Abstract
PURPOSE
UGT1A1*28 confers a higher risk of toxicity in patients treated with irinotecan. Patients with *1/*1 and *1/*28 genotypes might tolerate higher than standard doses of irinotecan. We aimed to identify the maximum tolerated dose (MTD) of irinotecan in mCRC patients with *1/*1 and *1/*28 genotypes treated with FOLFIRI plus bevacizumab, and to determine whether bevacizumab alters irinotecan pharmacokinetics.
EXPERIMENTAL DESIGN
Previously untreated mCRC patients (25 *1/*1; 23 *1/*28) were given FOLFIRI plus bevacizumab every two weeks. The irinotecan dose was escalated using a 3+3 design in each genotype group as follows: 260, 310, 370 mg/m2. The MTD was the highest dose at which < 4/10 patients had a dose-limiting toxicity (DLT). Pharmacokinetics of irinotecan and SN-38 were measured on days 1–3 (without bevacizumab) and 15–17 (with bevacizumab).
RESULTS
For *1/*1 patients, 2 DLTs were observed among 10 patients at 310 mg/m2, while 370 mg/m2 was not tolerated (2 DLTs in 4 patients). For *1/*28 patients, 2 DLTs were observed among 10 patients at 260 mg/m2, while 310 mg/m2 was not tolerated (4 DLTs in 10 patients). Neutropenia and diarrhea were the most common DLTs. Changes in the AUCs of irinotecan and SN-38 associated with bevacizumab treatment were marginal.
CONCLUSIONS
The MTD of irinotecan in FOLFIRI plus bevacizumab is 310 mg/m2 for UGT1A1 *1/*1 patients and 260 mg/m2 for *1/*28 patients. Bevacizumab does not alter the pharmacokinetics of irinotecan. The antitumor efficacy of these genotype-guided doses should be tested in future studies of mCRC patients treated with FOLFIRI plus bevacizumab.
INTRODUCTION
Irinotecan is approved by the U.S. Food and Drug Administration for the treatment of metastatic colorectal cancer (mCRC) and is also active in treating a variety of solid tumors. FOLFIRI is irinotecan plus infusional 5-fluorouracil/leucovorin (5-FU/LV), and when combined with bevacizumab, is one of the standard first-line treatment options for patients with metastatic colorectal cancer (mCRC).1 In the U.S., the recommended dose of irinotecan in FOLFIRI is 180 mg/m2 every two weeks based on a dose-finding study2.
Irinotecan has significant adverse effects, including myelosuppression and delayed-type diarrhea. The UGT1A1*28 allele confers reduced UGT1A1-mediated inactivation of SN-38, the active metabolite of irinotecan, and has been associated with severe neutropenia.3–5 The current U.S. prescribing information warns that the UGT1A1 *28/*28 genotype is a risk factor for neutropenia and states that a dose reduction should be considered in these patients.6
Since the early dose-finding studies of irinotecan were conducted in heavily pretreated patients without knowledge of the UGT1A1*28 genotype, it is reasonable to hypothesize that patients with the *1/*1 and *1/*28 genotypes might be able to tolerate more than the standard dose of irinotecan. Dose-escalation studies in mCRC patients treated with FOLFIRI are concordant in demonstrating that irinotecan doses higher than the standard ones are safe in the every other week schedule.7,8 Higher than standard doses have also been tolerated in the every three weeks schedule.9
Despite this collective evidence, the effect of adding a biologic to genotype-guided dosing of FOLFIRI is unknown. The safe doses of irinotecan in *1/*1 and *1/*28 patients treated with FOLFIRI plus bevacizumab have not yet been identified. We performed a dose-finding study in first-line mCRC patients treated with FOLFIRI plus bevacizumab to find the MTD of irinotecan in *1/*1 and *1/*28 patients. A prior study had found that bevacizumab was associated with a higher rate of grade ≥ 3 neutropenia (21% vs. 14%) when added to irinotecan and 5-FU in mCRC10, and a pharmacokinetic sub-study reported a 33% increase in the AUC0–5h of SN-38 with bevacizumab.11 Therefore, we also tested whether the administration of bevacizumab alters the pharmacokinetics of irinotecan and SN-38.
METHODS
The study was conducted at three sites (University of Chicago, Chicago, IL, USA; Centro di Riferimento Oncologico, Aviano, Italy; San Filippo Neri, Rome, Italy), and the protocol was approved by the Institutional Review Board of each participating site. All patients signed a written informed consent before entering the study. The clinicaltrials.gov identifier was .
Patient eligibility
Patients with histologically or cytologically confirmed diagnosis of mCRC were enrolled. Eligibility criteria included: UGT1A1 *1/*1 and *1/*28 genotypes, no prior chemotherapy for metastatic disease, age ≥ 18 years, absolute neutrophil count (ANC) ≥ 1,500/μl, platelets ≥ 100,000/μl, ECOG performance status of 0 or 1, creatinine clearance < 1.5 x the upper limit of normal (ULN), ALT and AST < 2.5 x the ULN (< 5 x the ULN in the presence of liver metastases), and total serum bilirubin < 1.6 mg/dl. Patients with the UGT1A1 *28/*28 genotype or carriers of other UGT1A1 alleles (*6, *36 (TA5), *37 (TA8)) were not eligible.
Study objectives
The primary objective was to determine the MTD of irinotecan in FOLFIRI plus bevacizumab during cycle 1 in *1/*1 and *1/*28 genotype patients. MTD was defined as the highest dose at which less than 4 of 10 patients had a DLT. DLT was defined as a grade ≥ 4 hematologic toxicity or a grade ≥ 3 non-hematologic toxicity during cycle 1 despite maximal supportive measures (such as anti-diarrheals and anti-emetics) according to the NCI Common Terminology Criteria for Adverse Events (version 3.0). Secondary objectives included: 1) the evaluation of pharmacokinetics of irinotecan and SN-38 with and without bevacizumab during cycle 1; and 2) the effect of higher doses of irinotecan and genotype on the efficacy of FOLFIRI plus bevacizumab as determined by objective response rate (ORR) and progression-free survival (PFS).
Drug administration and dose escalation
Based on the results of a prior study with FOLFIRI7, the starting dose of irinotecan for the *1/*1 and *1/*28 patients was 260 mg/m2 administered as an intravenous infusion over 90 minutes on days 1 and 15, with leucovorin 200 mg/m2 administered concomitantly (a lower dose than the standard 400 mg/m2 but one that has been used in many prior studies7,12). 5-FU was administered as a 400 mg/m2 bolus right after the end of the irinotecan infusion, followed by 2,400 mg/m2 over a 46 hour continuous infusion on days 1 and 15. Bevacizumab was given at 5 mg/kg over a 15–30 minute intravenous infusion (after an initial 90 minute infusion without a reaction) on days 1 and 15, with the exception of cycle 1 (when it was given on days 3 and 15). One cycle was 28 days. Before starting irinotecan, patients were pre-treated with atropine 0.5 mg, dexamethasone 20 mg, and ondansetron 8 mg (all given intravenously). Diarrhea was promptly treated with loperamide 4 mg oral intake at the onset, and then with 2 mg oral intake every 2 hours, until the patient was diarrhea free for at least 12 hours. Growth factors (i.e., G-CSF) were allowed only in patients who had grade ≥ 3 neutropenia during previous cycles.
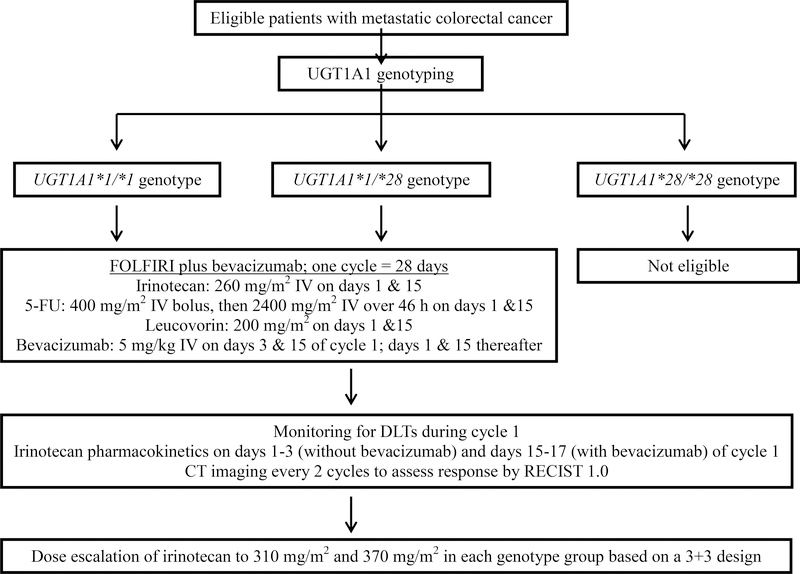
Under a 3+3 design, three patients were enrolled at each dose level (260, 310, 370 mg/m2) in each genotype cohort. If DLT was observed in none of them, the dose was escalated and 3 additional patients were treated at the next dose level. If DLT was observed in 1 of 3 patients, 3 additional patients were enrolled at the same dose level and the escalation to the next dose level continued only if DLT occurred in < 2 of 6 patients. If DLT was observed in > 1/3 of patients treated at any dose level, dose escalation was stopped. Ten patients total were then enrolled at the MTD to assess the safety and the inter-patient pharmacokinetic variability of higher doses of irinotecan. If < 4 of 10 patients experienced a DLT, this dose level was declared the MTD. No intra-patient dose escalation was allowed. A schema of the study is shown in Figure 1.
Figure 1.
Schema of the study.
UGT1A1 genotyping assay
Genotyping from peripheral blood was done separately at the University of Chicago and at Centro di Riferimento Oncologico in Aviano, Italy. In Chicago, genomic DNA was amplified for the promoter region of the UGT1A1 gene that contains the polymorphic TA site and sized by capillary electrophoresis, as previously described.13 Patients treated in Rome and Aviano, Italy, were genotyped in Aviano by pyrosequencing (Biotage, Uppsala, Sweden) using the High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH, Mannheim, Germany) and forward (5′-CCTGCTACCTTTGTGGACTGACA-3′) and reverse (5′-TCCTGCCAGAGGTTCGCC-3′) 5′-biotinylated PCR primers. The reaction was performed in a 50-μL volume with 2 mmol/L MgCl2, deoxynucleotide triphosphates 125 μmol/L each, 200 nmol/L each primer, and 1 unit of Taq polymerase for 35 cycles of amplification (15 s at 94°C, 30 s at 53.5°C, and 30 s at 72°C) obtaining a 98- to 100-bp fragment. The sequencing primer was 5′-TTCGCCCTCTCCTACTTAT-3′. Positive controls were used for quality control.
Pharmacokinetics of irinotecan and interaction with bevacizumab
The effect of bevacizumab on the pharmacokinetics of irinotecan and SN-38 was evaluated during cycle 1. In the same patient, drug levels were measured in the absence (days 1–3) and presence of bevacizumab (days 15–17). Serial blood samples were collected into heparinized tubes before irinotecan administration, and at 1, 2, 2.25, 2.5, 3, 4, 6, 8, 10, 26, and 50 hours following the start of the irinotecan infusion.
The total plasma concentration of irinotecan and SN-38 were determined by high-performance liquid chromatography-tandem mass spectrometry in a central laboratory for all patient samples (Aviano, Italy).14 Non-compartmental analysis was used for calculating the pharmacokinetic parameters. A linear-log trapezoidal numerical integration method was used to calculate the area under the irinotecan and SN-38 plasma concentration-time curves from time 0 to the last sampling time (AUClast). Irinotecan clearance was calculated as dose/AUClast.
Efficacy and toxicity assessments
Blood counts were measured at baseline, weekly during cycle 1, and within 48 hours before each administration during following cycles. Clinical evaluation, hematologic, hepatic and renal function tests were performed at baseline and within 48 hours before each irinotecan administration, during which patients were questioned about nausea and vomiting, mucositis, diarrhea, malaise, and appetite. Computed-tomography scans of measurable lesions were assessed at baseline and repeated every two cycles, with a minimum of one on-treatment CT required to be evaluable for efficacy (unless there was clinical disease progression). Objective tumor response, limited to those patients with measurable disease at enrollment, was assessed according to RECIST (version 1.0).15 PFS was measured from the time of drug administration to the occurrence of progressive disease or death, whichever came first.
Toxicity was classified and graded according to the U.S. NCI’s Common Terminology Criteria for Adverse Events (version 3.0). Patients were treated at the full dose of irinotecan if the following criteria were met: recovery to grade 1 or better from any non-hematological toxicity, absolute neutrophil count ≥ 1,500/μl, and platelet count ≥ 100,000/μl. Patients experiencing hematologic grade 4 toxicity or non-hematologic grade 3–4 toxicity were allowed to continue irinotecan at a lower dose, based on the physician’s assessment, without 5-FU and/or bevacizumab dosage modifications. Treatment was discontinued because of disease progression, intolerable side effects, patient refusal, or physician discretion.
Statistical analysis
Irinotecan and SN-38 pharmacokinetic differences when irinotecan was administrated alone and in combination with bevacizumab were tested by the non-parametric Wilcoxon signed rank matched pairs test. The effects of irinotecan dose and UGT1A1 genotype on PFS were estimated using the Kaplan-Meier estimator, and differences were tested using the log-rank test. ORRs (complete + partial response) between groups were compared using Fisher’s exact test for count data. For all comparisons, a two-sided p value < 0.05 is considered significant, not adjusted for multiple comparisons due to the exploratory nature of the analyses.
RESULTS
Patient characteristics and dose escalation
A total of 48 patients were enrolled in the study from 2009–2013, 25 with UGT1A1 *1/*1 genotype and 23 with *1/*28 genotype. Ninety-four percent of patients had ECOG performance status of 0. Patient characteristics are shown in Table 1. The dose of irinotecan was escalated from 260 to 310 and eventually to 370 mg/m2 in both *1/*1 and *1/*28 patients (Table 2).
Table 1.
Patient characteristics (n = 48 patients who received at least one dose of protocol therapy).
| Number of evaluable patients | |
| For safety* | 47 |
| For pharmacokinetics | 47 |
| For efficacy | 33 |
| Accrual site | |
| Aviano, Italy | 28 (58%) |
| Chicago, USA | 17 (35%) |
| Rome, Italy | 3 (6%) |
| Age, years | |
| Median, range | 56, 32–76 |
| Sex | |
| Male | 28 (58%) |
| Female | 20 (42%) |
| Race | |
| White | 41 (85%) |
| Black | 6 (13%) |
| Asian | 1 (2%) |
| Body-surface area (BSA), m2 | |
| Median, range | 1.80, 1.42–2.52 |
| Total bilirubin, mg/dL | |
| Mean ± SD, range | 0.46 ± 0.24, 0.17–1.2 |
| Prior adjuvant chemotherapy | |
| Yes** | 9 (19%) |
| No | 39 (81%) |
| ECOG performance status | |
| 0 | 45 (94%) |
| 1 | 3 (6%) |
| Primary site | |
| Colon | 37 (77%) |
| Rectum | 11 (23%) |
| Number of metastatic sites | |
| 1 | 29 (60%) |
| ≥ 2 | 19 (40%) |
| Surgery with curative intent | |
| Yes | 23 (48%) |
| No | 25 (52%) |
One patient was not evaluable for safety because his treatment was delayed for personal reasons in the absence of DLT.
4 patients with rectal cancer had received prior chemoradiotherapy.
Table 2.
Dose escalation of irinotecan and observed DLTs in patients treated with FOLFIRI plus bevacizumab.
| Irinotecan dose (mg/m2) | *1/*1 patients (DLTs) | *1/*28 patients (DLTs) |
|---|---|---|
| 260 | 10 (1) Grade 3 diarrhea |
10 (2) Grade 3 arrythmia Grade 4 neutropenia |
| 310 | 10 (2) Grade 3 diarrhea x 2 |
10 (4) Grade 3 diarrhea Grade 3 mucositis Grade 4 neutropenia x 2 |
| 370 | 4 (2) Grade 3 nausea/vomiting Grade 5 neutropenic sepsis |
3 (2) Grade 3 diarrhea Grade 4 neutropenia x 2 |
In the *1/*28 patients, 370 mg/m2 was not tolerated (2 DLTs out of 3 patients), and the 310 mg/m2 cohort was expanded to 10 patients, where 4 DLTs were observed. Hence, the 260 mg/m2 cohort was expanded to 10 patients, and because 2 DLTs were observed among those 10 patients, 260 mg/m2 was declared the MTD in *1/*28 patients.
In the *1/*1 patients, 2 DLTs (both diarrhea) occurred among the first 3 patients treated at 310 mg/m2. The 260 mg/m2 was expanded to 10 patients and only 1 DLT was observed. Since the 260 mg/m2 dose level was well tolerated, the protocol was amended to allow re-escalation of the dose and expansion of the 310 mg/m2 cohort to 10 patients. Since no additional patients at 310 mg/m2 had a DLT (total 2 DLTs out of 10 patients), the dose was further escalated to 370 mg/m2 as pre-specified in the amendment. At 370 mg/m2, 2 DLTs occurred among the first 4 patients treated, including a death from neutropenic sepsis. Hence, the 310 mg/m2 dose was declared the MTD in *1/*1 patients.
The most common DLTs were neutropenia (6 of 13; 46%) and diarrhea (5 of 13; 38%). Three of 6 neutropenia DLTs were febrile neutropenia. The other two DLTs were grade 3 mucositis and grade 3 arrhythmia (Table 2). Common (> 10% of patients) and/or severe toxicities during the first cycle of therapy are reported in Supplementary Table 1.
The median number of treatment cycles was 6 (range 0.5–10.5). In the 20 *1/*1 and *1/*28 patients treated at the MTD, the median number of treatment cycles was 6 (range 1.5–10), and 65% of the patients treated at the MTD did not require a dose reduction of irinotecan. The doses of 5-FU and bevacizumab were not modified.
Pharmacokinetics of irinotecan/SN-38 and interactions with bevacizumab
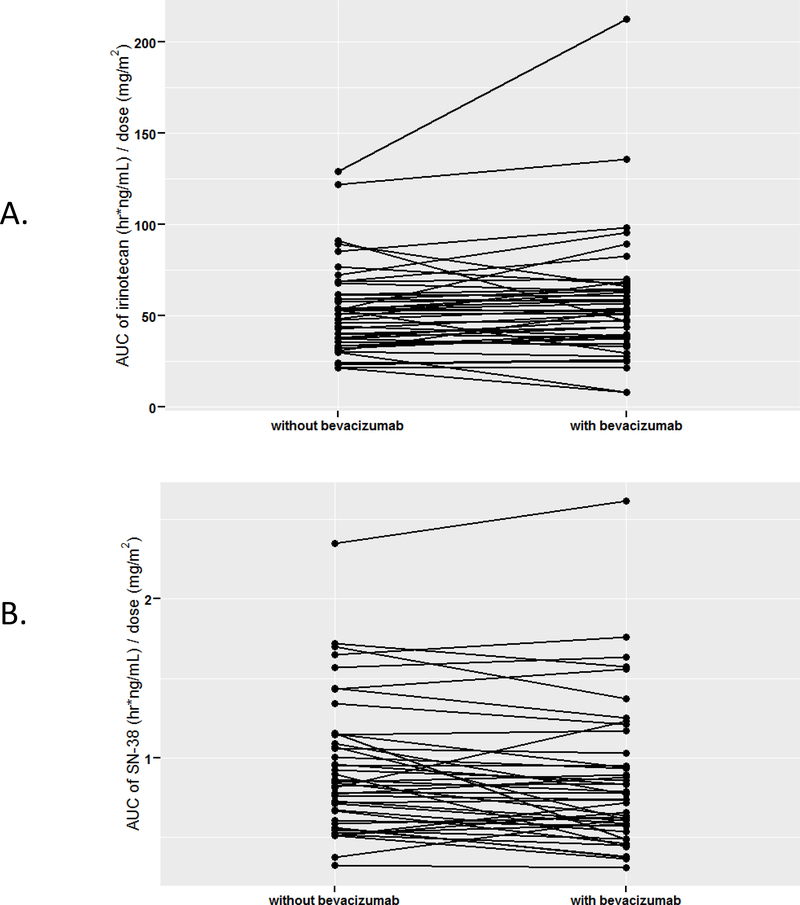
Pharmacokinetics were obtained from 47 patients (Table 3; Figure 2). Four patients did not receive a second dose of irinotecan due to DLTs, so they were excluded from the analyses regarding interactions with bevacizumab. No major differences were observed in all pharmacokinetic parameters of irinotecan and SN-38 in the presence or absence of bevacizumab. Irinotecan clearance was slightly higher when administered with bevacizumab compared to without bevacizumab (p = 0.06). The presence of bevacizumab was also associated with very small changes in the dose-adjusted AUClast of irinotecan (increased, p = 0.05) and SN-38 (reduced, p = 0.03) (Table 3; Figure 2). There were no differences (p > 0.05) in dose-adjusted Cmax and AUClast of irinotecan and SN-38 between patients who experienced a DLT and those who did not.
Table 3. Summary of irinotecan and SN-38 pharmacokinetic parameters with and without bevacizumab (n = 43 patients with data evaluable for pharmacokinetics).
Dose adjustments were made for Cmax and AUClast because 5 patients received a lower dose on day 15 (with bevacizumab) compared to day 1 (without bevacizumab). Comparisons were made using the Wilcoxon signed rank matched pairs test. CV, coefficient of variation.
| Mean, range (% CV) without bevacizumab | Mean, range (% CV) with bevacizumab | p | ||
|---|---|---|---|---|
| Irinotecan | Cmax (ng/mL)/Dose (mg/m2) | 7.2, 3.3–14.1 (39) | 7.8, 1.2–27.4 (50) | 0.08 |
| Tmax (h) | 2.1, 1.0–4.0 (21) | 1.9, 1.0–3.0 (24) | 0.23 | |
| AUClast (hr*ng/mL)/Dose (mg/m2) | 53.2, 21.6–129 (45) | 57.0, 8.2–213 (59) | 0.05 | |
| t1/2 (h) | 8.8, 5.3–14.3 (18) | 9.2, 5.4–12.0 (16) | 0.48 | |
| CL (L/h/m2) | 22.4, 7.7–46.3 (42) | 23.5, 4.7–122 (76) | 0.06 | |
| SN-38 | Cmax (ng/mL)/Dose (mg/m2) | 0.07, 0.02–0.22 (57) | 0.06, 0.02–0.17 (58) | 0.10 |
| Tmax (h) | 2.5, 1.0–6.0 (49) | 2.1, 1.0–6.0 (55) | 0.05 | |
| AUClast (hr*ng/mL)/Dose (mg/m2) | 0.93, 0.33–2.3 (46) | 0.86, 0.31–2.6 (53) | 0.03 | |
| t1/2 (h) | 25.4, 7.8–148 (96) | 28.8, 8.4–178 (88) | 0.02 |
Figure 2. AUClast of irinotecan (A) and SN-38 (B), normalized for the irinotecan dose, with and without concomitant treatment with bevacizumab.
Each black line represents an individual subject.
Tumor response and PFS
Thirty-three patients were evaluable for efficacy. The reasons for being non-evaluable for efficacy were: DLT or other early toxicity for which patients were taken off study (n = 7), lack of measurable lesions (n = 4), or missing data regarding the response to therapy (lost to follow-up; n =4). One patient with extensive liver and lymph node metastases and *1/*1 genotype treated at 310 mg/m2 (dose reduced to 233 mg/m2 after a DLT of grade 3 diarrhea) had a complete response that has been durable for 5 years since the start of therapy (as of CT scan dated September 21, 2015).
The ORR was 33% (13 of 40 patients), including patients with early dropout due to toxicity as non-responders. The overall median PFS was 9.0 months (95% CI = 6.6 – 13.1 months). PFS curves do not clearly separate by UGT1A1 genotype, and a trend of PFS with irinotecan dose is not clearly evident (Supplementary Figure 1).
DISCUSSION
This phase I trial has identified the safe doses of irinotecan that can be administered in mCRC patients treated with FOLFIRI in combination with bevacizumab according to their UGT1A1*28 genotype. The results support that irinotecan can be safely administered at doses up to 310 mg/m2, compared to the standard dose of 180 mg/m2. Such a genotype-directed strategy has been demonstrated to be effective in personalizing irinotecan dosing in various regimens7–9 which have now been surpassed by combinations with biologics like bevacizumab. The present study determines the dosing of irinotecan in a highly used, standard of care treatment for front-line therapy in mCRC.
The MTD of genotype-directed irinotecan was 260 mg/m2 for *1/*28 patients, and 310 mg/m2 for *1/*1 patients. As reported before7–9, the two genotypes confer different risk of toxicity and the *1/*28 patients do not tolerate the same dose as *1/*1 patients. The present phase I study did not determine the safe doses of irinotecan in patients with the *28/*28 genotype (and this is a limitation) because of their low frequency and the feasibility of accruing these patients in timely fashion.
One observation that is worth making is that the MTDs for FOLFIRI plus bevacizumab are lower than those for FOLFIRI alone. In a previous phase I with a similar design in front-line mCRC patients7, the MTDs were 370 and 310 mg/m2, respectively. It is difficult to say whether this downward shift in the MTDs is caused by a pharmacodynamic effect of bevacizumab or other factors, as the two studies cannot be compared head to head. In the current study, neutropenia was the most common DLT, and in placebo-controlled trials, bevacizumab has been found to increase the rate of grade ≥ 3 neutropenia when added to the IFL regimen in mCRC from 14 to 21%.10 This effect does not seem to be specific to irinotecan/5-FU, as similar increases have been observed in NSCLC patients treated with paclitaxel/carboplatin (grade 4 neutropenia increased from 17 to 26%).11 Among the causes of treatment-related death by bevacizumab combined with chemotherapy, neutropenia was not a significant factor.16
The analysis of the pharmacokinetics of irinotecan in our study seems to rule out that the lower MTDs in FOLFIRI plus bevacizumab compared to those of FOLFIRI alone are due to an interaction between bevacizumab and irinotecan increasing systemic exposure to irinotecan or SN-38. Pharmacokinetic parameters of exposure (Cmax and AUClast) are essentially unchanged (Table 3), and the p values of the differences cannot be interpreted as statistically significant since they have not been corrected for multiple comparisons. A limited sampling pharmacokinetic substudy of the phase III IFL study in mCRC10 suggested a 33% increase in the AUC0–5h of SN-38 associated with the bevacizumab treatment.11 This difference has not been confirmed in three formal drug-drug interaction studies, including ours.17, 18
This study, even with the addition of bevacizumab, concludes that a genotype-directed strategy leads to safe administration of higher doses of irinotecan. However, there are several limitations. Because this study enrolled previously untreated (for metastatic disease) patients with excellent performance status (94% ECOG 0), the impact of pretreatment regimens and worse performance status should be evaluated in larger studies of more heterogeneous populations, to test whether the higher doses are truly due to a more favorable genotype. It is likely that other genetic variants in UGT1A and/or SLCO1B1 might affect irinotecan disposition, and evaluation of these might improve the predictive power of genotyping.19–21 Finally, 7 of 47 patients (15%) were not evaluable due to DLTs despite a genotype-guided dosing strategy, suggesting that other factors affecting irinotecan pharmacodynamics and factors unrelated to irinotecan pharmacology are also important for tolerability.
It should be realized that efficacy was not the primary endpoint of this and other studies.7–9 The hypothesis that higher irinotecan dosing might confer increased efficacy of FOLFIRI plus bevacizumab should be tested as the primary endpoint, targeting a clinically meaningful improvement.22 Based on the MTDs from the current study, a phase II clinical trial in front-line mCRC where irinotecan is dosed at 310 and 260 mg/m2 in patients with the *1/*1 and *1/*28 genotypes (respectively) is currently ongoing and led by the University of North Carolina Lineberger Comprehensive Cancer Center ().23
After the initial proof-of-concept studies with either FOLFIRI or single-agent irinotecan7–9, this study provides evidence that higher than standard doses of irinotecan can be safely given in FOLFIRI plus bevacizumab for patients with UGT1A1 *1/*1 and *1/*28 genotypes. While this suggests that the majority of patients may currently be underdosed with respect to irinotecan, the role of routine assessment of UGT1A1 genotyping for dose individualization is not established yet, because it is awaiting results on efficacy. With the advent of genomic screening in cancer patients and pre-emptive genotyping for pharmacogenomics prediction24, available genetic data on the UGT1A1*28 status of patients in medical records can be utilized to reach the goal of improving precision in irinotecan-based therapies.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
UGT1A1 is an enzyme critical for elimination of SN-38, the active metabolite of irinotecan, and it is known that the UGT1A1*28 allele is associated with greater toxicity. The safe dose of irinotecan was established based on studies that did not take into consideration the UGT1A1 genotype. Subsequent studies have shown that higher than standard doses of irinotecan can be tolerated in metastatic colorectal cancer patients receiving FOLFIRI (5-fluorouracil, leucovorin, irinotecan). This study established the maximum tolerated dose of irinotecan in metastatic colorectal cancer patients receiving FOLFIRI plus bevacizumab (a standard first-line regimen), and evaluated whether the administration of bevacizumab alters the pharmacokinetics of irinotecan and SN-38. *1/*1 patients and *1/*28 patients tolerated 310 mg/m2 and 260 mg/m2 respectively, providing justification for future studies evaluating whether these higher doses result in greater efficacy than the standard dose of 180 mg/m2.
ACKNOWLEDGMENT
This work was supported by the “Associazione Italiana per la Ricerca sul Cancro” (AIRC) [Special Program Molecular Clinical Oncology, 5×1000 (No.12214)]
References
- 1.Benson AB 3rd, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, et al. Colon cancer, version 3.2014. J Natl Compr Canc Netw 2014;12:1028–59. [DOI] [PubMed] [Google Scholar]
- 2.Ducreux M, Ychou M, Seitz JF, Bonnay M, Bexon A, Armand JP, et al. Irinotecan combined with bolus fluorouracil, continuous infusion fluorouracil, and high-dose leucovorin every two weeks (LV5FU2 regimen): a clinical dose-finding and pharmacokinetic study in patients with pretreated metastatic colorectal cancer. J Clin Oncol 1999;17:2901–8. [DOI] [PubMed] [Google Scholar]
- 3.Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 2004;22:1382–8. [DOI] [PubMed] [Google Scholar]
- 4.Toffoli G, Cecchin E, Corona G, Russo A, Buonadonna A, D’Andrea M, et al. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J Clin Oncol 2006;24:3061–8. [DOI] [PubMed] [Google Scholar]
- 5.Hoskins JM, Goldberg RM, Qu P, Ibrahim JG, McLeod HL. UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst 2007;99:1290–5. [DOI] [PubMed] [Google Scholar]
- 6.Pfizer. Camptosar (irinotecan) prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020571s048lbl.pdf (accessed December 3, 2015)
- 7.Toffoli G, Cecchin E, Gasparini G, D’Andrea M, Azzarello G, Basso U, et al. Genotype-driven phase I study of irinotecan administered in combination with fluorouracil/leucovorin in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcuello E, Paez D, Pare L, Salazar J, Sebio A, del Rio E, et al. A genotype-directed phase I-IV dose-finding study of irinotecan in combination with fluorouracil/leucovorin as first-line treatment in advanced colorectal cancer. Br J Cancer 2011;105:53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Innocenti F, Schilsky RL, Ramirez J, Janisch L, Undevia S, House LK, et al. Dose-finding and pharmacokinetic study to optimize the dosing of irinotecan according to the UGT1A1 genotype of patients with cancer. J Clin Oncol 2014;32:2328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. [DOI] [PubMed] [Google Scholar]
- 11.Genentech. Avastin (bevacizumab) prescribing information. http://www.gene.com/download/pdf/avastin_prescribing.pdf (accessed December 3, 2015)
- 12.Budai B, Nagy T, Lang I, Hitre E. The use of high dose d,l-leucovorin in first-line bevacizumab+mFOLFIRI treatment of patients with metastatic colorectal cancer may enhance the antiangiogenic effect of bevacizumab. Angiogenesis 2013;16:113–21. [DOI] [PubMed] [Google Scholar]
- 13.Iyer L, Das S, Janisch L, Wen M, Ramirez J, Karrison T, et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J 2002;2:43–7. [DOI] [PubMed] [Google Scholar]
- 14.Marangon E, Posocco B, Mazzega E, Toffoli G. Development and validation of a high-performance liquid chromatography-tandem mass spectrometry method for the simultaneous determination of irinotecan and its main metabolites in human plasma and its application in a clinical pharmacokinetic study. PLoS One 2015;10:e0118194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- 16.Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA 2011;305:487–94. [DOI] [PubMed] [Google Scholar]
- 17.Suenaga M, Fuse N, Yamaguchi T, Yamanaka Y, Motomura S, Matsumoto H, et al. Pharmacokinetics, safety, and efficacy of FOLFIRI plus bevacizumab in Japanese colorectal cancer patients with UGT1A1 gene polymorphisms. J Clin Pharmacol 2014;54:495–502. [DOI] [PubMed] [Google Scholar]
- 18.Denlinger CS, Blanchard R, Xu L, Bernaards C, Litwin S, Spittle C, et al. Pharmacokinetic analysis of irinotecan plus bevacizumab in patients with advanced solid tumors. Cancer Chemother Pharmacol 2009;65:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cecchin E, Innocenti F, D’Andrea M, Corona G, De Mattia E, Biason P, et al. Predictive role of the UGT1A1, UGT1A7 and UGT1A9 genetic variants and their haplotypes on the outcome of metastatic colorectal cancer patients treated with fluorouracil, leucovorin, and irinotecan. J Clin Oncol 2009;27:2457–65. [DOI] [PubMed] [Google Scholar]
- 20.Carlini LE, Meropol NJ, Bever J, Andria ML, Hill T, Gold P, et al. UGT1A7 and UGT1A9 polymorphisms predict response and toxicity in colorectal cancer patients treated with capecitabine/irinotecan. Clin Cancer Res 2005;11:1226–36. [PubMed] [Google Scholar]
- 21.Crona DJ, Ramirez J, Qiao W, de Graan AJ, Ratain MJ, van Schaik RH, et al. Clinical validity of new genetic biomarkers of irinotecan neutropenia: an independent replication study. Pharmacogenomics J 2016;16:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis LM, Bernstein DS, Voest EE, Berlin JD, Sargent D, Cortazar P, et al. American Society of Clinical Oncology perspective: Raising the bar for clinical trials by defining clinically meaningful outcomes. J Clin Oncol 2014;32:1277–80. [DOI] [PubMed] [Google Scholar]
- 23.Williams GR, Innocenti F, Wood WA, Patel JN, Sherrill GB, O’Neil BH, et al. Genotype-directed phase II study of irinotecan dosing in metastatic colorectal cancer (mCRC) patients receiving FOLFIRI + bevacizumab: The GENIC study [abstract]. J Clin Oncol 2015;33 Suppl;TPS3621. [Google Scholar]
- 24.Gillis NK, Patel JN, Innocenti F. Clinical implementation of germ line cancer pharmacogenetic variants during the next-generation sequencing era. Clin Pharmacol Ther 2014;95:269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.