Abstract
Neutrophils, also known as polymorphonuclear leukocytes (PMNs), have long been considered as the short-lived, nonspecific white cells that form pus—and also happen to kill invading microbes. Indeed, neutrophils were often neglected (and largely not considered) as immune cells. This historic view of neutrophils has changed considerably over the past several decades, and we know now that, in addition to playing the predominant role in the clearance of bacteria and fungi, they play a major role in shaping the host response to infection and immune system homeostasis. The change in our view of the role of neutrophils in the immune system has been due in large part to the study of these cells in vitro. Such work has been made possible by new and/or improved methods and approaches used to investigate neutrophils. These methods are the focus of this volume.
Keywords: Polymorphonuclear leukocyte, Granulocyte, Neutrophil methods
1. Introduction
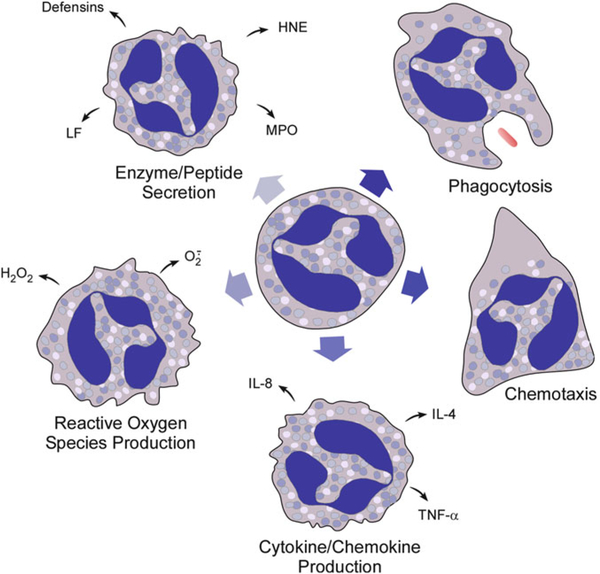
This valuable and unique book contains a compendium of methods and reviews that does much more than allow one to study the biology of neutrophils. What makes this collection of contributions so special is that it highlights and facilitates using the neutrophil as a simple, pure, single primary cell suspension model to study a remarkable array of generalized cellular functions (priming, chemotaxis and transmigration, adhesion, phagocytosis, degranulation, oxygen radical production, apoptosis, extracellular trap formation), biochemical pathways (GTPase activation, phospholipid metabolism, calcium transients, ion channel regulation, phosphorylation events, adhesion molecule regulation), as well as specialized functions and molecules important to host defense against infection, the mediation and resolution of inflammation, and cytokine/chemokine modulation of immunity (see Fig. 1). Consideration of the array of chapter topics evokes some of the past history of inquiry into how neutrophils function and how we evolved into the current widespread use of the neutrophil as a convenient model system for studying so many types of cellular processes and biochemical pathways.
Fig. 1.
Illustration of key neutrophil functions. Note that, for production reactive oxygen species, secretion of granule components, and production of cytokines and chemokines, only a few representative molecules are shown. HNE human neutrophil elastase, IL-8 interleukin-8, IL-4 interleukin-4, LF lactoferrin, MPO myeloperoxidase, TNF-α tumor nectosis factor-α
2. Historical Overview
Only a few decades ago, in the 1970s and even the early 1980s, the biology and pathophysiology of the neutrophil was a boutique area of study involving a relatively small number of laboratories and investigators internationally. These investigators all tended to know each other and most of the active investigators in the field of neutrophil biology could easily meet together at the biannual Gordon Research Conference on Phagocytes. Even as recently as the early 1980s, “real” immunologists were investigators who delineated the subtypes and life cycle of lymphocytes, and within this scheme the only phagocytes of significance for lymphocyte immunologists were the monocytes. This was because only monocytes, which are long-lived, and not neutrophils, which are short-lived, were thought to be capable of antigen presentation, differentiation into tissue macrophages and other fixed tissue cell types, or capable of any significant protein synthesis, including production of potent immune modulating factors. The relatively recently coined phase, “innate immunity” encompasses in part the recent growing appreciation of the special role of neutrophils in host defense, immune regulation, and regulation of inflammation, reflecting a vast body of new knowledge about how the neutrophil functions and affects the classic lymphocyte-oriented area of immunity encompassed by the term “acquired immunity” (reviewed in ref. 1).
Although the rapid ameboid migration of neutrophils to sites of inflammation and their unique capacity to surround and engulf foreign bodies have been known since the early twentieth century, it was only in the 1960s that it was generally appreciated that neutrophils produced microbicidal activated oxygen products or contained other nonoxidative potently microbicidal substances (e.g., see refs. 2–4). It was only in the late 1960s and early 1970s that a more detailed understanding of the different types of granules was delineated (e.g., see refs. 5, 6) and in the 1980s and 1990s that studies delineated the biochemistry of a large array of specialized cationic microbicidal proteins and a more complete understanding of the many proteolytic enzymes that were contained in those granules (reviewed in ref. 7). Only in the late 1980s and early 1990s were the biochemical details of the phagocyte oxidase delineated in fine detail (reviewed in refs. 8–10). Although investigators studying the biochemistry of non-muscle actin in cell motility performed much of the critical early research in lower eukaryotic organisms, translation of this work to mammalian tissues was largely performed in the 1980s and 1990s in neutrophils and monocytes (e.g., see refs. 11, 12).
Since the human tritium tracer studies of the 1960s, it has been appreciated that, when neutrophils emerge from bone marrow to peripheral blood, the half-life in blood is only 6–10 h and even shorter in infected patients and that the lifespan in tissues is 3 days or less (e.g., see refs. 5, 13, 14). This provided a basis for considering neutrophils as end-stage cells only minimally more capable of anabolic processes than erythrocytes. This impression was further engendered by the observations that neutrophils, as compared to other cell types, produce energy for survival primarily through anaerobic metabolism, reserving most use of oxygen for production of superoxide in the context of the stimulated microbicidal respiratory burst [15]. There is a paucity of mitochondria and ribosomes in neutrophils compared, for example, to monocytes, and most investigators in the 1970s assumed that mature neutrophils in blood or tissues were devoid of significant protein synthetic capacity, functioning entirely on the store of enzymes and other proteins that were contained within their granules, membranes, and cytoplasm as these cells emerged from the bone marrow. It was not that investigators viewed the neutrophil as inactive, since, after all, these are cells capable of remarkably rapid amoeboid migration, rapid engulfment of microorganisms, a prodigious respiratory burst-induced production of superoxide, and extremely rapid degranulation into phagosomes (reviewed in ref. 16). However, more recent studies have demonstrated unequivocally that neutrophils are capable of significant stimulated production of new proteins (e.g., see refs. 17–19); of note is production of a number of chemokines, in particular the production of large amounts of interleukin 8 [20]. This provides one important area of evidence for the important interface between the neutrophil component of innate immunity and the classic area of acquired immunity (e.g., see ref. 1).
One of the specialized motile properties of neutrophils is chemotaxis, and the delineation in the 1970s of bacteria-derived formyl peptides as chemotactic for neutrophils [21], in particular the discovery of the simple formylated tripeptide, formyl-methionyl-leucinyl-phenyalanine (fMLF), as a potent chemoattractant, began the process of making the neutrophil, in the 1980s, a model of choice for investigators interested in delineating a large array of biochemical signaling pathways whose diverse enzymes and regulatory proteins are still being worked out (e.g., see refs. 22, 23). Formylated peptides were shown to induce chemotaxis, but they also induced degranulation, which was associated metabolically with an ionized calcium transient, changes in electric potential similar to neural signaling, phosphorylations, metabolism of GTP, and metabolism of certain membrane phospholipids (e.g., see refs. 24–26). This was the beginning of the use of the neutrophil as the model system of choice for an increasing number of investigators delineating many types of newly identified biochemical signaling pathways, including the G-protein-coupled signaling pathway and the large array of small GTPases of the Ras and Rho families that regulate so many cell functions (e.g., see refs. 27–30).
Although it was appreciated that neutrophils have a relatively short lifespan following release from the bone marrow, the final fate of “old” neutrophils remained a mystery until the emergence of the new paradigm of apoptosis defined the process of regulated cell death as a final stage in the differentiation of cells and delineated it from trauma, toxin-induced, or immune-induced cell necrosis [17, 31–33]. The recent application of the apoptosis paradigm to neutrophils has been used to explain how some processes lead to resolution of infection or inflammation without tissue damage by allowing neutrophil apoptosis to occur. The apoptotic process prevents release of the cytotoxic and proteolytic contents of the neutrophil by facilitating phagocytosis of apoptotic neutrophils by macrophages and dendritic cells through engagement of “death receptors” [32, 33]. Not only does this facilitate “cleanup” and resolution of infection and inflammation without tissue damage, but it also probably comprises an important interface of innate with acquired immunity in that the engagement of death receptors modulates the function of antigen presenting cells, and the contents of the phagocytosed apoptotic neutrophils include components of killed microorganisms, which are processed by antigen presenting cells. Thus, it is appropriate that there is a chapter in this volume on neutrophil apoptosis. Apoptosis of neutrophils can be replaced prematurely by necrosis and release of the potent proteolytic enzymatic contents of neutrophils by microorganisms that contain toxins that lyse the neutrophil (reviewed in ref. 34), by autoimmune processes such as antibodies to neutrophils or their contents that induce lysis or phagocytosis of neutrophils before normal apoptotic processes can occur or by other causes of cytolysis. One such cytolytic process can result in the extracellular extrusion of the nucleus to form weblike DNA structures called neutrophil extra-cellular traps (NETs), which are coated with histones and cationic granule proteins [35]. These interesting structures ensnare bacteria and fungi—and some reports suggest they kill bacteria—and can thereby contribute to host defense. The idea that DNA released from neutrophils can contribute to innate immunity was unheard of until NET discovery in 2004 [35].
Neutrophils are the primary mediators of the rapid innate host defense against most bacterial and fungal pathogens that occurs before the complex humoral and lymphocyte cellular processes of acquired immunity can be brought to bear on an infection. The importance of the neutrophil in this process is highlighted by the fact that iatrogenic neutropenia from cancer chemotherapy or reactions to cytotoxic drugs is the most common severe immune deficiency associated with significant morbidity encountered in medical practice [36–39]. Inherited forms of neutropenias are also associated with significant risk of infection from bacteria and fungi. As discussed in this volume, there are a number of single gene disorders affecting primarily specific neutrophil functions such as defects in the phagocyte oxidase responsible for the group of diseases with a common phenotype called chronic granulomatous disease (CGD) or defects in the CD18 β integrin responsible for leukocyte adhesion deficiency (LAD). The groups of bacterial and fungal organisms typically infecting patients with severe neutropenia, CGD, or LAD overlap a little, but in general are different and distinct, suggesting strongly that there are an array of microbicidal defense systems and molecules used by the neutrophil in host defense and that different systems have evolved to provide specific defense against different organisms. Also of note is that CGD patients have excessive inflammation and a tendency to develop a variety of autoimmune disorders including inflammatory bowel disease and poor wound healing, suggesting that the superoxide or hydrogen peroxide products of the respiratory burst may play an important role in the resolution of inflammation and in wound healing [40]. LAD patients have large nonhealing ulcers, also suggesting a role for neutrophils in wound healing, since LAD neutrophils have trouble migrating into tissues [41].
3. Future Prospects
Despite strong evidence in a variety of model systems of inflammation that superoxide dismutase, which catabolizes superoxide; catalase, which catabolizes hydrogen peroxide; or dimethylsulfoxide (DSMO), which scavenges hydroxyl radical, protect against tissue injury from neutrophil-mediated tissue damage, there remains considerable controversy regarding the role of neutrophil products of oxidative metabolism in mediating any of the tissue damage syndromes associated with autoimmune disease or septic shock (e.g., see refs. 42–45). Stronger evidence exists for actual clinical benefit accruing from blocking the activity a number of the proteolytic enzymes derived from neutrophils in a variety of autoimmune or other disease processes (reviewed in ref. 46). Because of this, discovery and development of antiproteolytic small molecules active against specific proteases found in neutrophils are part of the anti-inflammatory drug development programs of a number of pharmaceutical companies (e.g., see refs. 47, 48). Neutrophils are also a source of a number of small potent antimicrobial proteins and peptides that could be developed into new classes of antibiotics, and this is another area of interest to pharmaceutical development (reviewed in ref. 49). Finally, recent advances in the area of induced pluripotent stem cells (iPSCs), which are embryonic-like stem cells that can be reprogrammed into many types of cells, including neutrophils, present an opportunity for long-term treatment of neutrophil disorders. Thus, the chapters in this series are likely to be of utility not only broadly to investigators interested in using the neutrophil as a model system, to investigators specifically interested in neutrophil function, or to investigators studying the interface between innate and acquired immunity but will also be important to investigators developing new classes of natural biologicals as antibiotics or developing new classes of anti-inflammatory agents.
References
- 1.Hoebe K, Janssen E, Beutler B (2004) The interface between innate and adaptive immunity. Nat Immunol 5:971–974 [DOI] [PubMed] [Google Scholar]
- 2.Babior BM, Kipnes RS, Curnutte JT (1973) Biological defense mechanisms: production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest 52:741–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klebanoff SJ (1967) Iodination of bacteria: a bactericidal mechanism. J Exp Med 126: 1063–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehrer RI, Hanifin J, Cline MJ (1969) Defective bactericidal activity in myeloperoxidase-deficient human neutrophils. Nature 223:78–79 [DOI] [PubMed] [Google Scholar]
- 5.Bainton DF, Ullyot JL, Farquhar MG (1971) The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med 134:907–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bainton DF, Farquhar MG (1968) Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. I. Histochemical staining of bone marrow smears. J Cell Biol 39:286–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borregaard N, Cowland JB (1997) Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89:3503–3521 [PubMed] [Google Scholar]
- 8.Segal AW, Abo A (1993) The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci 18:43–47 [DOI] [PubMed] [Google Scholar]
- 9.Babior BM (1999) NADPH oxidase: an update. Blood 93:1464–1476 [PubMed] [Google Scholar]
- 10.Clark RA (1990) The human neutrophil respiratory burst oxidase. J Infect Dis 161:1140–1147 [DOI] [PubMed] [Google Scholar]
- 11.Zigmond SH (1978) Chemotaxis by polymorphonuclear leukocytes. J Cell Biol 77:269–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Southwick FS, Stossel TP (1983) Contractile proteins in leukocyte function. Semin Hematol 20:305–321 [PubMed] [Google Scholar]
- 13.Fliedner TM, Cronkite EP, Robertson JS (1964) Granulocytopoiesis. I. Senescence and random loss of neutrophilic granulocytes in human beings. Blood 24:402–414 [PubMed] [Google Scholar]
- 14.Athens JW, Haab OP, Raab SO et al. (1961) Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest 40:989–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi F, Zatti M (1964) Changes in the metabolic pattern of polymorphonuclear leukocytes during phagocytosis. Br J Exp Pathol 45:548–559 [PMC free article] [PubMed] [Google Scholar]
- 16.Nauseef WM, Clark RA (2000) Granulocytic phagocytes In: Mandell GL, Bennett JP, Dolin R (eds) Basic principles in the diagnosis and management of infectious diseases, 5th edn. Churchill Livingstone, New York, pp 89–112 [Google Scholar]
- 17.Kobayashi SD, Voyich JM, Buhl CL, Stahl RM, DeLeo FR (2002) Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc Natl Acad Sci U S A 99:6901–6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theilgaard-Mönch K, Knudsen S, Follin P, Borregaard N (2004) The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J Immunol 172:7684–7693 [DOI] [PubMed] [Google Scholar]
- 19.Zhang XQ, Kluger Y, Nakayama Y et al. (2004) Gene expression in mature neutrophils: early responses to inflammatory stimuli. J Leukoc Biol 75:358–372 [DOI] [PubMed] [Google Scholar]
- 20.Strieter RM, Kasahara K, Allen RM et al. (1992) Cytokine-induced neutrophil-derived interleukin- 8. Am J Pathol 141:397–407 [PMC free article] [PubMed] [Google Scholar]
- 21.Schiffmann E, Corcoran BA, Wahl SM (1975) N -formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A 72:1059–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyderman R, Goetzl EJ (1981) Molecular and cellular mechanisms of leukocyte chemo-taxis. Science 213:830–835 [DOI] [PubMed] [Google Scholar]
- 23.Allen RA, Jesaitis AJ, Cochrane CG (1990) The N-formyl peptide receptor In: Cochrane CG, Gimbrone MA (eds) Cellular and molecular mechanisms of inflammation: receptors of inflammatory cells, structure–-function relationships. Academic, San Diego, pp 83–112 [Google Scholar]
- 24.O’Flaherty JT, Showell HJ, Ward PA (1977) Influence of extracellular Ca2+ and Mg2+ on chemotactic factor-induced neutrophil aggregation. Inflammation 2:265–276 [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN, Broekman MJ, Korchak HM, Smolen JE, Marcus AJ, Weissmann G (1983) Changes in phosphatidylinositol and phosphatidic acid in stimulated human neutrophils. Relationship to calcium mobilization, aggregation and superoxide radical generation. Biochim Biophys Acta 762:420–428 [DOI] [PubMed] [Google Scholar]
- 26.McPhail LC, Clayton CC, Snyderman R (1984) A potential second messenger role for unsaturated fatty acids: activation of Ca++- dependent protein kinase. Science 224:622–625 [DOI] [PubMed] [Google Scholar]
- 27.Aharoni I, Pick E (1990) Activation of the superoxide-generating NADPH oxidase of macrophages by sodium dodecyl sulfate in a soluble cell-free system: evidence for involvement of a G protein. J Leukoc Biol 48: 107–115 [DOI] [PubMed] [Google Scholar]
- 28.Quinn MT, Parkos CA, Walker L, Orkin SH, Dinauer MC, Jesaitis AJ (1989) Association of a ras-related protein with cytochrome b of human neutrophils. Nature 342:198–200 [DOI] [PubMed] [Google Scholar]
- 29.Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW (1991) Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353:668–670 [DOI] [PubMed] [Google Scholar]
- 30.Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM (1991) Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac2. Science 254:1512–1515 [DOI] [PubMed] [Google Scholar]
- 31.Serhan CN, Savill J (2005) Resolution of inflammation: the beginning programs the end. Nat Immunol 6:1191–1197 [DOI] [PubMed] [Google Scholar]
- 32.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C (1989) Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest 83:865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whyte MK, Meagher LC, MacDermot J, Haslett C (1993) Impairment of function in aging neutrophils is associated with apoptosis. J Immunol 150:5124–5134 [PubMed] [Google Scholar]
- 34.DeLeo FR (2004) Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 9:399–413 [DOI] [PubMed] [Google Scholar]
- 35.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–1535 [DOI] [PubMed] [Google Scholar]
- 36.Tobias JD, Schleien C (1991) Granulocyte transfusions – a review for the intensive care physician. Anaesth Intensive Care 19: 512–520 [DOI] [PubMed] [Google Scholar]
- 37.Froland SS (1984) Bacterial infections in the compromised host. Scand J Infect Dis Suppl 43:7–16 [PubMed] [Google Scholar]
- 38.Bodey GP, Buckley M, Sathe YS, Freireich EJ (1966) Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 64:328–340 [DOI] [PubMed] [Google Scholar]
- 39.Dale DC, Guerry D, Wewerka JR, Bull JM, Chusid MJ (1979) Chronic neutropenia. Medicine (Baltimore) 58:128–144 [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi SD, Voyich JM, Braughton KR et al. (2004) Gene expression profiling provides insight into the pathophysiology of chronic granulomatous disease. J Immunol 172:636–643 [DOI] [PubMed] [Google Scholar]
- 41.Bunting M, Harris ES, McIntyre TM, Prescott SM, Zimmerman GA (2002) Leukocyte adhesion deficiency syndromes: adhesion and tethering defects involving beta 2 integrins and selectin ligands. Curr Opin Hematol 9:30–35 [DOI] [PubMed] [Google Scholar]
- 42.Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247 [DOI] [PubMed] [Google Scholar]
- 43.Rahman I, Biswas SK, Kode A (2006) Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol 533:222–239 [DOI] [PubMed] [Google Scholar]
- 44.Temple MD, Perrone GG, Dawes IW (2005) Complex cellular responses to reactive oxygen species. Trends Cell Biol 15:319–326 [DOI] [PubMed] [Google Scholar]
- 45.Weiss SJ (1989) Tissue destruction by neutrophils. N Engl J Med 320:365–376 [DOI] [PubMed] [Google Scholar]
- 46.Altieri DC (1995) Proteases and protease receptors in modulation of leukocyte effector functions. J Leukoc Biol 58:120–127 [DOI] [PubMed] [Google Scholar]
- 47.Zaidi SH, You XM, Ciura S, Husain M, Rabinovitch M (2002) Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation 105:516–521 [DOI] [PubMed] [Google Scholar]
- 48.Zeiher BG, Matsuoka S, Kawabata K, Repine JE (2002) Neutrophil elastase and acute lung injury: prospects for sivelestat and other neutrophil elastase inhibitors as therapeutics. Crit Care Med 30:S281–S287 [DOI] [PubMed] [Google Scholar]
- 49.Ganz T (2004) Antimicrobial polypeptides. J Leukoc Biol 75:34–38 [DOI] [PubMed] [Google Scholar]