Abstract
Adropin is a secreted polypeptide that has been demonstrated to serve an important role in protecting the vascular endothelium. Pharmacological activation of pro-survival kinases, such as PI3K-Akt and ERK1/2, are involved in the reperfusion injury salvage kinase (RISK) pathway. In the present study, the effects of adropin in cardiomyocyte injury induced by simulated ischemia/reperfusion (SI/R) were assessed. Additionally, the current study also assessed the mechanisms that govern SI/R in a H9c2 cardiomyoblast cell model. Cell viability was measured using an MTT assay. Cell injury was assessed using creatine kinase MB measurements. Apoptosis was assessed using flow cytometry and caspase-3 activity. The inflammatory response was measured using tumor necrosis factor α and interleukin-10 expression. Oxidative stress was assessed using malondialdehyde and superoxide dismutase. The expression levels of Akt, ERK1/2, glycogen synthase kinase 3β (GSK3β), Bcl-2 and Bax were determined using western blot analysis. The results of the current study revealed that moderate-dose adropin increased cell viability, reduced early apoptosis and caspase-3 activity, promoted Bcl-2 expression, inhibited Bax and increased the Bcl-2/Bax ratio. Adropin significantly increased the phosphorylation of Akt, ERK1/2 and GSK3β, whereas inhibitors of PI3K and ERK1/2, respectively, LY294002 and PD98059, abolished the cardioprotective role of adropin. Furthermore, no significant difference was observed in phosphorylated-STAT3/total-STAT3 expression between the adropin and SI/R groups and Janus kinase 2 inhibitor AG490 did not significantly inhibit the protective role of adropin. These results indicate that adropin exerts a protective effect against SI/R injury through the RISK pathway instead of the survivor activating factor enhancement pathway.
Keywords: myocardial reperfusion injury, postconditioning, adropin, reperfusion injury salvage kinase pathway
Introduction
The most effective strategy to reduce acute myocardial ischemic injury and subsequent mortality is to promptly recover coronary reflow using thrombolytic therapy or percutaneous intervention (1). However, reperfusion can induce myocardial ischemia reperfusion injury (MIRI). The inflammatory response, oxidative stress and cell apoptosis are considered to be critical factors associated with mediating the effects of MIRI (2–4). Targeting these factors is important in the prevention and reduction of MIRI.
Adropin, first described by Kumar et al (5) in 2008, is a secreted protein and an endogenous biologically active substance encoded for by an energy homeostasis-associated gene. Lovren et al (6) demonstrated that adropin is expressed in the endothelial cells of the umbilical veins and coronary arteries. The aforementioned study also revealed that adropin may exhibit nonmetabolic properties, which includes the regulation of endothelial function through the upregulation of endothelial nitric oxide synthase (eNOS) via the PI3K-Akt and ERK1/2, which are the two major components of the reperfusion injury salvage kinase (RISK) pathway. The RISK pathway represents one of the most important survival mechanisms against ischemic reperfusion injury. Apart from the RISK pathway, the survivor activating factor enhancement (SAFE) pathway also serves a role in ischemic postconditioning. The major components of the SAFE pathway are TNF-α and Janus kinase (JAK), which phosphorylates the transcription factor STAT3. Additionally, adropin has been revealed to improve murine limb perfusion and elevate capillary density following the induction of hindlimb ischemia (6). Clinical research has demonstrated that adropin is associated with a variety of metabolic risk factors. Butler et al (7) demonstrated that plasma adropin levels are negatively associated with obesity and insulin resistance. Celik et al (8) revealed that serum adropin levels were negatively associated with cardiac X syndrome due to coronary microvascular perfusion dysfunction and that low serum adropin levels were an independent risk factor of X syndrome. Adropin has been revealed to be negatively correlated with inflammatory biomarker-C reactive protein and it has been demonstrated that patients with severe atherosclerosis exhibit lower adropin levels (9). These results indicated that adropin may influence the anti-inflammatory response and reduce atherosclerosis (9). Yang et al (10) demonstrated that adropin reduces endothelial cell permeability and modulates ischemia-induced blood-brain barrier injury. However, to the best of our knowledge, the role of adropin in myocardial reperfusion injury has not yet been assessed.
In the current study, a hypoxia/reoxygenation model was established in neonatal rat cardiomyoblast cells (H9c2) to simulate ischemia/reperfusion (SI/R) injury. The effect of adropin on SI/R injury and the mechanisms that govern this effect were subsequently assessed.
Materials and methods
Cell culture
H9c2 cells were obtained from the Type Culture Collection of the Chinese Academy of Sciences. Cells were passaged up to 4 times and were cultured in DMEM (GE Healthcare Life Sciences) containing 10% (v/v) heat-inactivated FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin (GE Healthcare Life Sciences) and 100 µg/ml streptomycin (GE Healthcare Life Sciences), under a 5% CO2 atmosphere at 37°C.
H9c2 cells subjected to hypoxia/reoxygenation induced injury
Hypoxia was induced as described previously (11). H9c2 cells were cultured to 70–80% confluency, fresh DMEM without FBS was subsequently added and the cells were transferred to a triple gas incubator with either hypoxic (5% CO2, 1% O2 and 94% N2) or SI/R (hypoxia: 5% CO2, 1% O2 and 94% N2, followed by reoxygenation: 5% CO2, 21% O2 and 74% N2) settings. A hypoxia/reoxygenation model was established and cells were divided into 11 groups. All groups except the control group were treated with hypoxic conditions for 12 h and reoxygenation for 24 h. Postconditioning of cardiomyocytes was achieved as follows: At the end of 12 h of hypoxia, the cells were initially received different doses of adropin and then returned to the reoxygenated condition for another 24 h. The groups were classified as follows: i) Control group, normoxic conditions (37°C, 5% CO2, 21% O2, 71% N2); ii) SI/R group; iii) SI/R + low dose adropin (10 ng/ml; Phoenix Pharmaceuticals, Inc.), in which adropin was added prior to reoxygenation (adropin-L); iv) SI/R + moderate dose adropin group (25 ng/ml; adropin-M); v) SI/R + high dose adropin group (50 ng/ml; adropin-H); vi) LY294002 group, 40 µmol/l PI3K specific inhibitor LY294002 (Sigma-Aldrich; Merck KGaA) was added to the medium prior to hypoxia as described previously (12); vii) adropin + LY294002 group, in which 40 µmol/l LY294002 and 25 ng/ml adropin were added to the medium prior to hypoxia (12) and reoxygenation, respectively; viii) PD98059 group, in which 25 µmol/l ERK1/2-specific inhibitor PD98059 (Sigma-Aldrich; Merck KGaA) was added to the medium (12) prior to hypoxia; ix) adropin + PD98059 group, in which 25 µmol/l PD98059 and 25 ng/ml adropin were added to the medium (12) prior to hypoxia and reoxygenation, respectively; x) AG490 group, in which 100 µmol/l JAK2 inhibitor AG490 (Sigma-Aldrich; Merck KGaA) was added to the medium prior to hypoxia as described previously (13); xi) adropin + AG490 group, in which 100 µmol/l AG490 and 25 ng/ml adropin were added to the medium (13) prior to hypoxia and reoxygenation, respectively.
MTT measurement of cell viability
A total of 1×105 H9c2 cells/ml were seeded into a 96-well culture plate and incubated at 5% CO2 and 37°C for 24 h. Cell viability was determined using an MTT assay. At 12 h following reoxygenation, 20 µl MTT solution was added into each well (5 mg/ml) and plates were incubated for 4 h at 37°C. A microplate reader was used to measure the absorbance at a wavelength of 490 nm.
ELISA assay and colorimetry
The expression of creatine kinase MB (CK-MB; cat. no. H197), tumor necrosis factor α (TNF-α; cat. no. H052) and interleukin (IL)-10 (cat. no. H009) were measured using ELISA assay kits (Nanjing Jiancheng Bioengineering Institute). Malondialdehyde (MDA; cat. no. A003-4) and superoxide dismutase (SOD; cat. no. A001-1) concentrations were determined using colorimetry kits according to manufacturer's protocols (Nanjing Jiancheng Bioengineering Institute). The experiment was performed at least three times and CK-MB level was expressed as IU/l. TNF-α and IL-10 levels were expressed as pg/ml. The MDA level and SOD were expressed as nmol/mg protein and as U/mg protein, respectively.
Apoptosis analysis
Early cell apoptosis was measured using flow cytometry. The analysis of phosphatidylserine on the outer apoptotic cell membranes was performed using annexin-V-fluorescein and propidium iodide (Annexin-V-FLUOS Staining kit; Roche Diagnostics). Collected cells were rinsed with ice-cold PBS and resuspended in 250 µl of binding buffer and ~1–5×105 cells were analyzed in each of the samples. A total of 100 µl annexin-V-FLUOS labeling solution was added to the cells, which were then incubated for 15 min at 25°C. The cells were analyzed using FlowJo software (version 10.4.1; BD FACScanto II; Becton, Dickinson and Company).
Measurement of caspase-3 activity
Caspase-3 activity was measured using a colorimetric activity assay kit (Ac-DEVD-pNA; Beyotime Institute of Biotechnology) according to manufacturer's protocol. In brief, cells were lysed in ice-cold lysis buffer, placed on ice for 15 min, then centrifuged at 4°C for 15 min at 16,000 × g and supernatant was subsequently incubated with caspase-3 substrate on a 96-well plate. Protein concentration was determined using Bradford protein assay kit (cat. no. P0006; Beyotime Institute of Biotechnology). Caspase-3 activity was determined using a microplate reader at a wavelength of 405 nm.
Western blot analysis
H9c2 cells were washed with PBS, enzymatically dissociated with the use of trypsin (HyClone; GE Healthcare Life Sciences), and prepared in lysis buffer with protease inhibitor cocktail (cat. no. P0013B; Beyotime Institute of Biotechnology). Protein quantification was measured by using a BCA protein assay kit (cat. no. P0012; Beyotime Institute of Biotechnology). Equal quantities of protein (30 µg/lane) from whole cell lysates of cultured H9c2 cells were separated by 10% SDS-PAGE and transferred to a PVDF membrane. Following blocking with 5% BSA for 1 h at room temperature for binding non-specific sites, membranes were incubated with primary antibodies overnight at 4°C. The following primary antibodies were used: Phosphorylated (p)-Akt polyclonal antibody (1:1,000; cat. no. YP0864) and Akt polyclonal antibody (1:1,000; cat. no. YT0173) were purchased from ImmunoWay Biotechnology Company. p-ERK 1/2 monoclonal antibody (1:1,000; cat. no. sc-136521), ERK 1/2 monoclonal antibody (1:1,000; cat. no. sc-514302), p-STAT3 antibody (1:1000; cat. no. sc-7993) and STAT3 antibody (1:1,000; cat. no. sc-8019) were purchased from Santa Cruz Biotechnology, Inc. P-GSK3β antibody (1:1,000; cat. no. ab131097), GSK3β antibody (1:5,000; cat. no. ab32391), Bcl-2 antibody (1:1,000; cat. no. ab59348) and Bax antibody (1:1,000; cat. no. ab32503) were purchased from Abcam. Following incubation with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:5,000; cat. no. ZB-5301; OriGene Technologies, Inc.) or HRP-conjugated goat anti-mouse IgG (1:5,000; cat. no. ZB-2305; OriGene Technologies, Inc.) at 37°C for 1 h, the signals were detected with Pierce™ ECL Western Blotting Substrate kit (cat. no. 32209, Pierce; Thermo Fisher Scientific, Inc.) and bands were subsequently quantified using Quantity One software (version 4.6.2; Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation. Comparisons between groups were performed using one-way ANOVA with Student-Newman-Keuls correction for multiple comparisons. Statistical analyses were performed using SPSS version 13.0 (SPSS Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of adropin dose on cell viability and CK-MB levels
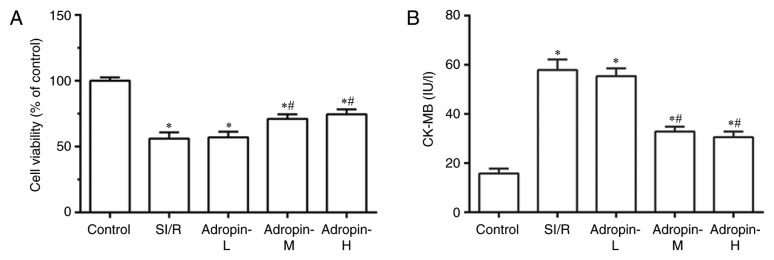
Cell viability was examined using a MTT assay and CK-MB levels were measured to assess cardiomyocyte injury. As presented in Fig. 1A and B, SI/R group cell viability was significantly reduced (P<0.001) and CK-MB levels significantly increased (P<0.001) compared with the control group. Cell viability was significantly higher and CK-MB levels were significantly lower in the adropin-M and adropin-H groups when compared with the SI/R group. In addition, no significant difference in cell viability and CK-MB levels was observed between the adropin-M and adropin-H groups. The adropin-L group did not exhibit any significant effect on cell viability or CK-MB expression when compared with the SI/R group, indicating that moderate and high adropin levels can reduce SI/R injury. The subsequent experiments were performed using moderate-dose adropin as the adropin group.
Figure 1.
Effect of adropin on cell viability. (A) Cell viability and (B) CK-MB of H9c2 cells subjected to SI/R. *P<0.001 vs. control and #P<0.001 vs. SI/R. Results are representative of three independent experiments. CK-MB, creatine kinase MB; SI/R, simulated ischemia/reperfusion; L, low; M, medium; H, high.
Effect of adropin on myocardial apoptosis
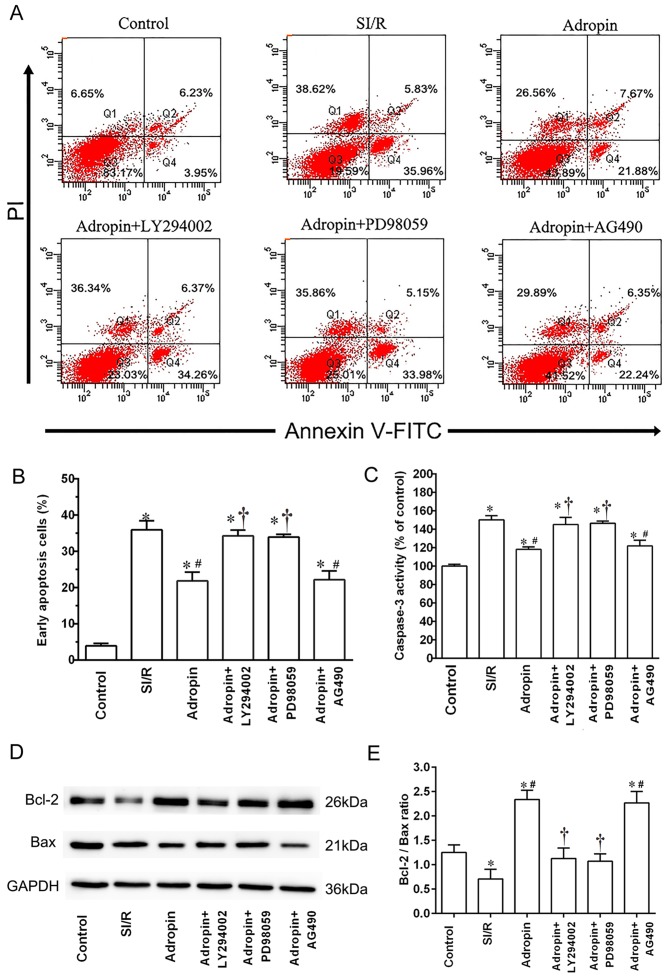
Flow cytometry was used to assess myocardial apoptosis and caspase-3 activity subsequent to reoxygenation (Fig. 2). The SI/R group exhibited a higher early apoptosis rate (P<0.001) and higher caspase-3 activity (P<0.001) when compared with the control group (Fig. 2B and C, respectively). Moderate-dose adropin exhibited a significantly lower early apoptosis rate (P<0.001) and caspase-3 activity (P<0.001) compared with the SI/R group. Additionally, LY294002 and PD98059 significantly reversed the protective effects of adropin on apoptosis rate (P<0.001) and significantly increased caspase-3 activity (P<0.001) compared with the adropin group (Fig. 2A-C). However, AG490 exhibited no significant effect on early apoptosis rate or caspase-3 activity when compared with the adropin group (Fig. 2A-C).
Figure 2.
Effect of adropin on cell apoptosis and caspase-3 activity. (A and B) Apoptotic rates were quantified by annexin V-FITC/PI double staining cytometry. Viable cells are annexin V-FITC−/PI− (Q3); annexin V-FITC+/PI− cells (Q4) are early in the apoptotic process; annexin V-FITC+/PI+ cells (Q2) are late in the apoptotic process; and necrotic cells are Annexin V-FITC−/PI+ (Q1). (C) Caspase-3 activity analysis. *P<0.001 vs. control; #P<0.001 vs. SI/R; †P<0.001 vs. adropin. Results are representative of three independent experiments. (D) Western blot analysis and (E) subsequent quantification of Bcl-2 and Bax expression. Mean ± standard deviation of relative Bcl-2/Bax ratios are presented. Data were normalized to loading control GAPDH. *P<0.05 vs. control; #P<0.05 vs. SI/R; †P<0.05 vs. adropin. PI, propidium iodide; SI/R, simulated ischemia/reperfusion.
Western blot analysis was used to detect the effect of adropin and the aforementioned inhibitors on the Bcl-2/Bax ratio. As presented in Fig. 2D and E, the SI/R group had a significantly lower Bcl-2/Bax ratio compared with the control group (P<0.05). When compared with the SI/R group, the adropin group exhibited a significantly higher Bcl-2/Bax ratio (P<0.05). Additionally, the adropin + LY294002 and adropin + PD98059 groups exhibited significantly lower Bcl-2/Bax ratios (P<0.05) compared with the adropin-only group. However, no significant differences were determined in the Bcl-2/Bax ratio between the adropin and adropin + AG490 group (Fig. 2D and E).
Effects of different doses of adropin on the inflammatory response
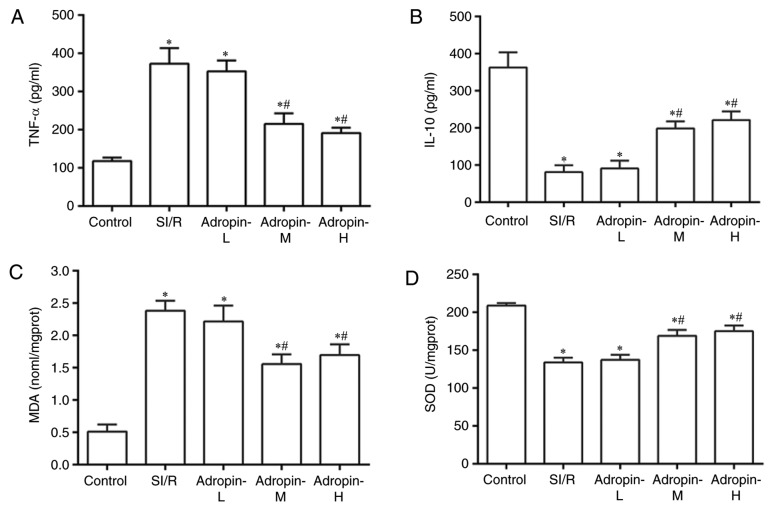
The inflammatory response was assessed using TNF-α and IL-10 expression measurements (Fig. 3A and B, respectively). TNF-α levels significantly increased (P<0.001) and IL-10 levels decreased (P<0.001) in the SI/R group compared with the control group. The adropin-M and adropin-H groups exhibited significantly reduced TNF-α expression (P<0.001) and significantly increased IL-10 expression (P<0.001) when compared with the SI/R group. In addition, no significant difference was determined in TNF-α and IL-10 expression levels between the adropin-M and adropin-H groups (P>0.05). The adropin-L group did not affect TNF-α or IL-10 levels compared with the control group, suggesting that moderate and high concentrations of adropin can protect the heart by alleviating the inflammatory response.
Figure 3.
Effects of adropin on the inflammatory response and oxidative stress. Effect of adropin on (A) TNF-α, (B) IL-10, (C) MDA and (D) SOD of H9c2 cells subjected to SI/R. *P<0.001 vs. control and #P<0.001 vs. SI/R. Results are representative of three independent experiments. TNF-α, tumor necrosis factor α; IL-10, interleukin 10; MDA, malondialdehyde; SOD, superoxide dismutase; SI/R, simulated ischemia/reperfusion; L, low; M, medium; H, high.
Effects of different doses of adropin on oxidative stress
Oxidative stress was examined by measuring MDA levels and SOD activity (Fig. 3C and D, respectively). MDA levels significantly increased (P<0.001) and SOD activity was significantly reduced in the SI/R group (P<0.001) compared with the control group. The adropin-M and adropin-H groups exhibited reduced MDA levels (P<0.001) and exhibited higher SOD activity (P<0.001) compared with the SI/R group. The results indicated that adropin may inhibit lipid peroxide production and increase scavenging superoxide radical activity. No significant difference in MDA levels and SOD activity were determined between the adropin-M and adropin-H groups. Adropin-L did not reduce MDA levels or increase SOD activity when compared with the SI/R group, demonstrating the dose-dependent role of adropin in the antioxidative effect.
Reperfusion injury salvage kinase (RISK) pathway is associated with the reduction of SI/R injury by adropin
The results of the present study demonstrated that adropin inhibited myocardial injury induced by SI/R in a dose-dependent manner. The Adropin-M group (the minimum optimal concentration) was used as the Adropin group in subsequent experiments to further assess the molecular mechanisms associated with the reduction of adropin in SI/R injury.
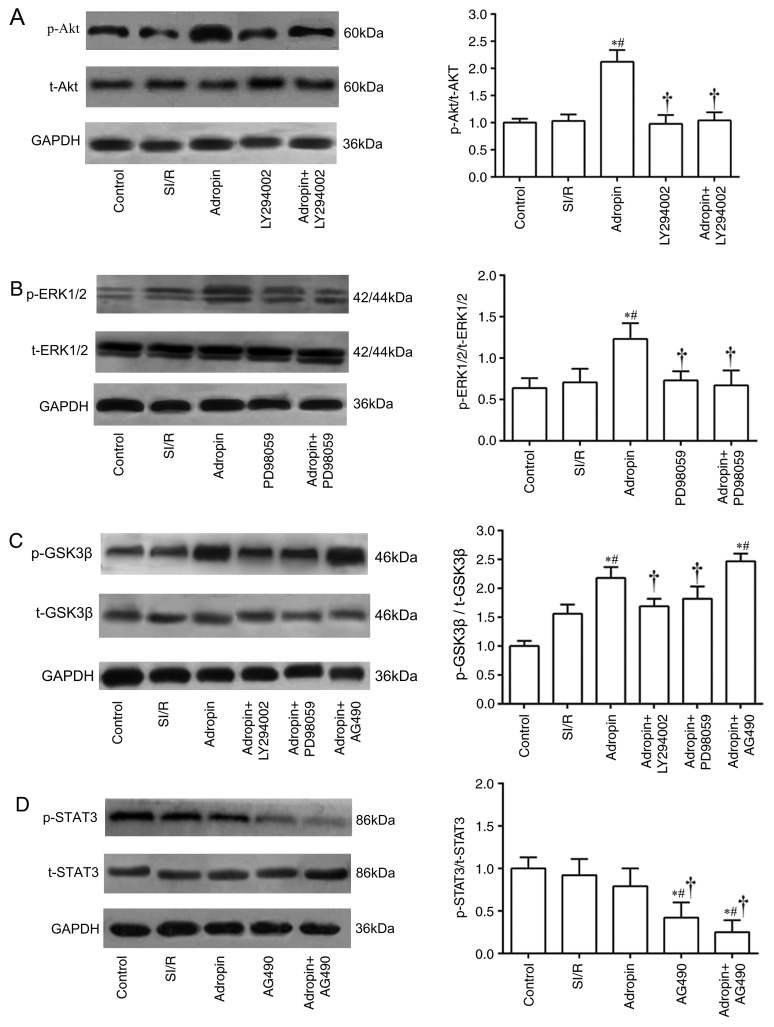
As presented in Fig. 4A, the adropin group induced a significant elevation in p-Akt/t-Akt ratio (P<0.05) compared with the SI/R group. LY294002 group exhibited a significantly decreased p-Akt/t-Akt ratio when compared with the adropin group (P<0.05). In addition, the adropin group had a significantly higher p-ERK1/2/t-ERK1/2 ratio (P<0.05) compared with the SI/R group. PD98059 exhibited a significantly decreased p-ERK1/2/t-ERK1/2 ratio compared with the adropin group (Fig. 4B). Furthermore, adropin significantly increased p-GSK3β/t-GSK3β ratio compared with the SI/R group (P<0.05), which was partially but significantly reversed by additive treatments with LY294002 (P<0.05) or PD98059 (P<0.05). However, the adropin and adropin + AG490 groups demonstrated no significant difference in the p-GSK3β/t-GSK3β ratio (Fig. 4C). Compared with the control, the AG490 and adropin + AG490 groups significantly inhibited the phosphorylation levels of STAT3 (P<0.05). Notably, adropin and control groups exhibited no difference in the p-STAT3/t-STAT ratio (Fig. 4D).
Figure 4.
Effect of SI/R and adropin on the activation of the RISK pathway in H9c2 cells. Expression and subsequent quantification of (A) p-Akt and t-Akt, (B) p-ERK1/2 and t-ERK1/2, (C) p-GSK3β and t-GSK3β and (D) p-STAT3 and t-STAT3. Data are presented as the mean ± standard deviation. Results were normalized to loading control GAPDH. *P<0.05 vs. control; #P<0.05 vs. SI/R. †P<0.05 vs. adropin. P, phosphorylated; t, total; GSK3β, glycogen synthase kinase 3β; SI/R, simulated ischemia/reperfusion.
Discussion
Adropin is a newly identified endogenous bioactive substance that serves an important role in energy metabolism. Lovren et al (6) demonstrated that adropin may directly affect endothelial cells and may possess nonmetabolic properties, including the protection of endothelial function through the RISK pathway. Adropin upregulates eNOS and increases the production of NO through the PI3K-Akt and ERK1/2 pathways. Adropin also serves a role in improving murine limb perfusion and elevating capillary density after ischemia (6). Exogenous adropin reduces insulin resistance and metabolic disorders, protects endothelial cells and attenuates organ ischemia (6,10). These results indicate that adropin may also be associated with ischemia reperfusion injury and may serve a cardioprotective role in MIRI.
Apoptosis is an important factor in the pathogenesis of MIRI (14,15). Mitochondria serve a central role in apoptosis regulation and control cytochrome C release through channels formed by Bcl-2 gene family expression, which is a key mechanism that regulates apoptosis (14). The inhibition of myocardial apoptosis can prevent myocardial cell loss and delay the occurrence of heart failure (16,17). In the present study, the results indicated that adropin treatment after hypoxia induction can inhibit hypoxia/reoxygenation-induced injury in H9c2 cells. Adropin reduced the proportion of early apoptosis in myocardial cells, decreased the activity of caspase-3, reduced the expression of Bax gene and increased Bcl-2 gene expression. These results demonstrated that adropin can reduce SI/R injury by regulating the mitochondrial apoptosis pathway.
A number of inflammatory factors including TNF-α, IL-1, IL-6 and IL-8 are released by myocardium subjected to ischemia-reperfusion (18,19). Oxidative stress also serves an important role in myocardial injury located in the infarcted and reperfused myocardium (20,21). Myocardial cells generate numerous reactive oxygen species during the ischemia-reperfusion process and increase TNF-α synthesis, which can lead to an increase in the apoptosis cascade reaction, the interactions between inflammatory and endothelial cells and intracellular calcium overload (22). Various novel antioxidants have been associated with renal protection through the antioxidative and antiapoptotic pathways (23,24). A previous study (9) has demonstrated that adropin is negatively correlated with the inflammatory marker C reactive protein. In patients with severe coronary atherosclerosis, adropin serum level is low (9), which indicates that adropin possesses a potential anti-inflammatory effect. In the current study, moderate and high concentrations of adropin were indicated to reduce the inflammatory response and oxidative stress during SI/R injury. Additionally, adropin was revealed to inhibit SI/R-induced myocardial injury by reducing early myocardial apoptosis, inflammatory response and oxidative stress, and increasing myocardial cell viability.
In 2007, Yellon et al (25) proposed a new cardioprotective strategy to reduce MIRI at the early stages of reperfusion by targeting the RISK-mitochondrial permeability transition pathway (mPTP). This study revealed that ischemic or pharmacological postconditioning prior to reperfusion can activate RISK or inhibit mPTP opening to limit infarct size and reduce MIRI (25). Ischemic and pharmacological postconditioning invoke the activation of signal transduction cascades by autacoids triggers and eventually inhibit the opening of mPTP (26). However, pharmacological postconditioning performed prior to continuous reperfusion is operable in clinical practice and can avoid mechanical manipulation and associated complications (26). The activation of the RISK signaling pathway (PI3K/Akt and ERK1/2) may serve a role in cardioprotection in myocardial reperfusion and therefore, this pathway may become an important drug target (27). The activation of PI3K and its downstream target (Akt) is also associated with myocardial reperfusion injury (28,29). In the ischemic myocardium, the phosphorylation of Akt can inhibit myocardial apoptosis and promote the cell survival pathway (30). Additionally, ERK1/2 is an important kinase of the RISK pathway and its activation in myocardial ischemia/reperfusion is beneficial to reduce apoptosis and to help recover cardiac function (31).
In addition to the RISK pathway, the survivor activating factor enhancement (SAFE) pathway has been revealed to be an additional pro-survival signaling pathway associated with the early reperfusion period and is composed of TNF-α and STAT-3 (32). mPTP is the downstream effector of the SAFE and RISK pathways (32). mPTP may be the common final effector of cardioprotective effects exhibited by pre and postconditioning (27). Furthermore, complex crosstalk between RISK and SAFE pathways may exist.
The current study assessed whether the RISK and SAFE pathways are associated with the role of adropin in the reduction of SI/R injury in cardiomyocytes. The results demonstrated that a moderate concentration of adropin significantly increased the phosphorylation of Akt and ERK1/2 and these results are consistent with Lovren et al (6). It was also revealed that adropin can promote the phosphorylation of GSK3β (a prosurvival signaling pathway downstream target protein). PI3K specific inhibitor LY294002 or ERK1/2 inhibitor PD98059 also significantly inhibited the cardioprotective effects of adropin, indicating that these effects may be dependent on the PI3K/Akt and ERK1/2 pathway. The adropin treatment did not significantly increase the phosphorylation of STAT3, which is the most important target of the SAFE pathway (33). STAT3 is also the substrate of JAK2 kinase. The JAK2 kinase specific inhibitor, AG490, did not significantly inhibit the protective role of adropin in SI/R injury.
In conclusion, the results of the present study demonstrate that adropin reduces SI/R injury in H9c2 myocardial cells through the RISK pathway (PI3K/Akt and ERK1/2) by activating the downstream target GSK3β to regulate the mitochondrial apoptosis. However, the SAFE pathway (JAK-STAT3) was not indicated to be associated with the exhibited myocardial protection. The current study may provide a potential therapeutic target for ischemia reperfusion injury and a theoretical basis for the clinical use of adropin.
Although H9c2 cells have been widely used in the study of cardiovascular disease, these studies may not accurately represent the in vivo reaction of normal myocardial cells to drug treatments. In the current study, the effects of adropin were only assessed in relation to a few inflammatory factors. However, other inflammatory factors such as leukocyte adhesion, aggregation and inflammatory stimulation signals and their receptors have not been involved. Reactive oxygen species (ROS) levels were not directly assessed and ROS scavenger was also not used. Therefore, the mechanisms underlying the changes in SOD and MDA levels observed in the current study following treatment with adropin remain to be determined.
Acknowledgements
Not applicable.
Funding
The current study was mainly supported by The National Natural Science Foundation of China (grant no. 81500352) and partially by the Natural Science Foundation of Fujian Province of China (grant no. 2016J05186), the Program for New Century Excellent Talents in Fujian Province University (grant no. 2015B021), the Medical Elite Cultivation Program of Fujian (grant no. 2015-ZQN-ZD-12), the Youth Foundation of Fujian Provincial Health and Family Planning Commission of China (grant no. 2015-1-40) and the Science Foundation for Distinguished Young Scholars of Fujian Province (grant no. 2013J06015).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LW and JF performed data analysis, wrote the manuscript and contributed to the critical revision of the manuscript. LW, XY and CX conducted the experiments and statistical analysis. LC performed data analysis and contributed to the critical revision of the manuscript. All authors are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation. 2001;103:2296–2302. doi: 10.1161/01.CIR.103.18.2296. [DOI] [PubMed] [Google Scholar]
- 3.Kaminski KA, Bonda TA, Korecki J, Musial WJ. Oxidative stress and neutrophil activation-the two keystones of ischemia/reperfusion injury. Int J Cardiol. 2002;86:41–59. doi: 10.1016/S0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 4.Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, Lips DJ, Doevendans PA. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza RA, Chouljenko VN, Kousoulas KG, Rogers PM, Kesterson RA, Thearle M, et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008;8:468–481. doi: 10.1016/j.cmet.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta M, Al-Omran M, Teoh H, Verma S. Adropin is a novel regulator of endothelial function. Circulation 122 (11 Suppl) 2010:S185–S192. doi: 10.1161/CIRCULATIONAHA.109.931782. [DOI] [PubMed] [Google Scholar]
- 7.Butler AA, Tam CS, Stanhope KL, Wolfe BM, Ali MR, O'Keeffe M, St-Onge MP, Ravussin E, Havel PJ. Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. J Clin Endocrinol Metab. 2012;97:3783–3791. doi: 10.1210/jc.2012-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celik A, Balin M, Kobat MA, Erdem K, Baydas A, Bulut M, Altas Y, Aydin S, Aydin S. Deficiency of a new protein associated with cardiac syndrome X; called adropin. Cardiovasc Ther. 2013;31:174–178. doi: 10.1111/1755-5922.12025. [DOI] [PubMed] [Google Scholar]
- 9.Wu L, Fang J, Chen L, Zhao Z, Luo Y, Lin C, Fan L. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin Chem Lab Med. 2014;52:751–758. doi: 10.1515/cclm-2013-0844. [DOI] [PubMed] [Google Scholar]
- 10.Yang C, DeMars KM, Hawkins KE, Candelario-Jalil E. Adropin reduces paracellular permeability of rat brain endothelial cells exposed to ischemia-like conditions. Peptides. 2016;81:29–37. doi: 10.1016/j.peptides.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Ito H, Adachi S, Akimoto H, Nishikawa T, Kasajima T, Marumo F, Hiroe M. Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ Res. 1994;75:426–433. doi: 10.1161/01.RES.75.3.426. [DOI] [PubMed] [Google Scholar]
- 12.Singla DK, Singla RD, McDonald DE. Factors released from embryonic stem cells inhibit apoptosis in H9c2 cells through PI3K/Akt but not ERK pathway. Am J Physiol Heart Circ Physiol. 2008;295:H907–H913. doi: 10.1152/ajpheart.00279.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogata Y, Takahashi M, Ueno S, Takeuchi K, Okada T, Mano H, Ookawara S, Ozawa K, Berk BC, Ikeda U, et al. Antiapoptotic effect of endothelin-1 in rat cardiomyocytes in vitro. Hypertension. 2003;41:1156–1163. doi: 10.1161/01.HYP.0000064342.30653.24. [DOI] [PubMed] [Google Scholar]
- 14.Scarabelli TM, Knight R, Stephanou A, Townsend P, Chen-Scarabelli C, Lawrence K, Gottlieb R, Latchman D, Narula J. Clinical implications of apoptosis in ischemic myocardium. Curr Probl Cardiol. 2006;31:181–264. doi: 10.1016/j.cpcardiol.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res. 2007;100:41–49. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- 16.Fu J, Huang H, Liu J, Pi R, Chen J, Liu P. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol. 2007;568:213–221. doi: 10.1016/j.ejphar.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Song JQ, Teng X, Cai Y, Tang CS, Qi YF. Activation of Akt/GSK-3beta signaling pathway is involved in intermedin(1–53) protection against myocardial apoptosis induced by ischemia/reperfusion. Apoptosis. 2009;14:1299–1307. doi: 10.1007/s10495-009-0398-7. [DOI] [PubMed] [Google Scholar]
- 18.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/S0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 19.Marx N, Neumann FJ, Ott I, Gawaz M, Koch W, Pinkau T, Schömig A. Induction of cytokine expression in leukocytes in acute myocardial infarction. J Am Coll Cardiol. 1997;30:165–170. doi: 10.1016/S0735-1097(97)00116-2. [DOI] [PubMed] [Google Scholar]
- 20.Kumar D, Jugdutt BI. Apoptosis and oxidants in the heart. J Lab Clin Med. 2003;142:288–297. doi: 10.1016/S0022-2143(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 21.Kumar D, Lou H, Singal PK. Oxidative stress and apoptosis in heart dysfunction. Herz. 2002;27:662–668. doi: 10.1007/s00059-002-2430-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhao ZQ. Oxidative stress-elicited myocardial apoptosis during reperfusion. Curr Opin Pharmacol. 2004;4:159–165. doi: 10.1016/j.coph.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Liang R, Zhao Q, Jian G, Cheng D, Wang N, Zhang G, Wang F. Tanshinone IIA attenuates contrast-induced nephropathy via Nrf2 activation in rats. Cell Physiol Biochem. 2018;46:2616–2623. doi: 10.1159/000489688. [DOI] [PubMed] [Google Scholar]
- 24.Kong Y, Yin J, Cheng D, Lu Z, Wang N, Wang F, Liang M. Antithrombin III attenuates AKI following acute severe pancreatitis. Shock. 2018;49:572–579. doi: 10.1097/SHK.0000000000000946. [DOI] [PubMed] [Google Scholar]
- 25.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 26.Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R, Working Group of Cellular Biology of Heart of European Society of Cardiology Postconditioning and protection from reperfusion injury: Where do we stand? Position paper from the working group of cellular biology of the heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 27.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: Targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Zhu M, Feng J, Lucchinetti E, Fischer G, Xu L, Pedrazzini T, Schaub MC, Zaugg M. Ischemic postconditioning protects remodeled myocardium via the PI3K-PKB/Akt reperfusion injury salvage kinase pathway. Cardiovasc Res. 2006;72:152–162. doi: 10.1016/j.cardiores.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong SC. Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:427–436. doi: 10.1016/j.cardiores.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 30.Mullonkal CJ, Toledo-Pereyra LH. Akt in ischemia and reperfusion. J Invest Surg. 2007;20:195–203. doi: 10.1080/08941930701366471. [DOI] [PubMed] [Google Scholar]
- 31.Jeong JJ, Ha YM, Jin YC, Lee EJ, Kim JS, Kim HJ, Seo HG, Lee JH, Kang SS, Kim YS, Chang KC. Rutin from Lonicera japonica inhibits myocardial ischemia/reperfusion-induced apoptosis in vivo and protects H9c2 cells against hydrogen peroxide-mediated injury via ERK1/2 and PI3K/Akt signals in vitro. Food Chem Toxicol. 2009;47:1569–1576. doi: 10.1016/j.fct.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 32.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 33.Lecour S. Activation of the protective survivor activating factor enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.