chemotherapy benefit and high RS, and the limited follow-up and consequent potential for bias in reporting events beyond 5 years.
Conclusions
Rates of distant recurrence after local therapy reflect the underlying recurrence risk, the benefit from adjuvant endocrine therapy, and benefit from adjuvant chemotherapy, the latter of which has little impact on nonrecurrence events such as contralateral breast cancer or second primary cancers.11,12,13 High RS of 26 to 100 is prognostic not only for high distant recurrence rates of approximately 30% at 10 years when treated with endocrine therapy alone, but also greater effectiveness of chemotherapy in reducing the risk of distant recurrence. The findings from this analysis add to the evidence base supporting the use of the 21-gene RS assay to guide the use of adjuvant chemotherapy in patients with hormone receptor–positive, ERBB2-negative, axillary node-negative breast cancer.
Trial Protocol.
eTable 1. Chemotherapy Regimens
eTable 2. Number and Type of Events
eTable 3. 9-Year Event Rates
eFigure. Duration of Follow up by Treatment Arm
Data Sharing Statement.
References
- 1.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817-2826. doi: 10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 2.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726-3734. doi: 10.1200/JCO.2005.04.7985 [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Barlow WE, Shak S, et al. ; Breast Cancer Intergroup of North America . Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55-65. doi: 10.1016/S1470-2045(09)70314-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geyer CE Jr, Tang G, Mamounas EP, et al. 21-Gene assay as predictor of chemotherapy benefit in HER2-negative breast cancer. NPJ Breast Cancer. 2018;4:37. doi: 10.1038/s41523-018-0090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26(5):721-728. doi: 10.1200/JCO.2007.15.1068 [DOI] [PubMed] [Google Scholar]
- 6.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111-121. doi: 10.1056/NEJMoa1804710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373(21):2005-2014. doi: 10.1056/NEJMoa1510764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380(25):2395-2405. doi: 10.1056/NEJMoa1904819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127-2132. doi: 10.1200/JCO.2006.10.3523 [DOI] [PubMed] [Google Scholar]
- 10.Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer: an overview. BMC Med. 2015;13:195. doi: 10.1186/s12916-015-0439-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peto R, Davies C, Godwin J, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432-444. doi: 10.1016/S0140-6736(11)61625-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamounas EP, Tang G, Liu Q. The importance of systemic therapy in minimizing local recurrence after breast-conserving surgery: the NSABP experience. J Surg Oncol. 2014;110(1):45-50. doi: 10.1002/jso.23609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertelsen L, Bernstein L, Olsen JH, et al. ; Women’s Environment, Cancer and Radiation Epidemiology Study Collaborative Group . Effect of systemic adjuvant treatment on risk for contralateral breast cancer in the Women’s Environment, Cancer and Radiation Epidemiology Study. J Natl Cancer Inst. 2008;100(1):32-40. doi: 10.1093/jnci/djm267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eTable 1. Chemotherapy Regimens
eTable 2. Number and Type of Events
eTable 3. 9-Year Event Rates
eFigure. Duration of Follow up by Treatment Arm
Data Sharing Statement.