Abstract
Botulinum toxin A (BTX-A) is a promising therapeutic modality against trigeminal neuralgia (TN) with certain controversies pertaining to its application. To provide further information on factors influencing the treatment outcomes of BTX-A, a retrospective study with 152 patients with TN treated with BTX-A was performed. The starting time and duration of the therapeutic effect, as well as side effects, of BTX-A in the treatment of TN were analyzed by sex, age, course of disease, number of branches and injected dose. A total of 136 patients exhibited symptom improvement within 2 weeks following BTX-A treatment as evaluated using a visual analog scale (VAS). The effect of BTX-A was sustained throughout the initial 6 months of the follow-up and was demonstrated to persist for as long as 28 months. Female sex, short disease course and high injection dose (>70 units) were associated with lower long-term VAS scores. Patients receiving short-term medium-(50–70 units) or high-dose injections were more likely to be completely cured. Patients with a median disease course (1–10 years) or multiple branches were more likely to exhibit facial asymmetry. Based on the stratified analysis, female patients with a median disease course (1–10 years) exhibited a higher incidence of side effects and male patients achieved better treatment outcomes with high BTX-A doses. BTX-A effectively alleviated patients with TN in both short or long term, although the treatment efficacy may depend on patient characteristics.
Keywords: botulinum toxin, sex differences, long-term outcome, trigeminal neuralgia
Introduction
Trigeminal neuralgia (TN) is a type of severe chronic pain characterized by brief electric shock-like pains in one or more divisions of the trigeminal nerve (1). The disorder typically occurs in the middle- or advanced-aged population; however, young adults, particularly those with multiple sclerosis, may still present with TN (2). In general, TN is evoked by stimulation in the ‘trigger points’ of the face and attacks may occur repeatedly in a short period of time (3). A number of potential causes regarding the etiology of TN have been proposed, including epileptic seizures in the trigeminal structures of the brainstem, trigeminal root compression, arteriovenous malformations and aneurysms (4). At present, two major treatment modalities for TN are being applied in clinical settings: Pharmacotherapy and neurosurgical procedures (5). Although surgical treatment may be more likely to cure TN, the majority of patients opt for pharmacotherapy due to the reduced risk associated with it. A number of pharmacotherapies have been successfully applied in clinical settings to relieve patients from the impairments of TN (6). Among the types of pharmacotherapy offered, botulinum toxin A (BTX-A) has been increasingly reported to successfully control TN in recent years, and has received considerable attention in the subject area of pain management (7).
BTX-A is one of the botulinum neurotoxins produced by Clostridium botulinum. The agent exerts its function by inhibiting acetylcholine release at nerve-muscle junctions, causing relaxation of the muscle (8,9). Therefore, BTX-A has been widely used in cosmetology and the treatment of dysmyotonia (10), and has exhibited promising effects in the treatment of headaches (11,12). In 2002, Micheli et al (13) initially reported the effect of BTX-A to ameliorate TN. A number of subsequent studies supported the beneficial effects of BTX-A in the treatment of TN (7,14). As a result, Medicines and Healthcare Products Regulatory Agency in the UK and the US Food and Drug Administration approved Botox® (Allergan, Inc.) for the treatment of pains in adults with chronic migraine. However, the application of BTX-A for the treatment of TN remains controversial, with discrepancies concerning the optimal injection dose, starting time of therapeutic effects, long-term therapeutic effects and side effects (15,16). Although meta-analyses based on randomized controlled trials have confirmed the benefits of BTX-A in treating TN, the conclusions were limited by the small number of trials and patients available that supported the safety and efficacy of BTX-A in the treatment of TN (15,16). Therefore, in the present study, a comprehensive retrospective analysis was performed. The study cohort consisted of 152 subjects and was established to assess the effect of BTX-A on TN by different grouping strategies. The results of the present study supplement the results of previous studies exploring the treatment efficacy and side effects of BTX-A for TN.
Patients and methods
Patient recruitment
An open retrospective study was performed on 161 TN patients treated with BTX-A. Patients who were treated with BTX-A at the Department of Neurology of the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China) from June 2011 to March 2016 were retrospectively enrolled in the current study. All subjects enrolled in the analysis were diagnosed with classical TN according to the current version of the International Classification of Headache Disorders (17) and detailed information on the clinicopathological characteristics, including age, sex, number of branches affected by TN, course of disease, injection dose, number of injections, starting time of therapeutic effect, duration of therapeutic effect and side effects, was available. Each patient underwent magnetic resonance imaging or computed tomography to exclude the presence of structural pathology. Complete blood count, electrocardiogram, liver function tests, renal function tests and other diagnostic tests were performed prior to the trial to exclude coagulopathy and severe dysfunction of major organs, including the heart, liver and kidney. Patients with any disease that may have represented a risk associated with BTX-A (including myasthenia gravis, motor neuron disease or Lambert-Eaton syndrome), infections or skin problems at any of the injection sites, use of drugs that damaged the neuromuscular junction within 7 days prior to study entry (e.g. quinine, aminoglycosides or penicillamine), significantly unstable medical diseases, a history of significant psychiatric disorder or a history of substance dependence or abuse were excluded from the present study. Furthermore, females who were pregnant, nursing, planning a pregnancy during the study or unable or unwilling to use a reliable form of contraception during the study were also excluded. Follow-up was performed for ≥6 months for all patients and the longest follow-up time was 28 months. Based on the data collected, 9 patients lost to follow-up within 6 months following treatment were excluded from the database and the final cohort for analysis consisted of 152 subjects.
The purpose and safety aspects of the study were explained to all patients and written informed consent was included in the documents of each patient. The present study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China) for the screening, inspection and data collection of the patients. All protocols were in accordance with the provisions of the Declaration of Helsinki.
Treatment strategy
BTX-A crystals (HengLi®; Lanzhou Institute of Biological Products Co., Ltd.; 100 units Clostridium botulinum type A neurotoxin complex supplemented with 5 mg gelatin, 25 dextran and 25 mg saccharose) was diluted with 2 ml 0.9% saline to 50 U/ml prior to use. The injections of BTX-A were administered intradermally and/or submucosally at the trigger sites using a 1-ml syringe with a 0.45×16-mm needle (Shandong Weigao Group Medical Polymer Co., Ltd.) by experienced clinicians, and the injection depth was −0.1 cm. For patients requiring injection at multiple sites, 15–25 injection sites in total were located and a distance of 15–20 mm was set between them. Each site received one injection with 1.25–5.0 units (0.025–0.1 ml) BTX-A during the first course of the treatment and the patients' response to the treatment was monitored. For certain patients who did not exhibit any symptom improvement within 2 weeks following the initial injection, additional injections were administered at the same sites (each site received one injection with 1.25–5.0 units BTX-A during the second course of the treatment). Patients who received a total dose of <40 units were assigned to the low-dose group, patients who received a total dose from 40–70 units were stratified into the medium-dose group and those who received a total dose of >70 units were classified into the high-dose group.
Efficacy and safety assessment
The extent of pain was evaluated using a visual analog scale (VAS) method (18): Patients were requested to rate their pain by marking a representative point on a panel with a length of 10 cm and 11 equidistant points, where 0 indicated ‘no pain’ and 10 represented the ‘most severe pain’ (the difference between females and males in rating/perceiving pain was not taken into consideration in the present study). Regarding the treatment efficacy, a reduction in the VAS score by <50% following treatment was classified as ‘ineffective’, 50–70% as ‘effective’ and >75% as ‘significantly effective’ and 100% as ‘totally effective’. For different analysis strategies, the overall treatment effect was defined as the percentage of patients with a VAS score reduction by ≥50%. Side effects (short-term facial asymmetry, twisted mouth, and ptosis) associated with BTX-A treatment were also recorded in detail.
Statistical analysis
Continuous data with non-normal distribution (assessed by Kolmogorov-Smirnov test) were reported as the median (interquartile range). Categorical data were represented as the number of cases (percentage). Wilcoxon's signed-rank test and the Kruskal-Wallis test were employed for analysis of variables with a non-normal distribution. χ2 and Fisher's exact tests were used to determine whether differences existed between different groups of categorical indices by sex, age, number of branches affected by TN and dose of injections. All tests were two-tailed and P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using R, version 3.3 (19).
Results
Patient characteristics
The patients were treated by BTX-A between June 2011 and March 2016, and a total of 161 patients were enrolled in the present study. The follow-up period for each patient was ≥6 months and 9 subjects lost to follow-up were excluded from the database. The final cohort for analysis comprised 152 patients, including 87 females (57.2%) and 65 males (42.8%). The median age of the females was 59 years (age range, 31–91 years) and that of males was 60 years (age range, 39–89 years); no statistically significant difference was determined in the age distribution between different sexes (P=0.65). The average disease course of the females was 68 months (range, 1–240 months) and that of the males was 60 months (range, 2–300 months), and no significant difference was observed between the two groups (P=0.11).
Efficacy results
The beneficial effect of BTX-A on TN, indicated by a decrease in the degree of pain represented by the VAS score, was observed as early as 1 week following the first injection. For patients whose symptoms were not improved within 2 weeks following the first injection, additional doses were administered at the same site as the first injection. In the present study, 50 patients received additional injections and 41 achieved pain alleviation (82%). Following treatment with BTX-A, 136 patients exhibited symptom improvement within 2 weeks, including 31 cases of effective (20.4%), 21 cases of significantly effective (13.8%), and 83 cases of completely effective treatment (54.6%). The overall effective rate in the present study was 89.4%.
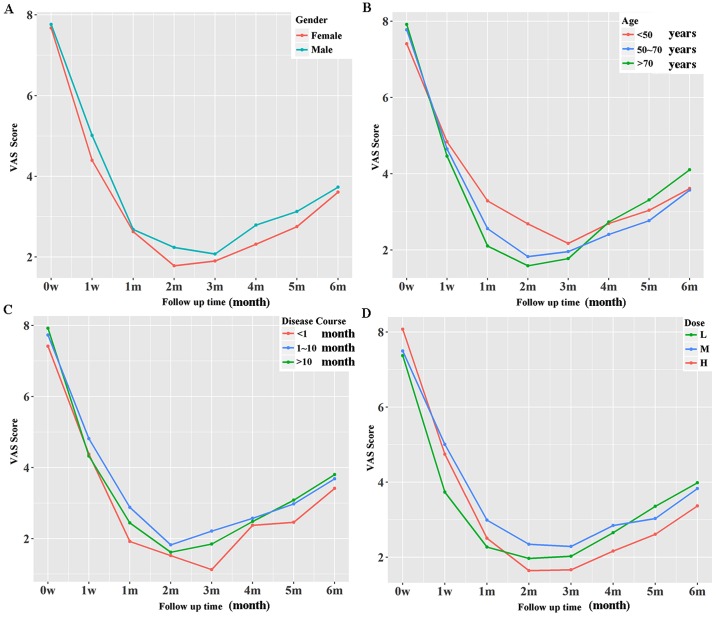
The effect of BTX-A was sustained throughout the initial 6 months of the follow-up. No significant differences in overall treatment efficacy were detected when the VAS data of the initial 6 months were analyzed by sex, age, disease course or injection dose (Fig. 1). However, female sex, short disease course and high injection dose were associated with lower VAS scores in a longer period (≥2 months), although this was not statistically significant (Fig. 1).
Figure 1.
Long-term treatment effect of botulinum toxin A on trigeminal neuralgia represented by VAS scores. It was indicated that (A) female sex resulted in lower VAS scores during the entire follow-up period. (B) No influence of age on the long-term treatment effect was observed. (C) A short disease course and (D) a high injection dose resulted in lower VAS scores during the entire follow-up period. VAS, visual analog scale; L, low; M, medium; H, high.
In the present study, a reduction in the VAS score by >50% was considered as ‘effective’. To further explore factors influencing the treatment effect of BTX-A, changes in the VAS score were analyzed with regard to sex, age, disease course, branch number and injection dose. As presented in Table I, sex, age, disease course and branch number did not influence the starting time of the effect, the peak time of the effect, the average lasting time of the effect or the overall efficacy of BTX-A. However, the injection dose affected the treatment efficacy: patients who received injections of medium-(21.1%) or high-dose (22.7%) BTX-A exhibited higher completely treated rates compared with the low-dose group (11.2%) although no statistical significance was observed regarding patient distribution in treatment efficacy (Table I).
Table I.
Therapeutic effect of botulinum toxin A by sex, age, disease course, number of branches and injection dose (n=152).
| Sex | Age (years) | Disease course (months) | Number of branches | Injection dose | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Male | Female | P-value | <50 | 50–70 | >70 | P-value | <1 | 1–10 | >10 | P-value | 1 | >1 | P-value | L | M | H | P-value |
| Effect starting timea | 7 | 7 | 0.33 | 7 | 7 | 7 | 0.44 | 7 | 7 | 7 | 0.71 | 7 | 7 | 0.99 | 7 | 7 | 7 | 0.97 |
| (6) | (1) | (1.7) | (4) | (4.5) | (5) | (4) | (2) | (3) | (4) | (3) | (5) | (7) | ||||||
| Effect peak timea | 24 | 30 | 0.34 | 22.5 | 28 | 28 | 0.42 | 21.5 | 28 | 29 | 0.83 | 28 | 27 | 0.96 | 23.5 | 27.5 | 28 | |
| (27) | (30) | (31) | (34) | (22) | (30) | (34) | (20) | (30) | (24) | (28) | (34) | (27) | ||||||
| Lasting timea | 5 | 5 | 0.99 | 4 | 5.5 | 5 | 0.20 | 5 | 5 | 4.7 | 0.56 | 5 | 5 | 0.57 | 4 | 4.5 | 6 | 0.22 |
| (8) | (6.5) | (6) | (8) | (9) | (9) | (6) | (7) | (9) | (2) | (7) | (4) | (8) | ||||||
| Efficacyb | 0.96 | 0.24 | 0.57 | 0.63 | 0.07 | |||||||||||||
| Ineffective | 8 | 9 | 7 | 8 | 2 | 1 | 14 | 2 | 14 | 3 | 2 | 12 | 3 | |||||
| (5.3%) | (5.9%) | (4.6%) | (5.3%) | (1.3%) | (1%) | (9.2%) | (1.3%) | (9.2%) | (2.0%) | (1.3%) | (7.9%) | (2.0%) | ||||||
| Effective | 14 | 17 | 4 | 22 | 5 | 3 | 21 | 7 | 21 | 10 | 7 | 10 | 14 | |||||
| (9.2%) | (11.2%) | (2.6%) | (14.5%) | (3.3%) | (2.0%) | (13.8%) | (4.6%) | (13.8%) | (6.6%) | (4.6%) | (6.6%) | (9.2%) | ||||||
| Significant | 9 | 12 | 2 | 16 | 3 | 5 | 12 | 4 | 14 | 7 | 2 | 6 | 13 | |||||
| (5.9%) | (7.9%) | (1.3%) | (10.5%) | (2.0%) | (3.3%) | (7.9%) | (2.6%) | (9.2%) | (4.6%) | (1.3%) | (3.9%) | (8.6%) | ||||||
| Complete | 34 | 49 | 21 | 48 | 14 | 15 | 55 | 13 | 62 | 21 | 17 | 32 | 34 | |||||
| (22.4%) | (32.2%) | (13.8%) | (31.6%) | (9.2%) | (9.9%) | (36.2%) | (8.6%) | (40.8%) | (13.8%) | (11.2%) | (21.1%) | (22.7%) | ||||||
Values are expressed as the median (interquartile range).
Values are expressed as the number of cases (percentage). L, low; M, medium; H, high.
The long-term effect of BTX-A on TN was also investigated in the present study. Cases from the BTX-A effective cohort were followed up each month following the 7th month after BTX-A treatment. Among the available patients (n=58) that were followed up, no significant increases in the VAS score were recorded, which may indicate the long-term control of TN by BTX-A.
Safety
BTX-A treatment may cause facial asymmetry. In the present study, 21 patients (13.8%) experienced short-term facial asymmetry, whereas no cases of twisted mouth or ptosis were observed (Table II). The incidence of side effects did not vary with the sex, age or injection dose; however, the data indicated that the disease course and number of branches did influence the incidence of side effects, with patients with a median disease course (duration, 1–10 months) exhibiting a higher occurrence of side effects associated with BTX-A injection. Furthermore, side effects of BTX-A were also influenced by the number of branches, in that multiple branches contributed to a higher incidence, later occurrence and longer duration of side effects (Table II). Patients who received one injection of BTX-A were also compared with those who received >1 injections. Additional injections did not result in a higher occurrence of side effects of BTX-A (P=0.76), since 13 out of 102 patients who received no additional injections were afflicted with side effects compared with 8 in 50 patients with additional injections afflicted with side effects.
Table II.
SEs associated with botulinum toxin A treatment by sex, age, disease course, number of branches and injection dose (n=152).
| Sex | Age (years) | Disease course (months) | Number of branches | Injection dose | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Male | Female | P-value | <50 | 50–70 | >70 | P-value | <1 | 1–10 | >10 | P-value | 1 | >1 | P-value | L | M | H | P-value |
| SEsa | 0.48 | 0.60 | 0.05 | 0.01 | 0.16 | |||||||||||||
| No | 58 | 73 | 28 | 81 | 22 | 23 | 83 | 25 | 102 | 29 | 26 | 54 | 51 | |||||
| (38.2%) | (48.0%) | (18.4%) | (53.3%) | (14.5%) | (15.1%) | (54.6%) | (16.4%) | (67.1%) | (19.1%) | (17.1%) | (35.5%) | (33.6%) | ||||||
| Yes | 7 | 14 | 6 | 13 | 2 | 1 | 19 | 1 | 9 | 12 | 2 | 6 | 13 | |||||
| (4.6%) | (9.2%) | (3.9%) | (8.6%) | (13.2%) | (1.%) | (12.5%) | (1.0%) | (5.9%) | (7.9%) | (1.3%) | (3.9%) | (8.6%) | ||||||
| SE observed time (days)b | 0 (0) | 0 (2) | 0.26 | 0 (0) | 0 (0) | 0 (0) | 0.63 | 0 (0) | 0 (0) | 0 (0) | 0.14 | 0 (0) | 0 (7) | 0.01 | 0 (0) | 0(0) | 0 (0) | 0.21 |
| SE lasting time (days)b | 0 (0) | 0 (0) | 0.22 | 0 (0) | 0 (0) | 0 (0) | 0.60 | 0 (0) | 0 (0) | 0 (0) | 0.11 | 0 (0) | 0 (25) | 0.01 | 0 (0) | 0 (0) | 0 (0) | 0.21 |
Values are expressed as the median (interquartile range).
Values are expressed as the number of cases (percentage). L, low; M, medium; H, high. SE observed time, the time period between the first BTX treatment and the appearance of side effects.
Stratified analysis
To provide more comprehensive information regarding the treatment of TN with BTX-A, a stratified analysis by sex and age was performed. Based on the results, it was observed that the age distribution of females influenced the treatment outcome of BTX-A: Younger subjects (<70 years) exhibited higher sensitivity to treatment with BTX-A (P=0.02; Table III). It was also observed that the disease course was significantly associated with the incidence of side effects in the female patients (P<0.05; Table III). In males, the disease course had no significant influence on side effect-associated parameters (Table IV), indicating that the association of the disease course with side effects in the entire cohort was primarily due to the significant association in female patients. Furthermore, in males, a higher injection dose was associated with a longer effect duration (P<0.01) and a higher incidence of successful treatment (P=0.06; Table IV). When the patients were stratified by age, the effect duration of BTX-A increased with the injection dose in patients younger than 50 years (P=0.09; Table V).
Table III.
Treatment outcomes of botulinum toxin A by age, disease course, and injection dose in females (n=87).
| Age (years) | Disease course (months) | Injection dose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | <50 | 50–70 | >70 | P-value | <1 | 1–10 | >10 | P-value | L | M | H | P-value |
| Effect starting timea | 7 (8) | 7 (3) | 7 (1) | 0.14 | 7 (2) | 7 (2) | 7 (1) | 0.69 | 7.5 (3) | 7 (5) | 7 (1) | 0.51 |
| Effect peak time (days)a | 20 (31) | 30 (29) | 28.5 (36) | 0.14 | 21 (21) | 30 (33) | 31 (18) | 0.72 | 37 (30) | 30 (35) | 29 (21) | 0.70 |
| Lasting time (days)a | 4 (6) | 6 (7) | 4.5 (3) | 0.17 | 5 (10) | 5 (5) | 4.5 (5) | 0.44 | 7.5 (7) | 5 (4) | 5 (5) | 0.50 |
| Efficacyb | 0.02 | 0.71 | 0.49 | |||||||||
| Ineffective | 5 (3.3%) | 2 (1.3%) | 2 (1.3%) | 0 (0.0%) | 8 (5.3%) | 1 (1.0%) | 0 (0.0%) | 6 (3.9%) | 3 (2.0%) | |||
| Effective | 1 (1.0%) | 15 (9.9%) | 1 (1.0%) | 3 (2.0%) | 11 (7.2%) | 3 (2.0%) | 3 (2.0%) | 7 (4.6%) | 7 (4.6%) | |||
| Significant | 1 (1.0%) | 10 (6.6%) | 1 (1.0%) | 3 (2.0%) | 6 (3.9%) | 3 (2.0%) | 1 (1.0%) | 4 (2.6%) | 7 (4.6%) | |||
| Complete | 13 (8.6%) | 28 (18.4%) | 8 (5.3%) | 7 4.6%) | 31 (20.4%) | 11 (7.2%) | 12 (7.9%) | 18 (11.8%) | 19 (12.5%) | |||
| SEsb | 0.39 | 0.05 | 0.19 | |||||||||
| No | 15 (9.9%) | 48 (31.6%) | 10 6.6%) | 13 (8.5%) | 43 (28.3%) | 17 (11.2%) | 15 (9.9%) | 31 (20.4%) | 27 (17.8%) | |||
| Yes | 5 (3.3%) | 7 (4.6%) | 2 (1.3%) | 0 (0.0%) | 13 (8.6%) | 1 (1.0%) | 1 (1.0%0 | 4 2.6%) | 9 (5.9%) | |||
| SE observed time (days)a | 0 (2) | 0 (0) | 0 (0) | 0.57 | 0 (0) | 0 (0) | 0 (0) | 0.12 | 0 (0) | 0 (0) | 0 (0) | 0.26 |
| SE lasting time (days)a | 0 (5) | 0 (0) | 0 (0) | 0.61 | 0 (0) | 0 (0) | 0 (0) | 0.12 | 0 (0) | 0 (0) | 0 (5) | 0.22 |
Values are expressed as the median (interquartile range).
Values are expressed as the number of cases (percentage). L, low; M, medium; H, high. SE observed time, the time period between the first BTX treatment and the appearance of side effects.
Table IV.
Treatment outcomes of botulinum toxin A by age, disease course, and injection dose in males (n=65).
| Age (years) | Disease course (months) | Injection dose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | <50 | 50–70 | >70 | P-value | <1 | 1–10 | >10 | P-value | L | M | H | P-value |
| Effect starting timea | 7.5 (3) | 7 (5) | 9 (7) | 0.56 | 8 (5) | 7 (5) | 7 (5) | 0.90 | 7 (5) | 7 (5) | 7 (6) | 0.50 |
| Effect peak time (days)a | 26 (38) | 21 (31) | 28 (9) | 0.85 | 22 (38) | 25 (28) | 25 (17) | 0.86 | 18 (12) | 24 (27) | 28 (35) | 0.25 |
| Lasting time (days)a | 3.5 (6) | 5 (8) | 6 (8) | 0.34 | 4 (9) | 5 (7) | 5 (9) | 0.97 | 3.5 (3) | 3.5 (5) | 6 (7) | 0.00 |
| Efficacyb | 0.79 | 0.19 | 0.06 | |||||||||
| Ineffective | 2 (1.3%) | 6 (3.9%) | 0 (0.0%) | 1 (1.0%) | 6 (3.9%) | 1 (1.0%) | 2 (1.3%) | 6 (3.9%) | 0 (0.0%) | |||
| Effective | 3 (2.0%) | 7 (4.6%) | 4 (2.6%) | 0 (0.0%) | 10 (6.6%) | 4 (2.6%) | 4 (2.6%) | 3 (2.0%) | 7 (4.6%) | |||
| Significant | 1 (1.0%) | 6 (3.9%) | 2 (1.3%) | 2 (1.3%) | 6 (3.9%) | 1 (1.0%) | 1 (1.3%) | 2 (1.3%) | 6 (3.9%) | |||
| Complete | 8 (5.2%) | 20 (13.2%) | 6 (3.9%) | 8 (5.2%) | 24 (15.8%) | 2 (1.3%) | 5 (3.3%) | 14 (9.2%) | 15 (9.8%) | |||
| SEsb | 0.47 | 0.83 | 0.87 | |||||||||
| No | 13 (8.6%) | 33 (21.7%) | 12 (7.9%) | 10 (6.6%) | 40 (26.3%) | 8 (5.3%) | 11 (7,2%) | 23 (15.1%) | 24 (15.8%) | |||
| Yes | 1 (1.0%) | 6 (3.9%) | 0 (0.0%) | 1 (1.0%) | 6 (3.9%) | 0 (0.0%) | 1 (1.0%) | 2 (1.3%) | 4 (2.6%) | |||
| SE observed time (days)a | 0 (0) | 0 (0) | 0 (0) | 0.28 | 0 (0) | 0 (0) | 0 (0) | 0.55 | 0 (0) | 0 (0) | 0 (0) | 0.73 |
| SE lasting time (days)a | 0 (0) | 0 (0) | 0 (0) | 0.31 | 0 (0) | 0 (0) | 0 (0) | 0.53 | 0 (0) | 0 (0) | 0 (0) | 0.76 |
Values are expressed as the median (interquartile range).
Values are expressed as the number of cases (percentage). L, low; M, medium; H, high. SE observed time, the time period between the first BTX treatment and the appearance of side effects.
Table V.
Treating outcomes of botulinum toxin A by sex, disease course, and injection dose in patients younger than 50 years (n=34).
| Sex | Disease course (months) | Injection dose | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Male | Female | P-value | <1 | 1–10 | >10 | P-value | L | M | H | P-value |
| Effect starting timea | 7.5 (3) | 7 (7) | 0.22 | 7.5 (1) | 7 (3) | 7 (5) | 0.87 | 7 (3) | 7 (1) | 8 (1) | 0.62 |
| Effect peak time (days)a | 26 (17) | 20.5 (31) | 0.30 | 27 (29) | 23 (29) | 9 (24) | 0.58 | 22 (27) | 16.5 (44) | 30 (21) | 0.82 |
| Lasting time (days)a | 3.5 (6) | 4 (6) | 0.83 | 7.5 (8) | 4 (6) | 3 (2) | 0.22 | 4 (5) | 3 (4) | 6 (6) | 0.09 |
| Efficacyb | 0.54 | 0.88 | 0.49 | ||||||||
| Ineffective | 2 (1.3%) | 5 (3.3%) | 0 (0.0%) | 6 (3.9%) | 1 (1.0%) | 1 (1.0%) | 4 (2.6%) | 2 (1.3%) | |||
| Effective | 3 (2.0%) | 1 (1.0%) | 0 (0.0%) | 4 (2.6%) | 0 (0.0%) | 1 (1.0%) | 0 (0.0%) | 3 (2.0%) | |||
| Significant | 1 (1.0%) | 1 (1.05) | 0 (0.0%) | 2 (1.3%) | 0 (0.0%) | 1 (1.0%) | 0 (0.0%) | 1 (1.0%) | |||
| Complete | 8 (5.3%) | 13 (8.6%) | 4 (2.6%) | 15 (9.9%) | 2 (1.3%) | 6 (3.9%) | 8 (5.2%) | 7 (4.6%) | |||
| SEsb | 0.36 | 0.76 | 0.39 | ||||||||
| No | 13 (8.6%) | 15 (9.9%) | 4 (2.6%) | 12 (7.9%) | 3 (2.0%) | 8 (5.3%) | 11 (7.2%) | 9 (5.9%) | |||
| Yes | 1 (1.0%) | 5 (3.3%) | 0 (0.0%) | 6 (3.9%) | 0 (0.0%) | 1 (1.0%) | 1 (1.0%) | 4 (2.6%) | |||
| SE observed time (days)a | 0 (0) | 0 (1) | 0.18 | 0 (0) | 0 (0) | 0 (0) | 0.40 | 0 (0) | 0 (0) | 0 (7) | 0.30 |
| SE lasting time (days)a | 0 (5) | 0 (0) | 0.21 | 0 (0) | 0 (0) | 0 (0) | 0.40 | 0 (0) | 0 (0) | 0 (21) | 0.33 |
Values are expressed as the median (interquartile range).
Values are expressed as the number of cases (percentage). L, low; M, medium; H, high. SE observed time, the time period between the first BTX treatment and the appearance of side effects.
Discussion
BTX-A has been widely used to protect patients against neuropathic pain, including TN (20). A number of clinical studies and meta-analyses have suggested that BTX-A is beneficial in the treatment of TN when compared with placebo in terms of the proportion of responders and the VAS score at the end of the follow-up (15,16,21,22). However, a number of controversies regarding the clinical application of BTX-A remain. Compared with previous studies, the present study performed a more comprehensively stratified analysis. Consistent with previous results, the treatment of BTX-A decreased the VAS score with only one administration. For patients insensitive to one injection of BTX-A, repeated injection led to better control of pain with no significant increase in the incidence of side effects. The results also demonstrated that patient sex, age or the number of branches had no significant influence on the starting time of the effect, the peak time of the effect, average effect duration, incidence of side effects, starting time of side effects and side effect duration. Furthermore, medium- and high-dose injections of BTX-A were more likely to completely control the pain of the patients, even though statistical significance was not observed (P=0.07). Conversely, a median disease course (duration, 1–10 months) and multiple branches contributed to a higher incidence of side effects, and multiple branches were associated with later occurrence of side effects and longer side effect duration.
Previous studies have indicated that the effect of BTX-A may last as long as 2 years (23); however, whether the long-term effect of BTX-A is influenced by the patients' characteristics has remained elusive. Therefore, the follow-up was performed for up to 28 months. It was indicated that within the initial 6 months following BTX-A treatment, females, patients with a short disease course and patients with a high injection dose reported lower VAS scores. In the short-term, higher injection doses of BTX-A did not influence the VAS scores; however, the effect of high-dose BTX-A receded at a slower rate than that of medium or low doses. Considering that treatment with a higher dose of BTX-A did not lead to a higher incidence of side effects, treatment of patients with TN with relatively higher doses of BTX-A may contribute to a greater duration of pain control. With regard to the dose range, Fabregat et al (24) suggested values from 50–100 units to achieve optimal treatment outcomes. Zhang et al (21) indicated that the lower (25 units) and the high dose (75 units) were similar in terms of short-term efficacy. These conclusions were validated in the present study: Different doses of BTX-A injection did not influence the short-term treatment outcomes and higher doses of BTX-A contributed to a more stable long-term control of pain due to TN.
To provide more specific information for the application of BTX-A in clinical settings, stratified analyses by sex and age were also performed. The majority of parameters investigated in the present study exhibited similar patterns with different stratifications by sex or age. However, certain notable trends were observed: For instance, female patients younger than 70 years were more sensitive to BTX-A treatment. Furthermore, the disease course influenced the incidence of side effects primarily in female patients, whereas in male patients, the disease course was not significantly associated with changes in side effects, indicating that the application of BTX-A in female patients with TN with a median disease course (duration, 1–10 months) should be more conservative. Higher BTX-A injection doses were more likely to successfully treat TN in male patients. The mechanisms responsible for the anti-TN effect of BTX-A remain to be elucidated. In addition to its effect on muscle contraction by inhibiting the release of acetylcholine, the direct analgesic action of BTX-A has also been observed (25). Previous studies have also indicated that BTX-A may alleviate neurogenic inflammation in sensory terminals by reducing the release of glutamate, substance P and calcitonin gene-associated peptide, and selectively inhibiting C-fibers and the transient receptor potential vanilloid (TRPV)1 receptor (26,27). The distinct sensitivities to BTX-A under different stratifications may also be attributable to the existence of BTX-A-sensitive and -insensitive C-fibers and the TRPV1 receptor (27).
Collectively, the present results demonstrated that the efficacy of BTX-A in the treatment of TN may persist for as long as 28 months. Furthermore, based on the present stratified analyses, it was demonstrated that female patients with a median disease course (duration, 1–10 months) exhibited a higher incidence of side effects following treatment with BTX-A and males achieved better treatment outcomes with high BTX-A doses. Given that the clinical response to treatment with BTX-A varies in all individual patients, the present study provided insight regarding the treatment outcomes of BTX-A and supported the long-term efficacy of the treatment. To validate the present results, adequately powered studies investigating the effect of BTX-A in other populations are required in the future.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National Natural Science Foundation of China (grant nos. 81701271, 81771397 and 81701295).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
HZ prepared the manuscript and analyzed the data. YL analyzed the data. NX, XC, CC and HX performed the data collection. YZ designed the experiment and revised the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China) for the screening, inspection and data collection of the patients. All protocols were in accordance with the provisions of the Declaration of Helsinki.
Patient consent for publication
The purpose and safety aspects of the study were explained to all patients and written informed consent was included in the documents of each patient.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Olesen J, Steiner TJ. The International classification of headache disorders, 2nd edn (ICDH-II) J Neurol Neurosurg Psychiatry. 2004;75:808–811. doi: 10.1136/jnnp.2003.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katusic S, Beard CM, Bergstralth E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann Neurol. 1990;27:89–95. doi: 10.1002/ana.410270114. [DOI] [PubMed] [Google Scholar]
- 3.Ngeow WC, Nair R. Injection of botulinum toxin type A (BOTOX) into trigger zone of trigeminal neuralgia as a means to control pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e47–e50. doi: 10.1016/j.tripleo.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: The ignition hypothesis. Clin J Pain. 2002;18:4–13. doi: 10.1097/00002508-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Cheshire WP. Trigeminal neuralgia: For one nerve a multitude of treatments. Expert Rev Neurother. 2007;7:1565–1579. doi: 10.1586/14737175.7.11.1565. [DOI] [PubMed] [Google Scholar]
- 6.Merrison AF, Fuller G. Treatment options for trigeminal neuralgia. BMJ. 2003;327:1360–1361. doi: 10.1136/bmj.327.7428.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo-Alvarez F, Hernando de la Barcena I, Marzo-Sola ME. Botulinum toxin in trigeminal neuralgia. Med Clin (Barc) 2017;148:28–32. doi: 10.1016/j.medcli.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 8.Humeau Y, Doussau F, Grant NJ, Poulain B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie. 2000;82:427–446. doi: 10.1016/S0300-9084(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 9.Pearce LB, First ER, Maccallum RD, Gupta A. Pharmacologic characterization of botulinum toxin for basic science and medicine. Toxicon. 1997;35:1373–1412. doi: 10.1016/S0041-0101(96)00180-8. [DOI] [PubMed] [Google Scholar]
- 10.Lian YJ, Wei HL, Zhang BA, et al. Treatment of 795 facial spasm and local dystonia with Botulinum toxin A. J Zhengzhou Univ (Med Sci) 2009;44:3. [Google Scholar]
- 11.Aurora SK, Winner P, Freeman MC, Spierings EL, Heiring JO, DeGryse RE, VanDenburgh AM, Nolan ME, Turkel CC. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358–1373. doi: 10.1111/j.1526-4610.2011.01990.x. [DOI] [PubMed] [Google Scholar]
- 12.Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, Silberstein SD, Brin MF, PREEMPT 2 Chronic Migraine Study Group OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804–814. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- 13.Micheli F, Scorticati MC, Raina G. Beneficial effects of botulinum toxin type a for patients with painful tic convulsif. Clin Neuropharmacol. 2002;25:260–262. doi: 10.1097/00002826-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Wu CJ, Lian YJ, Zheng YK, Zhang HF, Chen Y, Xie NC, Wang LJ. Botulinum toxin type A for the treatment of trigeminal neuralgia: Results from a randomized, double-blind, placebo-controlled trial. Cephalalgia. 2012;32:443–450. doi: 10.1177/0333102412441721. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Guan X, Fan L, Li M, Liao Y, Nie Z, Jin L. Therapeutic efficacy and safety of botulinum toxin type A in trigeminal neuralgia: A systematic review. J Headache Pain. 2013;14:72. doi: 10.1186/1129-2377-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morra ME, Elgebaly A, Elmaraezy A, Khalil AM, Altibi AM, Vu TL, Mostafa MR, Huy NT, Hirayama K. Therapeutic efficacy and safety of botulinum toxin a therapy in trigeminal neuralgia: A systematic review and meta-analysis of randomized controlled trials. J Headache Pain. 2016;17:63. doi: 10.1186/s10194-016-0651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Headache Classification Subcommittee of the International Headache Society, corp-author. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):S9–S160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS. Pain measurement: Understanding existing tools and their application in the emergency department. Emerg Med (Fremantle) 2001;13:279–287. doi: 10.1046/j.1035-6851.2001.00230.x. [DOI] [PubMed] [Google Scholar]
- 19.Ross I, Robert G. R: A language for data analysis and graphics. J Computational Graphical Statistics. 1996;5:299–314. doi: 10.1080/10618600.1996.10474713. [DOI] [Google Scholar]
- 20.Zakrzewska JM. Botulinum toxin type A for trigeminal neuralgia-we have the prima facie evidence. Cephalalgia. 2012;32:1156–1157. doi: 10.1177/0333102412459577. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Lian Y, Ma Y, Chen Y, He C, Xie N, Wu C. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: Observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J Headache Pain. 2014;15:65. doi: 10.1186/1129-2377-15-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Lian YJ, Chen Y, Zhang HF, Ma YQ, He CH, Wu CJ, Xie NC, Zheng YK, Zhang Y. Therapeutic effect of botulinum toxin-a in 88 patients with trigeminal neuralgia with 14-month follow-up. J Headache Pain. 2014;15:43. doi: 10.1186/1129-2377-15-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen JH, Lian YJ, Zheng YK. Effect of botulinum toxin type a on clssic trigeminal neuralgia. Chin J Rehab Med. 2011;26:2. [Google Scholar]
- 24.Fabregat G, De Andrés J, Villanueva-Pérez VL, Asensio-Samper JM. Subcutaneous and perineural botulinum toxin type A for neuropathic pain: A descriptive review. Clin J Pain. 2013;29:1006–1012. doi: 10.1097/AJP.0b013e31827eafff. [DOI] [PubMed] [Google Scholar]
- 25.Klein AW. The therapeutic potential of botulinum toxin. Dermatologic Surgery. 2004;30:452–455. doi: 10.1097/00042728-200403000-00030. [DOI] [PubMed] [Google Scholar]
- 26.Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26:785–793. doi: 10.1016/j.neuro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Gazerani P, Pedersen NS, Staahl C, Drewes AM, Arendt-Nielsen L. Subcutaneous Botulinum toxin type A reduces capsaicin-induced trigeminal pain and vasomotor reactions in human skin. Pain. 2009;141:60–69. doi: 10.1016/j.pain.2008.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.