Abstract
Background
Hypoxia promotes cancer progression. Hypoxia-inducible factor-1α (HIF-1α) has been reported to enhance tumor invasion and metastasis via activating downstream genes, such as matrix metalloproteinases (MMPs). The purpose of this study was to explore the probable roles of HIF-1α and MMP13 in the invasion and metastasis of ovarian cancer under hypoxic conditions.
Material/Methods
The expression of HIF-1α and MMP13 protein were detected with immunohistochemistry staining in ovarian cancer tissues, metastatic lesions, and normal fallopian tissues. Ovarian cancer A2780 cells were cultured under normoxic condition and hypoxic condition. mRNA and protein expression of HIF-1α and MMP13 were detected by RT-PCR and Western blot analysis. The effects of siRNA against HIF-1α on MMP13 expression were examined by RT-PCR and Western blot analysis. Transwell invasion assays were performed to test the invasive ability of A2780 cells.
Results
Immunohistochemistry staining showed significantly higher expression of HIF-1α and MMP13 protein in ovarian cancer tissues and metastatic lesions than in normal fallopian tissues. HIF-1α and MMP13 expression were closely related. After exposure to hypoxia, mRNA and protein levels of HIF-1α and MMP13 were upregulated. siRNA effectively inhibited HIF-1α expression and MMP13 expression. The number of invading A2780 cells decreased after HIF-1α was silenced.
Conclusions
This study suggests that HIF-1α promotes ovarian cancer cell invasion through a MMP13 mechanism. It might be an effective strategy targeting HIF-1α - MMP13 to inhibit invasion and metastasis of ovarian cancer.
MeSH Keywords: Cell Hypoxia, Neoplasm Invasiveness, Neoplasm Metastasis, Ovarian Neoplasms
Background
Ovarian cancer is the second most common malignant tumor of the female reproductive system, and the leading cause of death of gynecological malignancies, performing a threat to women’s lives [1]. To improve the therapeutic effect of ovarian cancer, considerable efforts have been made, but 5-year survival rate remains at only 30% [2], and the death of most patients due to distant metastasis [3]. Despite all this, the pathogenesis of distant metastasis is still poorly understood. Therefore, a detailed understanding of metastasis mechanism might help develop new therapeutic strategy for ovarian cancer.
Hypoxia is the basic characteristic of the vast majority of solid tumors [4]and it can contribute to accelerate malignant progression [5], tumor cell proliferation and metastasis [6,7]. Some researchers have reported that hypoxia enhanced extracellular matrix (ECM) invasion in vitro [8,9]. Clinical studies also demonstrated that hypoxia is correlated with cancer metastatic potential [10]. Hypoxia-inducible factor 1α (HIF-1α), a pivotal factor in regulating the intracellular oxygen metabolism, is up-regulated under hypoxic environment which is associated with tumor cell proliferation[11], invasion[12] and metastasis [4,13].
Tumor cells invade the ECM through secreting proteolytic enzymes like matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases, to digest ECM [14, 15]. MMP13, one of the collagenases plays a significant role in tumor invasion and metastasis as a result of their power to degrade native fibrillar collagen [16]. MMP13 is related to invasion and metastasis in various malignant tumors [17–19]. However, the regulation of MMP13 expression remains largely undetermined [20]. Although MMP13 have no hypoxic response elements, there are some evidence that it may be activated by hypoxia [21,22].
The aim of the present study is to elucidate the effect of hypoxia-induced HIF-1α on the expression of MMP13 and the invasive potential in ovarian cancer cells in hypoxia environment.
Material and Methods
Tissue specimens
A total of 91 formalin fixed and paraffin embedded tissues were obtained from Qilu hospital of Shandong University, Shandong, China and Shandong Provincial Hospital, Shandong, China. There were 36 ovarian cancer, 26 metastatic lesions and 31 normal fallopian tissues. Among them, 15 ovarian cancer cases were paired with metastatic lesions and fallopian tissues from the same patient. The patient’ age ranged from 34 years to 78 years with a mean age of 56.9 years. Tissue identification and histological diagnosis of ovarian cancer, metastatic lesion and fallopian were confirmed by gynecologic pathologists. All ovarian cancers were high-grade serous adenocarcinoma. Metastatic lesions were located in omentum or peritoneum. The human subject research protocol was approved by the institutional review board.
Immunohistochemistry stain and score
The immunohistochemistry (IHC) detection was performed and quantified as described previously [23]. The mouse monoclonal antibody HIF-1α and rabbit polyclonal MMP13 was obtained from Abcam. Brown granules in cytoplasm or nucleus represented positive IHC results. Semi-quantitative score was assessed by 2 independent observers. Score representing the percentage of positive stained cells were graded as follows [24]: 0 (no positive tumor cells), 1 (5–25%), 2 (26–50%), 3 (51–75%), 4 (>75%). The intensity of staining was determined as follows: 0 (no staining), 1 (weak brown), 2 (moderate brown), and 3 (dark brown). The staining intensity score and the percentage score were then multiplied to obtain the total immunostaining score (TIS). TIS 1~4 means low expression, while TIS 6, 8 means moderately expression and 9, 12 means high expression.
Cell culture
Ovarian cancer A2780 cell line was obtained from the Shandong Academy of Medical Sciences (Jinan, China). These cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (HyClone; UK) in a humidified incubator containing 95% air and 5% CO2 at 37°C. After plating, cells were grown in hypoxia chamber with 1% oxygen.
siRNA transfection
The siRNAs against HIF-1α (sequence 1: 5′-CGGCGAAGTAA AGAATCTGAA-3′, sequence 2: 5′-GCAGACTCAAATACAAGAA-3′, sequence 3: 5′-TGTGAGTTCGCATCTTGAT-3′) were designed and synthesized by Shanghai GenePharma gene (Shanghai, China). The non-silencing siRNA that does not target any known mammalian gene (sequence: 5′-UUCUUCGAACGUGUCACGUTT-3′) was used as negative control. The siRNA was transfected into the cells using Lipofectamine 2000 (Life, USA) according to the manufacturer’s instructions. After 48 h of transfection, the cells were harvested for further experiments.
Western blot analysis
Cells were harvested and prepared using RIPA buffer (Beyotime, China) according to the manufacturer’s instructions. Total protein extracts were separated by 10% SDS-PAGE and transferred onto polyvinylidene membrane (Millipore, USA). Membranes were blocked with 5% non-fat milk for 2 h at 37°C and then incubated for overnight at 4°C with appropriate primary antibody as follows: anti-Hif-1α (1: 1000, Abcam), and anti-MMP13 (1: 3000, Abcam). After washing 3 times with Tris-buffered saline containing Tween-20, membranes were incubated with secondary mouse or rabbit IgG antibodies conjugated to peroxidase (1: 5000, ZSJQ-BIO, China) for 1 h at 37°C. Immunoreactive signals were visualized using the Protein Detector 5-bromo-4-chloro-Western blotting Kit (Beyotime, China). GAPDH signal was used as a control protein for quantification. Optical density of individual bands was measured using ImageJ software.
Reverse-transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using RNA isolation reagent (TIANGEN BIOTECH, China) following the manufacturer’s instructions and was reverse transcribed for cDNA synthesis using qPCR-RT Kit (Toyobo, Japan) in a 10-μl reaction system. The sequences of the primers are as follows:
HIF-1α forward, 5′-TGATACCAAAACCAATACACCTC-3′ and
reverse 5′-TGCTGAATTCACACAGTCACAAC-3′;
MMP13 forward, 5′-TTGAGGATACAGGCAAGACT-3′ and
reverse, 5′-TGGAAGTATTACCCCAAATG-3′;
GAPDH forward, 5′-ATTCATCTCTCCTCTCCCA-3′ and
reverse, 5′-GTTGGTGGTTGGTACTGT-3′.
PCR reaction was conducted in a total volume of 10 μl, containing 5μl SYBR Green (Toyobo, Japan). PCR amplification cycles were performed as follows: 95°C for 10 s, 40 cycles at 95°C for 5 s, and 60°C. GAPDH was used as a housekeeping control. The results were quantified using the ΔCt method: Expression ratio=2−ΔCt.
Transwell migration assay
Matrigel invasion assays were performed using transwell polycarbonate filters with 24 wells in 8.0-μm pore size (Corning, USA). Cells at 8×104/200 μl in serum-free medium were seeded into the upper chambers pre-coated with Matrigel (BD Biosciences, USA), while 600 μl medium with 10% fetal bovine serum was added to the lower chamber. Following incubation at 37°C for 24 h, cells above the membranes were removed with a cotton swab and the cells migrated to the lower side were fixed in methanol for 20 min at room temperature, and then stained with 0.5% crystal violet for 15 min. Cells that had migrated to the lover surface were photographed and counted.
Statistical analysis
All statistical analyses were performed with SPSS software (SPSS, version 13.0, USA). The data were analyzed using unpaired student’s t-test. All results were expressed as the mean ± standard deviation. Pearson correlation analysis was done. p<0.05 was considered to indicate a statistically significant difference.
Results
The protein expression of HIF-1α and MMP13 with IHC detection
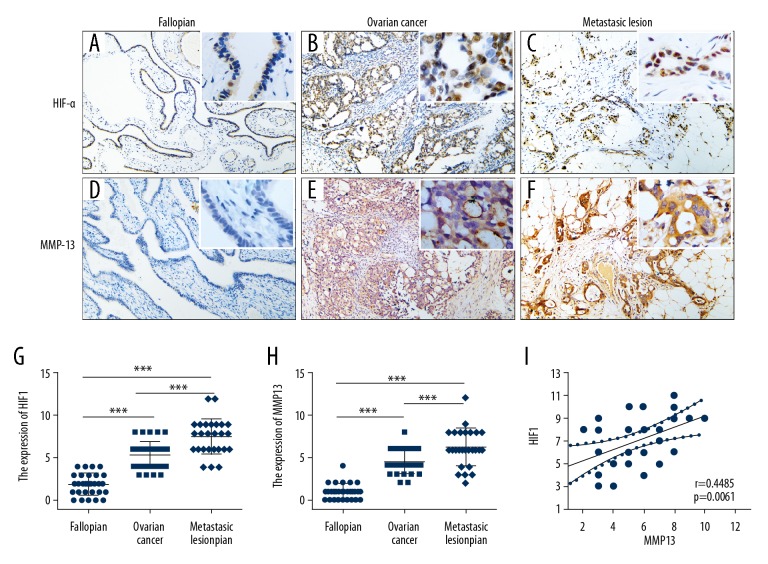
HIF-1α was expressed in all 36 ovarian carcinoma and 26 metastasis lesions. HIF-1α was expressed in cytoplasm and degraded rapidly at normal oxygen level. Hypoxic microenvironment, HIF-1α expression was steadily and transferred into nucleus playing its regulatory role. So brown stain in normal fallopian cytoplasm was not included in the scoring system. The positive cases showed nucleus localization. Moderate-strong intensity was respectively 58.33% and 80.77% in carcinoma and metastasis lesions, only weak immunoreaction intensity was observed in 22 of 31 fallopian tissues (Figure 1A–1C). The average TIS of HIF-1α in ovarian cancer, metastasis lesion and fallopian were respectively 5.33±0.26, 7.5±0.42 and 1.9±0.23. Statistical analysis showed significant difference of HIF-1α expression in ovarian carcinoma, metastasis lesion and fallopian (P<0.001) (Figure 1G). MMP13 was also expressed in all 36 ovarian carcinoma and 26 metastasis lesions. Cytoplasmic localization was observed in all positive cases. Moderate-strong intensity was respectively 30.56% and 76.92% in carcinoma and metastasis lesions. No staining or weak intensity was found in 31 fallopian tissues (Figure 1D–1F). In addition to epithelial cells, locally mesenchymal cells also expressed MMP13. The average TIS of MMP13 in ovarian cancer, metastasis lesion and fallopian were respectively 4.42±0.22, 6.23±0.43 and 0.97±0.16. There was significant difference of MMP13 expression level in ovarian carcinoma, metastasis lesion and fallopian (p<0.001) (Figure 1H). Statistical analysis confirmed a significant correlation between HIF-1α and MMP13 expression in metastasis lesions (r=0.4485, p=0.0061) (Figure 1I).
Figure 1.
The protein expression of HIF-1α and MMP13 with IHC detection. (A–C) HIF-1α protein expression pattern of fallopian, ovarian cancer, and metastasis lesion. (D–F) MMP13 protein expression pattern of fallopian, ovarian cancer, and metastasis lesion. (G, H) Statistical analysis showed a significant difference in HIF-1α and MMP13 expression. (I) A significant correlation was found between HIF-1α and MMP13 expression in metastasis lesion. *** p<0.001.
The expression of HIF-1α and MMP13in A2780 cell line in hypoxia environment
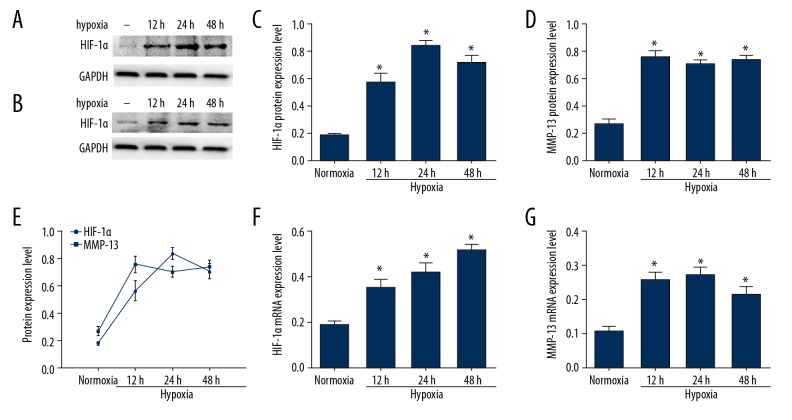
Ovarian cancer cell line A2780 was subjected to hypoxia for various time periods (12 h, 24 h, 48 h), the mRNA expression of HIF-1α and MMP13 were determined by real-time PCR. In normoxia environment, the expression level of HIF-1α was low. Hypoxia increased HIF-1α mRNA expression in a time-dependent manner, which was significantly increased after exposure to hypoxia for 48 h as shown in Figure 2A. Hypoxia increased protein expression of HIF-1α in time dependently, which were consistent with the change of mRNA (Figure 2C, 2F). In hypoxic environment, MMP13 mRNA and protein levels were up-regulated significantly in A2780 cells as shown in Figure 2B, 2D, 2G (P<0.05). Pearson correlation coefficient of HIF-1α expression and MMP13 expression was 0.8813 (P<0.001) (Figure 2E).
Figure 2.
Hypoxia-induced expression of HIF-1α and MMP13 in ovarian cancer A2780 cells. (A, C) HIF-1α protein expression after culturing in normoxia and hypoxic conditions. (F) HIF-1α mRNA levels in A2780 cells after exposure to normoxia and hypoxia. (B, D) MMP-13 protein expression. (G) MMP13 mRNA levels in A2780 cells after exposure to normoxia and hypoxia. Values are expressed as the mean ± standard deviation. * p<0.05 versus normoxia group. (E) Correlation analysis between HIF-1α and MMP13 expression under hypoxic condition.
siRNA transfection and Transwell migration assay
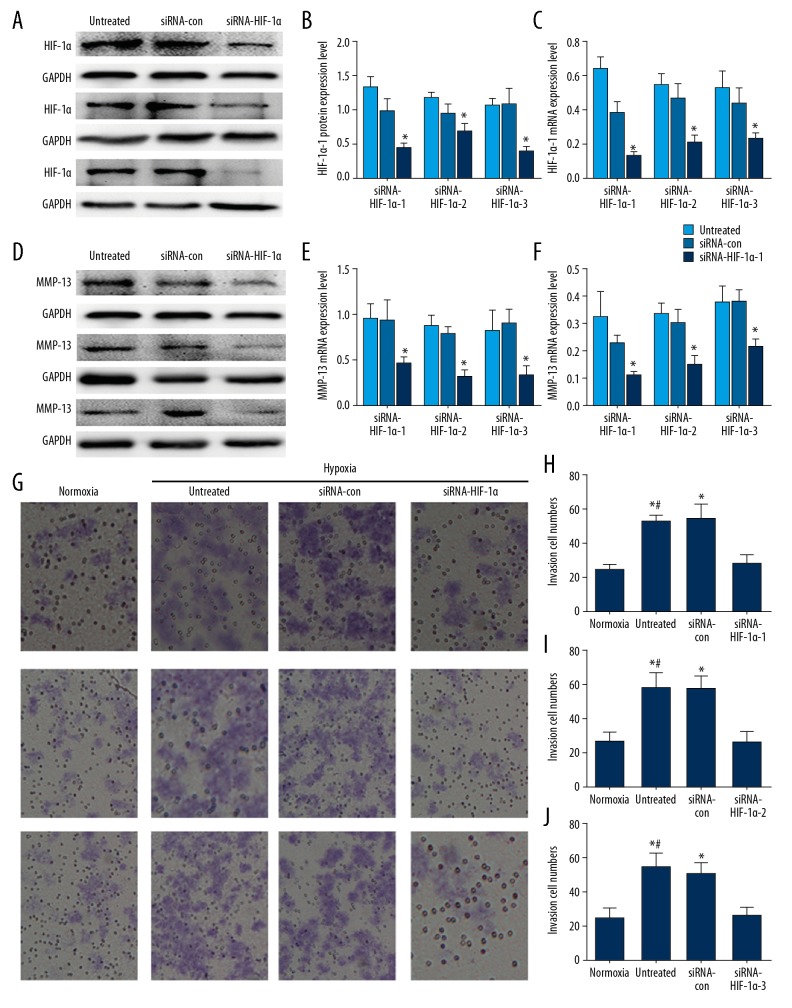
HIF-1α siRNA markedly decreased the mRNA and protein levels of the targeted HIF-1α subunit. The inhibition rate of mRNA level was 80% (Figure 3C). The inhibition rate of protein level was above 60% (Figure 3A, 3B). Transfection with HIF-1α siRNA simultaneously inhibited MMP13 expression (Figure 3D–3F) (P<0.05). Transwell migratory assays showed the migratory capacities of A2780 cells changed after being cultured in hypoxia combined with siRNA transfection (Figure 3G–3J). The migration of A2780 cells was increased after exposed to hypoxia for 24 h compared with that cultured in normoxia condition (P<0.05). A significantly decrease of cell migration was observed in the siRNA-HIF-1α group with hypoxia (hypoxia+siRNA-con vs. hypoxia+siRNA-HIF-1α (P<0.05). There is no difference between siRNA-control and hypoxia group.
Figure 3.
siRNA transfection and Transwell migration assay. (A, B) Western blot analysis was performed to test HIF-1α protein expression culturing in hypoxia after silencing HIF-1α. (C) RT-PCR was used to detect the effects of HIF-1α knockdown on the expression of HIF-1α mRNA levels in A2780 cells under hypoxic conditions. (F) After transfected with HIF-1α siRNA, MMP13 mRNA was downregulated. (D, E) MMP13 protein had the same change. The data are presented as the mean ± standard deviation. * p<0.05 versus normoxia group or the control siRNA group. (G–J) Suppressed effects of HIF-1α siRNA on the invasion potency of ovarian cancer A2780 cells. Invaded cells were counted in 5 microscopic fields per well. * p<0.05 versus the normoxia group. # p<0.05 versus the untreated group and the control siRNA group.
Discussion
Hypoxia is a common feature of chronic diseases, including inflammation and cancer. HIF-1α is a mediator for hypoxia and regulates O2 homeostasis during physiological and pathophysiological responses in vivo. Under hypoxia conditions, HIF-1α transfers to nuclei, as a transcription factor, and activates gene expression. HIF-1α has been found to activate more than 100 downstream genes to respond O2 level changes. HIF-1α inhibits MMP-13 expression by blocking TCF4-β-catenin interaction in chondrocytes, thus reducing less cartilage degradation in OA. Specific loss of HIF1α exacerbated MMP13 expression and cartilage destruction [25]. HIF-2α expression was higher in osteoarthritic cartilages, induces the mRNA expression of MMP13 [26]. Although these contradictory results have emerged in the study of arthritis, there is a close relationship between HIF and MMP13 under hypoxic environment. The relationship between HIF-1α and MMP13 in ovarian cancer metastasis is also deserve exploring.
Our IHC results showed that the expression of HIF-1α in ovarian high-grade serous adenocarcinoma was higher than in normal fallopian. Its expression in metastatic foci was further elevated. Expression level of HIF-1α in normoxia culture of A2780 ovarian cancer cells was low, but after cultured in hypoxic environment, its expression was significantly increased in time dependently. These results hinted that HIF-1α was a significant molecular factor in ovarian cancer under hypoxic microenvironment. High-grade serous adenocarcinoma is the most common ovarian cancer. We also detected one endometrioid carcinoma and one clear cell carcinoma with moderately stain of HIF-1α protein. Recent studies have shown that over-expression of HIF-1α is associated with ovarian cancer tissue type, FIGO staging, histological type, lymph node metastasis and five-year survival rate [27].
MMPs degrade a diverse group of the extracellular matrix (ECM), including collagen, laminin, fibronectin, vitronectin, gelatins and proteoglycans. Thereby, MMPs may be the key regulatory point in invasion and migration of cancer cells and thus in metastasis and tumorigenesis. MMP13, also known as collagenase-3, can act on wide spectrum of substrates, including collagens, versican, and fibronectin, and so on. Hantke et al. [28] detected the protein levels of MMP13 in ascitic fluids of 30 ovarian cancer patients. According to MMP13 values, these patients were stratified into two subpopulations, one population with short survival (median 16 months) and one with long overall survival (median 36 months). MMP13 level in ascitic fluid was seemed to be associated with shorter survival. Thus, MMP13 could be a potential prognostic factor and plays an important role in ovarian cancer metastasis [29,30].
In our study, IHC detection showed MMP13 protein was highly expressed in metastasis lesion than in cancer and fallopian. Furthermore, there was a significant correlation between HIF-1α and MMP13 expression in metastasis lesions. The expression of MMP13 in ovarian cancer cells under hypoxia condition of A2780 cells was significantly higher than normoxic condition, which was significantly correlated with the expression of HIF-1α. The down-regulation of HIF-1α expression with siRNA inhibited MMP13 expression in hypoxic environment, suggesting further that HIF-1α can regulate the expression of MMP13 in ovarian cancer cells. Although the hypoxic culture enhanced A2780 cell invasion, HIF-1α siRNA interference decreased MMP13 expression and invasion ability. It seems that HIF-1α is a key factor influencing the invasion and metastasis of ovarian cancer, modulatingMMP13 expression in hypoxic environment. We put forward the new HIF-1α-MMP13 pathway of the invasion and metastasis of ovarian cancer. Initial stages of ovarian cancer often have no clinically symptom, and most cases are diagnosed at advanced stage with metastasis. Therefore, a detailed understanding of the mechanisms of HIF-1α-MMP13 involved in invasion and metastasis of ovarian cancer might help develop new therapeutic strategy.
Conclusions
This study suggests that HIF-1α promotes ovarian cancer cell invasion through a MMP13 mechanism. We found that after exposure to hypoxia, both mRNA and protein levels of HIF-1α and MMP13 were up-regulated. Interference of HIF-1α expression effectively inhibited MMP13 expression, as well as invasion and metastasis of ovarian cancer cells. In conclusion, it might be an effective strategy targeting HIF-1α - MMP13 to inhibit invasion and metastasis of ovarian cancer. Further molecular experiments are needed to confirm this finding.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (81301789), the Shandong Province Natural Science Foundation (ZR2011HQ013), and the Key Research and Development Projects in Shandong Province, China (2016GSF201150)
Conflicts of interest
None.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Brun JL, Feyler A, Chene G, et al. Long-term results and prognostic factors in patients with epithelial ovarian cancer. Gynecol Oncol. 2000;78:21–27. doi: 10.1006/gyno.2000.5805. [DOI] [PubMed] [Google Scholar]
- 3.Dinkelspiel HE, Champer M, Hou J, et al. Long-term mortality among women with epithelial ovarian cancer. Gynecol Oncol. 2015;138:421–28. doi: 10.1016/j.ygyno.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hockel M, Vaupel P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 6.Araos J, Sleeman JP, Garvalov BK. The role of hypoxic signalling in metastasis: Towards translating knowledge of basic biology into novel anti-tumour strategies. Clin Exp Metastasis. 2018;35:563–99. doi: 10.1007/s10585-018-9930-x. [DOI] [PubMed] [Google Scholar]
- 7.Lewis DM, Pruitt H, Jain NC, et al. A feedback loop between hypoxia and matrixstress relaxation increases oxygen-axis migration and metastasis in sarcoma. Cancer Res. 2019;79(8):1981–95. doi: 10.1158/0008-5472.CAN-18-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jing SW, Wang YD, Kuroda M, et al. HIF-1α contributes to hypoxia-induced invasion and metastasis of esophageal carcinomavia inhibiting E-cadherin and promoting MMP-2 expression. Acta Med Okayama. 2012;66(5):399–407. doi: 10.18926/AMO/48964. [DOI] [PubMed] [Google Scholar]
- 9.Munoz-Najar UM, Neurath KM, Vumbaca F, Claffey KP. Hypoxia stimulates breast carcinoma cell invasion through MT1-MMP and MMP-2 activation. Oncogene. 2006;25:2379–92. doi: 10.1038/sj.onc.1209273. [DOI] [PubMed] [Google Scholar]
- 10.Nobre AR, Entenberg D, Wang Y, et al. The different routes to metastasis via hypoxia-regulated programs. Trends Cell Biol. 2018;28(11):941–56. doi: 10.1016/j.tcb.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HJ, Ryu JM, Jung YH, et al. Novel pathway for hypoxia-induced proliferation and migration in human mesenchymal stem cells: Involvement of HIF-1alpha, FASN, and mTORC1. Stem Cells. 2015;33:2182–95. doi: 10.1002/stem.2020. [DOI] [PubMed] [Google Scholar]
- 12.Tian Q, Xue Y, Zheng W, et al. Overexpression of hypoxia-inducible factor 1alpha induces migration and invasion through Notch signaling. Int J Oncol. 2015;47:728–38. doi: 10.3892/ijo.2015.3056. [DOI] [PubMed] [Google Scholar]
- 13.Nagaraju GP, Bramhachari PV, Raghu G, El-Rayes BF. Hypoxia inducible factor-1α: Its role in colorectal carcinogenesis and metastasis. Cancer Lett. 2015;366:11–18. doi: 10.1016/j.canlet.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Pego ER, Fernández I, Núñez MJ. Molecular basis of the effect of MMP-9 on the prostate bone metastasis: A review. Urol Oncol. 2018;36:272–82. doi: 10.1016/j.urolonc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237:273–81. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- 16.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–90. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen BS, Rank F, Lopez JM, et al. Collagenase-3 expression in breast myofibroblasts as a molecular marker of transition of ductal carcinoma in situ lesions to invasive ductal carcinomas. Cancer Res. 2001;61:7091–100. [PubMed] [Google Scholar]
- 18.Uria JA, Balbin M, Lopez JM, et al. Collagenase-3 (MMP13) expression in chondrosarcoma cells and its regulation by basic fibroblast growth factor. Am J Pathol. 1998;153:91–101. doi: 10.1016/S0002-9440(10)65549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang SH, Law CH, Kuo PH, et al. MMP13 is involved in oral cancer cell metastasis. Oncotarget. 2016;7:17144–61. doi: 10.18632/oncotarget.7942. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Kotepui M, Punsawad C, Chupeerach C, et al. Differential expression of matrix metalloproteinase-13 in association with invasion of breast cancer. Contemp Oncol (Pozn) 2016;20:225–28. doi: 10.5114/wo.2016.61565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu OY, Hou MF, Yang SF, et al. Cobalt chloride-induced hypoxia modulates the invasive potential and matrix metalloproteinases of primary and metastatic breast cancer cells. Anticancer Res. 2009;29:3131–38. [PubMed] [Google Scholar]
- 22.Ishizuka S, Sakai T, Hiraiwa H, et al. Hypoxia-inducible factor-2alpha induces expression of type X collagen and matrix metalloproteinases 13 in osteoarthritic meniscal cells. Inflamm Res. 2016;65:439–48. doi: 10.1007/s00011-016-0926-1. [DOI] [PubMed] [Google Scholar]
- 23.Luo Y, Li M, Zuo X, et al. β-catenin nuclear translocation induced by HIF-1α overexpression leads to the radioresistance of prostate cancer. Int J Oncol. 2018;52(6):1827–40. doi: 10.3892/ijo.2018.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenza GL. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985) 2000;88:1474–80. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 25.Bouaziz W, Sigaux J, Modrowski D, et al. Interaction of HIF1α and β-catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proc Natl Acad Sci USA. 2016;113(19):5453–58. doi: 10.1073/pnas.1514854113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito T, Fukai A, Mabuchi A, et al. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med. 2010;16(6):678–86. doi: 10.1038/nm.2146. [DOI] [PubMed] [Google Scholar]
- 27.Jin Y, Wang H, Liang X, et al. Pathological and prognostic significance of hypoxia-inducible factor 1alpha expression in epithelial ovarian cancer: A meta-analysis. Tumour Biol. 2014;35:8149–59. doi: 10.1007/s13277-014-2059-x. [DOI] [PubMed] [Google Scholar]
- 28.Hantke B, Harbeck N, Schmalfeldt B, et al. Clinical relevance of matrix metalloproteinase-13 determined with a new highly specific and sensitive ELISA in ascitic fluid of advanced ovarian carcinoma patients. Biol Chem. 2003;384:1247–51. doi: 10.1515/BC.2003.137. [DOI] [PubMed] [Google Scholar]
- 29.Zhao H, Bo Q, Wang W, et al. CCL17-CCR4 axis promotes metastasis via ERK/MMP13 pathway in bladder cancer. J Cell Biochem. 2018 doi: 10.1002/jcb.27494. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Orive-Ramos A, Seoane S, Ocaña A, et al. Regulation of theprometastatic neuregulin-MMP13 axis by SRC family kinases: Therapeuticimplications. Mol Oncol. 2017;11(12):1788–805. doi: 10.1002/1878-0261.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]