Abstract
Background
Kyphoplasty (KP) is a palliative treatment for patients with metastatic vertebral tumors. The distribution pattern of cement affects safety and efficacy. The distribution pattern of cement has not been previously reported for patients with metastatic vertebral tumors.
Material/Methods
From January 2013 to December 2017, patients with metastatic vertebral tumors who met our criteria were divided into cement fusion (n=91) and separation (n=97) groups. Visual analogue scale (VAS) and middle vertebral height (MVH) were evaluated preoperatively, postoperatively, and 1 year after surgery. Spinal Instability Neoplastic Score, fluoroscopy time, operation time, cement volume, cement leakage, and vertebral fractures were recorded and evaluated.
Results
Compared with the fusion group, the separation group had significantly different (P<0.001) operation time, fluoroscopy time, and cement volume. Compared with preoperative status, VAS and MVH were significantly improved 3 days postoperatively and 1 year postoperatively in both groups (P<0.001). The difference in cement leakage between the 2 groups (P<0.05) and in the number of adjacent vertebral fractures between the 2 groups (P<0.05) were significant.
Conclusions
The distribution patterns of the bone cement had a good analgesic effect and preventive effect on vertebral collapse. However, the separation of bone cement may be safer.
MeSH Keywords: Bone Cements, Kyphoplasty, Palliative Care, Spine
Background
With the development of social economy and medical technology, the survival time of cancer patients is significantly prolonged [1]. The spine is the most common site of bone metastasis in patients with cancer, and the incidence of metastatic vertebral tumors is increasing continuously [2]. In the United States, more than 1 million patients with cancer suffer from spinal metastasis each year, with the highest incidence of thoracic vertebrae (70%), followed by lumbar vertebrae (20%) and cervical vertebrae (10%) [3,4]. Therefore, metastatic vertebral cancer is an important disease in terms of social economy and human health.
Considering the invasion of tumor tissue, patients with metastatic vertebral tumors often suffer from low back pain, spinal cord compression, and the risk of vertebral collapse [5]. Analgesics, chemotherapy, radiotherapy, hormone therapy, and other conservative treatments are occasionally ineffective or are effective only for short-term treatment [6]. Surgical decompression is the first choice for treatment of metastatic vertebral tumors with spinal cord compression. For patients without symptoms of compression, minimally invasive surgery is mainly a palliative treatment to relieve pain and prevent vertebral collapse. Compared with open surgery, minimally invasive surgery has the advantages of low trauma, low bleeding, and short hospital stay. In view of the short life expectancy and poor tolerance of patients with advanced cancer, traditional open surgery is generally not used [7]. At present, vertebral augmentation (vertebroplasty and kyphoplasty) is considered to be the preferred palliative surgical treatment for patients with spinal metastatic tumors [8]. Kyphoplasty (KP) is used to restore vertebral height through an inflatable balloon to reduce the pressure of cement injection and the risk of leakage [9].
The distribution pattern of cement may affect the therapeutic effect and spinal stability [10]. Most relevant studies have described the cement distribution in osteoporotic vertebral compressive fractures, and the study of cement distribution involving metastatic vertebral tumors has not been reported. Osteoporotic vertebral compression fractures generally result in loss of anterior vertebral height, while tumors generally invade the posterior vertebral body [11]. Therefore, the cement distribution in osteoporotic vertebral compression fractures may not be suitable for the treatment of metastatic vertebral tumors. In practice, bilateral cement fusion or separation has different clinical effects in patients with metastatic vertebral tumors. In the present study, we retrospectively analyzed the relationship between different cement distribution patterns (fusion or separation) and short-term clinical outcomes in patients with metastatic vertebral tumors after KP.
Material and Methods
Patients
This study was supported and approved by the Ethics Committee of the First Affiliated Hospital of Soochow University. We reviewed the records of patients with metastatic vertebral tumors treated with KP in our hospital from January 2013 to December 2017. The inclusion criteria were metastatic vertebral tumors, diagnosed by pathology or cytology, ability to maintain prone position for at least 2 h, a Karnofsky performance score of more than 60, expected survival time of more than 1 year according to the evaluation of oncologists, no more than 2 metastases, and absence of other serious diseases. The exclusion criteria were infections, psychiatric disorders, coagulation disorders, diagnosis with primary malignant vertebral tumors, compression in spinal cord or nerve roots, and loss to follow-up. All follow-up patients were transferred to the Oncology Department for anticancer treatment after KP treatment.
Grouping
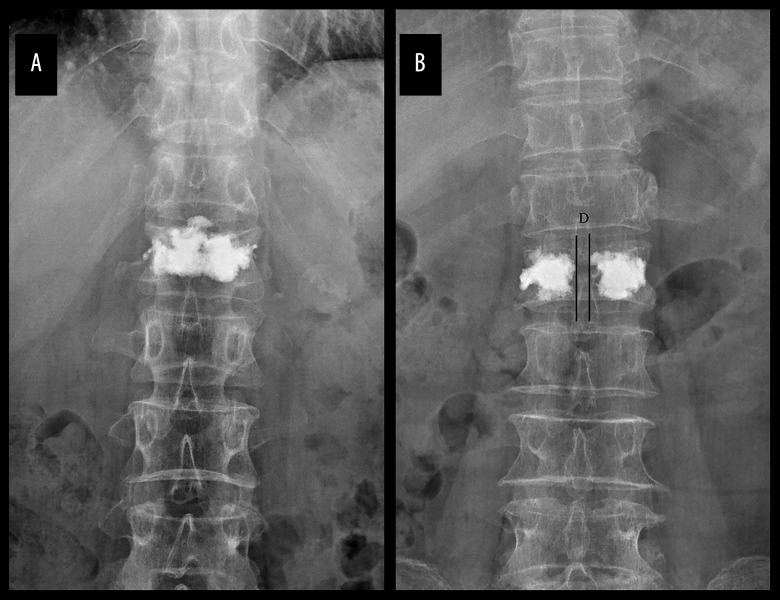
According to the distribution of positive and lateral X-ray bone cement after surgery, patients who met the criteria were screened and divided into 2 groups: group A had bilateral cement fusion (Figure 1A) and group B had bilateral cement separation (Figure 1B).
Figure 1.
Postoperative X-ray films of the 2 bone cement distribution patterns. (A) Group A: bilateral cement fusion; (B) Group B: bilateral cement separation.
Surgical operation
The entire KP procedure was performed by 2 senior spine surgeons. The anesthetized patient was positioned prone on the operating table. Pedicle puncture was guided by C-arm fluoroscopy. The puncture needle tip reached the internal edge of the pedicle and puncturing obliquity was generally at 19° to 22° in T6–L5 under frontal fluoroscopy, and the puncture needle tip reached between the anterior and posterior 1/4 of the midline of the vertebral body under lateral fluoroscopy, which demonstrated that the puncture procedure was successful. After successful puncture, an inflatable balloon was inserted to restore the height and shape of the vertebral body. Finally, bone cement was slowly and carefully injected into the vertebral body. After the KP procedure, the patients were monitored for 6 h.
Clinical evaluation
Middle vertebral height (MVH) was measured by X-ray lateral films from case data and outpatient reexamination preoperatively. The visual analogue scale (VAS) was used to evaluate the degree of back pain, and the pain increased with the increase in numerical value through the horizontal line from 0 to 10. The Spinal Instability Neoplastic Score (SINS) is a reliable and reproducible evaluation of spinal stability. The spacing between cement in the separation group was measured (D). Operative time, fluoroscopy time, and cement volume were recorded during the operation. Cement leakage and vertebral fractures were assessed 1 year postoperatively.
Statistical analysis
The average deviation and standard deviation of surgical time, fluoroscopy time, cement volume, SINS, MVH, and VAS were calculated and analyzed using SPSS software (SPSS 23.0, USA). The basic characteristics and result evaluation parameters of the 2 groups of MVH and VAS were compared by the t test of the group design data. The chi-squared test was used to compare the cement leakage and adjacent vertebral fractures in the 2 groups. When P<0.05, the difference was significant.
Results
From January 2013 to December 2017, after screening for inclusion and exclusion criteria, 204 patients met our requirements. Among these patients, 10 died of potential diseases within 1 year, and 6 failed to complete follow-up for other reasons. A total of 188 patients completed the 1-year follow-up in Table 1. In group A (fusion group), the average age of the 91 patients (49 females and 42 males) was 69.25±8.45 years. In group B (separation group), the average age of the 97 patients (53 females and 44 males) in the was 68.03±8.94 years.
Table 1.
Demographics of both groups.
| Parameters | Fusion | Separation | P |
|---|---|---|---|
| Patients | |||
| Number | 91 | 97 | – |
| Age (years) | 69.25±8.45 | 68.03±8.94 | >0.05 |
| Sex (F/M) | 49/42 | 53/44 | >0.05 |
| Follow-up (months) | 12 | 12 | – |
| SINS | 8.05±0.92 | 7.96±0.79 | >0.05 |
| Space between cement (D) | – | 6.04±1.11 | |
| KP | |||
| Operation time (minutes) | 26.84±4.87 | 23.51±3.91 | <0.001 |
| Fluoroscopy time (minutes) | 12.03±2.24 | 10.23±1.67 | <0.001 |
| Injected cement volume (mL) | 4.14±1.10 | 3.44±0.90 | <0.001 |
| VAS | |||
| Preoperatively | 7.43±0.85 | 7.23±0.88 | >0.05 |
| 3 days postoperatively | 2.77±0.76* | 2.76±0.84* | >0.05 |
| 1 year postoperatively | 2.64±0.72* | 2.63±1.10* | >0.05 |
| MVH | |||
| Preoperatively | 22.53±3.91 | 21.98±3.71 | >0.05 |
| 3 days postoperatively | 25.97±3.38* | 25.82±3.44* | >0.05 |
| 1 year postoperatively | 26.09±3.32* | 26.45±3.47* | >0.05 |
| Complications | |||
| Cement leakage | 8 | 1 | <0.05 |
| Adjacent vertebral fracture | 16 | 5 | <0.05 |
P<0.001 compared to preoperative value.
In the fusion group, the SINS score was 8.05±0.92, the operation time was 26.84±4.87 min, the fluoroscopy time was 12.03±2.24 min, and the volume of bone cement was 4.14±1.10 ml. The VAS score decreased from 7.43±0.85 preoperatively to 2.77±0.76 postoperatively (P<0.001) and remained at 2.64±0.72 1 year after KP. MVH increased from 22.53±3.91 mm preoperatively to 25.97 ±3.38 mm postoperatively (P<0.001) and re-mained at 26.09±3.32 mm 1 year after KP.
In the separation group, the SINS score was 7.96±0.79, the operation time was 23.51±3.91 min, the fluoroscopy time was 10.23±1.67 min, and the volume of bone cement was 3.44±0.90 ml. The space between cement in the separa-tion group was 6.04±1.11 mm. The VAS scores decreased from 7.23±0.88 preoperatively to 2.76±0.84 postoperatively (P<0.001) and remained at 2.63±1.10 1 year after KP. MVH increased from 21.98±3.71 mm preoperatively to 25.82±3.44 mm postoperatively (P<0.001) and remained at 26.45±3.47 mm 1 year after KP.
Compared with the fusion group, the operation time, fluoroscopy time, and cement volume in the separation group were significantly different (P<0.001). Compared with preoperative scores, VAS and MVH were significantly improved in both groups 3 days postoperatively and 1 year postoperatively (P<0.001). However, no serious complications related to cement leakage (e.g., pulmonary embolism and spinal cord compression) were found. The difference in cement leakage between the fusion and separation groups was significant (P<0.05). During the 1-year follow-up period, 21 patients suffered from adjacent vertebral fractures, including 16 patients in the fusion group and 5 patients in the separation group. The difference in the incidence of adjacent vertebral fractures between the 2 groups 1 year postoperatively was significant (P<0.05).
Discussion
Cancer metastases to the spine can lead to severe back pain, pathological vertebral fractures, and spinal cord compression [12]. The mechanism of back pain caused by metastatic vertebral tumors is mainly tumor invasion and mechanical instability. Pain caused by tumor invasion mainly occurs at night or in the early morning, possibly due to inflammatory mediators or from the tumor pulling the periosteum. Mechanical pain is mainly caused by structural damage, thereby leading to spine instability. The pain is related to movement and aggravates with increased axial load of the spine [13,14].
Treatment for patients with metastatic vertebral tumors is generally palliative, mainly to improve quality of life and reduce or eliminate pain [15]. The development of minimally invasive technology provides a new way to solve the aforementioned problems. Compared with nonsurgical treatment, KP can significantly reduce or eliminate pain, prevent vertebral collapse, and improve quality of life [16]. The mechanism of cement in the treatment of metastatic vertebral tumors is speculated to be the following [17–20]: (1) bone cement stabilization: the fracture is fixed to avoid nerve stimulation by compression and reduce the pain caused by inflammatory mediators; (2) exothermic effect: bone cement releases heat to destroy tumor tissue and nerve endings; (3) monomer toxicity: methyl methacrylate unpolymerized monomer has toxic effects on surrounding tumor tissue; (4) damage to blood supply: bone cement destroys tumor-nourishing blood vessels, thereby leading to the ischemic necrosis of the tumor tissue; and (5) occupancy effect: bone cement occupies space and oppresses tumor tissue to cause necrosis. In our study, there was obvious pain relief after KP in both groups, indicating that KP is effective for pain relief. However, the difference in pain relief between the 2 groups was insignificant. During the 1-year follow-up, the shape of the vertebral body remained intact, which proved that cement has an extremely good supporting effect and prevents collapse of the diseased vertebral body caused by tumor invasion.
Chen et al. showed that unipedicular and bipedicular KP can significantly improve the total stiffness of the compressed fractured vertebral bodies. However, the degree of increased stiffness still showed differences, in which unipedicular KP only restored the stiffness to the prefracture state, while bipedicular KP increased the stiffness to a significantly higher level than the prefracture state [21]. Liebschner et al. found that the strength of the vertebral body can be restored by the bipedicular and unipedicular injection of cement, but considerable stiffness can be obtained in the condition of symmetrical distribution. These researchers also believed that the volume fraction of cement was more valuable than the absolute volume [22]. Zhang et al. reported that compared with other distribution patterns, patients with cement connecting the upper and lower endplates have the lowest recompression rate, and further research proved that cement distribution is closely related to recompression [23]. When cement fills the cancellous part of the vertebral body and connects the upper and lower endplates, it can provide considerable support in the vertical direction. Load should be transferred uniformly through the upper endplate, filled cement, and lower endplate, in sequence. Thus, the possibility of recompression is extremely low [24]. Kim et al. indicated that stiffness of compressed vertebral bodies requires only 30% of the volume of bone cement to return to normal range. When the volume of cement is more than 30%, the hardness increases further, which may lead to adjacent vertebral fracture [25]. Tanigawa et al. found that patients with a sponge-like filling pattern had fewer new adjacent compression fractures than those with a compact and solid cement filling pattern [26]. He et al. reported a significant correlation between cement distribution patterns and adjacent vertebral fractures after surgery [27]. The aforementioned studies revealed that cement distribution plays an important role.
In the present study, we analyzed the efficacy and safety of 2 different cement distribution patterns – fusion and separation. Compared with the preoperative state, the fusion and separation groups both had an excellent analgesic effect but there was no significant difference between groups in the analgesic effect. In view of the higher incidence of adjacent vertebral fracture in the fusion group than in the separation group, and the significant difference in the volume of cement between the 2 groups, the cement volume in the fusion group may have caused significant changes in the biomechanical properties of the vertebral body after KP, which makes the vertebral body prone to fracture.
Cement leakage is the most common complication of KP, and a meta-analysis conducted by Li et al. reported that the overall leakage rate in KP is approximately 14% [28]. The factors that affect cement leakage include cement viscosity, bone cement volume vertebral wall integrity, bone porosity, injection cavity size, and bone pore size [29–32]. For patients with vertebral wall defect, a little bone cement should be injected into the normal area around the tumor lesion as an anchor to prevent the cement from moving after surgery [33]. Another challenging method is the graded infusion technique, which is described as follows: the first step is to inject high-viscosity cement to block the defect, and the second step is to inject low-viscosity cement to fill the vertebral body; the challenge is the difficulty in determining the injury of the vertebral wall and the precise location of the needle [34]. Another method is the incremental temperature cement delivery system. The viscosity of cement is related to both time and temperature. The cement injected into the vertebral body solidifies faster than that left in situ (body temperature is approximately 37°C, and operating room temperature is approximately 20°C). Therefore, the next injection of bone cement can be safe when we wait approximately 1–2 min after the first injection, when cement becomes viscous at the vertebral body defect [35]. For separated cements, several injection techniques are commonly used. First, bipedicular injection is better than unipedicular injection. Second, the small amount of cement that should be injected slowly should not cross the midline of the vertebral body. Finally, in the metastatic vertebral body, although the vertebral wall is generally damaged, the unfilled part of the separated cement can solve this problem effectively. In the present study, cement leakage, which was mainly attributed to the good technique of our senior physicians, was relatively low in the both groups. The difference in cement leakage between the fusion and separation groups 1 year postoperatively was significant. The fact that the cement volume in the fusion group was larger than that in the separation group may be related to the cement volume. Ren et al. reported that the cement volume is less than or equal to 4.17 ml, which not only achieves satisfactory curative effect, but also reduces the possibility of cement leakage [36].
The present study has several limitations. First, the study was retrospective and produced less evidence than prospective studies. Second, our study only included patients receiving KP, without controlled or alternative treatment such as vertebroplasty. Finally, we studied the effect of the intervention 1 year after surgery. Further research is needed to determine the cement distribution for the treatment of the metastatic vertebral tumors.
Conclusions
Both cement distribution patterns have good analgesic effect and prevent vertebral collapse. However, given the relatively higher incidence of cement leakage and adjacent vertebral fractures in the fusion group, separation may be safer than fusion.
Acknowledgements
Thanks to all patients involved in this study from the First Affiliated Hospital of Soochow University.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Cai Z. A preliminary study of the safety and efficacy of radiofrequency ablation with percutaneous kyphoplasty for thoracolumbar vertebral metastatic tumor treatment. Med Sci Monit. 2014;20:556–63. doi: 10.12659/MSM.889742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toma CD, Dominkus M, Nedelcu T, et al. Metastatic bone disease: A 36-year single-centre trend-analysis of patients admitted to a tertiary orthopaedic surgical department. J Surg Oncol. 2007;96(5):404–10. doi: 10.1002/jso.20787. [DOI] [PubMed] [Google Scholar]
- 3.Molina CA, Gokaslan ZL, Sciubba DM. Diagnosis and management of metastatic cervical spine tumors. Orthop Clin North Am. 2012;43(1):75–87. doi: 10.1016/j.ocl.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Guarnieri G, Izzo R, Muto M. Current trends in mini-invasive management of spine metastases. Interv Neuroradiol. 2015;21(2):263–72. doi: 10.1177/1591019915582366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Wang Y, Zhao L, et al. Effectiveness and safety of percutaneous vertebroplasty in the treatment of spinal metastatic tumor. Pak J Med Sci. 2017;33(3):675–79. doi: 10.12669/pjms.333.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Liu H, Pi B, et al. Clinical evaluation of percutaneous kyphoplasty in the treatment of osteolytic and osteoblastic metastatic vertebral lesions. Int J Surg. 2016;30:161–65. doi: 10.1016/j.ijsu.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Itagaki MW, Talenfeld AD, Kwan SW, et al. Percutaneous vertebroplasty and kyphoplasty for pathologic vertebral fractures in the Medicare population: Safer and less expensive than open surgery. J Vasc Interv Radiol. 2012;23(11):1423–29. doi: 10.1016/j.jvir.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Kırcelli A, Çöven İ. Percutaneous balloon kyphoplasty vertebral augmentation for compression fracture due to vertebral metastasis: A 12-month retrospective clinical study in 72 patients. Med Sci Monit. 2018;24:2142–48. doi: 10.12659/MSM.909169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun ZY, Li XF, Zhao H, et al. Percutaneous balloon kyphoplasty in treatment of painful osteoporotic occult vertebral fracture: A retrospective study of 89 cases. Med Sci Monit. 2017;23:1682–90. doi: 10.12659/MSM.903997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin WC, Lu CH, Chen HL, et al. The impact of preoperative magnetic resonance images on outcome of cemented vertebrae. Eur Spine J. 2010;19(11):1899–906. doi: 10.1007/s00586-010-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CS, Jung CH. Metastatic spinal tumor. Asian Spine J. 2012;6(1):71–87. doi: 10.4184/asj.2012.6.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkegaard AO, Sørensen ST, Ziegler DS, et al. Percutaneous vertebroplasty is safe and effective for cancer-related vertebral compression fractures. Dan Medical J. 2018;65(10):A5509–13. [PubMed] [Google Scholar]
- 13.Bouza C, López-Cuadrado T, Cediel P, et al. Balloon kyphoplasty in malignant spinal fractures: A systematic review and meta-analysis. BMC Palliat Care. 2009;8(1):12. doi: 10.1186/1472-684X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez L, Perez-Higueras A, Quinones D, et al. Vertebroplasty in the treatment of vertebral tumors: Postprocedural outcome and quality of life. Eur Spine J. 2003;12(4):356–6. doi: 10.1007/s00586-003-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho JH, Ha JK, Hwang CJ, et al. Patterns of treatment for metastatic pathological fractures of the spine: The efficacy of each treatment modality. Clin Orthop Surg. 2015;7(4):476–82. doi: 10.4055/cios.2015.7.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aparisi F. Vertebroplasty and kyphoplasty in vertebral osteoporotic fractures. Semin Musculoskelet Radiol. 2016;20(04):382–91. doi: 10.1055/s-0036-1592431. [DOI] [PubMed] [Google Scholar]
- 17.Tseng YY, Lo YL, Chen LH, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of pain induced by metastatic spine tumor. Surg Neurol. 2008;70:S78–83. doi: 10.1016/j.surneu.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 18.He SC, Teng GJ, Deng G, et al. Repeat vertebroplasty for unrelieved pain at previously treated vertebral levels with osteoporotic vertebral compression fractures. Spine. 2008;33(6):640–47. doi: 10.1097/BRS.0b013e318166955f. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez L, Perez-Higueras A, Quinones D, et al. Vertebroplasty in the treatment of vertebral tumors: Postprocedural outcome and quality of life. Eur Spine J. 2003;12(4):356–60. doi: 10.1007/s00586-003-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr JD, Barr MS, Lemley TJ, et al. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine. 2000;25(8):923–28. doi: 10.1097/00007632-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 21.Chen BL, Li YQ, Xie DH, et al. Comparison of unipedicular and bipedicular kyphoplasty on the stiffness and biomechanical balance of compression fractured vertebrae. Eur Spine J. 2011;20(8):1272–80. doi: 10.1007/s00586-011-1744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebschner MAK, Rosenberg WS, Keaveny TM. Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine. 2001;26(14):1547–54. doi: 10.1097/00007632-200107150-00009. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Wang Q, Wang L, et al. Bone cement distribution in the vertebral body affects chances of recompression after percutaneous vertebroplasty treatment in elderly patients with osteoporotic vertebral compression fractures. Clin Interv Aging. 2017;12:431–36. doi: 10.2147/CIA.S113240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MJ, Lindsey DP, Hannibal M, et al. Vertebroplasty versus kyphoplasty: Biomechanical behavior under repetitive loading conditions. Spine. 2006;31(18):2079–84. doi: 10.1097/01.brs.0000231714.15876.76. [DOI] [PubMed] [Google Scholar]
- 25.Kim JM, Shin DA, Byun DH, et al. Effect of bone cement volume and stiffness on occurrences of adjacent vertebral fractures after vertebroplasty. J Korean Neurosurg Soc. 2012;52(5):435–40. doi: 10.3340/jkns.2012.52.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanigawa N, Komemushi A, Kariya S, et al. Relationship between cement distribution pattern and new compression fracture after percutaneous vertebroplasty. Am J Roentgenol. 2007;189(6):348–52. doi: 10.2214/AJR.07.2186. [DOI] [PubMed] [Google Scholar]
- 27.He D, Lou C, Yu W, et al. Cement distribution patterns are associated with recompression in cemented vertebrae after percutaneous vertebroplasty: A retrospective study. World Neurosurg. 2018;120:e1–e7. doi: 10.1016/j.wneu.2018.06.113. [DOI] [PubMed] [Google Scholar]
- 28.Lee MJ, Dumonski M, Cahill P, et al. Percutaneous treatment of vertebral compression fractures: A meta-analysis of complications. Spine. 2009;34(11):1228–32. doi: 10.1097/BRS.0b013e3181a3c742. [DOI] [PubMed] [Google Scholar]
- 29.Bohner M, Gasser B, Baroud G, et al. Theoretical and experimental model to describe the injection of a polymethylmethacrylate cement into a porous structure. Biomaterials. 2003;24(16):2721–30. doi: 10.1016/s0142-9612(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 30.Lin D, Hao J, Li L, et al. Effect of bone cement volume fraction on adjacent vertebral fractures after unilateral percutaneous kyphoplasty. Clin Spine Surg. 2017;30(3):E270–75. doi: 10.1097/BSD.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 31.Yan-Sheng H, Chao-Yuan G, Hang F, et al. Bone cement-augmented short-segment pedicle screw fixation for Kümmell disease with spinal canal stenosis. Med Sci Monit. 2018;24:928–35. doi: 10.12659/MSM.905804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YS, Hao DJ, Feng H, et al. Comparison of percutaneous kyphoplasty and bone cement-augmented short-segment pedicle screw fixation for management of Kümmell disease. Med Sci Monit. 2018;24:1072–79. doi: 10.12659/MSM.905875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun H, Yang Z, Xu Y, et al. Safety of percutaneous vertebroplasty for the treatment of metastatic spinal tumors in patients with posterior wall defects. Eur Spine J. 2015;24(8):1768–77. doi: 10.1007/s00586-015-3810-8. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Pan J, Sun Z, et al. Percutaneous augmented instrumentation of unstable thoracolumbar burst fractures: Our experience in preventing cement leakage. Eur Spine J. 2012;21(7):1410–12. doi: 10.1007/s00586-012-2278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H, Liu H, Wang S, et al. Review of percutaneous kyphoplasty in China. Spine (Phila Pa 1976) 2016;41(Suppl 19):B52–58. doi: 10.1097/BRS.0000000000001804. [DOI] [PubMed] [Google Scholar]
- 36.Ren H, Shen Y, Zhang YZ, et al. Correlative factor analysis on the complications resulting from cement leakage after percutaneous kyphoplasty in the treatment of osteoporotic vertebral compression fracture. J Spinal Disord Tech. 2010;23(7):e9–15. doi: 10.1097/BSD.0b013e3181c0cc94. [DOI] [PubMed] [Google Scholar]