Key Points
Question
Is there a difference in functional outcome at 3 months between patients who receive general anesthesia or procedural sedation during stroke thrombectomy?
Findings
In this individual patient data meta-analysis of 3 randomized clinical trials that included 368 patients with acute ischemic stroke in the anterior circulation, the use of general anesthesia during thrombectomy, compared with procedural sedation, was significantly associated with less disability at 3 months (common odds ratio for categorical shift in the modified Rankin Scale score, 1.58).
Meaning
General anesthesia during thrombectomy, compared with procedural sedation, was associated with less disability at 3 months after ischemic stroke, although the findings should be interpreted tentatively because the individual trials analyzed were single-center trials and disability was the primary outcome in only 1 trial.
Abstract
Importance
General anesthesia during thrombectomy for acute ischemic stroke has been associated with poor neurological outcome in nonrandomized studies. Three single-center randomized trials reported no significantly different or improved outcomes for patients who received general anesthesia compared with procedural sedation.
Objective
To detect differences in functional outcome at 3 months between patients who received general anesthesia vs procedural sedation during thrombectomy for anterior circulation acute ischemic stroke.
Data Source
MEDLINE search for English-language articles published from January 1, 1980, to July 31, 2019.
Study Selection
Randomized clinical trials of adults with a National Institutes of Health Stroke Scale score of at least 10 and anterior circulation acute ischemic stroke assigned to receive general anesthesia or procedural sedation during thrombectomy.
Data Extraction and Synthesis
Individual patient data were obtained from 3 single-center, randomized, parallel-group, open-label treatment trials with blinded end point evaluation that met inclusion criteria and were analyzed using fixed-effects meta-analysis.
Main Outcomes and Measures
Degree of disability, measured via the modified Rankin Scale (mRS) score (range 0-6; lower scores indicate less disability), analyzed with the common odds ratio (cOR) to detect the ordinal shift in the distribution of disability over the range of mRS scores.
Results
A total of 368 patients (mean [SD] age, 71.5 [12.9] years; 163 [44.3%] women; median [interquartile range] National Institutes of Health Stroke Scale score, 17 [14-21]) were included in the analysis, including 183 (49.7%) who received general anesthesia and 185 (50.3%) who received procedural sedation. The mean 3-month mRS score was 2.8 (95% CI, 2.5-3.1) in the general anesthesia group vs 3.2 (95% CI, 3.0-3.5) in the procedural sedation group (difference, 0.43 [95% CI, 0.03-0.83]; cOR, 1.58 [95% CI, 1.09-2.29]; P = .02). Among prespecified adverse events, only hypotension (decline in systolic blood pressure of more than 20% from baseline) (80.8% vs 53.1%; OR, 4.26 [95% CI, 2.55-7.09]; P < .001) and blood pressure variability (systolic blood pressure >180 mm Hg or <120 mm Hg) (79.7 vs 62.3%; OR, 2.42 [95% CI, 1.49-3.93]; P < .001) were significantly more common in the general anesthesia group.
Conclusions and Relevance
Among patients with acute ischemic stroke involving the anterior circulation undergoing thrombectomy, the use of protocol-based general anesthesia, compared with procedural sedation, was significantly associated with less disability at 3 months. These findings should be interpreted tentatively, given that the individual trials examined were single-center trials and disability was the primary outcome in only 1 trial.
This meta-analysis pools individual patient data to estimate the association between receipt of general anesthesia vs procedural sedation and 3-month disability among patients with acute ischemic stroke undergoing mechanical thrombectomy from 3 randomized trials.
Introduction
Mechanical thrombectomy is standard of care for eligible patients with acute ischemic stroke caused by large vessel occlusion in the anterior circulation.1 An unresolved matter is whether the choice of anesthetic strategy affects functional outcome. Proposed advantages of general anesthesia are patient immobilization, pain management, and airway protection. Disadvantages of general anesthesia are potential compromises in cerebral hemodynamics and intervention delay. Proposed benefits of procedural sedation include clinical monitoring, stable hemodynamics, and a potentially shorter procedure. The disadvantages of procedural sedation, including unprotected airways and patient movement, may promote procedural complications and prolong the intervention. Retrospective studies2,3,4,5,6 and meta-analyses7,8 have suggested poorer functional outcome and higher mortality in patients who receive general anesthesia than procedural sedation. Differences in sedative agents (eg, type, dosage) were suggested to be associated with differences in functional outcome.9 However, these studies have been limited by selection bias (ie, confounding by indication) because patients with more severe stroke and poorer clinical presentation were more likely to receive general anesthesia. Furthermore, these nonrandomized studies did not focus primarily on details of anesthetic strategy.
Three single-center randomized clinical trials, SIESTA (Sedation vs Intubation for Endovascular Stroke Treatment; NCT02126085),10 ANSTROKE (Anesthesia During Stroke; NCT01872884),11 and GOLIATH (General or Local Anaesthesia in Intra Arterial Therapy; NCT02317237),12 have demonstrated results in functional outcome that were not significantly different between the 2 anesthesia regimens or better for patients receiving general anesthesia. However, all 3 studies lacked generalizability because of their single-center design, and 2 studies were limited by the choice of surrogate outcome parameters (early neurological improvement and infarct growth).
Because mechanical thrombectomy has become the mainstay of therapy for patients with acute large vessel occlusion stroke, the need to clarify the optimal anesthetic strategy is imperative. This study was an individual patient data meta-analysis that was performed to assess the association between anesthetic strategy and the 3-month functional outcome in patients undergoing mechanical thrombectomy for acute ischemic stroke.
Methods
Selection Criteria
A literature search limited to English-language articles published from January 1, 1980, to July 31, 2019, in MEDLINE using the terms “randomized (randomised) controlled trial,” “endovascular stroke treatment,” “stroke thrombectomy,” “conscious sedation,” “procedural sedation,” “awake sedation,” “local an(a)esthesia,” and “general an(a)esthesia” as subject headings and text words in various combinations was conducted by 1 author (S.S.). The inclusion criteria for the study search were inclusion of adult participants with a National Institutes of Health Stroke Scale (NIHSS) score of at least 10 and acute ischemic stroke in the anterior circulation, who were randomized to receive general anesthesia vs procedural sedation during thrombectomy; informed consent provided by the patient or legal representative; and decision for thrombectomy according to local protocols and standardized in-hospital protocols for neuroanesthesia and physiology targets. The exclusion criteria for the study search included studies with participants with radiologic ambiguity concerning infarction and vessel occlusion, acute ischemic stroke in the posterior circulation, or additional intracerebral hemorrhage (project outline in the Supplement).
The results of the literature search were presented by 1 author (S.S.) to the trial executive committee (composed of the 2 lead representatives of each trial [6 individuals in total]) in a joint meeting in July 2018 in Gothenburg, Sweden, and a telephone conference in July 2019 to discuss the search results and reach a consensus for inclusion by resolving potential conflicts by discussion. The collaborators of the SIESTA, ANSTROKE, and GOLIATH Association (SAGA) reached the consensus that only 3 trials met all formal inclusion criteria to be included in the meta-analysis (eFigure 1 in the Supplement) and agreed to aggregate individual patient data from these 3 trials. Risk of bias was assessed using the Cochrane ROB-2 tool by 2 independent methodologist raters who resolved any disagreement by discussion.
All recruited patients or their legal representatives had provided informed consent according to each trial protocol, and all trials had been approved by their respective local ethics committee. The decision for patients to undergo mechanical thrombectomy was made based on local treatment protocols. Prespecified anesthetic protocols, including physiology targets, for procedural sedation and general anesthesia, the latter with tracheal intubation, have previously been reported in detail for each of the trials.10,11,12 The Institute of Medical Biometry and Statistics at the University of Heidelberg, Germany, supervised the creation of a unified database and established a statistical analysis plan a priori that was agreed on by the principal investigators of the trials.
All patients aged 18 years or older presenting with an NIHSS score of at least 10 and acute ischemic stroke in the anterior circulation who were recruited in any of these 3 trials were included in the analysis.
A few parameters from the original trials were additionally adapted or recalculated from the raw data; for details, see the statistical analysis plan (project outline in the Supplement). That protocol of the meta-analysis was prospectively designed and prespecified prior to the start of any data analysis by the trial collaborators, but not registered.
Outcomes
The primary outcome was the degree of disability, measured via modified Rankin Scale (mRS) score (range, 0-6; lower scores indicate less disability), at 3 months analyzed with the common odds ratio (cOR) to detect ordinal shift in the distribution of disability over the range of the mRS.
A total of 15 secondary outcomes were analyzed, including 4 clinical, 2 imaging, and 9 care process outcomes, as well as 5 adverse events. The clinical secondary outcomes were the improvement of NIHSS score after 24 hours (range 0-42; higher scores indicate more severe neurological deficits; a difference of 4 points was considered to be clinically relevant),13 the dichotomization of mRS score to functional independence (mRS score of 0-2), and ambulatory and capable of bodily self-care or better (mRS score of 0-3) after 3 months, as well as in-hospital mortality. Imaging secondary outcomes included successful recanalization, measured via the modified thrombolysis in cerebral infarction (mTICI) scale score (range 0-3; lower scores indicate less perfusion), and infarct growth. The process of care outcomes included time intervals of the periprocedural workflow. Adverse events included intervention-associated complications, delayed intubation, and antibiotics for suspected pneumonia. These and all other secondary outcomes are listed in eTable 2 in the Supplement and were specified in the statistical analysis plan (project outline and eTable 2 in the Supplement).
Data Analysis
The primary outcome was the mRS score 3 months after stroke onset, which is measured on an ordinal scale. The following question at test was defined:
| H0: cORGeneralAnesthesia,ProcedualSedation = 1 H1: cORGeneralAnesthesia,ProcedualSedation ≠ 1 |
in which the result of the equation is the cOR between the general anesthesia group and the procedural sedation group. The null hypothesis (H0) was tested by implementing an individual patient data meta-analysis (1-stage approach). Treatment group relation to the primary end point was evaluated in a mixed cumulative proportional logistic regression model with adjustment for 7 baseline patient features, including age, sex, neurological deficit (NIHSS score), prestroke disability (mRS score), extent of early brain ischemic change (Alberta stroke program early computed tomography score [ASPECTS]), location of occlusion, and hemispheric laterality, with adjustment for treatment with intravenous alteplase (yes or no).
The proportional odds assumption was assessed using the score χ2 test. A random intercept for the study was included to cluster the participants with the study they were recruited into.14 The prespecified analytic plan included a fixed-effect meta-analysis as well as a random-effect meta-analysis with respect to the treatment effect. Treatment group was planned to be considered either in the fixed-effect model or in the random-effect model to assess differences between the 2 models. However, the models did not reach convergence after including a random treatment effect (presumably because of the low number of studies). Therefore, only the results obtained in the model with a fixed treatment effect over studies (and a random intercept) are presented with an overall 2-sided significance level. The Kenward-Roger approximation was used when calculating the degrees of freedom.
In addition, a 2-stage approach was applied.15 In the first stage, a proportional odds model for each study was estimated that included the same variables as the main model. In the second stage, a meta-analysis was performed based on the treatment effects estimated in the first stage. Imputation for missing data was not performed because only 3 values were missing (for ASPECTS). In the primary analysis, patients were analyzed according to their randomization group in each trial. All patients were included. Further details regarding this statistical approach are described in the reports and publications of each trial.10,11,12
Heterogeneity between the trials was assessed by estimating τ2 (obtained in the 2-stage approach). Furthermore, an additional model included an interaction term between the original study and the group variable. In this model, no random effects were included, but study was taken as an additional fixed factor.
Several sensitivity and additional analyses were conducted. First, the primary analysis was repeated using a per-protocol and an as-treated population. In the per-protocol analysis, patients with major protocol violations, including violations of inclusion or exclusion criteria, treatment according to randomization group, and group treatment protocols, were excluded. In the as-treated analysis, patients who switched treatment groups (ie, from procedural sedation to general anesthesia) were analyzed in the group of the treatment that the patients actually received. In addition, an analysis was performed in which patients who switched treatment groups were excluded. Second, the primary outcome was dichotomized (mRS score of 0-2 vs 3-6 as well as mRS score of 0-3 vs 4-6), whereby a logistic mixed-effects model was applied including the same fixed and random effects as in the primary analysis. Third, the following subgroups were considered: (1) baseline NIHSS score less than or equal to 17 vs greater than 17, (2) age 70 years or younger vs older than 70 years, (3) male vs female sex, (4) yes or no recombinant tissue-type plasminogen activator, (5) onset to door time (median split), and (6) ASPECTS less than 8 vs 8 to 10. An interaction term with the group variable was included in the primary analysis model (separately for each subgroup) to test for differences in the group effect.
The secondary outcomes (eg, peri-interventional aspects of feasibility and adverse events; project outline in the Supplement) were analyzed using mixed-effects models adjusted as necessary. The same fixed-effect and random-effect models were included. The cOR for group or the adjusted mean difference between the groups obtained from the fitted mixed models are presented as appropriate.
Due to the expectedly skewed data distribution (with many small values and only a few high values), all variables measuring time intervals were log-transformed before the individual patient data meta-analysis model was applied.
An overall 2-sided significance level of α = .05 was applied. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Analyses were done using SAS, version 9.4 (SAS Institute) and R, version 3.5.1, with the additional forestplot (version 1.7.2), ordinal (version 2019.3-9), and meta (version 4.9-4) packages.
Results
Patient Characteristics
Three trials10,11,12 met all formal inclusion criteria and were included in the meta-analysis. The specific aspects of each trial are summarized in Table 1 and eTable 1 in the Supplement. All 3 trials were considered to have low risk of bias (eFigure 2 in the Supplement).
Table 1. Characteristics of Studies Included in a Meta-analysis Examining the Association of General Anesthesia vs Procedural Sedation With Functional Outcome Among Patients With Acute Ischemic Stroke Undergoing Thrombectomy.
| Trial Characteristics | SIESTA10 | ANSTROKE11 | GOLIATH12 |
|---|---|---|---|
| Inclusion criteria | Age ≥18 y | Age ≥18 y | Age ≥18 y |
| NIHSS score ≥10 | NIHSS score ≥10 | NIHSS score ≥10 | |
| Acute ischemic stroke in anterior circulation | Acute ischemic stroke in anterior circulation | Acute ischemic stroke in anterior circulation | |
| Waiver of consent before randomization and deferred informed consent by the patient or a legal representative after mechanical thrombectomy | Oral consent prior to randomization from patient or relative and written consent after treatment from patient or relative | Waiver of consent prior randomization and deferred informed consent by the patient or a legal representative after mechanical thrombectomy | |
| Exclusion criteria and reasons for exclusion | Radiologic ambiguity concerning infarction and vessel occlusion (n = 1561) Additional clinical and radiologic signs of occlusion of a vessel other than those listed under inclusion criteria Additional intracerebral hemorrhage Severe agitation on admission (n = 42) 2 Simultaneous thrombectomies and 1 not managed by neurointensivists (n = 35) Obvious loss of airway protective reflexes and/or vomiting on admission (n = 7) Obviously difficult or known difficult airway Known intolerance of certain medication for sedation and/or analgesia Other (n = 11) |
Radiologic ambiguity concerning infarction and vessel occlusion (n = 17) Additional clinical and radiologic signs of an occlusion of a vessel other than those listed under inclusion criteria (n = 29) Additional intracerebral hemorrhage Not eligible because of anesthesiological concerns (n = 59) Could not receive mechanical thrombectomy within 8 h from symptoms first observed NIHSS score <14 in left-sided stroke Premorbid mRS score of ≥4 Informed consent not timely obtained or died (n = 39) Inclusion failure (n = 11) Other (n = 60) |
Radiologic ambiguity concerning infarction and vessel occlusion (n = 1105) Additional clinical and radiologic signs of occlusion of a vessel other than those listed under inclusion criteria (n = 107) Additional intracerebral hemorrhage Known intolerance of certain medication for sedation and/or analgesia Could not receive mechanical thrombectomy within 6 h from symptoms first observed (n = 35) Premorbid mRS score >2 Initial infarct volume on MRI of >70 mL (n = 62) Contraindications to MRI (n = 18) Intubated at arrival (n = 7) Included in other studies (n = 3) Other (n = 36) |
| No. of patients approached | 1808 | 321 | 1501 |
| No. of patients randomized | 152 | 106 | 128 |
| No. of patients analyzed | 150 | 90 | 128 |
| Primary end point | Change in NIHSS score after 24 h | mRS after 3 mo | Infarct growth measured via MRI |
| Reasons for conversion from procedural sedation to general anesthesia | Agitation and/or movement Aspiration Respiratory failure Vomiting Other |
Agitation and/or movement Aspiration Puncture of common carotid artery |
Agitation Aspiration Respiratory failure Vomiting |
Abbreviations: ANSTROKE, Anesthesia During Stroke; GOLIATH, General or Local Anesthesia in Intra Arterial Therapy; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SIESTA, Sedation vs Intubation for Endovascular Stroke Treatment.
All 368 randomized patients from the 3 trials (Table 1) were included in the meta-analysis, including 183 (49.7%) patients who were assigned to receive general anesthesia and 185 (50.3%) who were assigned to receive procedural sedation. Details of patient characteristics from each individual trial have been published previously (Table 2).10,11,12 There were no major protocol violations, with the exception of 1 patient who was randomized to receive general anesthesia and was mistakenly given procedural sedation. The as-treated analysis included 203 (55.2%) patients in the general anesthesia group (82 [54.7%] from SIESTA, 52 [57.8%] from ANSTROKE, and 69 [53.9%] from GOLIATH) and 165 (44.8%) in the procedural sedation group (68 [45.3%] from SIESTA, 38 [42.2%] from ANSTROKE, and 59 [46.1%] from GOLIATH).
Table 2. Baseline Characteristics of Patients With Acute Ischemic Stroke in a Study of the Association of General Anesthesia vs Procedural Sedation During Thrombectomy With Functional Outcome.
| Characteristic | No. (%) | |
|---|---|---|
| General Anesthesia (n = 183) | Procedural Sedation (n = 185) | |
| Demographic Characteristics | ||
| Age, mean (SD), y | 71.5 (12.1) | 71.5 (13.8) |
| Sex | ||
| Men | 110 (60.1) | 95 (51.4) |
| Women | 73 (39.9) | 90 (48.6) |
| Patients included from each study | ||
| SIESTA | 73 (39.9) | 77 (41.6) |
| ANSTROKE | 45 (24.6) | 45 (24.3) |
| GOLIATH | 65 (35.5) | 63 (34.1) |
| Vascular risk factors | ||
| Hypertension | 119 (65.0) | 108/184 (58.7) |
| Atrial fibrillation | 78 (42.6) | 81/184 (44.0) |
| Hyperlipidemia | 73/182 (40.1) | 75 (40.5) |
| Diabetes mellitus | 35 (19.1) | 33/184 (17.9) |
| Smoker | 33 (18.0) | 41/182 (22.5) |
| Pretreatment imaging | (n = 183) | (n = 182) |
| ASPECTSa | ||
| 6-10 (Less advanced infarction) | 163 (89.1) | 159 (87.4) |
| <6 | 20 (10.9) | 23 (12.6) |
| Median (IQR) | 8 (7-10) | 8 (6-10) |
| Scores at Admission | ||
| Premorbid mRSb | ||
| 0 (No symptoms) | 133 (72.7) | 132 (71.4) |
| 1 | 23 (12.6) | 30 (16.2) |
| 2 | 15 (8.2) | 16 (8.6) |
| >2 | 12 (6.6) | 7 (3.8) |
| NIHSS score on admission, median (IQR)c | 18 (14-21) | 17 (14-20) |
| Occlusion | ||
| Localization of occlusion | ||
| Single ICA | 8 (4.4) | 12 (6.5) |
| Single ICA-Td | 27 (14.8) | 32 (17.3) |
| Single MCA | 102 (55.8) | 114 (61.6) |
| M1 | 81 (44.3) | 96 (51.9) |
| M2 | 21 (11.5) | 18 (9.7) |
| Tandem | 46 (25.1) | 27 (14.6) |
| ICA + ICA-Td | 15 (8.2) | 7 (3.8) |
| ICA + M1 | 29 (15.8) | 14 (7.6) |
| ICA + M2 | 2 (1.1) | 6 (3.2) |
| Occlusion side right | 73 (39.9) | 94 (50.8) |
| Reperfusion treatments, No. (%) | ||
| Pre–mechanical thrombectomy IV tPA | 129 (70.5) | 132 (71.4) |
| Onset to door time, median (IQR), min | 129.5 (67.0-195.0) | 110.0 (65.0-190.0) |
Abbreviations: ANSTROKE, Anesthesia During Stroke; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; GOLIATH, General or Local Anesthesia in Intra Arterial Therapy; ICA, internal carotid artery; IQR, interquartile range; IV tPA, intravenous thrombolysis; MCA, middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SIESTA, Sedation vs Intubation for Endovascular Stroke Treatment.
ASPECTS is a measure of the extension of a stroke. Score ranges from 0-10, with higher scores indicating fewer early ischemic changes and more normal tissue.
mRS scores range from 0-6, with 0 indicating no symptoms; 1, no clinically relevant disability; 2, slight disability (able to look after own affairs without assistance but not to full extent); 3, moderate disability (requires some help but able to walk unassisted); 4, moderately severe disability (requires assistance and unable to walk unassisted); 5, severe disability (requires constant nursing care); and 6, death.
NIHSS classifies neurological deficit from 0 (no deficit) to 42 (most severe deficit).
ICA-T was defined as occlusion of the internal carotid artery below the bifurcation of the middle and the anterior cerebral artery.
In the pooled study population, the mean (SD) age was 71.5 (12.9) years and the baseline median (interquartile range) NIHSS score was 17 (14-21). A total of 70.5% of the general anesthesia group and 71.4% of the procedural sedation group received intravenous thrombolysis with recombinant tissue-type plasminogen activator prior to mechanical thrombectomy.
Outcome-relevant risk factors were evenly distributed between the treatment groups. Baseline demographic and clinical characteristics are displayed in Table 2 and divided for the 3 trials in eTable 3 in the Supplement.
Primary Outcome
The 3-month mean mRS score was 2.8 (95% CI, 2.5-3.1) in the general anesthesia group vs 3.2 (95% CI, 3.0-3.5) in the procedural sedation group (difference, 0.43 [95% CI, 0.03-0.83]).
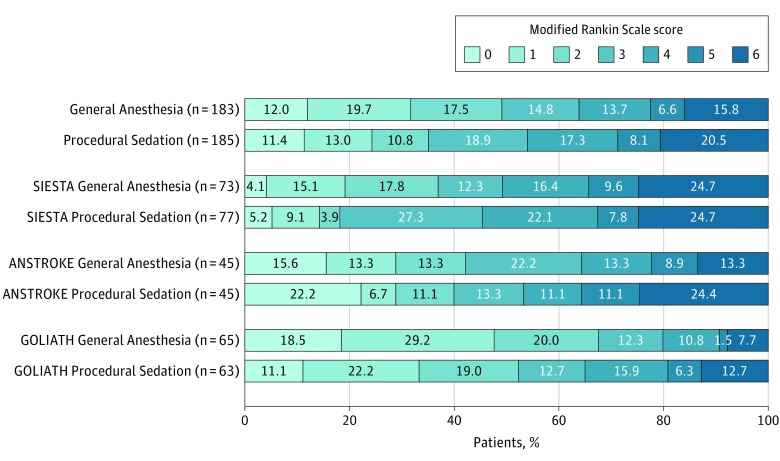
The main analysis of the primary outcome showed significantly different results in favor of the general anesthesia group (cOR, 1.58 [95% CI, 1.09-2.29]; P = .02) (Table 3; eTable 4 and eFigure 3 in the Supplement). The result of the score χ2 test to assess the proportional assumption was not significant (P = .51), which indicates that the proportional odds assumption is acceptable. The heterogeneity (obtained in the 2-step approach) was estimated as 0. If not stated otherwise, all results are based on the chosen individual patient data meta-analysis models and are, therefore, adjusted results. Figure 1 shows the mRS category distribution of the meta-analysis as well as the individual trials according to treatment groups.
Table 3. Non–Time-Related Outcomes in a Study of the Association of General Anesthesia vs Procedural Sedation With Functional Outcome Among Patients With Acute Ischemic Stroke Undergoing Thrombectomy.
| Non–Time-Related Outcomes | General Anesthesia (n = 183) | Procedural Sedation (n = 185) | Absolute Difference (95% CI)a | General Anesthesia vs Procedural Sedation OR (95% CI)b | P Valueb |
|---|---|---|---|---|---|
| Primary Outcome | |||||
| mRS after 3 moc,d | 1.58 (1.09 to 2.29)e | .02 | |||
| Secondary Outcomes, No. (%) | |||||
| mRS score 0-2 after 3 mo | 90 (49.2) | 65 (35.1) | 14.0 (4.1 to 24.0) | 2.16 (1.31 to 3.54) | .003 |
| mRS score 0-3 after 3 mo | 117 (63.9) | 100 (54.1) | 9.9 (−0.1 to 19.9) | 1.73 (1.06 to 2.82) | .03 |
| In-hospital mortality | 14 (7.7) | 15 (8.1) | −0.5 (−6.0 to 5.0) | 0.75 (0.32 to 1.75) | .51 |
| Early neurological improvement in NIHSSf | −6.6 (−8.0 to −5.3) | −5.4 (−6.7 to −4.2) | −1.2 (−3.0 to 0.7) | −1.11 (−2.90 to 0.68)g | .22 |
| Successful reperfusion (mTICI score of 2b or 3)h | 133 (72.7) | 117 (63.2) | 9.4 (−0.1 to 18.9) | 1.84 (1.12 to 3.01) | .02 |
| Infarction growth, mLi | (n = 110) | (n = 108) | |||
| Median (IQR) | 15.0 (3.5 to 49.9) | 21.0 (4.5 to 72.5) | −11.2 (−32.4 to 10.0) | −14.8 (−35.5 to 6.0)g | .17 |
| Mean (SD) | 44.3 (76.8) | 55.5 (82.9) | |||
| Hypotension (<20% of baseline) | 143 (80.8) | 95 (53.1) | 27.7 (18.4 to 37.1) | 4.3 (2.6 to 7.1) | <.001 |
| Systolic blood pressure variability (>180 or <120 mm Hg) | 145 (79.7) | 114 (62.3) | 17.4 (8.2 to 26.5) | 2.4 (1.5 to 3.9) | <.001 |
| Start of antibiotics within 72 h for suspected pneumoniaj | 34 (18.6) | 36 (19.5) | −0.9 (−8.9 to 7.1) | 0.85 (0.5 to 1.46) | .66 |
| Delayed extubation, No./total (%)k,l | 35/108 (32.4) | 5/55 (9.1) | 23.3 (11.7 to 35.0) | ||
| Total intervention-associated complications, No./total (%)l | 10/118 (8.5) | 17/122 (13.9) | −5.5 (−13.4 to 2.5) | ||
| Selected intervention-associated complicationsl,m,n | |||||
| ICH/SAH | 1 (0.8) | 5 (4.1) | |||
| Groin | 3 (2.5) | 1 (0.8) | |||
Abbreviations: ASPECTS, Alberta Stroke Program Early Computed Tomography Score; ICH, intracerebral hemorrhage; IQR, interquartile range; mRS, modified Rankin Scale; mTICI, modified thrombolysis in cerebral infarction; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; SAH, subarachnoid hemorrhage.
The absolute mean difference or difference between percentages.
OR and common OR were adjusted for the following variables: age, sex, NIHSS score on admission, premorbid mRS scores, ASPECTS, infarction side, intravenous thrombolysis. All P values were calculated using adjusted models as well.
mRS scores range from 0-6, with 0 indicating no symptoms; 1, no clinically relevant disability; 2, slight disability (able to look after own affairs without assistance but not to full extent); 3, moderate disability (requires some help but able to walk unassisted); 4, moderately severe disability (requires assistance and unable to walk unassisted); 5, severe disability (requires constant nursing care); and 6, death.
Intention-to-treat analysis.
Common OR.
Early neurological improvement was prespecified as the difference between NIHSS score after 24 hours and baseline NIHSS score on admission (range, 0-42; higher scores indicating more severe neurological deficits). Mean values and the 95% CIs per group are provided.
Adjusted mean difference.
mTICI scale range, 0-3; 0 indicates antegrade flow beyond the occlusion; 1, minimal perfusion; 2a, perfusion of <50% of the vascular distribution of the occluded artery; 2b, perfusion of ≥50 of the vascular distribution of the occluded artery; and 3, complete perfusion.
Variables were log-transformed before calculating the P value. The same adjusted model as described previously was used. Negative values were due to possible decrease in initial diffusion weight imaging signals.
Start of antibiotics within 72 hours for suspected pneumonia were data from ventilated-associated pneumonia in SIESTA (Sedation vs Intubation for Endovascular Stroke Treatment) and antibiotics for suspected pneumonia in ANSTROKE (Anesthesia During Stroke) and GOLIATH (General or Local Anesthesia in Intra Arterial Therapy).
Delayed extubation was prespecified as extubation >2 hours after cessation of sedation and analgesia.
Individual patient data meta-analysis models did not converge.
May not sum to total complications due to selection and overlap.
Data available from SIESTA and ANSTROKE only; no absolute difference is presented due to the very low numbers.
Figure 1. Functional Outcome at 90 Days for the Intention-to-Treat Population in a Study of the Association of General Anesthesia vs Procedural Sedation With Functional Outcome Among Patients With Acute Ischemic Stroke Undergoing Thrombectomy.
The modified Rankin Scale (mRS) score ranges from 0-6, with 0 indicating no symptoms; 1, no clinically relevant disability; 2, slight disability (able to look after own affairs without assistance but not to full extent); 3, moderate disability (requires some help but able to walk unassisted); 4, moderately severe disability (requires assistance and unable to walk unassisted); 5, severe disability (requires constant nursing care); and 6, death. Each cell corresponds to a score on the modified Rankin Scale; the width of the cell indicates the proportion of patients with equivalent scores, and the percentage of patients is shown within the cell. Distribution of mRS categories was additionally tested using the Mann-Whitney U test (P = .41).
Sensitivity Analyses Related to the Primary Outcome
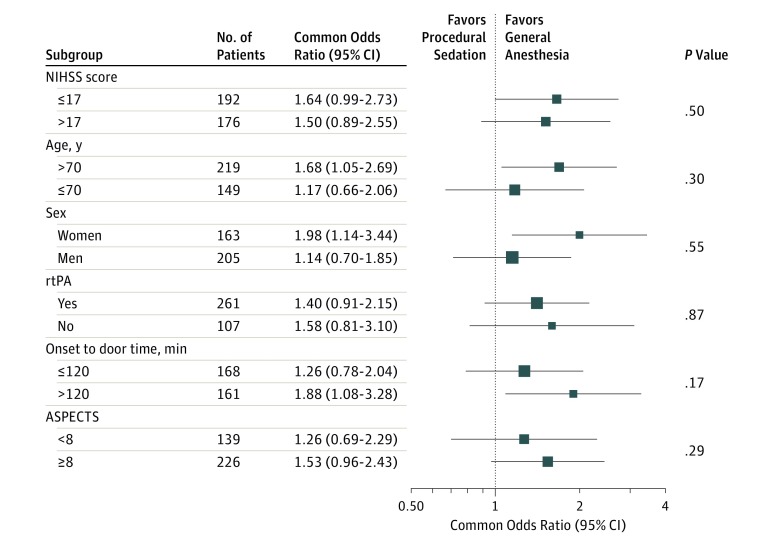
The results of the per-protocol analysis, from which only 1 patient had to be excluded, were consistent with the main analysis (cOR, 1.60 [95% CI, 1.10- 2.32]; P = .01). The as-treated analysis for the primary outcome did not show any significant difference between the groups (cOR, 1.20 [95% CI, 0.83-1.74]; P = .34) (eFigure 4 and 5 in the Supplement). In the subgroup analyses, no significant differences between any predefined subgroups were identified (Figure 2; eTable 5 and eFigure 6 in the Supplement).
Figure 2. Subgroup Analyses for the Primary Outcome for the Intention-to-Treat Population in a Study of the Association of General Anesthesia vs Procedural Sedation With Functional Outcome Among Patients With Acute Ischemic Stroke Undergoing Thrombectomy.
Definitions of the Alberta Stroke Program Early Computed Tomography Score (ASPECTS), and National Institutes of Health Stroke Scale (NIHSS) are included in the footnotes in Table 1. Size of the data markers is proportional to the precision of the estimates (ie, the area is proportional to the inverse of the squared standard errors). An interaction term between treatment group × the subgroup variable was included in the model (separately for each subgroup) to calculate P values. rtPA indicates recombinant tissue-type plasminogen activator.
Secondary Outcomes
Dichotomization of the mRS score at 3 months for functional independence (mRS score of 0-2 vs 3-6) showed a significant difference, with more functional independence in the general anesthesia group than in the procedural sedation group (49.2% vs 35.1%; odds ratio [OR], 2.16 [95% CI, 1.31-3.54]; P = .003), and a smaller, but statistically significant, difference between general anesthesia and procedural sedation when the mRS score was dichotomized to ambulatory and capable of bodily self-care or better (mRS score 0-3 vs 4-6) (63.9% vs 54.1%; OR, 1.73 [95% CI, 1.06-2.82]; P = .03). When a study × treatment interaction term was included in the primary analysis, the results were consistent (see eTable 5 in the Supplement). In-hospital mortality was not significantly different between the groups (14 patients [7.7%] in the general anesthesia group vs 15 [8.1%] in the procedural sedation group; OR, 0.75 [95% CI, 0.32-1.75]; P = .51). An analysis of the mRS score after 3 months that did not include the patients who converted from the procedural sedation group to the general anesthesia group showed no significant difference between the groups (cOR, 1.37 [95% CI, 0.93-2.02]; P = .11) (eTable 6 in the Supplement).
The early improvement in neurological function was not significantly different between the groups; the mean NIHSS score decreased from 17.7 at baseline to 11.1 after 24 hours in the general anesthesia group and from 17.4 at baseline to 11.9 after 24 hours in the procedural sedation group (adjusted mean difference, −1.11 [95% CI, −2.90 to 0.68]; P = .22) (eTable 5 in the Supplement).
Successful reperfusion (mTICI grades 2b or 3) was achieved more often in the general anesthesia group than in the procedural sedation group (72.7% vs 63.3%; OR, 1.84 [95% CI, 1.12-3.01]; P = .02). Infarction growth was not significantly different between the groups (44.3 mL in the general anesthesia group vs 55.5 mL in the procedural sedation group; adjusted mean difference, −14.8 [95% CI, −35.5 to 6.0]; P = .17). Table 3 and Table 4 summarize the results for all clinical primary and secondary outcomes.
Table 4. Time-Related Primary and Secondary Outcome Results in a Study of the Association of General Anesthesia vs Procedural Sedation With Functional Outcome Among Patients With Acute Ischemic Stroke Undergoing Thrombectomy.
| Time-Related Outcomes | Median (IQR)a | Difference of Medians (95% CI)b | P Value | |
|---|---|---|---|---|
| General Anesthesia (n = 183) | Procedural Sedation (n = 185) | |||
| Length of stay in ICU, h | 17.9 (2.0 to 42.4) | 0.0 (0.0 to 24.5) | 17.9 (7.4 to 22.4) | <.001 |
| Duration of mechanical ventilation, h | 3.8 (1.9 to 7.0) (n = 108) | 0.0 (0.0 to 0.0) (n = 55) | 3.8 (3.25 to 4.5) | <.001 |
| Door to groin puncture, min | 75 (60 to 91) | 69 (53 to 90) | 6 (0 to 15) | .04 |
| Computed tomography to angiography suite arrival, min | 49 (29 to 68) | 41 (28 to 62) | 8 (2 to 12) | .22 |
| Angiography suite arrival to groin puncture, min | 23 (14 to 30) | 15 (10 to 24) | 8 (5 to 11) | <.001 |
| Onset to groin puncture, min | 180 (136 to 255) | 170 (133 to 240) | 10 (−11 to 32) | .04 |
| Onset to reperfusion, min | 241.5 (200 to 348.5) | 257 (197 to 325) | −15.5 (−45 to 26.5) | .53 |
| Door to reperfusion, min | 150 (113.5 to 200) | 165 (119 to 207) | −15 (−30 to 5) | .86 |
| Groin puncture to reperfusion, min | 51.5 (31 to 90) | 70.5 (33.5 to 105) | −19 (−31 to −4) | .15 |
| Duration of intervention, min | 157.5 (82 to 232) | 160 (97 to 225) | −2.5 (−29 to 26.5) | .19 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
Variables were log-transformed before calculating the P value. The same adjusted model as described above was used.
A nonparametric bootstrap approach was used to calculate 95% CIs.
Care Process and Adverse Events
Most time intervals and durations of the procedures as well as intervention-associated complications did not differ significantly between the groups. Twenty-one patients (11.5%) randomized to the procedural sedation group were later converted to the general anesthesia group on an emergency basis. Reasons for the change were severe agitation (n = 9), aspiration (n = 1), respiratory insufficiency (n = 1), direct puncture of the common carotid artery (n = 4), and other (n = 6) (eTable 10 in the Supplement). A separate descriptive analysis of these 21 patients revealed no clinically important features with regard to their baseline characteristics (eTable 11 in the Supplement). In terms of their outcome, 11 of these patients (52.4%) reached an mTICI score of at least 2b, 1 (4.8%) achieved an mRS score of 0 to 2, and 6 (28.6%) had an mRS score of 0 to 3 after 3 months (eTable 12 in the Supplement). Baseline characteristics of the main analysis population that did not include patients assigned to the procedural sedation group who received general anesthesia are displayed in eTable 13 in the Supplement. An adjusted subgroup analysis of patients with successful reperfusion and mTICI scores of at least 2b (cOR 1.3 [95% CI, 0.75-2.26]; P = .36) and mTICI scores less than 2b (cOR, 1.40 [95% CI, 0.86-2.26]; P = .18) showed no significant difference in mRS score after 3 months between the groups (eTables 14 and 15 in the Supplement). The detailed reperfusion outcome results are displayed in eTable 16 in the Supplement.
The median time intervals were slightly yet significantly shorter in the procedural sedation group than the general anesthesia group for angiography suite arrival to groin puncture (15 vs 23 min; P < .001), onset to groin puncture (170 vs 180 min; P = .04), and door to groin puncture (69 vs 75 min; P = .04).
The length of stay in the intensive care unit was longer in the general anesthesia group than the procedural sedation group (46.3 vs 25.0 h; P < .001), as was the duration of mechanical ventilation (20.4 vs 5.2 h; P < .001).
Among adverse events, the decline in systolic blood pressure of more than 20% from baseline was significantly more common in the general anesthesia group than the procedural sedation group (80.8% vs 53.1%; OR, 4.26 [95% CI, 2.55-7.09]; P < .001). Patients who received general anesthesia also experienced more variability in systolic blood pressure than patients who received procedural sedation, as demonstrated by the percentage of patients with values greater than 180 mm Hg and less than 120 mm Hg (79.7% vs 62.3%; OR, 2.42 [95% CI, 1.49-3.93]; P < .001). Other adverse events, including intervention-associated complications, were not different between groups (Table 3).
Discussion
In this meta-analysis of individual patient data from 3 single-center trials with a total of 368 patients with stroke in the anterior circulation who were randomized to receive either general anesthesia or procedural sedation during mechanical thrombectomy, functional outcome at 3 months was significantly better for patients who received general anesthesia. The difference in mean mRS scores between the groups was modest, although more patients in the general anesthesia group compared with the procedural sedation group had mRS scores of 0 to 2 after 3 months.
These findings are in contrast with the results of previous retrospective studies that reported worse functional outcome in patients with acute ischemic stroke who received general anesthesia compared with those who received procedural sedation.5,7,8 Post hoc analyses of 2 large thrombectomy trials found that general anesthesia was associated with a significant decrease in thrombectomy-related improvement of functional outcome.5,16 A meta-analysis of individual data from 1764 patients enrolled in multiple mechanical thrombectomy trials (patients were randomized to undergo mechanical thrombectomy or medical treatment alone) by the HERMES collaboration showed a better functional outcome after mechanical thrombectomy with a non–general anesthesia approach compared with a general anesthesia approach (mRS score of 0-2 at 90 days: 50% vs 40%).8 However, a common limitation of these studies was that the NIHSS score at baseline was often significantly higher for patients receiving general anesthesia,2,3,6,17 and other baseline parameters were largely unbalanced between treatment groups, thus increasing the risk for bias and confounding. None of these studies randomized patients based on anesthetic approach to either general anesthesia or procedural sedation, and no detailed information on anesthesia and hemodynamic management was provided, which largely limits their interpretability. A cohort study18 and a randomized thrombectomy trial19 have not confirmed detrimental effects of general anesthesia, thus suggesting equipoise based on observational or secondary data.
The clinical benefits associated with general anesthesia in the present analysis may be primarily related to the higher rates of reperfusion in the general anesthesia group (72.7% in the general anesthesia group vs 63.3% in procedural sedation group with mTICI scores of 2b or 3). This finding suggests that general anesthesia is associated with a more optimal procedural condition for performing mechanical thrombectomy (eg, because of the absence of patient movement). A post hoc analysis of SIESTA suggested advantages for several technical aspects and a shorter procedure time with general anesthesia.20
Retrospective studies7 have suggested that the time delay associated with intubation may have led to worse outcome for patients in the general anesthesia group. This meta-analysis, however, showed either no significant or no clinically relevant differences in most prespecified time intervals and procedure durations. Although several time intervals were shorter in the procedural sedation group, no significant differences were found in groin puncture to reperfusion time or in duration of intervention between the treatment groups, and the mean time delay caused by general anesthesia was only about 6 minutes. This is probably a consequence of the standardized workflow instituted in all 3 randomized clinical trials, which may not have been present in individual centers participating in previous nonrandomized studies.
The benefits in functional outcome associated with general anesthesia were observed, although more hypotension and blood pressure variability occurred in patients who received general anesthesia, which may appear counterintuitive. Hypotensive episodes during induction of general anesthesia and before recanalization have been associated with poorer outcome in retrospective mechanical thrombectomy studies.3,21,22 In all 3 trials, strict blood pressure targets were applied and have minimized more extreme variations in blood pressure. Milder hypotensive episodes, although significantly different between groups, may not have sufficiently offset the reperfusion advantage that general anesthesia seemed to confer. It is possible that the general anesthesia–associated hypotension observed in previous retrospective studies was more severe and detrimental, as was seen in MR CLEAN.23 In post hoc analyses of the single trials focusing on blood pressure, SIESTA and GOLIATH, there was no association between outcome and blood pressure variations within their protocol limits.24,25 Whether other potentially neuroprotective effects of general anesthesia (eg, hypothermia, normocarbia, drug effects) may have played a role cannot be determined from this analysis.
The more favorable outcome results associated with general anesthesia, however, were no longer statistically significant when the patients who converted from procedural sedation to general anesthesia on an emergency basis were excluded for the sensitivity analysis or analyzed according to the group they crossed over to. This latter as-treated analysis, albeit cautiously interpreted, demonstrated no significant difference in functional outcome between the groups. Hence, the patients who were converted from procedural sedation to general anesthesia may have diluted the positive association observed between general anesthesia and the primary outcome. Despite the lack of benefit from general anesthesia seen in the as-treated analysis, none of the analyses showed any sign that procedural sedation was associated with a more favorable primary outcome compared with general anesthesia.
To the best of our knowledge, this is the first meta-analysis of randomized trials focused on peri-interventional anesthetic management during mechanical thrombectomy, and it provides the largest body of pooled prospective data to date. The strengths of this study include standardized treatment of both groups in all 3 trials analyzed with only minor differences across the trials, making the groups comparable; the use of data from 3 independent international study groups; similar study designs and protocols of all 3 trials with prespecified end points; and few missing data and no patients lost to follow-up.
These findings suggest that general anesthesia, compared with procedural sedation, was associated with a more favorable functional outcome and that it may be advantageous provided that strict hemodynamic and anesthetic protocols are in place and performed by clinicians and nurses trained for that particular setting. Further research is needed to identify clinical and radiologic factors that could predict when primary general anesthesia is necessary to reduce the number of emergency conversions from procedural sedation to general anesthesia. Given the likelihood that the patient population and anesthetic strategies investigated in this analysis differ from those in other countries, a large, multicenter RCT including at least a basic agreement on standards of procedure and physiology parameter targets directed at functional outcome after 3 months is necessary.
Limitations
This study has several limitations. First, as a meta-analysis of only 3 randomized single-center trials, power to assess cross-center differences was limited. Second, the relatively small sample size of the single trials may have reduced the power to detect moderate but clinically relevant differences in some outcomes, which particularly applies to the subgroup analyses. Third, every trial had different prespecified primary outcomes; in 2 of them, long-term function was only a secondary outcome. Fourth, all 3 trials had limited racial/ethnic diversity among their study population and enrolled predominantly white European patients. Fifth, despite a run-in phase of more than 6 months establishing both general anesthesia and procedural sedation in all 3 centers in parallel prior to randomization it cannot be ruled out that interventionalists still performed more comfortably while patients were receiving general anesthesia, which was preferred before. Sixth, current definitions of general anesthesia and procedural sedation are heterogeneous and allow for a diverse choice of drugs and measures that were only partially covered in these trials.
Conclusions
Among patients with acute ischemic stroke in the anterior circulation undergoing thrombectomy, the use of protocol-based general anesthesia compared with procedural sedation was significantly associated with less disability at 3 months. These findings should be interpreted tentatively because the individual trials analyzed were single-center trials and disability was the primary outcome in only 1 trial.
Figure e1: PRISMA diagram of the SAGA meta-analysis (for SIESTA, ANSTROKE and GOLIATH)
Table e1: Trial-specific aspects of SIESTA, ANSTROKE, and GOLIATH
Table e2: List of all prespecified secondary outcomes
Figure e2: Risk of bias assessment according to the ROB-2 tool from the Cochrane group
Table e3: Baseline characteristics of the three single center trials SIESTA, AnStroke and GOLIATH
Table e4: Adjusted analysis for the primary outcome including study treatment interaction term
Figure e3: Forest plot of the primary outcome categorical shift of mRS after 3 months in SIESTA, ANSTROKE, and GOLIATH for the intention-to-treat population
Figure e4: Forest plot of the primary outcome as the shift of mRS in SIESTA, ANSTROKE, and GOLIATH for the as-treated population
Figure e5: Functional outcome at 90 days for the as-treated population
Table e5: Adjusted analysis for the improvement of NIHSS after 24 hours of the intention-to-treat population
Figure e6: Subgroup analyses for primary outcome for the as-treated population
Table e6: Adjusted analysis for the mRS after 3 months without the patients converted from Procedural Sedation to General Anesthesia
Table e7: Adjusted analysis for the mRS 5 and 6 after 3 months for CS vs GA
Table e8: Adjusted analysis for in-house mortality of the intention-to-treat population
Table e9: Adjusted analysis for start of antibiotic treatment with 72 hours because of a suspected pneumonia of the intention-to-treat population
Table e10: Evaluation of crossover from assigned treatment
Table e11: Baseline characteristics of the patients converted from Procedural Sedation to General Anesthesia
Table e12: Primary outcome and selected secondary outcomes of the patients converted from Procedural Sedation to General Anesthesia
Table e13: Baseline characteristics of the intention-to-treat population without the patients converted from Procedural Sedation to General Anesthesia
Table e14: Adjusted subgroup analysis of reperfusion with mTICI ≥ 2b for the mRS after 3 months
Table e15: Adjusted subgroup analysis of reperfusion with mTICI < 2b for the mRS after 3 month
Table e16: Detailed reperfusion outcome results
Project outline
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Jumaa MA, Zhang F, Ruiz-Ares G, et al. . Comparison of safety and clinical and radiographic outcomes in endovascular acute stroke therapy for proximal middle cerebral artery occlusion with intubation and general anesthesia versus the nonintubated state. Stroke. 2010;41(6):1180-1184. doi: 10.1161/STROKEAHA.109.574194 [DOI] [PubMed] [Google Scholar]
- 3.Davis MJ, Menon BK, Baghirzada LB, et al. ; Calgary Stroke Program . Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012;116(2):396-405. doi: 10.1097/ALN.0b013e318242a5d2 [DOI] [PubMed] [Google Scholar]
- 4.van den Berg LA, Koelman DL, Berkhemer OA, et al. ; MR CLEAN pretrial study group; Participating centers . Type of anesthesia and differences in clinical outcome after intra-arterial treatment for ischemic stroke. Stroke. 2015;46(5):1257-1262. doi: 10.1161/STROKEAHA.115.008699 [DOI] [PubMed] [Google Scholar]
- 5.Berkhemer OA, van den Berg LA, Fransen PS, et al. ; MR CLEAN investigators . The effect of anesthetic management during intra-arterial therapy for acute stroke in MR CLEAN. Neurology. 2016;87(7):656-664. doi: 10.1212/WNL.0000000000002976 [DOI] [PubMed] [Google Scholar]
- 6.Abou-Chebl A, Yeatts SD, Yan B, et al. . Impact of general anesthesia on safety and outcomes in the endovascular arm of Interventional Management of Stroke (IMS) III trial. Stroke. 2015;46(8):2142-2148. doi: 10.1161/STROKEAHA.115.008761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinjikji W, Murad MH, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anesthesia during endovascular acute ischemic stroke treatment: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36(3):525-529. doi: 10.3174/ajnr.A4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell BCV, van Zwam WH, Goyal M, et al. ; HERMES collaborators . Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol. 2018;17(1):47-53. doi: 10.1016/S1474-4422(17)30407-6 [DOI] [PubMed] [Google Scholar]
- 9.Sivasankar C, Stiefel M, Miano TA, et al. . Anesthetic variation and potential impact of anesthetics used during endovascular management of acute ischemic stroke. J Neurointerv Surg. 2016;8(11):1101-1106. doi: 10.1136/neurintsurg-2015-011998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schönenberger S, Uhlmann L, Hacke W, et al. . Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316(19):1986-1996. doi: 10.1001/jama.2016.16623 [DOI] [PubMed] [Google Scholar]
- 11.Löwhagen Hendén P, Rentzos A, Karlsson JE, et al. . General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the AnStroke trial (anesthesia during stroke). Stroke. 2017;48(6):1601-1607. doi: 10.1161/STROKEAHA.117.016554 [DOI] [PubMed] [Google Scholar]
- 12.Simonsen CZ, Yoo AJ, Sørensen LH, et al. . Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2018;75(4):470-477. doi: 10.1001/jamaneurol.2017.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazighi M, Serfaty JM, Labreuche J, et al. ; RECANALISE investigators . Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8(9):802-809. doi: 10.1016/S1474-4422(09)70182-6 [DOI] [PubMed] [Google Scholar]
- 14.Thomas AK, Gordon LT, Cernasov PM, Bulevich JB. The effect of testing can increase or decrease misinformation susceptibility depending on the retention interval. Cogn Res Princ Implic. 2017;2(1):45. doi: 10.1186/s41235-017-0081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke DL, Ensor J, Riley RD. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med. 2017;36(5):855-875. doi: 10.1002/sim.7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eker OF, Saver JL, Goyal M, et al. ; SWIFT PRIME investigators . Impact of anesthetic management on safety and outcomes following mechanical thrombectomy for ischemic stroke in SWIFT PRIME cohort. Front Neurol. 2018;9:702. doi: 10.3389/fneur.2018.00702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols C, Carrozzella J, Yeatts S, Tomsick T, Broderick J, Khatri P. Is periprocedural sedation during acute stroke therapy associated with poorer functional outcomes? J Neurointerv Surg. 2010;2(1):67-70. doi: 10.1136/jnis.2009.001768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slezak A, Kurmann R, Oppliger L, et al. . Impact of anesthesia on the outcome of acute ischemic stroke after endovascular treatment with the solitaire stent retriever. AJNR Am J Neuroradiol. 2017;38(7):1362-1367. doi: 10.3174/ajnr.A5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bracard S, Ducrocq X, Mas JL, et al. ; THRACE investigators . Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138-1147. doi: 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 20.Pfaff JAR, Schönenberger S, Nagel S, et al. . Effect of general anesthesia versus conscious sedation for stroke thrombectomy on angiographic workflow in a randomized trial: a post hoc analysis of the SIESTA trial. Radiology. 2018;286(3):1016-1021. doi: 10.1148/radiol.2017171002 [DOI] [PubMed] [Google Scholar]
- 21.Löwhagen Hendén P, Rentzos A, Karlsson JE, et al. . Hypotension during endovascular treatment of ischemic stroke is a risk factor for poor neurological outcome. Stroke. 2015;46(9):2678-2680. doi: 10.1161/STROKEAHA.115.009808 [DOI] [PubMed] [Google Scholar]
- 22.Whalin MK, Halenda KM, Haussen DC, et al. . Even small decreases in blood pressure during conscious sedation affect clinical outcome after stroke thrombectomy: an analysis of hemodynamic thresholds. AJNR Am J Neuroradiol. 2017;38(2):294-298. doi: 10.3174/ajnr.A4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treurniet KM, Berkhemer OA, Immink RV, et al. ; MR CLEAN investigators . A decrease in blood pressure is associated with unfavorable outcome in patients undergoing thrombectomy under general anesthesia. J Neurointerv Surg. 2018;10(2):107-111. doi: 10.1136/neurintsurg-2017-012988 [DOI] [PubMed] [Google Scholar]
- 24.Schönenberger S, Uhlmann L, Ungerer M, et al. . Association of blood pressure with short- and long-term functional outcome after stroke thrombectomy: post hoc analysis of the SIESTA trial. Stroke. 2018;49(6):1451-1456. doi: 10.1161/STROKEAHA.117.019709 [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen M, Espelund US, Juul N, et al. . The influence of blood pressure management on neurological outcome in endovascular therapy for acute ischaemic stroke. Br J Anaesth. 2018;120(6):1287-1294. doi: 10.1016/j.bja.2018.01.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure e1: PRISMA diagram of the SAGA meta-analysis (for SIESTA, ANSTROKE and GOLIATH)
Table e1: Trial-specific aspects of SIESTA, ANSTROKE, and GOLIATH
Table e2: List of all prespecified secondary outcomes
Figure e2: Risk of bias assessment according to the ROB-2 tool from the Cochrane group
Table e3: Baseline characteristics of the three single center trials SIESTA, AnStroke and GOLIATH
Table e4: Adjusted analysis for the primary outcome including study treatment interaction term
Figure e3: Forest plot of the primary outcome categorical shift of mRS after 3 months in SIESTA, ANSTROKE, and GOLIATH for the intention-to-treat population
Figure e4: Forest plot of the primary outcome as the shift of mRS in SIESTA, ANSTROKE, and GOLIATH for the as-treated population
Figure e5: Functional outcome at 90 days for the as-treated population
Table e5: Adjusted analysis for the improvement of NIHSS after 24 hours of the intention-to-treat population
Figure e6: Subgroup analyses for primary outcome for the as-treated population
Table e6: Adjusted analysis for the mRS after 3 months without the patients converted from Procedural Sedation to General Anesthesia
Table e7: Adjusted analysis for the mRS 5 and 6 after 3 months for CS vs GA
Table e8: Adjusted analysis for in-house mortality of the intention-to-treat population
Table e9: Adjusted analysis for start of antibiotic treatment with 72 hours because of a suspected pneumonia of the intention-to-treat population
Table e10: Evaluation of crossover from assigned treatment
Table e11: Baseline characteristics of the patients converted from Procedural Sedation to General Anesthesia
Table e12: Primary outcome and selected secondary outcomes of the patients converted from Procedural Sedation to General Anesthesia
Table e13: Baseline characteristics of the intention-to-treat population without the patients converted from Procedural Sedation to General Anesthesia
Table e14: Adjusted subgroup analysis of reperfusion with mTICI ≥ 2b for the mRS after 3 months
Table e15: Adjusted subgroup analysis of reperfusion with mTICI < 2b for the mRS after 3 month
Table e16: Detailed reperfusion outcome results
Project outline