Key Points
Question
Does treatment with abemaciclib plus fulvestrant prolong the overall survival (OS) of patients with hormone receptor (HR)–positive, ERBB2 (formerly HER2)-negative advanced breast cancer who progressed during prior endocrine therapy?
Findings
In the randomized, placebo-controlled MONARCH 2 trial of 669 patients with HR-positive, ERBB2-negative advanced breast cancer, abemaciclib plus fulvestrant significantly improved median OS to 46.7 months compared with 37.3 months for patients receiving placebo plus fulvestrant.
Meaning
The addition of abemaciclib to fulvestrant provided a clinically meaningful median OS benefit of 9.4 months for patients with HR-positive, ERBB2-negative advanced breast cancer that had progressed on endocrine therapy.
This randomized clinical trial compared the effect of abemaciclib plus fulvestrant vs placebo plus fulvestrant on overall survival in women with hormone receptor–positive, ERBB2-negative advanced breast cancer that progressed during prior endocrine therapy.
Abstract
Importance
Statistically significant overall survival (OS) benefits of CDK4 and CDK6 inhibitors in combination with fulvestrant for hormone receptor (HR)–positive, ERBB2 (formerly HER2)-negative advanced breast cancer (ABC) in patients regardless of menopausal status after prior endocrine therapy (ET) has not yet been demonstrated.
Objective
To compare the effect of abemaciclib plus fulvestrant vs placebo plus fulvestrant on OS at the prespecified interim of MONARCH 2 (338 events) in patients with HR-positive, ERBB2-negative advanced breast cancer that progressed during prior ET.
Design, Setting, and Participants
MONARCH 2 was a global, randomized, placebo-controlled, double-blind phase 3 trial of abemaciclib plus fulvestrant vs placebo plus fulvestrant for treatment of premenopausal or perimenopausal women (with ovarian suppression) and postmenopausal women with HR-positive, ERBB2-negative ABC that progressed during ET. Patients were enrolled between August 7, 2014, and December 29, 2015. Analyses for this report were conducted at the time of database lock on June 20, 2019.
Interventions
Patients were randomized 2:1 to receive abemaciclib or placebo, 150 mg, every 12 hours on a continuous schedule plus fulvestrant, 500 mg, per label. Randomization was stratified based on site of metastasis (visceral, bone only, or other) and resistance to prior ET (primary vs secondary).
Main Outcomes and Measures
The primary end point was investigator-assessed progression-free survival. Overall survival was a gated key secondary end point. The boundary P value for the interim analysis was .02.
Results
Of 669 women enrolled, 446 (median [range] age, 59 [32-91] years) were randomized to the abemaciclib plus fulvestrant arm and 223 (median [range] age, 62 [32-87] years) were randomized to the placebo plus fulvestrant arm. At the prespecified interim, 338 deaths (77% of the planned 441 at the final analysis) were observed in the intent-to-treat population, with a median OS of 46.7 months for abemaciclib plus fulvestrant and 37.3 months for placebo plus fulvestrant (hazard ratio [HR], 0.757; 95% CI, 0.606-0.945; P = .01). Improvement in OS was consistent across all stratification factors. Among stratification factors, more pronounced effects were observed in patients with visceral disease (HR, 0.675; 95% CI, 0.511-0.891) and primary resistance to prior ET (HR, 0.686; 95% CI, 0.451-1.043). Time to second disease progression (median, 23.1 months vs 20.6 months), time to chemotherapy (median, 50.2 months vs 22.1 months), and chemotherapy-free survival (median, 25.5 months vs 18.2 months) were also statistically significantly improved in the abemaciclib arm vs placebo arm. No new safety signals were observed for abemaciclib.
Conclusions and Relevance
Treatment with abemaciclib plus fulvestrant resulted in a statistically significant and clinically meaningful median OS improvement of 9.4 months for patients with HR-positive, ERBB2-negative ABC who progressed after prior ET regardless of menopausal status. Abemaciclib substantially delayed the receipt of subsequent chemotherapy.
Trial Registration
ClinicalTrials.gov identifier: NCT02107703
Introduction
Most patients with metastatic breast cancer have tumors that are hormone receptor (HR)-positive and are initially treated with endocrine therapy (ET).1,2,3,4 Although ET is an efficacious and well-tolerated therapy in most patients, resistance to ET and subsequent disease progression remains a major challenge.2 In an effort to improve treatment options, cyclin-dependent kinase 4 and 6 (CDK4 and CDK6) inhibitors in combination with ET have emerged as a standard-of-care treatment for patients with HR-positive, ERBB2 (formerly HER2)-negative advanced breast cancer (ABC) (eg, inoperable locally advanced or metastatic breast cancer).5,6,7,8
Abemaciclib is an orally administered, potent, and selective small-molecule inhibitor of CDK4 and CDK6 that is 14 times more potent against CDK4 than CDK6 in enzymatic assays.1,6 In preclinical models, continuous exposure to abemaciclib resulted in sustained cell-cycle inhibition, which led to senescence and apoptosis, whereas short-term inhibition resulted in cell-cycle rebound.9 Abemaciclib is currently the only US Food and Drug Administration–approved inhibitor of CDK4 and CDK6 for the treatment of patients with HR-positive, ERBB2-negative ABC as monotherapy for endocrine refractory disease (MONARCH 1).6 Additionally, abemaciclib in combination with ET is approved for management of HR-positive, ERBB2-negative ABC both as initial therapy with an aromatase inhibitor10 (MONARCH 3) and after progression on ET with fulvestrant (MONARCH 2).1
MONARCH 2 was a phase 3 randomized, double-blind study of abemaciclib or placebo in combination with fulvestrant for patients with HR-positive, ERBB2-negative ABC whose disease had progressed on ET.1 Abemaciclib plus fulvestrant significantly improved progression-free survival (PFS) (median, 16.4 vs 9.3 months; HR, 0.553; 95% CI, 0.449-0.681; P < .001) and ORR (measurable disease, 48.1% vs 21.3%; P < .001) compared with placebo plus fulvestrant.1 At the time of PFS reporting, the OS data, an important secondary end point of this study, were immature. Herein we present the preplanned interim OS analysis of the MONARCH 2 trial at approximately 77% maturity (338 OS events of the planned 441).
Methods
Study Design and Treatment
MONARCH 2 was a global, randomized (2:1), double-blind, placebo-controlled phase 3 study of abemaciclib plus fulvestrant vs placebo plus fulvestrant in women with HR-positive, ERBB2-negative ABC who progressed during neoadjuvant or adjuvant ET, within 12 months after adjuvant ET, or while receiving first-line ET for ABC.1 The study was conducted in 142 centers in 19 countries.1 Randomization was stratified by metastatic site (visceral, bone only, or other) and ET resistance (primary or secondary). Within the stratification factors, permuted block randomization was used. Primary ET resistance was defined by the European Society for Medical Oncology guidelines and included patients whose disease relapsed during the first 2 years of neoadjuvant or adjuvant ET or progressed within the first 6 months of first-line ET for ABC. Patients who did not meet the criteria for primary ET resistance were defined as having secondary resistance.
Dosing has been previously described.1 Briefly, patients received abemaciclib (150 mg) or placebo twice daily each 28-day cycle plus fulvestrant (500 mg) by intramuscular injection on days 1 and 15 of the first cycle and on day 1 of each cycle thereafter. Treatment continued until progressive disease (PD), death, or withdrawal from the study for any other reason.
Patients
Eligible adult women of any menopausal state (premenopausal or perimenopausal women received a gonadotrophin-releasing hormone agonist) with a diagnosis of HR-positive, ERBB2-negative ABC and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 were enrolled from August 7, 2014, to December 29, 2015. Disease had to be measurable according to the Response Evaluation Criteria in Solid Tumors (RECIST Version 1.111) or non–measurable bone-only disease (eg, blastic, lytic, or mixed lytic). Patients were required to have PD while receiving neoadjuvant or adjuvant ET, within 12 months from the end of adjuvant ET, or while receiving first-line ET for ABC. Patients were ineligible if they received more than 1 line of ET or any prior chemotherapy for ABC. Other exclusion criteria included prior treatment with fulvestrant, everolimus, or CDK4 and CDK6 inhibitors, the presence of visceral crisis, or evidence or history of central nervous system metastasis.
The protocol (Supplement 1) was approved by ethical and institutional review boards at the participating institutions and all patients provided written informed consent prior to joining the study. This study was conducted in compliance with the Declaration of Helsinki, and the conduct of the trial was overseen by a steering committee. An independent data monitoring committee reviewed the safety data up to the primary analysis, and thereafter they were reviewed by the sponsor.
End Points
The primary end point was investigator-assessed PFS, analyzed from the time of randomization until PD or death. The secondary end point of OS was analyzed from the time of randomization until death. Exploratory end points included time to second disease progression (PFS2), time to chemotherapy (TTC), and chemotherapy-free survival (CFS) and were defined as follows: PFS2 was analyzed from time from randomization to discontinuation of first subsequent postdiscontinuation therapy or death (whichever is earlier). Time to chemotherapy was analyzed from randomization to initiation of first postdiscontinuation chemotherapy (censoring patients who died prior to initiation of chemotherapy). Chemotherapy-free survival was analyzed from randomization to initiation of first postdiscontinuation chemotherapy or death.
Efficacy and Safety Measures
Efficacy and safety measures have been previously described,1 including tumor measurements and bone scintigraphy. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria (CTCAE), version 4.0, and coded by MedDRA.
Statistical Analyses
All efficacy analyses were performed on the intent-to-treat (ITT) population. The family-wise type I error was controlled at .05 (2-sided), with a gate-keeping strategy between PFS and OS: only if PFS was significant would OS also be tested inferentially for significance. The study was powered for the primary end point of PFS.1 No power assumptions were made for the secondary end point of OS. For OS, the cumulative 2-sided type I error of .05 was maintained using the Lan-Demets method with the O’Brien-Fleming type α-spending function to account for multiplicity of interim and final analyses. The preplanned interim OS analysis was performed at 338 events (approximately 77% of the 441 events planned for the final analysis) using a stratified log-rank test. The 2-sided boundary P value for the interim analysis was .02. A stratified Cox proportional hazards model was used to estimate the treatment effect hazard ratio.
Unless otherwise indicated, all hypothesis tests were performed at the time of database lock on June 20, 2019, using a 2-sided, .05 level, and all CIs used a 95% confidence level. Interaction tests were performed using a Cox proportional hazards model.
Safety was assessed in all patients who received at least one dose of study drug. SAS version 9.2 or later (SAS Institute) was used for statistical analyses.
Results
Patients
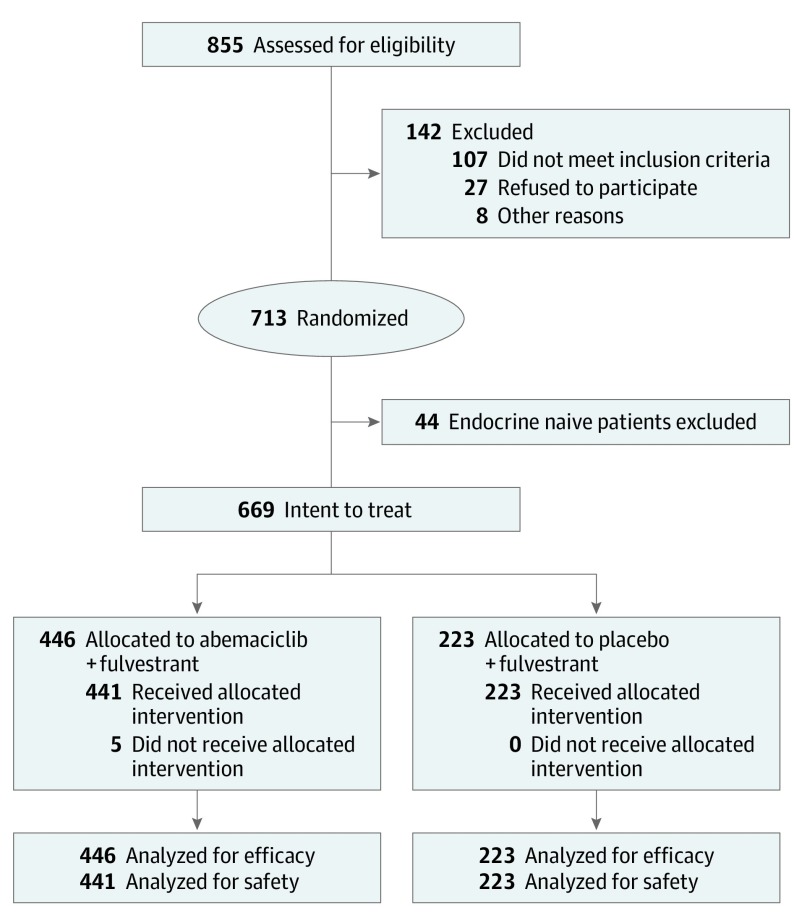
Between August 7, 2014, and December 29, 2015, 669 patients were enrolled and randomly assigned to receive abemaciclib plus fulvestrant (n = 446; median [range] age, 59 [32-91] years) or placebo plus fulvestrant (n = 223; median [range] age, 62 [32-87] years) (Figure 1). Baseline characteristics were well balanced (eTable 1 in Supplement 2). Most patients enrolled had visceral disease (n = 373), followed by bone-only disease (n = 180), and other sites of disease (eg, lymph nodes, soft tissue, skin) (n = 113). A total of 169 patients had primary ET resistance and 489 had secondary ET resistance.
Figure 1. CONSORT Diagram.
Overall Survival
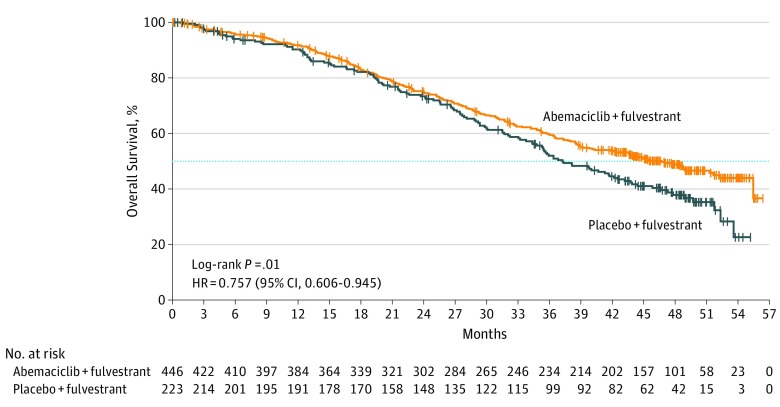
The cut off for the interim OS data analysis was June 20, 2019, at which time 338 deaths had occurred among 669 patients (abemaciclib arm, n = 211; placebo arm, n = 127). Median follow-up time was 47.7 months. The addition of abemaciclib to fulvestrant resulted in a statistically significant increase in OS compared with placebo plus fulvestrant (HR, 0.757; 95% CI, 0.606-0.945; P = .01) (Figure 2). Median OS was improved by 9.4 months, with a median OS of 46.7 months in the abemaciclib arm and 37.3 months in the placebo arm.
Figure 2. Kaplan-Meier Curve of Overall Survival in the Intent-to-Treat Population.
The number of events in the abemaciclib plus fulvestrant arm was 211 vs 127 in the placebo plus fulvestrant arm. Median overall survival in the abemaciclib plus fulvestrant arm was 46.7 months vs 37.3 months in the placebo plus fulvestrant arm. HR indicates hazard ratio.
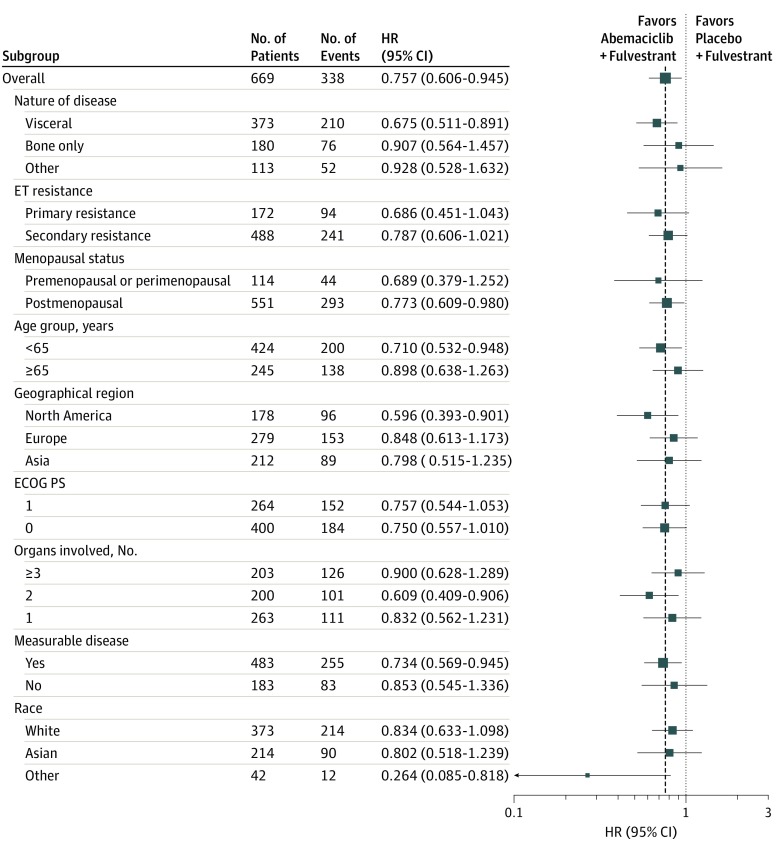
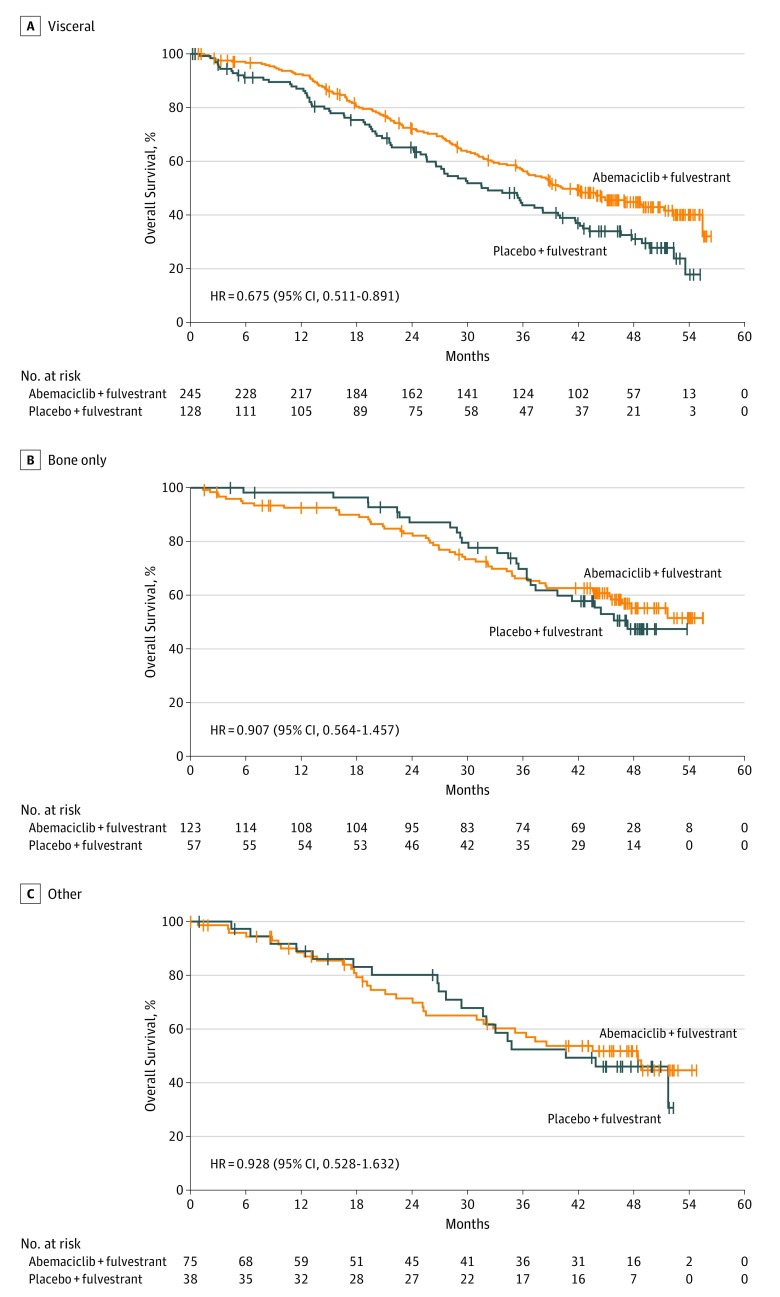
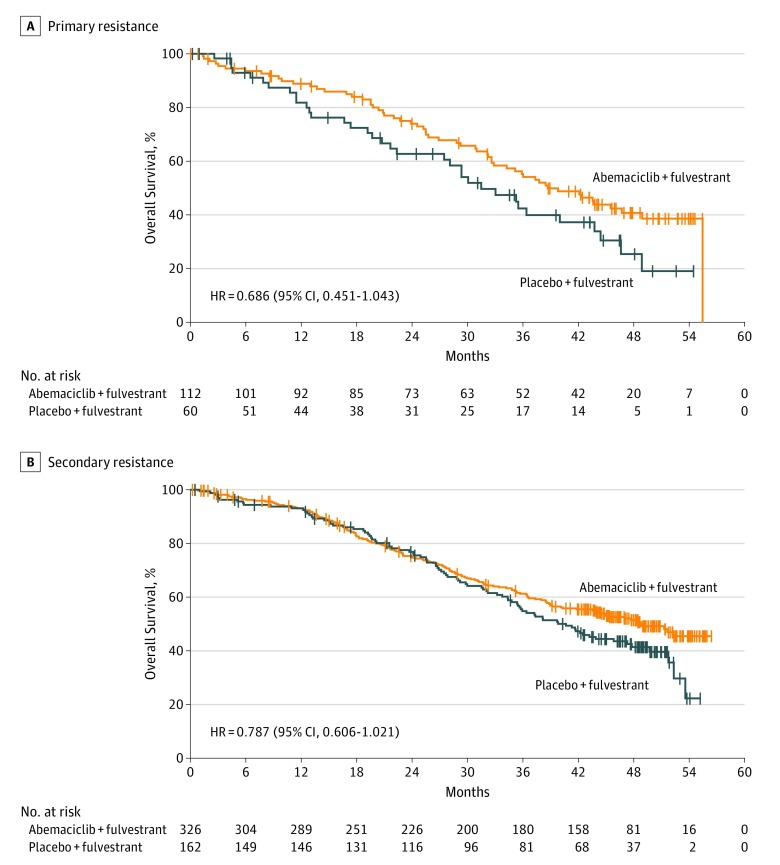
Improvement in OS was consistent among subgroups (Figure 3). Within the stratification factor of site of metastasis, earlier separation of the curves and a numerically larger effect were observed in patients with visceral disease (HR, 0.675; 95% CI, 0.511-0.891) (Figure 4A) compared with bone-only disease (HR, 0.907; 95% CI, 0.564-1.457) (Figure 4B) or other sites of disease (HR, 0.928; 95% CI, 0.528-1.632) (Figure 4C). However, no statistically significant interaction was observed. Similarly, within the stratification factor of endocrine resistance, earlier separation of the curves and a numerically larger effect were observed in patients with primary endocrine therapy resistance (HR, 0.686; 95% CI, 0.451-1.043) (Figure 5A) compared with patients with secondary endocrine therapy resistance (HR, 0.787; 95% CI, 0.606-1.021) (Figure 5B) but no statistically significant interaction was observed.
Figure 3. Subgroup Analysis of Overall Survival.
The hazard ratios (HRs) are for the abemaciclib arm vs placebo arm. The HRs are unstratified except for the overall survival (OS), which was stratified by metastatic site and endocrine therapy (ET) resistance. Overall survival HRs are indicated by diamonds, and 95% CIs are indicated by the crossing horizontal lines. The diamond size is proportional to patient subgroup population size. ECOG PS indicates Eastern Cooperative Oncology Group performance status.
Figure 4. Kaplan-Meier Curves of Overall Survival by Metastatic Site.
A, In patients with visceral disease, the median overall survival (mOS) was 40.3 months vs 32.2 months in the abemaciclib and placebo arms, respectively. B, The mOS in patients with bone-only disease was 47.3 months in the placebo arm and was not reached in the abemaciclib arm. C, In those with other sites of metastasis, mOS was 48.5 months vs 40.7 months in the abemaciclib and placebo arms, respectively. HR indicates hazard ratio.
Figure 5. Kaplan-Meier Curves of Overall Survival by Resistance to Endocrine Therapy.
A, The median overall survival (mOS) in patients with primary endocrine therapy resistance was 38.7 months vs 31.5 months in the abemaciclib arm vs placebo arm. B, In patients with secondary endocrine therapy resistance, mOS was 48.8 months vs 40.7 months in the abemaciclib vs placebo arms. HR indicates hazard ratio.
Analysis by menopausal status indicated consistent OS results for premenopausal or perimenopausal (HR, 0.689; 95% CI, 0.379-1.252) and postmenopausal (HR, 0.773; 95% CI, 0.609-0.980) women (Figure 3).
Treatment Duration
At the time of data cutoff, 77 (17.3%) of 446 patients in the abemaciclib arm and 8 (3.6%) of 223 patients in the placebo arm continued to receive study treatment. Patients in the abemaciclib arm received a mean of 18.9 cycles compared with 13.7 cycles in the placebo arm. Treatment for 2 years or more (26 cycles) was achieved in 126 (28.6%) patients in the abemaciclib arm and 33 (14.8%) in the placebo arm.
Postdiscontinuation Therapy
At the time of data cutoff, a total of 584 patients in the ITT population had discontinued study treatment. Of these, 461 (78.9%) received postdiscontinuation therapy: 281 patients (76.2%) in the abemaciclib arm compared with 180 patients (83.7%) in the placebo arm. Postdiscontinuation therapy was well balanced considering the number of patients remaining on study treatment in the abemaciclib arm (eFigure 1 in Supplement 2).
Of the 461 patients who received any postdiscontinuation therapy, the first subsequent therapy was chemotherapy for 209 patients (45.3%), single-agent ET for 119 patients (25.8%), and an everolimus-based therapy for 80 patients (17.4%). Median duration of chemotherapy was 4.4 months and 4.6 months in the abemaciclib arm and placebo arm, respectively. Median duration of single-agent ET was 5.3 months in the abemaciclib arm and 4.8 months in the placebo arm, and median duration of everolimus-based therapy was 4.5 months and 8.8 months in the abemaciclib arm and placebo arm, respectively. Overall, the duration of these classes of postdiscontinuation therapy was similar across arms with the exception that abemaciclib may have affected the duration of everolimus-based postdiscontinuation treatment options. However, because of the small sample size of the everolimus-based therapy group, further follow-up study is warranted.
Other Exploratory End Points
Consistent with the primary analysis,1 the updated PFS from this interim analysis was significantly improved by the addition of abemaciclib to fulvestrant (HR, 0.536; 95% CI, 0.445-0.645) (eFigure 2A in Supplement 2). Median PFS was 16.9 months in the abemaciclib arm and 9.3 months in the placebo arm. The 3-year PFS rate was 29.9% in the abemaciclib arm vs 10.1% in the placebo arm.
Time to second disease progression, TTC, and CFS were all statistically significantly prolonged with the addition of abemaciclib to fulvestrant. Median PFS2 was 23.1 months in the abemaciclib-treated arm vs 20.6 months in the placebo arm (HR, 0.675; 95% CI, 0.558-0.816) (eFigure 3 in Supplement 2). Median TTC (censoring patients who died prior to receiving chemotherapy) was 50.2 months in the abemaciclib arm vs 22.1 months in the placebo arm (HR, 0.625; 95% CI, 0.501-0.779) (eFigure 4A in Supplement 2). In the abemaciclib arm, 70 patients (15.7%) vs 41 patients (18.4%) in the placebo arm died prior to receiving any chemotherapy. Chemotherapy-free survival (including both chemotherapy and death as events) was 25.5 months in the abemaciclib arm vs 18.2 months in the placebo arm (HR, 0.638; 95% CI, 0.527-0.773) (eFigure 4B in Supplement 2).
Safety
No new safety signals were observed relative to the primary analysis. The type and relative frequency of AEs remained consistent with those in the primary analysis (eTable 2 in Supplement 2). Common hematologic AEs graded 3 or higher in the abemaciclib arm included neutropenia (n = 131 [29.9%]), anemia (n = 40 [9.1%]), and leukopenia (n = 49 [11.1%]). No new cases of febrile neutropenia were reported relative to the primary analysis (n = 6). Diarrhea was the most frequent nonhematologic AE reported in the abemaciclib arm with 64 (14.5%) CTCAE grade 3 events. Most diarrhea cases occurred during the first 4 weeks of abemaciclib initiation and were effectively managed using loperamide or dose adjustments; treatment discontinuation due to diarrhea remained infrequent (1.4%). Within the subset of patients who had been in the study for 1 year or more (abemaciclib arm, n = 240 vs placebo arm, n = 89), new treatment-emergent diarrhea events of any grade appearing after 1 year or more of treatment were reported in 68 patients (28.3%) in the abemaciclib arm vs 10 (11.2%) in the placebo arm.
Discussion
The MONARCH 2 study demonstrated that the addition of abemaciclib, dosed on a continuous, twice-daily schedule, to fulvestrant resulted in a statistically significant improvement in OS, a key secondary objective of this phase 3 study. Patients in the abemaciclib arm received a clinically meaningful median OS improvement of 9.4 months in this ET-resistant setting. To our knowledge, these data constitute the largest absolute OS benefit reported so far in a phase 3 clinical trial for HR-positive, ERBB2-negative ABC.12,13
The OS results of this prespecified interim analysis, including 338 OS events of the planned 441 events needed for the final analysis (77% maturity), met the predefined O’Brien-Fleming boundary for significance and are therefore definitive. Despite being generated from an interim analysis, the high degree of maturity of these data fosters their validity.
The clinically meaningful and statistically significant OS benefit of 9.4 months observed in the abemaciclib arm in the ITT population was consistent with the statistically significant PFS benefit observed in the primary analysis of MONARCH 2. The absolute PFS improvement of 7.6 months translated into a 9.4-month prolongation of OS, which exceeds the magnitude of the PFS benefit by almost 2 months. Similarly, PFS2, defined as the time measured from randomization to the end of the subsequent line of therapy after MONARCH 2 study treatment, demonstrated a statistically significant improvement in favor of the abemaciclib arm. This consistency in statistical significance across different clinically relevant end points further corroborates the strength of these interim data.
Having observed statistically significant OS benefit for the ITT population, further analyses for clinically relevant subgroups were conducted. Improvement in OS was consistent across the 2 stratification factor subgroups: site of metastasis and ET resistance. Interestingly, patients with visceral disease at baseline, a poor prognostic subgroup,14,15 had a numerically more pronounced OS effect relative to patients with nonvisceral disease (bone only or other sites), with separation between the abemaciclib and placebo arms occurring in the first 6 months. This is largely consistent with a previous exploratory PFS analysis across the abemaciclib phase 3 program, indicating that, among subgroups, patients with poor prognostic factors received the largest PFS benefit from the addition of abemaciclib to ET.16 In contrast to the OS data for patients with poor prognosis, the OS results for the better prognosis subgroups of bone-only disease and other sites of disease appear to be less mature, and further follow-up may be particularly warranted for these subgroups. Notably, the updated PFS data as an earlier end point showed a clear separation of the curves in favor of the abemaciclib arm for both the bone only (HR, 0.580; 95% CI, 0.398, 0.844) and other (HR, 0.683; 95% CI, 0.443, 1.053) subgroups (eFigures 2C and 2D in Supplement 2).
Overall survival subgroup analysis done in patients with primary vs secondary resistance to ET17,18 showed a numerically stronger OS effect (HR) in patients with primary endocrine resistance receiving abemaciclib plus fulvestrant, with separation between the abemaciclib and placebo arms occurring in the first year. This contrasts with recently published OS data for palbociclib and fulvestrant in the PALOMA-3 study for patients with HR-positive, ERBB2-negative ABC.13 Although it is important to note that the study’s eligibility criteria were not the same as in MONARCH 2, PALOMA-3 did not demonstrate a statistically significant OS improvement in the ITT population (HR, 0.81; 95% CI, 0.64-1.03; P = .09). A subgroup analysis in PALOMA-3 indicated a numerical OS improvement (HR, 0.72; 10.0-month median OS improvement) for patients with sensitivity to previous ET (defined as disease control for 24 weeks or more of prior ET for ABC or 24 months or more of adjuvant ET before recurrence) but not for patients without sensitivity to previous ET (HR, 1.14; no improvement in median OS).13 Similar divergent results have been observed for PFS in these subgroups in PALOMA-3 and MONARCH 2,1,19 suggesting a potential differential activity of abemaciclib in patients with primary ET resistance. For this steadily increasing group of patients who progress on or within 12 months of completing adjuvant ET,20,21 abemaciclib plus fulvestrant might be an attractive treatment option based on these data. Although the mechanism underlying the OS effects in the visceral and primary ET-resistant populations is unknown, relevant factors may include the ability of abemaciclib to be dosed continuously and its greater potency for CDK4 over CDK6 as demonstrated in enzymatic assays.1,6 Further studies may be warranted to confirm these observations prospectively.
Recently, ribociclib, another CDK4 and CDK6 inhibitor, reported a statistically significant OS improvement in combination with ET (nonsteroidal aromatase inhibitor or tamoxifen) in premenopausal or perimenopausal patients with HR-positive, ERBB2-negative ABC based on an interim (MONALEESA-7) analysis.12 In this study, the combination of ribociclib plus ET demonstrated a statistically significant HR of 0.71 (95% CI, 0.54-0.95). In MONARCH 2, about 17% of the enrolled patients (n = 114 of 669) were premenopausal or perimenopausal women. In this subgroup, abemaciclib plus fulvestrant demonstrated an OS HR of 0.689 (95% CI, 0.379-1.252), which was consistent with that of the postmenopausal group (HR, 0.773; 95% CI, 0.609-0.980) and the overall MONARCH 2 population. These data are consistent overall with the MONALEESA-7 data and demonstrate that premenopausal or perimenopausal patients with HR-positive, ERBB2-negative ABC also derived meaningful OS improvement from abemaciclib plus fulvestrant.
There was no obvious difference between the study arms regarding the frequency of postdiscontinuation treatment or the modalities administered. Postdiscontinuation therapies received were numerically higher in the placebo arm than in the abemaciclib arm (eFigure 1 in Supplement 2); however, considering the number of patients still on treatment in the abemaciclib arm, the numbers appeared to be balanced overall across the 2 treatment arms. The duration of the immediate subsequent line of therapy after the completion of study treatment was similar overall for both study arms, which indicates that standard treatments had similar efficacy following completion of study treatment. A total of 17% of patients (38 of 223) in the placebo arm received a CDK4 and CDK6 inhibitor as postdiscontinuation therapy. This “crossover” might have attenuated an even more significant OS benefit for the abemaciclib arm in MONARCH 2.
One important treatment consideration in HR-positive ABC is to postpone the use of chemotherapy for as long as possible in an effort to maintain and optimize quality of life for patients.22 Although this TTC difference is substantial, the concept of TTC is hampered by a lack of adjustment for patient death because patients who died prior to receiving chemotherapy were censored. An alternative measure for the effect of delaying the receipt of chemotherapy is the analysis of CFS, including patient death prior to receiving chemotherapy as an event. Abemaciclib plus fulvestrant also demonstrated a statistically significant improvement in CFS with an absolute improvement of 7.3 months. Taken together, TTC and CFS highlight a statistically significant advantage for patients who received abemaciclib plus fulvestrant.
There were no new safety signals observed for abemaciclib. The extended follow-up at this interim provides long-term safety data supporting a generally tolerable and manageable safety profile for abemaciclib plus fulvestrant. The incidence of any grade diarrhea AEs reported by patients 1 year or more after initiation of abemaciclib was more than 3-fold lower compared with the overall incidence of diarrhea. The long-term tolerability of abemaciclib plus fulvestrant is highlighted by the 77 patients still on investigational treatment for more than 3.5 years at the time of the interim analysis.
Limitations
The current interim analysis has limitations. Although these interim results are statistically definitive, it will nevertheless be important and clinically meaningful to further characterize the OS and other exploratory efficacy end points in this study, particularly in those subgroups of patients with more favorable prognostic factors (eg, patients with secondary endocrine resistance or bone-only disease) where it appears that the observation time might not yet be sufficient as the OS Kaplan-Meier curves only just start to separate (eg, patients with secondary endocrine resistance) or have not yet reached a median OS value (eg, patients with bone-only disease). Such analyses will be conducted as post-hoc analyses at the time of the originally planned final OS analysis for MONARCH 2. In combination with results from other studies (eg, MONARCH 310 and SONIA23 trials), these data may address the question of whether abemaciclib and other CDK4 and CDK6 inhibitors should be used as first-line treatment in combination with ET or following initial ET alone. In addition, another MONARCH 2 cohort, including ET naive patients will be evaluated in the future. Besides clinical trial data, real-world evidence studies will also be important to answer this clinically relevant question.
Conclusions
In conclusion, MONARCH 2 demonstrated a statistically significant and clinically meaningful improvement in OS by 9.4 months for abemaciclib plus fulvestrant in patients with HR-positive, ERBB2-negative ABC following progression on prior ET. This OS benefit was consistent across subgroups. Among subgroups, the strongest effects were observed in patients with poor prognostic factors such as visceral metastasis and primary ET resistance. No new safety signals were observed and abemaciclib plus fulvestrant significantly delayed the receipt of subsequent chemotherapy.
Trial Protocol
eTable 1. Patient and Disease Baseline Characteristics
eTable 2. Treatment-emergent Adverse Events
eFigure 1. Post Discontinuation Therapy
eFigure 2. Kaplan-Meier Plots of Updated Progression-free Survival
eFigure 3. Kaplan-Meier Plots of Time to Second Disease Progression (PFS2)
eFigure 4. Kaplan-Meier Plots of Time to Chemotherapy (TTC) and Chemotherapy-free Survival (CFS)
Data Sharing Statement
References
- 1.Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884. doi: 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 2.Milani A, Geuna E, Mittica G, Valabrega G. Overcoming endocrine resistance in metastatic breast cancer: Current evidence and future directions. World J Clin Oncol. 2014;5(5):990-1001. doi: 10.5306/wjco.v5.i5.990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016;34(25):3069-3103. doi: 10.1200/JCO.2016.67.1487 [DOI] [PubMed] [Google Scholar]
- 4.Lobbezoo DJ, van Kampen RJ, Voogd AC, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141(3):507-514. doi: 10.1007/s10549-013-2711-y [DOI] [PubMed] [Google Scholar]
- 5.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425-439. doi: 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- 6.Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase ii study of abemaciclib, a CDK4 and CDK6 Inhibitor, as a single agent, in patients with refractory HR+/HER2- metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218-5224. doi: 10.1158/1078-0432.CCR-17-0754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hortobagyi GN. Ribociclib for the first-line treatment of advanced hormone receptor-positive breast cancer: a review of subgroup analyses from the MONALEESA-2 trial. Breast Cancer Res. 2018;20(1):123. doi: 10.1186/s13058-018-1050-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rugo HS. Achieving improved survival outcomes in advanced breast cancer. N Engl J Med. 2019;381(4):371-372. doi: 10.1056/NEJMe1906236 [DOI] [PubMed] [Google Scholar]
- 9.Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32(5):825-837. doi: 10.1007/s10637-014-0120-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646. doi: 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 12.Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307-316. doi: 10.1056/NEJMoa1903765 [DOI] [PubMed] [Google Scholar]
- 13.Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926-1936. doi: 10.1056/NEJMoa1810527 [DOI] [PubMed] [Google Scholar]
- 14.Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19(12):2012-2019. doi: 10.1093/annonc/mdn424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leone BA, Vallejo CT, Romero AO, et al. Prognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosis. Breast Cancer Res Treat. 2017;161(3):537-548. doi: 10.1007/s10549-016-4066-7 [DOI] [PubMed] [Google Scholar]
- 16.Di Leo A, O’Shaughnessy J, Sledge GW Jr, et al. Prognostic characteristics in hormone receptor-positive advanced breast cancer and characterization of abemaciclib efficacy. NPJ Breast Cancer. 2018;4:41. doi: 10.1038/s41523-018-0094-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso F, Costa A, Senkus E, et al. Corrigendum to “3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3)” [Breast 31 (February 2017) 244-259]. [Breast 31 (February 2017) 244-259]. Breast. 2017;32:269-270. doi: 10.1016/j.breast.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 18.Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol. 2017;28(12):3111. doi: 10.1093/annonc/mdx036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner NC, Ro J, André F, et al. ; PALOMA3 Study Group . Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209-219. doi: 10.1056/NEJMoa1505270 [DOI] [PubMed] [Google Scholar]
- 20.Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO Clinical Practice Guideline focused update. J Clin Oncol. 2019;37(5):423-438. doi: 10.1200/JCO.18.01160 [DOI] [PubMed] [Google Scholar]
- 21.Pan H, Gray R, Braybrooke J, et al. ; EBCTCG . 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836-1846. doi: 10.1056/NEJMoa1701830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman PTM, Neven P, Sohn JH, et al. Health-related quality of life (HRQoL) in MONARCH 2: Abemaciclib plus fulvestrant in women with HR+, HER2- advanced breast cancer (ABC) who progressed on endocrine therapy. J Clin Oncol. 2019;36(15)(suppl):1049. doi: 10.1200/JCO.2018.36.15_suppl.1049 [DOI] [Google Scholar]
- 23.van Ommen-Nijhof A, Konings IR, van Zeijl CJJ, et al. ; SONIA study steering committee . Selecting the optimal position of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer - the SONIA study: study protocol for a randomized controlled trial. BMC Cancer. 2018;18(1):1146. doi: 10.1186/s12885-018-4978-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Patient and Disease Baseline Characteristics
eTable 2. Treatment-emergent Adverse Events
eFigure 1. Post Discontinuation Therapy
eFigure 2. Kaplan-Meier Plots of Updated Progression-free Survival
eFigure 3. Kaplan-Meier Plots of Time to Second Disease Progression (PFS2)
eFigure 4. Kaplan-Meier Plots of Time to Chemotherapy (TTC) and Chemotherapy-free Survival (CFS)
Data Sharing Statement