Abstract
Staphylococcus aureus is a commensal colonizer of both humans and animals, but also an opportunistic pathogen responsible for a multitude of diseases. In recent years, colonization of pigs by methicillin resistant S. aureus has become a problem with increasing numbers of humans being infected by livestock strains. In S. aureus colonization and virulence factor expression is controlled by the agr quorum sensing system, which responds to and is activated by self-generated, autoinducing peptides (AIPs). AIPs are also produced by coagulase negative staphylococci (CoNS) commonly found as commensals in both humans and animals, and interestingly, some of these inhibit S. aureus agr activity. Here, we have addressed if cross-communication occurs between S. aureus and CoNS strains isolated from pig nares, and if so, how properties such as host factor binding and biofilm formation are affected. From 25 pig nasal swabs we obtained 54 staphylococcal CoNS isolates belonging to 8 different species. Of these, none were able to induce S. aureus agr as monitored by reporter gene fusions to agr regulated genes but a number of agr-inhibiting species were identified including Staphylococcus hyicus, Staphylococcus simulans, Staphylococcus arlettae, Staphylococcus lentus, and Staphylococcus chromogenes. After establishing that the inhibitory activity was mediated via AgrC, the receptor of AIPs, we synthesized selective AIPs to explore their effect on adhesion of S. aureus to fibronectin, a host factor involved in S. aureus colonization. Here, we found that the CoNS AIPs did not affect adhesion of S. aureus except for strain 8325-4. When individual CoNS strains were co-cultured together with S. aureus we observed variable degrees of biofilm formation which did not correlate with agr interactions. Our results show that multiple CoNS species can be isolated from pig nares and that the majority of these produce AIPs that inhibit S. aureus agr. Further they show that the consequences of the interactions between CoNS and S. aureus are complex and highly strain dependent.
Keywords: Staphylococcus aureus, coagulase-negative staphylococci, colonization, agr, quorum sensing interaction, cross-talk
Introduction
Staphylococcus aureus is a common colonizer and opportunistic pathogen of both animals and humans. The increasing spread of antibiotic resistance among S. aureus strains is of major concern in the treatment of staphylococcal infections, with methicillin-resistant S. aureus (MRSA) in particular being a proven health risk to humans, causing skin and soft tissue infections, food poisoning, and even fatal systemic disease (Fridkin et al., 2005; Kourbatova et al., 2005; King et al., 2006). MRSA strains are commonly divided into community, hospital or livestock associated and in recent years, the transmission of livestock-associated (LA)-MRSA from animals to humans has become a public health concern particularly in Europe, North America and Asia where pig farming is extensive. Within the EU alone nearly 46% of pigs are colonized by strains of the most predominant LA-MRSA type namely the clonal complex 398 (CC398) (Khanna et al., 2008; Lewis et al., 2008; Van Duijkeren et al., 2008; Authority, 2009; Smith et al., 2009; Golding et al., 2010; Köck et al., 2013; Chuang and Huang, 2015). Studies have revealed a high prevalence of nasal MRSA carriage in pig slaughterhouse workers and pig farmers, indicating that working with MRSA-colonized pigs is the predominant risk factor (Lewis et al., 2008; Van Cleef et al., 2010).
In general, S. aureus colonization is a multifactorial process involving a number of adhesins or host binding proteins that are expressed by, and located on, the surface of the bacterium (Josse et al., 2017). Particularly fibronectin binding proteins have been reported to be important for internalization and uptake of S. aureus by keratinocytes; to be key in the adhesion of S. aureus to keratinocytes of atopic skin and also to contribute to biofilm formation by MRSA strains (Cho et al., 2001; Kintarak et al., 2004; O’Neill et al., 2008; Josse et al., 2017). In addition to colonization factors, S. aureus also expresses a multitude of toxins and other factors necessary for virulence and biofilm formation (Archer et al., 2011; Kobayashi et al., 2015). Production of both adhesins and toxins are controlled by the accessory gene regulator (agr) quorum sensing system with the former being produced at low bacterial cell densities and the latter at high cell densities (Yarwood and Schlievert, 2003). agr is composed of a two component system which senses a self-generated autoinducing peptide (AIP) that, by binding to the sensor histidine kinase AgrC, leads to phosphorylation of the AgrA response-regulator and expression of the main agr effector molecule, RNAIII. As cells enter stationary phase, RNAIII is responsible for the down-regulation of host binding proteins such as Protein A encoded by spa and the concomitant upregulation of toxins such as α-hemolysin encoded by hla (Queck et al., 2008; Wang et al., 2014; Le and Otto, 2015). An RNAIII-independent agr gene regulation pathway also exists, involving AgrA-mediated expression of a family of toxins called the phenol soluble modulins (PSMs) (Periasamy et al., 2012). These PSMs are important players in biofilm formation and dispersal linking agr and biofilm formation (Boles and Horswill, 2008; Periasamy et al., 2012). Interestingly agr varies between S. aureus strains and can be divided into four groups (AgrC-I-IV) where AIPs from the corresponding group lead to self-activation whereas AIPs from other groups lead to cross-inhibition (Otto et al., 2001; Olson et al., 2014; Le and Otto, 2015). This group specificity has lead to an interest in studying the inhibitory activity of non-cognate AIPs as antivirulence sources targeting agr (Canovas et al., 2016; Tal-Gan et al., 2016).
Humans and animals are also colonized with a variety of other staphylococcal species. In contrast to S. aureus they do not produce coagulase and thus are termed the coagulase negative staphylococci (CoNS). Commonly, the CoNS are not pathogens and their presence has been suggested to influence S. aureus colonization. For example, in humans it has been proposed that Staphylococcus epidermidis may prevent colonization by S. aureus (Iwase et al., 2010), whereas in pigs, S. aureus colonization was not observed in the presence of Staphylococcus sciuri, Staphylococcus cohnii, or Staphylococcus saprophyticus (Verstappen et al., 2017). Interestingly, CoNS also encode AIP-like molecules and some of these are able to inhibit S. aureus agr (Otto et al., 1999; Canovas et al., 2016; Gless et al., 2017, 2019; Mahmmod et al., 2018). This cross-talk has been suggested to be involved in the competition between S. aureus and S. epidermidis on the skin (Otto et al., 2001) and in preventing MRSA colonization (Paharik et al., 2017).
The agr-mediated interactions between species isolated from the same host niche remains largely unexplored. In the present study we have examined which staphylococcal species co-colonize the pig nares and assessed the extent to which isolated CoNS strains are able to inhibit S. aureus agr. We have addressed if agr mediated cross-species communication affects S. aureus binding to fibronectin as well as biofilm formation, both elements that may be important for host colonization. Our results suggest extensive cross-communication between CoNS and S. aureus colonizing the same host niche. A better understanding of the role of agr cross-talk between colonizing staphylococci may provide insightful information that can be used for future exploitation in S. aureus colonization interference and anti-virulence therapy.
Materials and Methods
Bacterial Strains and Growth Conditions
Strains used in this study are listed in Table 1. Unless otherwise stated, all bacterial strains were grown in Tryptone Soya Broth (TSB) from Oxoid, at a 1:10 volume/flask ratio, at 37°C with shaking at 200 rpm.
TABLE 1.
Strains in this study and their source.
| Strain | Description | References |
| 8325-4 | S. aureus producing AIP group I | Novick and Morse, 1967 |
| PC203 | S. aureus 8325-4, spa:lacZ | Chan and Foster, 1998 |
| PC322 | S. aureus 8325-4, hla:lacZ | Chan and Foster, 1998 |
| SH101F7 | S. aureus 8325-4, rnaIII:lacZ | Horsburgh et al., 2002 |
| RN10829/pagrC-I | S. aureus AgrC-I P3:blaZ | Nielsen et al., 2014 |
| RN10829/pagrC-II | S. aureus AgrC-II P3:blaZ | Gless et al., 2019 |
| RN10829/pagrC-III | S. aureus AgrC-III P3:blaZ | Gless et al., 2019 |
| RN10829/pagrC-IV | S. aureus AgrC-IV P3:blaZ | Gless et al., 2019 |
| RN10829/pagrC-I-R23H (AgrC const.) | S. aureus AgrC-I-R23H P3:blaZ | Geisinger et al., 2009 |
| RN6607 | S. aureus producing AIP group II | (Lab stock) |
| MW2 | S. aureus producing AIP group III | (Lab stock) |
| RN4850 | S. aureus producing AIP group IV | (Lab stock) |
| 61599 | LA-MRSA CC398 strain | Tang et al., 2017b |
| 8325-4Δagr | Transduction from S. aureus RN6911 | Canovas et al., 2016 |
| HG001 | S. aureus 8325-4, restored rsbU | Herbert et al., 2010 |
| HG003 | S. aureus 8325-4, restored rsbU and tcaR | Herbert et al., 2010 |
Sample Collection, Isolation, and Identification
Nasal swabs (E-Swab, Copan Diagnostics Inc., United States) were collected from the pig nasal cavity of randomly selected pigs (weighing 20–30 kg) at three organic farms in Denmark. It should be noted that no permission is required to sample the nostril of pigs according to the Danish Animal Experimentation Act § 1.2. Samples were sent within 24 h to the Department of Veterinary and Animal Sciences, University of Copenhagen, and analyzed on the day of arrival. In total, 25 samples from 25 pigs were analyzed. Swabs were suspended and diluted in saline solution and plated on SaSelectTM plates (Bio-Rad) for staphylococcal species isolation. Species identification was carried out by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) and tuf gene analysis by standard PCR-based methods (Hwang et al., 2011).
β-Galactosidase Plate Assay
This assay was performed as previously described (Nielsen et al., 2010; Bojer et al., 2017). Briefly, the fused reporter strains PC203 (spa:lacZ), PC322 (hla:lacZ), and SH101F7 (rnaIII:lacZ) all in the 8325-4 strain background, were grown in TSA agar supplemented with 150 μg/mL β-galactosidase substrate 5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside (X-gal) and 5 μg/mL erythromycin. Sixty microliter supernatants of the identified staphylococcal strains or TSB medium were added to premade wells in the plates. The plates were incubated at 37°C for 10–24 h (the incubation time varies depending on the different reporter strains) until the plates appeared blue.
β-Lactamase Assay
This method was carried out as previously described with minor modifications (Nielsen et al., 2014; Bojer et al., 2017). Briefly, the reporter strains RN10829/pagrC-I-IV (WT) and RN10829/pagrC-I-R23H (AgrC const.) were treated with a 1/10 volume of CoNS supernatant at OD600 = 0.35, followed by the addition of a 1/10 volume of AIP-I-IV containing supernatant obtained separately from S. aureus strain 8325-4, RN6607, MW2 and RN4850. For the experiment investigating whether CoNS supernatants can induce agr, external AIP-I-IV supernatants were added as activation controls only. Samples were obtained after incubating at 37°C for 1 h, and optical density at 600 nm was recorded. Samples were stored at −80°C before thawing to test for β-lactamase activity as described (Bojer et al., 2017). Activity was calculated as arbitrary units based on nitrocefin conversion velocities (Vmax, ΔOD486 nm/time) normalized to the sample cell densities.
Chemical Synthesis of AIPs
All AIPs were synthesized according to a previously reported protocol (Gless et al., 2017). Briefly, linear peptides were synthesized using automated 9-fluorenylmethyloxycarbonyl (Fmoc) solid-phase peptide synthesis (SPPS) on a Gly-ChemMatrix resin loaded with Fmoc-3-amino-4-(methylamino)-benzoic acid (Fmoc-MeDbz-OH). The last residue was incorporated as N-Boc protected amino acid. After SPPS, the MeDbz linker was converted to the N-acyl-benzimidazolinone (Nbz) species by treating the resin with 4-nitrophenyl-chloroformate in dichloromethane followed by a solution of i-Pr2NEt in dimethylformamide. The activated Nbz-resin was then treated with a trifluoroacetic acid (TFA) solution to cleave protecting groups and after excessive washing, swelled in cyclization buffer (phosphate buffer, 0.2 M, pH 6.8 in 50% acetonitrile) and incubated at 50°C for 2 h. The AIP containing solution was separated from the resin and the desired AIP purified by preparative reverse-phase high performance liquid chromatography (RP-HPLC). Full characterization of all synthetic AIPs has been reported previously (Gless et al., 2019). The sequences and quality of the synthetic AIPs can be found in Supplementary Table S1.
Adhesion Assay
This assay was carried out as previously described (Baldry et al., 2016a). Ninety-six well plates were pre-coated with 100 μL/well of 10 μg/mL fibronectin (Fibronectin from human plasma, F2006, Sigma-Aldrich) and incubated for up to 24 h with mild shaking at 4°C. Respective overnight cultures of S. aureus strains 8325-4, 61599 (CC398 strain), HG001, HG003 and two S. aureus pig isolates (from this study) were diluted 1:100 and grown till OD600 = 0.5 in fresh TSB medium, after which the bacteria were treated with the synthesized AIPs belonging to Staphylococcus hyicus (10–4 mM), Staphylococcus simulans (10–4 mM), and Staphylococcus chromogenes (10–3 mM) separately, and grown at 37°C with shaking until OD600 = 1.7. The concentrations resulting in 100% inhibitory effect on the agr system were chosen according to their IC50 values. After removing and washing, untreated and treated S. aureus were added to fibronectin-coated wells and incubated statically at 37°C for 1 h. To avoid the toxic effect of DMSO on bacterial growth, the final solvent concentration of DMSO was maintained at 0.2% (v/v) for all experimental and control cultures. After removing the non-adhered bacteria and washing the wells, the attached bacteria were fixed with 2.5% glutaraldehyde in PBS statically for 1 h at 37°C. Binding activity of S. aureus was quantified by measuring the OD570 absorbance of resuspension in 96% ethanol after staining with 0.1% crystal violet at room temperature for 30 min. Arbitrary binding units were calculated by dividing the crystal violet absorption OD by the bacterial cell density of 1.7.
Static Biofilm Assay
As previously described (Nielsen et al., 2012; Goetz et al., 2017) and with minor modifications, overnight cultures were adjusted to OD600 = 0.2 in TSB and then further diluted 1:100 in 66%TSB supplemented with 0.2% glucose. A total of 200 μL of the bacterial suspensions were added to wells where either S. aureus 8325-4 WT, 8325-4Δagr (agr– strain), CoNS alone, or a 1:1 ratio of S. aureus + CoNS was added. After a 24–30 h incubation period, the medium was removed from each well; the plates were washed and allowed to air dry. Dried biofilms were stained with 125 μL of 0.1% crystal violet solution for 30 min, washed three times with PBS and allowed to dry. To quantify the biofilm formation, the stained biofilm was solubilized in 200 μL of 95% ethanol for 10–15 min and 100 μL were transferred to a new microtiter plate, after which the absorbance was measured at 590 nm. In this assay, three biological replicates were performed with eight technical replicates per experiment. Parallel samples were set for CFU quantification by subsequent plating on SaSelectTM plates (Bio-Rad).
DNA Sequence Analysis
From purified DNA a sequencing library was generated using Nextera XT (Illumina) followed by (2 × 150 bp) paired-end sequencing on a NextSeq (Illumina) instrument. Genome sequences were de novo assembled using skesa with default settings (Souvorov et al., 2018). From assembled draft genomes the species were identified using the tuf gene, which has previously been described to discriminate between Staphylococcus species (McMurray et al., 2016; Strube et al., 2018).
Statistical Analysis
Where applied, we used a 1-way ANOVA analysis (GraphPad Prism version 7.04 software; GraphPad Software Inc., La Jolla, CA, United States). Differences were considered statistically significant at P < 0.05.
Results
Nasal Colonization of S. aureus and Other Staphylococcal Species
To investigate CoNS strains colonizing pig nares we collected nasal swabs from 25 pigs originating from Danish organic pig farms and isolated staphylococcal species on Sa SelectTM plates. In total, 384 isolates were obtained of which 75 were identified by MALDI-TOF MS. tuf gene analysis and genome sequencing were performed to further verify some of the strains (Hwang et al., 2011; Loonen et al., 2012). Of the 75 isolates 21 were identified as S. aureus; corresponding to just over half of the swabs being positive for S. aureus (52%; 13/25 pigs). The remaining 54 isolates were identified and classified into 8 CoNS species originating from 20 of the 25 pigs (Table 2). Staphylococcus sciuri (40%) was the most dominant amongst the CoNS isolated, followed by Staphylococcus lentus (24%), Staphylococcus xylosus (24%), S. simulans (20%), S. hyicus (16%), Staphylococcus arlettae (16%), S. chromogenes (8%), and finally Staphylococcus agnetis (4%). These results show that there is substantial variation among pigs with respect to staphylococcal colonization and that they are commonly colonized by more than one species.
TABLE 2.
Frequency and presence of eight staphylococcal species isolated from 25 pigs.
| Pig No. | % Isolation frequency – species identity – species isolated/pig | ||||||||
| 40% | 24% | 24% | 16% | 16% | 20% | 8% | 4% | 52% | |
| S. sciuri | S. lentus | S. xylosus | S. hyicus | S. arlettae | S. simulans | S. chromogenes | S. agnetis | S. aureus | |
| 1 | X | X | X | ||||||
| 2 | X | X | X | ||||||
| 3 | X | X | X | ||||||
| 4 | X | X | X | X | |||||
| 5 | X | X | X | ||||||
| 6 | X | X | |||||||
| 7 | X | X | |||||||
| 8 | X | X | |||||||
| 9 | X | ||||||||
| 10 | X | ||||||||
| 11 | X | ||||||||
| 12 | X | ||||||||
| 13 | X | ||||||||
| 14 | X | X | |||||||
| 15 | X | X | |||||||
| 16 | X | ||||||||
| 17 | X | X | |||||||
| 18 | X | X | X | ||||||
| 19 | X | X | |||||||
| 20 | X | X | |||||||
| 21 | X | X | X | ||||||
| 22 | X | X | |||||||
| 23 | X | X | |||||||
| 24 | X | X | |||||||
| 25 | X | ||||||||
Rows indicate the presence of staphylococci in the individual pigs. Columns contain the different staphylococcal species identified.
S. aureus Virulence Factor Expression Is Affected by CoNS Strains
Based on previous reports of cross-communication between S. aureus and CoNS strains we hypothesized that S. aureus interacts via agr with the surrounding microbial consortia including the resident CoNS. Therefore, the secreted products of the isolated CoNS strains were screened for their ability to modulate S. aureus agr using a previously established reporter assay. This assay is based on three reporter strains where the promoters of RNAIII, hla and spa, respectively, are fused to lacZ (Nielsen et al., 2010). Upon induction of agr such as is observed during entry into stationary growth phase, promoters of both RNAIII and hla will be induced while that of spa will be repressed. Therefore, after incorporation of the reporter strains together with the LacZ substrate into the agar plates they will become blue when containing the RNAIII and hla reporter strain fusions, but will remain colorless when containing the spa reporter strain after overnight incubation. Conversely, if an agr inhibiting compound has been added to a well in the agar plate reduced expression of hla and RNAIII but increased expression of spa will be observed. As seen in Figure 1, the extent to which CoNS supernatants affected the S. aureus agr (agr-I) system varied between species and in some cases even within species. Interestingly, while S. sciuri was the most prevalent species in the swabs, none of the supernatants from isolates of this species affected the S. aureus agr system. In contrast, isolates belonging to S. hyicus, S. simulans, and S. lentus species contained the most isolates with S. aureus agr modulation capabilities. These findings show that CoNS display varying ability to repress the S. aureus agr and that such repression is commonly observed.
FIGURE 1.
Effect on virulence factor expression of S. aureus by CoNS culture supernatant. TSA agar plates (with erythromycin and X-gal) containing the hla:lacZ (PC322; Eryr) (plates A1–A4), the rnaIII:lacZ (SH101F7; Eryr) (plates B1–B4), or the spa:lacZ (PC203; Eryr) (plates C1–C4) reporter strains of S. aureus were used to screen the cell-free overnight culture supernatants from 55 isolates. Sixty microliter of supernatant or TSB (as a negative control) were added to the wells in the plates. The supernatants in wells are from S. hyicus (wells 1–7 and 51); S. sciuri (wells 8–16 and 52–60); S. simulans (wells 17–21, 23 and 28); S. lentus (wells 24–27 and 29–32); S. arlettae (wells 33–36); S. xylosus (wells 37–43); S. agnetis (well 44); S. chromogenes (well 45); UI shown in well 22 stands for an unidentified isolate. The plates were incubated at 37°C for 10–24 h (until zones appeared). The assay was performed three times as biological replicates. This figure is representative of one set of screening plates.
Effect of CoNS Strains on S. aureus agr Groups I–IV
In the agar plate assay (Figure 1) we had determined the inhibitory activity of CoNS strains in a S. aurues strain belonging to AgrC group I. To determine if the CoNS AIPs are able to inhibit agr in S. aureus strains carrying the AgrC groups II to IV, and to obtain a quantative measure of the inhibitory effect we employed β-lactamase reporter strains that monitor expression of the RNAIII P3 promoter in cells expressing AgrC groups I to IV. As these strains have been engineered so that they do not produce intrinsic AIPs, induction of agr requires addition of supernatants from strains producing the corresponding AIP group (Nielsen et al., 2014). In this system, the activity of the reporter strains were measured in the presence or absence of cell-free, overnight culture supernatants of our CoNS isolates. Importantly, all the CoNS supernatants that displayed an agr-inhibitory activity in the plate assay also inhibited RNAIII expression in S. aureus strains carrying agr groups I, II, and III, whereas the inhibitory potential against group IV was only marginal (Figures 2A1–D2). Further we confirmed the notion that the CoNS AIPs affect S. aureus agr via competitive inhibition of AgrC as we saw little to no inhibition of the P3 promoter in a reporter strain encoding a constitutively active AgrC variant of agr group I that displays kinase activity in the absence of inducing AIP (Geisinger et al., 2009) (Figures 2E1,E2).
FIGURE 2.
AgrC-mediated interference of RNAIII expression by CoNS supernatants. Reporter strains RN10829 (P2-agrA: P3-blaZ)/pagrC-I-IV (WT) (A1–D2) and RN10829 (P2-agrA: P3-blaZ)/pagrC-I-R23H (AgrC const.) (E1,E2) were grown to OD600 = 0.35, and exposed to 1/10 volume supernatant of CoNS and 1/10 external inducing AIP-I-IV supernatant. After 45 min incubation, RNAIII expression was assessed using the nitrocefin hydrolysis method and analyzed for relative β-lactamase activity by nitrocefin conversion. The numbers displayed on the X-axis correspond to those in Figure 1: No. 1–7 and 51 (S. hyicus); No. 8–16 and 52–60 (S. sciuri); No. 17–21, 23 and 28 (S. simulans); No. 24–27 and 29–32 (S. lentus); No. 33–36 (S. arlettae); No. 37–43 (S. xylosus); No. 44 (S. agnetis); No. 45 (S. chromogenes). Each CoNS species is represented by a different color. Each column is representative of at least three biological replicates and the error bars represent the standard deviation.
In addition to inhibition, we were also interested in exploring whether any of the staphylococcal supernatants could induce S. aureus agr activity. To this end we tested the ability of our CoNS isolates to induce S. aureus agr using the same β-lactamase reporters of the S. aureus agr groups, but in this case the staphylococcal supernatants were used as presumptive inducers omitting induction by the cognate AIP. Our results show that none of the CoNS supernatants were capable of activating any of the four S. aureus agr groups (Supplementary Figure S1). These results show that CoNS strains interfere with agr induction by competing with the S. aureus AIPs for AgrC binding and that they generally have inhibitory activity toward S. aureus agr.
Dual Species Biofilm Involving S. aureus and CoNS
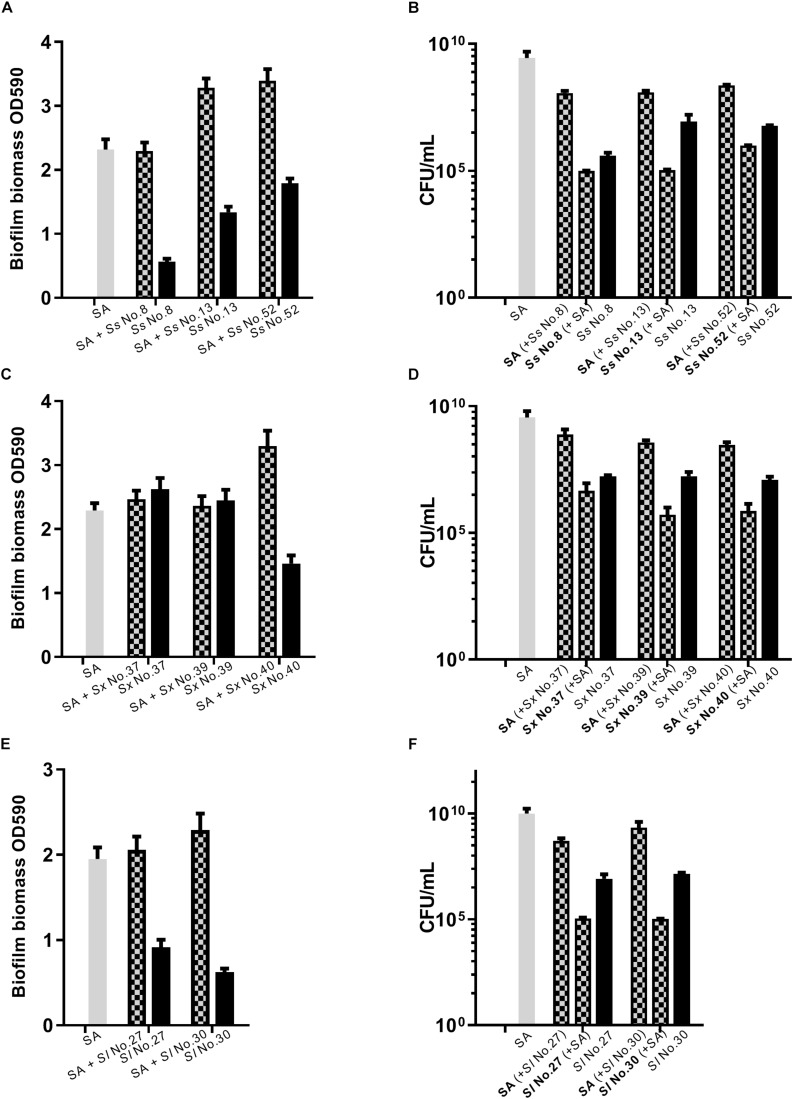
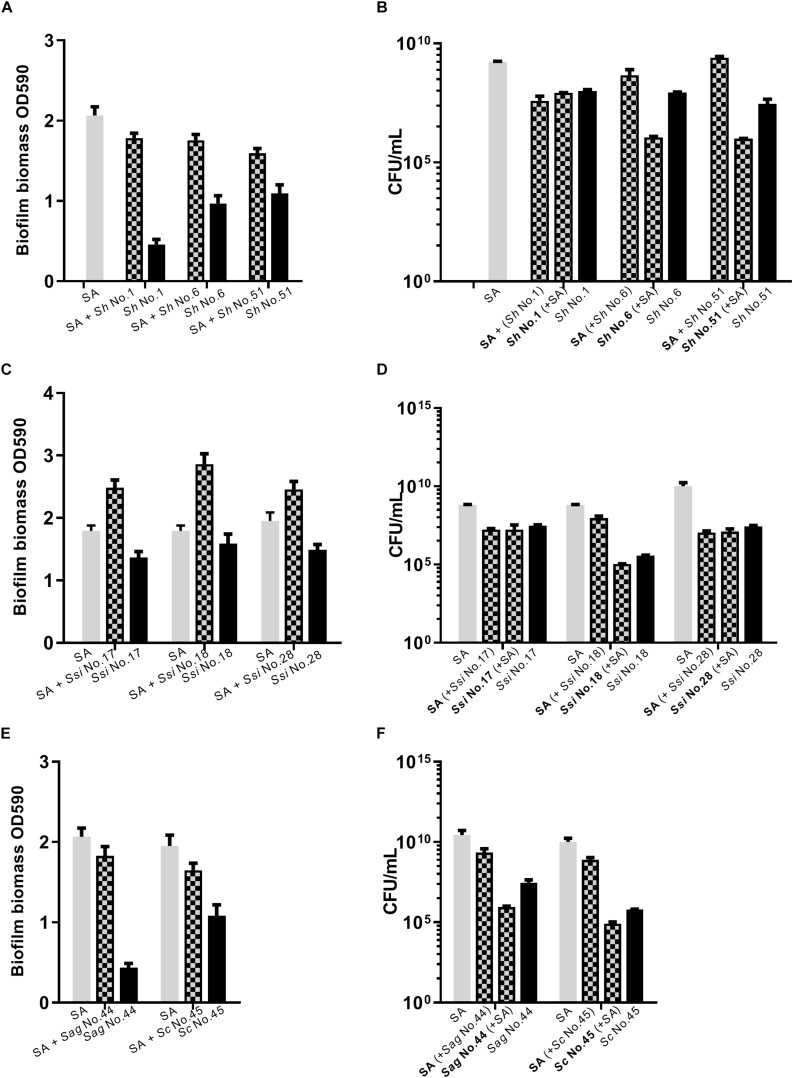
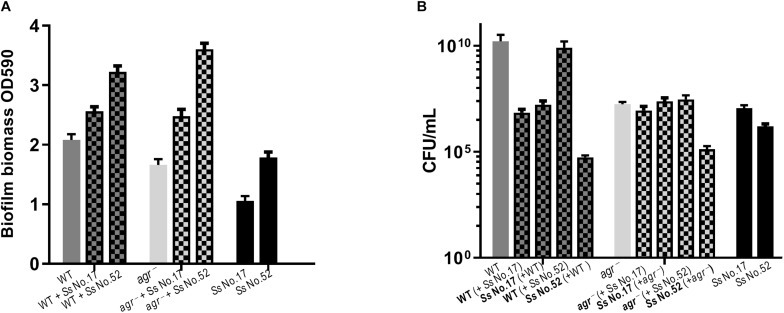
As S. aureus agr is known to influence biofilm formation (Le and Otto, 2015), we asked if CoNS strains potentially producing agr repressing peptides affected biofilm formation. When grown individually, the CoNS strains were less robust at forming biofilm than S. aureus (Figures 3, 4 and Supplementary Figure S2). From these we selected 1-3 CoNS strains from each species to examine biofilm formation in the presence of S. aureus. While the biofilm biomass was quantified by crystal violet staining, bacterial composition of these dual-species biofilms was determined by inspection of CFU on SaSelectTM plates. In all, we tested biofilm formation for eight combinations where the CoNS species had no inhibitory effect on S. aureus agr (Figure 3), eight combinations for those CoNS species with a strong S. aureus agr inhibitory effect (Figure 4), and another three combinations with CoNS strains with varying agr inhibitory effects (Supplementary Figure S2). When examining the composition of dual-species biofilm (Figures 3B,D, 4B,D and Supplementary Figure S2B), both species were represented. For 8 out of the 19 dual-species combinations we observed increased biofilm biomass when compared to biofilm formation by individual strains. Importantly, these grouped almost evenly into the agr cross-inhibition group (5 out of 10) and the non-inhibitory group (3 out of 9). While this data already indicated that the increased biofilm in dual species biofilms was independent of agr cross-talk, we sought to consolidate this finding further. For this we chose one strain capable of agr cross-inhibition (S. simulans No. 17) and one strain from the non-inhibitory group (S. sciuri No. 52), and analyzed their effect on biofilm formation of a S. aureus agr mutant strain. These data indicate that the absence of a functional agr in S. aureus did not influence biofilm formation when mixed with CoNs strains (Figure 5). Collectively our data show that the presence of both S. aureus and CoNS may in some instances enhance biofilm formation when compared to that formed by the individual strains, but in those cases it is unrelated to agr mediated interactions.
FIGURE 3.
Dual species biofilm formation between S. aureus and CoNS strains not displaying agr inhibitory activity. For dual-species biofilms, S. aureus 8325-4 (SA) was co-cultured together with one of S. sciuri (Ss, A,B), S. xylosus (Sx, C,D) and S. lentus (Sl, E,F) and biofilm biomass (A,C,E) or CFU (B,D,F) were determined as indicated by mix color bars and compared to biofilms formed by the individual species (SA indicated by gray bars and CoNS by black bars).
FIGURE 4.
Dual species biofilm formation between S. aureus and CoNS strains displaying agr cross-inhibition. For dual-species biofilms, S. aureus 8325-4 (SA) was co-cultured together with one of S. hyicus (Sh, A,B), S. simulans (Ssi, C,D), S. agnetis and S. chromogenes (Sag/Sc, E,F) and biofilm biomass (A,C,E) or CFU (B,D,F) were determined as indicated by mix color bars and compared to biofilms formed by the individual species (SA indicated by gray bars and CoNS by black bars).
FIGURE 5.
Biofilm formation does not correlate with agr inhibition. For dual-species biofilms, S. aureus 8325-4 WT or agr– (Δagr) mutant strains were co-cultured together with either S. simulans (Ss No. 17) or S. sciuri (Ss No. 52) and biofilm biomass (A) or CFU (B) were determined as indicated by mix color bars and compared to biofilms formed by the individual species (SA indicated by gray bars and CoNS by black bars).
Strain-Specific Enhancement of S. aureus Adherence to Fibronectin in the Presence of Synthesized CoNS AIPs
As inhibition of S. aureus agr leads to increased expression of surface adhesion proteins recognizing host factors, we were curious to see whether the addition of synthesized AIPs from CoNS would lead to increased binding of S. aureus to host factor. To address this, we studied the fibronectin binding capacity of different S. aureus strains namely the laboratory strain 8325-4 (CC8), the livestock associated CC398 strain 61599 (Tang et al., 2017b) as well as two S. aureus strains identified from the pig nares together with either S. chromogenes (A) or S. hyicus and S. simulans (B). S. aureus A was classified as CC8 and S. aureus B as CC45. Strains belonging to both CC8 and CC45 have previously been found associated with live stock (Tang et al., 2017a). When these strains were separately treated with synthesized AIPs of S. hyicus, S. simulans, and S. chromogenes, that have been detected in a previous study (Gless et al., 2019), we observed a significant increase in S. aureus 8325-4 binding to fibronectin in the presence of the CoNS AIPs, in comparison to the vehicle (DMSO)-treated control (Figure 6). However, neither strain 61599 nor the pig-derived S. aureus isolates obtained in this study and tested here were affected by the presence of CoNS AIPs over the vehicle control. In consideration of the known regulatory defects of S. aureus 8325-4, we also examined the adherence of repaired strains HG001 (restored rsbU, an activator of SigB) and HG003 (restored rsbU and tcaR, an activator of protein A transcription) under the same condition (Herbert et al., 2010) (Supplementary Figure S3). Both of these strains showed a low adhesion to fibronectin. Thus, exposure to CoNS AIPs does not lead to increased binding to fibronectin except for strain 8325-4.
FIGURE 6.
Synthesized AIPs from S. hyicus (Sh AIP), S. simulans (Ss AIP), and S. chromogenes (Sc AIP) enhance S. aureus adherence to host factors fibronectin in vitro. S. aureus strains were separately treated with AIPs (10–4 mM S. hyicus, 10–4 mM S. simulans AIPs and 10–3 mM S. chromogenes), incubated and fixed in human fibronectin pre-coated 96-well plates. Crystal violet staining was performed to quantify the amount of S. aureus adhering to host factors via measurement of OD590. Results shown are representative of 3 independent experiments. Each bar represents the average of 8 biological replicates and the error bars represent the standard deviation. ∗P < 0.05; ∗∗∗P < 0.001; ****P < 0.0001.
Discussion
Coagulase negative staphylococci comprise a diverse group of staphylococcal species that largely are harmless colonizers of both humans and animals. For a given host several CoNS are commonly present and the composition varies both within and between host species (Nagase et al., 2002). Likewise, pigs have been reported to be colonized by a variety of CoNS with one study describing 10 species including S. hyicus, Staphylococcus haemolyticus, Staphylococcus warneri, S. simulans, S. xylosus, and S. sciuri to be isolated at approximately equal frequency (Nagase et al., 2002). Others document higher CoNS species numbers (between 18 and 20 different CoNS) including the afore-mentioned, as well as S. saprophyticus and S. cohnii (Schoenfelder et al., 2017; Verstappen et al., 2017). However, both the latter studies report a marked increase in S. sciuri prevalence over the other species amounting to between 30 and 46% of the total colonizing CoNS species (Schoenfelder et al., 2017; Verstappen et al., 2017). In our investigation we also found S. sciuri to be the most prevalent CoNS being isolated from 40% of the pigs followed by S. lentus and S. xylosus. Unlike the Verstappen study though, we did not isolate S. saprophyticus or S. cohnii which were the other most prominent species identified along with S. sciuri (Verstappen et al., 2017).
Interestingly CoNS strains appear to be common producers of AIP molecules that resemble the AIPs of the S. aureus agr quorum sensing system. Analogous to the cross-inhibition of agr that occurs between S. aureus strains belonging to different AgrC subgroups, the CoNS AIPs also tend to inhibit expression of agr controlled genes. Previously we showed that out of 52 staphylococcal isolates obtained from a common strain collection, 37 were capable of inhibiting agr of S. aureus representing 17 different CoNS species (Canovas et al., 2016). Here, we aimed to investigate the extent to which CoNS strains isolated form the same niche environment (i.e., from individual pigs) were able to repress S. aureus agr. Our results show that out of 25 pigs we isolated 8 different CoNS species of which 24 out of 54 strains had quorum quenching properties. Interestingly out of 18 tested S. sciuri strains none were able to repress agr. Similar was observed for S. xylosus whereas for S. lentus, which was present in 24% of the pigs, agr was repressed by some strains but unaffected by others. In contrast, all isolates of both S. hyicus and S. simulans displayed strong agr repressing activity. Using a constitutively active AgrC variant we were able to show that the CoNS strains likely repress agr through production of AIP-like molecules that are secreted to the culture supernatant and compete with S. aureus AIPs for binding to S. aureus AgrC, as opposed to other agr quorum quenching mechanisms such as via AgrA binding (Baldry et al., 2016b). This notion was confirmed by synthesis of selective CoNS AIP molecules. Furthermore, we observed correlation between the inhibitory potential of individual CoNS strains against S. aureus agr group I and the inhibition exerted on groups II and III, while the inhibition pattern was not clearly reflected on group IV. Low inhibitory activity against agr group IV for entities that are highly active against other groups have been described before (Gless et al., 2019). We also examined if any of the CoNS strains were able to induce the S. aureus agr system; however, none of the strains demonstrated such activity. For P. aeruginosa, analogs of quorum sensing molecules have been reported to induce quorum sensing (Smith et al., 2003) and we have observed that synthesized AIPs of S. schleiferi and Staphylococcus hominis are capable of inducing S. aureus AgrC group IV (Gless et al., 2019). However, cross-species induction of agr appears to be a rare phenomenon and the vast majority of agr modulating compounds interfere with quorum sensing induction (Hansen et al., 2018).
Previously it has been suggested that presence or absence of CoNS strains may correlate with S. aureus colonization. For example, Verstappen et al. observed a lower frequency of S. aureus colonization in the presence of S. sciuri, S. cohnii, or S. saprophyticus. We did not identify any S. cohnii or S. saprophyticus in our sampled pigs, and out of the 10 pigs positive for S. sciuri, 4 were co-isolated with S. aureus while 6 were not. However, we did observe that all S. arlettae and S. lentus isolates were colonizing pig nares where S. aureus was not found to be present. To investigate competitive behavior between CoNS strains and S. aureus we performed a series of dual-species biofilm studies based on the notion that agr has been reported to influence both biofilm formation and dispersal (Boles and Horswill, 2008; Periasamy et al., 2012). This rational was also made interesting by the recent observations by Gonzalez et al. that S. epidermidis secreted soluble products (when added to S. aureus cultures) inhibit S. aureus biofilm formation, but when the two species are co-inoculated and grow in physical contact they are capable of forming a robust dual-species biofilm (Gonzalez et al., 2018). Our data corroborate Gonzalez’s observations in that we also show robust biofilm formation in the dual-species setting with no evident out-competition of one over the other species. Such dual-species interactions can benefit both species in that they can persist in a colonizing state more robustly, as biofilms are extremely hard to eradicate by both the host and by antimicrobial therapies, and thus also providing a constant reservoir for possible S. aureus chronic infections (Archer et al., 2011). Moreover, even though we have observed the effect of interaction between S. aureus and CoNS on biofilm formation, no correlation to agr-inhibition was seen. Further studies are needed to better understand the complexity of these interactions.
In context of antibacterial therapy, cross-talk between staphylococci and S. aureus via agr has become a topic of interest. We recently showed that agr inhibition by AIP-like molecules reduces S. aureus induced lesions in an atopic dermatitis model (Baldry et al., 2018) and this was supported by the finding that CoNS strains reduce skin barrier damage by inhibiting production of proteases and phenol-soluble modulins secreted by S. aureus (Williams et al., 2019). Another study reports a synthetic AIP from Staphylococcus caprae that dramatically reduced dermonecrotic injury caused by S. aureus and reduced cutaneous bacterial burden relative to controls (Paharik et al., 2017). However, as inhibition of agr is associated with increased expression of surface adhesion proteins favoring host adhesion and immune evasion (Novick et al., 1993), one could speculate that CoNS strains may increase the ability of S. aureus to colonize. For only one strain of S. aureus, namely 8325-4, we observed a significant increase in adhesion to human fibroncetin in the presence of CoNS AIPs. This was not seen for the livestock associated MRSA strain 61599 belonging to CC398, the two S. aureus isolates obtained from pigs in the present study, nor for the strains HG001 or HG003 that are derivatives of 8325-4 and restored by rsbU or rsbU/tcaR regulatory genes. Thus, we could not consistently demonstrate an effect of CoNS AIPs on S. aureus binding to fibronectin. In conclusion, the interactions between coagulase-negative staphylococci and S. aureus are complex and involve both agr dependent and independent factors, which future studies will be required to elucidate.
Conclusion
We have conducted an investigation of the possible role of the agr cross-talk between S. aureus and CoNS strains isolated from the same colonizing location. We show that there are substantial variations with respect to species colonization amongst the pig hosts tested, as well as in S. aureus agr-modulation capacity of isolated CoNS species. Importantly, our results document multiple interactions between S. aureus and CoNS and they suggest that S. aureus adhesion and dual-species biofilm formation can indeed be influenced by CoNS in both agr-dependent and agr-independent manners.
Data Availability Statement
All datasets analyzed for this study are included in the manuscript/Supplementary Files.
Ethics Statement
No permission is required to sample the nostril of pigs according to the Danish Animal Experimentation Act § 1.2.
Author Contributions
PP, MB, MSB, and HI designed the study. PP, BG, SB, and CE-G conducted the experimental work. PP, MB, MSB, HI, CO, BG, PA, CE-G, and SB analyzed the data. PP, MB, MSB, BG, CO, and HI wrote the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02212/full#supplementary-material
References
- Archer N. K., Mazaitis M. J., Costerton J. W., Leid J. G., Powers M. E., Shirtliff M. E. (2011). Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2 445–459. 10.4161/viru.2.5.17724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authority E. F. S. (2009). Analysis of the baseline survey on the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008-Part A: MRSA prevalence estimates. EFSA J. 7:1376 10.2903/j.efsa.2009.1376 [DOI] [Google Scholar]
- Baldry M., Kitir B., Frøkiær H., Christensen S. B., Taverne N., Meijerink M., et al. (2016a). The agr inhibitors solonamide B and analogues alter immune responses to Staphylococccus aureus but do not exhibit adverse effects on immune cell functions. PLoS One 11:e0145618. 10.1371/journal.pone.0145618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldry M., Nielsen A., Bojer M. S., Zhao Y., Friberg C., Ifrah D., et al. (2016b). Norlichexanthone reduces virulence gene expression and biofilm formation in Staphylococcus aureus. PLoS One 11:e0168305. 10.1371/journal.pone.0168305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldry M., Nakamura Y., Nakagawa S., Frees D., Matsue H., Núñez G., et al. (2018). Application of an agr-specific antivirulence compound as therapy for Staphylococcus aureus-induced inflammatory skin disease. J. Infect. Dis. 218 1009–1013. 10.1093/infdis/jiy259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojer M. S., Baldry M., Ingmer H. (2017). “Protocols for screening antimicrobial peptides that influence virulence gene expression in Staphylococcus aureus,” in Antimicrobial Peptides. Methods in Molecular Biology, ed. Hansen P. (New York, NY: Humana Press; ), 387–394. 10.1007/978-1-4939-6737-7_28 [DOI] [PubMed] [Google Scholar]
- Boles B. R., Horswill A. R. (2008). Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 4:e1000052. 10.1371/journal.ppat.1000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canovas J., Baldry M., Bojer M. S., Andersen P. S., Grzeskowiak P. K., Stegger M., et al. (2016). Cross-talk between Staphylococcus aureus and other staphylococcal species via the agr quorum sensing system. Front. Microbiol. 7:1733. 10.3389/fmicb.2016.01733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. F., Foster S. J. (1998). The role of environmental factors in the regulation of virulence-determinant expression in Staphylococcus aureus 8325-4. Microbiology 144 2469–2479. 10.1099/00221287-144-9-2469 [DOI] [PubMed] [Google Scholar]
- Cho S.-H., Strickland I., Boguniewicz M., Leung D. Y. (2001). Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J. Allergy Clin. Immunol. 108 269–274. 10.1067/mai.2001.117455 [DOI] [PubMed] [Google Scholar]
- Chuang Y.-Y., Huang Y.-C. (2015). Livestock-associated meticillin-resistant Staphylococcus aureus in Asia: an emerging issue? Int. J. Antimicrob. Agents 45 334–340. 10.1016/j.ijantimicag.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Fridkin S. K., Hageman J. C., Morrison M., Sanza L. T., Como-Sabetti K., Jernigan J. A., et al. (2005). Methicillin-resistant Staphylococcus aureus disease in three communities. New Engl. J. Med. 352 1436–1444. [DOI] [PubMed] [Google Scholar]
- Geisinger E., Muir T. W., Novick R. P. (2009). “Agr receptor mutants reveal distinct modes of inhibition by staphylococcal autoinducing peptides,” in Proceedings of the National Academy of Sciences PNAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gless B. H., Bojer M. S., Peng P., Baldry M., Ingmer H., Olsen C. A. (2019). Identification of autoinducing thiodepsipeptides from staphylococci enabled by native chemical ligation. Nat. Chem. 11 1–7. 10.1038/s41557-019-0256-3 [DOI] [PubMed] [Google Scholar]
- Gless B. H., Peng P., Pedersen K. D., Gotfredsen C. H., Ingmer H., Olsen C. A. (2017). Structure–activity relationship study based on autoinducing peptide (aip) from dog pathogen S. schleiferi. Organ. Lett. 19 5276–5279. 10.1021/acs.orglett.7b02550 [DOI] [PubMed] [Google Scholar]
- Goetz C., Tremblay Y. D., Lamarche D., Blondeau A., Gaudreau A. M., Labrie J., et al. (2017). Coagulase-negative staphylococci species affect biofilm formation of other coagulase-negative and coagulase-positive staphylococci. J. Dairy Sci. 100 6454–6464. 10.3168/jds.2017-12629 [DOI] [PubMed] [Google Scholar]
- Golding G. R., Bryden L., Levett P. N., McDonald R. R., Wong A., Wylie J., et al. (2010). Livestock-associated methicillin-resistant Staphylococcus aureus sequence type 398 in humans, Canada. Emerg. Infect. Dis. 16:587. 10.3201/eid1604.091435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez T., Baatyrbek-kyzy A., Andersen H., Haslam D. B., Myers J. M. B., Hershey G. K. K., et al. (2018). Investigating the role of staphylococcal biofilms in the pathogenesis of pediatric atopic dermatitis. J. Allergy Clin. Immunol. 141:AB93. [Google Scholar]
- Hansen A. M., Peng P., Baldry M., Perez-Gassol I., Christensen S. B., Vinther J. M. O., et al. (2018). Lactam hybrid analogues of solonamide B and autoinducing peptides as potent S. aureus AgrC antagonists. Eur. J. Med. Chem. 152 370–376. 10.1016/j.ejmech.2018.04.053 [DOI] [PubMed] [Google Scholar]
- Herbert S., Ziebandt A.-K., Ohlsen K., Schäfer T., Hecker M., Albrecht D., et al. (2010). Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect. Immun. 78 2877–2889. 10.1128/IAI.00088-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh M. J., Aish J. L., White I. J., Shaw L., Lithgow J. K., Foster S. J. (2002). σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184 5457–5467. 10.1128/jb.184.19.5457-5467.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. M., Kim M. S., Park K. U., Song J., Kim E.-C. (2011). Tuf gene sequence analysis has greater discriminatory power than 16S rRNA sequence analysis in identification of clinical isolates of coagulase-negative staphylococci. J. Clin. Microbiol. 49 4142–4149. 10.1128/JCM.05213-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase T., Uehara Y., Shinji H., Tajima A., Seo H., Takada K., et al. (2010). Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465:346. 10.1038/nature09074 [DOI] [PubMed] [Google Scholar]
- Josse J., Laurent F., Diot A. (2017). Staphylococcal adhesion and host cell invasion: fibronectin-binding and other mechanisms. Front. Microbiol. 8:2433. 10.3389/fmicb.2017.02433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna T., Friendship R., Dewey C., Weese J. (2008). Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Vet. Microbiol. 128 298–303. 10.1016/j.vetmic.2007.10.006 [DOI] [PubMed] [Google Scholar]
- King M. D., Humphrey B. J., Wang Y. F., Kourbatova E. V., Ray S. M., Blumberg H. M. (2006). Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann. Int. Med. 144 309–317. [DOI] [PubMed] [Google Scholar]
- Kintarak S., Whawell S. A., Speight P. M., Packer S., Nair S. P. (2004). Internalization of Staphylococcus aureus by human keratinocytes. Infect. Immun. 72 5668–5675. 10.1128/iai.72.10.5668-5675.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S. D., Malachowa N., DeLeo F. R. (2015). Pathogenesis of Staphylococcus aureus abscesses. Am. J. Pathol. 185 1518–1527. 10.1016/j.ajpath.2014.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck R., Schaumburg F., Mellmann A., Köksal M., Jurke A., Becker K., et al. (2013). Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) as causes of human infection and colonization in Germany. PLoS One 8:e55040. 10.1371/journal.pone.0055040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourbatova E. V., Halvosa J. S., King M. D., Ray S. M., White N., Blumberg H. M. (2005). Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 clone as a cause of health care-associated infections among patients with prosthetic joint infections. Am. J. Infect. Control 33 385–391. 10.1016/j.ajic.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Le K. Y., Otto M. (2015). Quorum-sensing regulation in staphylococci–an overview. Front. Microbiol. 6:1174. 10.3389/fmicb.2015.01174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis H. C., Mølbak K., Reese C., Aarestrup F. M., Selchau M., Sørum M., et al. (2008). Pigs as source of methicillin-resistant Staphylococcus aureus CC398 infections in humans, Denmark. Emerg. Infect. Dis. 14:1383. 10.3201/eid1409.071576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonen A. J., Jansz A. R., Bergland J. N., Valkenburg M., Wolffs P. F., van den Brule A. J. (2012). Comparative study using phenotypic, genotypic and proteomics methods for identification of coagulase-negative staphylococci. J. Clin. Microbiol. 50 1437–1439. 10.1128/JCM.06746-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmmod Y. S., Klaas I. C., Svennesen L., Pedersen K., Ingmer H. (2018). Communications of Staphylococcus aureus and non-aureus Staphylococcus species from bovine intramammary infections and teat apex colonization. J. Dairy Sci. 101 7322–7333. 10.3168/jds.2017-14311 [DOI] [PubMed] [Google Scholar]
- McMurray C. L., Hardy K. J., Calus S. T., Loman N. J., Hawkey P. M. (2016). Staphylococcal species heterogeneity in the nasal microbiome following antibiotic prophylaxis revealed by tuf gene deep sequencing. Microbiome 4:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase N., Sasaki A., Yamashita K., Shimizu A., Wakita Y., Kitai S., et al. (2002). Isolation and species distribution of staphylococci from animal and human skin. J. Vet. Med. Sci. 64 245–250. 10.1292/jvms.64.245 [DOI] [PubMed] [Google Scholar]
- Nielsen A., Mansson M., Bojer M. S., Gram L., Larsen T. O., Novick R. P., et al. (2014). Solonamide B inhibits quorum sensing and reduces Staphylococcus aureus mediated killing of human neutrophils. PLoS One 9:e84992. 10.1371/journal.pone.0084992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen A., Nielsen K. F., Frees D., Larsen T. O., Ingmer H. (2010). Method for screening compounds that influence virulence gene expression in Staphylococcus aureus. Antimicrob. Agents Chemother. 54 509–512. 10.1128/AAC.00940-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L. N., Roggenbuck M., Haaber J., Ifrah D., Ingmer H. (2012). Diverse modulation of spa transcription by cell wall active antibiotics in Staphylococcus aureus. BMC Res. Notes 5:457. 10.1186/1756-0500-5-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Morse S. I. (1967). In vivo transmission of drug resistance factors between strains of Staphylococcus aureus. J. Exper. Med. 125:45. 10.1084/jem.125.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Ross H., Projan S., Kornblum J., Kreiswirth B., Moghazeh S. (1993). Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12 3967–3975. 10.1002/j.1460-2075.1993.tb06074.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. E., Todd D. A., Schaeffer C. R., Paharik A. E., Van Dyke M. J., Büttner H., et al. (2014). The Staphylococcus epidermidis agr quorum-sensing system: signal identification, cross-talk, and importance in colonization. J. Bacteriol. 196 3482–3493. 10.1128/JB.01882-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill E., Pozzi C., Houston P., Humphreys H., Robinson D. A., Loughman A., et al. (2008). A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J. Bacteriol. 190 3835–3850. 10.1128/JB.00167-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M., Echner H., Voelter W., Götz F. (2001). Pheromone cross-inhibition between Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 69 1957–1960. 10.1128/iai.69.3.1957-1960.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M., Süßmuth R., Vuong C., Jung G., Götz F. (1999). Inhibition of virulence factor expression in Staphylococcus aureus by the Staphylococcus epidermidis agr pheromone and derivatives. FEBS Lett. 450 257–262. 10.1016/s0014-5793(99)00514-1 [DOI] [PubMed] [Google Scholar]
- Paharik A. E., Parlet C. P., Chung N., Todd D. A., Rodriguez E. I., Van Dyke M. J., et al. (2017). Coagulase-negative staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe 22 746–756. 10.1016/j.chom.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy S., Joo H.-S., Duong A. C., Bach T.-H. L., Tan V. Y., Chatterjee S. S., et al. (2012). How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. U.S.A. 109 1281–1286. 10.1073/pnas.1115006109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queck S. Y., Jameson-Lee M., Villaruz A. E., Bach T.-H. L., Khan B. A., Sturdevant D. E., et al. (2008). RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 32 150–158. 10.1016/j.molcel.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfelder S. M., Dong Y., Feßler A. T., Schwarz S., Schoen C., Köck R., et al. (2017). Antibiotic resistance profiles of coagulase-negative staphylococci in livestock environments. Vet. Microbiol. 200 79–87. 10.1016/j.vetmic.2016.04.019 [DOI] [PubMed] [Google Scholar]
- Smith K. M., Bu Y., Suga H. (2003). Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem. Biol. 10 81–89. 10.1016/s1074-5521(03)00002-4 [DOI] [PubMed] [Google Scholar]
- Smith T. C., Male M. J., Harper A. L., Kroeger J. S., Tinkler G. P., Moritz E. D., et al. (2009). Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern US swine and swine workers. PLoS One 4:e4258. 10.1371/journal.pone.0004258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souvorov A., Agarwala R., Lipman D. J. (2018). SKESA: strategic k-mer extension for scrupulous assemblies. Genome Biol. 19:153. 10.1186/s13059-018-1540-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strube M. L., Hansen J. E., Rasmussen S., Pedersen K. (2018). A detailed investigation of the porcine skin and nose microbiome using universal and Staphylococcus specific primers. Sci. Rep. 8:12751. 10.1038/s41598-018-30689-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal-Gan Y., Ivancic M., Cornilescu G., Blackwell H. E. (2016). Characterization of structural elements in native autoinducing peptides and non-native analogues that permit the differential modulation of AgrC-type quorum sensing receptors in Staphylococcus aureus. Organ. Biomol. Chem. 14 113–121. 10.1039/c5ob01735a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Larsen J., Kjeldgaard J., Andersen P. S., Skov R., Ingmer H. (2017a). Methicillin-resistant and-susceptible Staphylococcus aureus from retail meat in Denmark. Int. J. Food Microbiol. 249 72–76. 10.1016/j.ijfoodmicro.2017.03.001 [DOI] [PubMed] [Google Scholar]
- Tang Y., Nielsen L. N., Hvitved A., Haaber J. K., Wirtz C., Andersen P. S., et al. (2017b). Commercial biocides induce transfer of prophage Φ13 from human strains of Staphylococcus aureus to livestock CC398. Front. Microbiol. 8:2418 10.3389/fmicb.2017.02418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cleef B., Broens E., Voss A., Huijsdens X., Züchner L., Van Benthem B., et al. (2010). High prevalence of nasal MRSA carriage in slaughterhouse workers in contact with live pigs in The Netherlands. Epidemiol. Infect. 138 756–763. 10.1017/S0950268810000245 [DOI] [PubMed] [Google Scholar]
- Van Duijkeren E., Ikawaty R., Broekhuizen-Stins M., Jansen M., Spalburg E., De Neeling A., et al. (2008). Transmission of methicillin-resistant Staphylococcus aureus strains between different kinds of pig farms. Vet. Microbiol. 126 383–389. 10.1016/j.vetmic.2007.07.021 [DOI] [PubMed] [Google Scholar]
- Verstappen K. M., Willems E., Fluit A. C., Duim B., Martens M., Wagenaar J. A. (2017). Staphylococcus aureus nasal colonization differs among pig lineages and is associated with the presence of other staphylococcal species. Front. Vet. Sci. 4:97. 10.3389/fvets.2017.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Zhao A., Novick R. P., Muir T. W. (2014). Activation and inhibition of the receptor histidine kinase AgrC occurs through opposite helical transduction motions. Mol. Cell 53 929–940. 10.1016/j.molcel.2014.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. R., Costa S. K., Zaramela L. S., Khalil S., Todd D. A., Winter H. L., et al. (2019). Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci. Transl. Med. 11:eaat8329. 10.1126/scitranslmed.aat8329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarwood J. M., Schlievert P. M. (2003). Quorum sensing in Staphylococcus infections. J. Clin. Invest. 112 1620–1625. 10.1172/jci20442 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets analyzed for this study are included in the manuscript/Supplementary Files.