Abstract
Background
Polycystic ovary syndrome (PCOS) is associated with low-grade inflammation, adipocyte hypertrophy, hyperglycemia, increased serum testosterone levels, and reduced lipolysis. This study aimed to investigate the role of interleukin-6 (IL-6) and IL-11 in the pathophysiology of adipocyte hypertrophy in a rat model of PCOS.
Material/Methods
The rat model of PCOS was developed using a subcutaneous injection of dehydroepiandrosterone (DHEA). Histology of the rat ovaries was used to confirm the development of PCOS. Serum levels of testosterone and glucose were measured. Immunohistochemistry, immunofluorescence, quantitative real-time polymerase chain reaction (qRT-PCR), and Western blot were performed to measure IL-6 and IL-11 in the rat model of PCOS. Cell proliferation was measured using the cell counting kit-8 (CCK-8) assay.
Results
Serum levels of testosterone and glucose and the expression of IL-6 and IL-11 were significantly increased in the rat model of PCOS via the activation of AKT/STAT3 signaling. Following IL-6 and IL-11 stimulation of mesenchymal adipocytes isolated from adipose tissue, IL-6 and IL-11 induced cell proliferation through the STAT3/AKT signaling pathway.
Conclusions
In a rat model of PCOS, increased expression of IL-6 and IL-11 was associated with the AKT/STAT3 pathway. Increased levels of IL-6 and IL-11 stimulated adipocytes from adipose tissue of the rat model, which promoted cell proliferation by activating AKT/STAT3 signaling.
MeSH Keywords: Adipose Tissue, Interleukin-6, Interleukin-11, Polycystic Ovary Syndrome
Background
Polycystic ovary syndrome (PCOS) is a common and complex endocrine disorder characterized by abnormal development of ovarian follicles, increased production of androgens, and insulin resistance. PCOS accounts for about 75% of all cases of anovulatory infertility, which affects between 5–20% of women of reproductive age [1]. Patients with PCOS also have metabolic disorders that include glucose intolerance, impaired lipid profiles, dysfunction of beta cells in the pancreas, and visceral obesity [2]. However, the etiology of this heterogonous syndrome remains unknown.
Increasing numbers of studies have shown that pro-inflammatory mediators are involved in the pathophysiology of PCOS, which is also characterized by the presence of low-grade chronic inflammation [3]. Several inflammatory cytokines have been reported to be linked with insulin resistance in women with PCOS [3]. Also, most women with PCOS are obese and visceral fat is involved in the occurrence of the pro-inflammatory environment found in patients with PCOS [4].
Interleukin (IL)-6, a regulator of inflammation that modulates the secretion of several cytokines, has influences ovarian function, ovulation, fertilization, and implantation in women with PCOS [5]. In PCOS, levels of IL-6 are increased in the serum and granulosa cells [3,6], and studies have suggested that increased IL-6 increment may be associated with hyperandrogenism and insulin resistance in PCOS [7]. IL-11, a member of the IL-6 family, and has a role in hematopoiesis, tissue repair, and carcinogenesis. IL-11 is involved in adipogenesis, the development of osteoclasts, platelet maturation, and neurogenesis [8]. The IL-11 receptor system is commonly expressed in both malignant and nonmalignant ovarian tissues [9]. IL-11 and IL-6 activate signal transducer and activator of transcription 3 (STAT3) through glycoprotein 130 (GP130) signaling and has previously been identified as a therapeutic target in gastrointestinal tumorigenesis [10].
Therefore, this study aimed to investigate the role of IL-6 and IL-11 in the pathophysiology of adipocyte hypertrophy in a rat model of PCOS.
Material and Methods
Animals and experimental protocols
Female rats were purchased from the Animal Experiment Center of the Chinese Academy of Science in Shanghai. All animals were treated in compliance with the guidelines for the care and use of animals approved by Xuzhou City Hospital of Traditional Chinese Medicine and were in accordance with the principles of laboratory animal care. All rats were maintained at cages under appropriate environment conditions at a temperature of between 20–24°C, with a 12-hourly light and dark cycle and with free access to food and water.
The rats were randomly assigned to two groups that included the control group (n=12) and the polycystic ovary syndrome (PCOS) group (n=12). PCOS was induced by a single subcutaneous injection of dehydroepiandrosterone (DHEA) (6 mg/100 g body weight) for 20 days. The success of the establishment of the PCOS model was shown by symptoms that included an irregular estrous cycle and persistent vaginal cornification for 8 days. The rats were euthanized by carbon dioxide (CO2) asphyxiation, and blood samples were collected, separated by centrifugation, and stored at −20°C before analysis.
Biochemical assay for blood glucose and testosterone
Blood glucose levels were measured by electrochemical analysis using the One Touch Ultra meter (Johnson & Johnson, New Brunswick, NJ, USA). Serum testosterone levels were determined using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. All samples were analyzed simultaneously.
Histology
Ovarian tissue samples were obtained from each group of rats, fixed in 10% formalin, and embedded in paraffin wax. For histology, tissue samples were sectioned at a thickness of 5 μm. Then sections were stained with hematoxylin and eosin (H&E) and examined by light microscopy. For immunohistochemistry, samples were sectioned at 4 μm in thickness and incubated with primary antibodies to β-catenin (Cell Signaling Technology, USA) at 4°C overnight, followed by incubation of horseradish peroxidase (HRP)-conjugated secondary antibody for 30 min at 37°C. All sections were photographed under a light microscope.
Immunohistochemistry and immunofluorescence
The expression of IL-6 and IL-11 in adipose tissues was determined by immunofluorescence assay. Briefly, adipose tissues were fixed in paraffin and sectioned. For immunohistochemistry, samples were sectioned at 4 μm thickness and incubated with primary antibodies against β-catenin (Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight, followed by incubation of horseradish peroxidase (HRP)-conjugated secondary antibody for 30 min at 37°C. All sections were photographed under light microscopy. For immunofluorescence, the tissue sections were blocked with 3% goat serum albumin (ZSGB-Biotechnology Co., Beijing, China) for 30 min and then washed with PBS, and bovine serum albumin (BSA). Tissue sections were incubated with primary antibodies to IL-6 and IL-11 at 4 °C overnight, respectively. The sections were incubated with the corresponding secondary antibodies for 1 hr, followed by incubation with 4′, 6-diamidino-2-phenylindole (DAPI) before embedding in mounting medium.
Isolation, culture, and treatment of mesenchymal adipocytes
The adipose tissues were dissociated at 37°C for 1 h with collagenase type IA solution (Sigma-Aldrich, St. Louis MO, USA). The cells were centrifuged at 1500 rpm for 5 min, and the pellet containing the stromal vascular fraction was collected. The stromal vascular fraction was treated with lysing buffer (0.154 M NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA-2Na) at room temperature and then centrifuged. The resulting cell pellet was washed with Hank’s solution, and then suspended in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, USA) supplemented with 10% fetal bovine serum (FBS) (Life Technologies, Carlsbad, CA, USA), 100 U/ml penicillin and 100 g/ml streptomycin (Life Technologies, Carlsbad, CA, USA). The mesenchymal adipocytes were seeded in culture wells and maintained at 37°C in a humidified atmosphere of 5% CO2. The isolated adipocytes were stimulated by increasing concentrations of IL-6 and IL-11 at 1 pg/ml, 5 pg/ml, 10 pg/ml, 50 pg/ml, 100 pg/ml, and 1000 pg/ml. The optimal concentrations of IL-6 and IL-11 were used for subsequent experiments.
Cell proliferation assay
The effects on cell proliferation of IL-6 and IL-11 were analyzed using the cell counting kit-8 (CCK-8) proliferation assay (Sigma-Aldrich, St. Louis MO, USA) according to the manufacturer’s instructions. The cells were seeded into 96-well plates at a density of 2×104 cells/well and incubated for 24 hr. IL-6 and IL-11 treatment was performed at different concentrations of 1 pg/ml, 5 pg/ml, 10 pg/ml, 50 pg/ml, 100 pg/ml, and 1000 pg/ml for 24 hr. Then, the supernatant was removed, and DMEM medium containing 10 μl of CCK-8 was added to each well and incubated at 37°C for 3 hr. Cell viability was determined by measuring the absorbance at 450 nm.
RNA isolation and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from adipose tissues using TRIzol reagent (ThermoFisher Scientific Inc., Carlsbad, CA) according to the manufacturer’s instructions. The cDNA was prepared with PrimeScript reverse transcriptase (Takara, Minato-ku, Tokyo, Japan). Specific primers were used to detect the mRNA expression of IL-6 and IL-11. Amplification of β-actin was used as the control. Then, qRT-PCR was performed using the SYBR PrimeScript RT-PCR Kit II (Takara, Minato-ku, Tokyo, Japan). The following primers were used:
IL-6 Forward: 5′-TCTCCACAAGCGCCTTGG -3′;
IL-6 Reverse: 5′-CTCAGGGCTGAGATGCCC-3′;
IL-11 Forward: 5′-GTGGCCAAGATACAGCTGTCGC-3′;
IL-11 Reverse: 5′-GGTAGGACAGTAGGTCCGCTC-3′;
β-actin Forward: 5′-CTGTCCCTGTATGCCTCTG-3′;
β-actin Reverse: 5′-ATGTCACGCACGATTTCC-3′.
Western blot
Adipose tissue from the rats was harvested and lysed with lysis buffer (20 mM Tris-HCL, 137 mM NaCl 1% Nonidet P-40, and 10% glycerol) supplemented with protease inhibitors and phosphatase inhibitors, and homogenized with an Ultra-Turrax homogenizer (IKA-Werke GmbH & Co., Germany). Cultured cells were lysed using lysis buffer. The final supernatants from cells and tissues were obtained by centrifugation at 1000×g for 10 min at 4°C. Protein concentrations were determined with the Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA).
Proteins were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred onto a polyvinylidene difluoride (PVDF) membrane, and blocked in 5% dried skimmed milk powder. After incubation overnight with the primary antibodies to IL-6, IL-11, p-mTOR, mTOR, p-stat3, stat3, p-AKT, AKT, CDK4, cyclin D1, and GAPDH (Cell Signaling Technology, Danvers, MA, USA), the membranes were incubated with HRP-conjugated mouse anti-rabbit secondary antibody (Santa Cruz Biotechnology Inc., Dallas, TX, USA) for 1.5 hr at room temperature. The bands were visualized using the enhanced chemiluminescence (ECL) detection (Amersham Biosciences Corp., Little Chalfont, UK). The ratio of the relevant protein was determined against GAPDH and was analyzed by ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data were analyzed using SPSS statistical software (SPSS lnc., Chicago, IL, USA). The data were presented as the mean ± standard deviation (SD). Student’s t-test and one-way analysis of variance (ANOVA) were used for statistical analysis. Differences were considered to be statistically significant at P<0.05.
Results
Establishment of the rat model of polycystic ovary syndrome (PCOS)
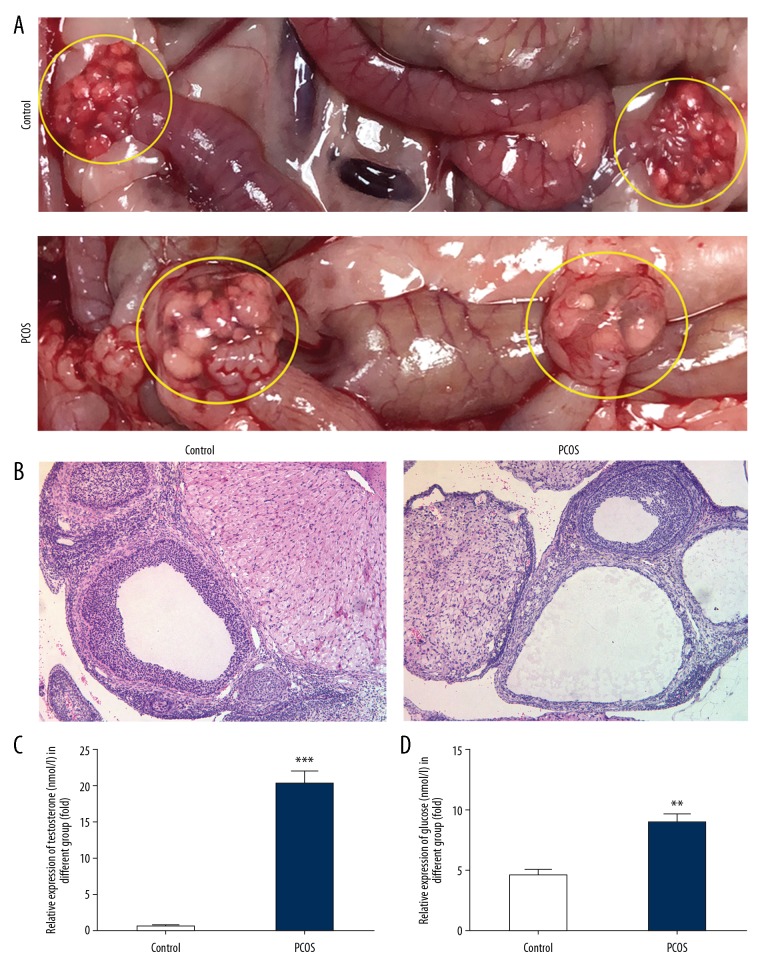
To investigate the role of IL-6 and IL-11 in PCOS, dehydroepiandrosterone (DHEA) was used to establish the rat model of PCOS. As shown in Figure 1A and 1B, 20 days following subcutaneous injection of DHEA, the ovaries of the rats in the PCOS group increased in volume and contained cystic dilated follicles. The ovaries in the rat control group had normal morphology with normal corpora lutea and antral follicles. Also, in the PCOS group, the levels of serum testosterone and glucose were significantly increased when compared with the control group (Figure 1C, 1D).
Figure 1.
(A–D) Establishment of the rat model of polycystic ovary syndrome (PCOS). The rats in the PCOS model group showed macroscopic appearances of PCOS in the ovaries (A), and histologically on hematoxylin and eosin (H&E) staining (B). Raised serum testosterone and glucose levels, which are also a feature of PCOS, were also detected. Data are presented as the mean ± standard deviation (SD). ** P<0.01, *** P<0.001 versus the control group.
The levels of IL-6 and IL-11 were increased in the rat model of PCOS
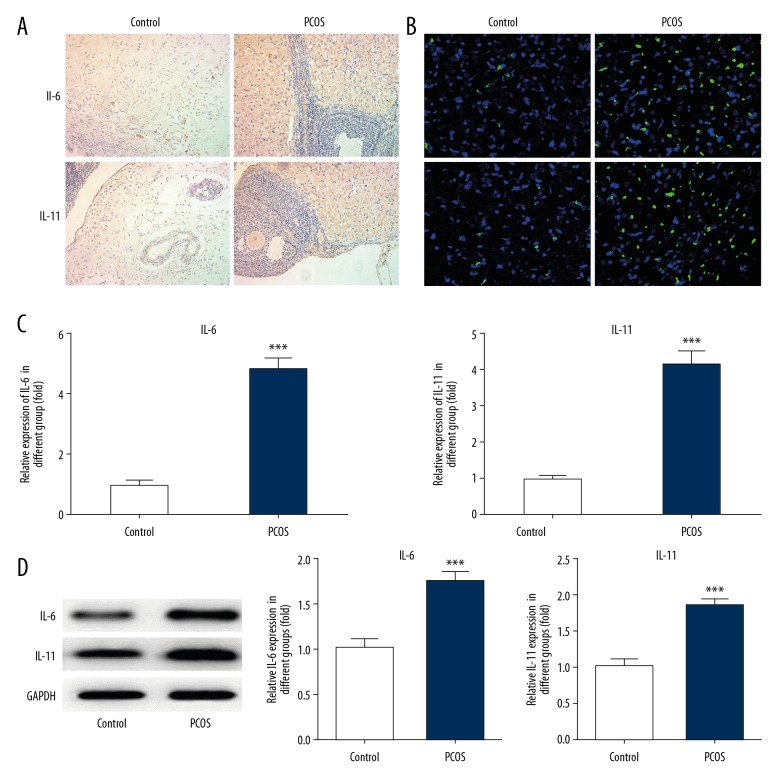
To determine whether the levels of IL-6 and IL-11were altered in the rat model of PCOS, quantitative real-time polymerase chain reaction (qRT-PCR), Western blot, immunohistochemistry, and immunofluorescence were performed. As shown in Figure 2, the expression of IL-6 and IL-11 were significantly increased in the PCOS group compared with the control group. Also, the mRNA level of IL-6 in the PCOS group was significantly increased by nearly 5-fold compared with the control group. IL-11 mRNA was also significantly increased in the PCOS group, and the protein expression levels of IL-6 and IL-11 were significantly increased in the PCOS group.
Figure 2.
Expression of IL-6 and IL-11 in the adipose tissue of rats in the model of polycystic ovary syndrome (PCOS). To determine the change in expression of IL-6 and IL-11 in PCOS, immunohistochemistry (A), immunofluorescence (B), quantitative real-time polymerase chain reaction (qRT-PCR) (C), and Western blot (D) were used to detect the expression of IL-6 and IL-11 in rat adipose tissue. Data are presented as the mean ± standard deviation (SD). *** P<0.001 versus the control group.
IL-6 and IL-11 promoted cell growth of adipocytes
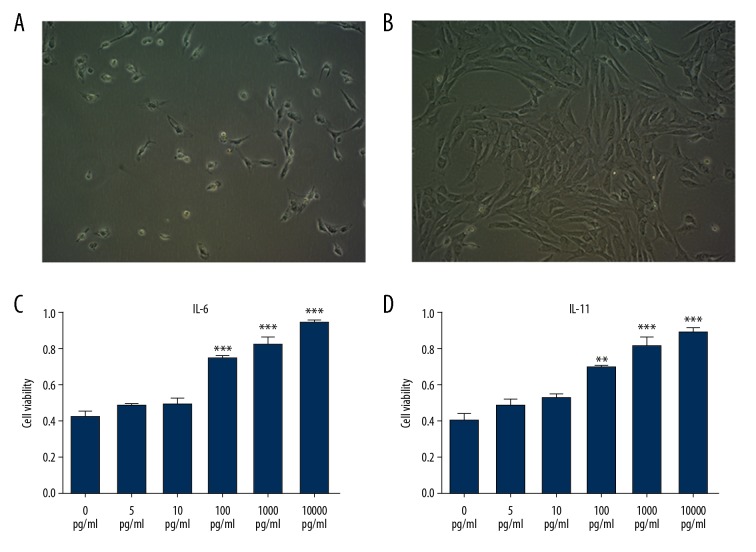
To further explore the underlying mechanism of IL-6 and IL-11 in PCOS, adipocytes were isolated and cultured from the rat adipose tissues. Rat adipocytes grew well (Figure 3A, 3B). When rat adipocytes were treated with increasing doses of IL-6 and IL-11, growth in culture was not affected when treated with IL-6 at 5 pg/ml and 10 pg/ml, while with the increased concentration of IL-6, the proliferation of adipocytes was significantly increased when adipocytes were treated with IL-6 at 100 pg/ml, 1000 pg/ml, and 10000 pg/ml (Figure 3C). The same effects were seen when the cells were treated with increasing concentrations of IL-11 (Figure 3D). The optimum dose of IL-6 and IL-11 that affected cell proliferation was 100 pg/ml, which was used for subsequent studies.
Figure 3.
Culture and treatment of adipocytes from rats in the model of polycystic ovary syndrome (PCOS). (A) Cells obtained from subcutaneous fat tissue of the rats. (B) The cells growth pattern as peaks and troughs. (C, D) Rat adipocytes were treated with IL-6 and IL-11.
STAT3/AKT signaling was activated in PCOS
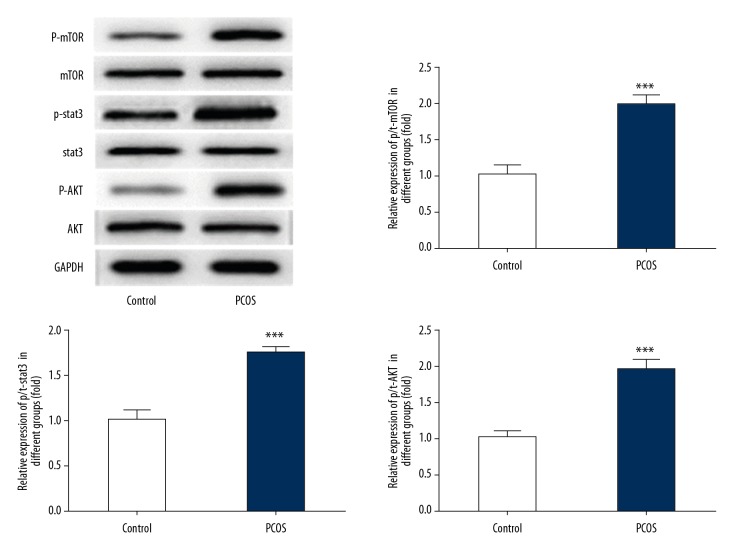
Figure 4 shows that the protein expressions of p-STAT3, p-protein kinase B (p-AKT), and p-mammalian target of rapamycin (p-mTOR) were significantly increased in the PCOS group, indicating that AKT/STAT3 signaling was activated in the rat model of PCOS, and AKT/STAT3 signaling might have a role in the progression of PCOS.
Figure 4.
STAT3/AKT signaling was activated in the rat model of polycystic ovary syndrome (PCOS). The relative protein expression of p-STAT3, p-AKT, p-mTOR of adipose tissue in the control group and the PCOS group were detected by Western blot, and the bands were quantified. Data are presented as the mean ± standard deviation (SD). *** P<0.001 versus the control group.
IL-6 and IL-11 modulated AKT/STAT3 signaling to regulate adipocyte proliferation
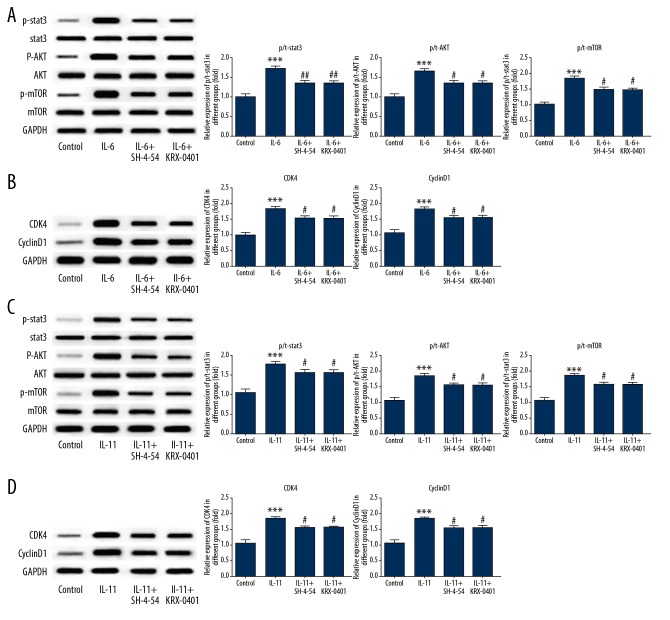
We investigated whether cell proliferation affected by IL-6 and IL-11 was dependent on AKT/STAT3 signaling. As shown in Figure 5A, following treatment of adipocytes with IL-6, the protein expression of p-STAT3, p-AKT, and p-mTOR were significantly increased, which was consistent with protein expression in the PCOS group. Also, the expression of the cell cycle-associated protein, cyclin D1, and cyclin-dependent kinase 4 (CDK4), were significantly increased (Figure 5B). Therefore, in the rat model of PCOS, IL-6 promoted the proliferation of adipocytes. To further investigate the role of IL-6 in adipocyte proliferation through AKT/STAT3 signaling, the STAT inhibitor, SH-4-54 (Selleckchem, Houston, TX, USA) and the AKT inhibitor, KRX-0401 (Selleckchem, Houston, TX, USA) were added to the adipocytes in culture, following treatment with IL-6. Following treatment with SH-4-54, the protein expression of p-STAT3 was reduced. Following treatment with KRX-0401, the protein expression of p-AKT and p-mTOR were reduced, and the protein expression of cyclin D1 and CDK4 were significantly reduced. These findings supported that the proliferative activity of IL-6 in rat adipocytes in the PCOS model group was dependent on STAT3/AKT signaling. When adipocytes were treated with IL-11, results were consistent with those found for IL-6 (Figure 5C, 5D). These results supported that the proliferative activity of IL-6 and IL-11 in rat adipocytes was associated with STAT3/AKT signaling.
Figure 5.
IL-6 and IL-11 modulated STAT3/AKT signaling to regulate adipocyte proliferation in the rat model of polycystic ovary syndrome (PCOS). After the stimulation of adipocytes by IL-6, the relative protein expression of p-STAT3, p-AKT, p-mTOR was detected by Western blot (A). The expression of cell cycle-related proteins, cyclin D1, and CDK4, were detected with Western blot (B). When the STAT3/AKT pathway was blocked, the relative protein expression of pathway and cell cycle-related proteins were examined by Western blot (A, B). Data are presented as the mean ± standard deviation (SD). *** P<0.001 versus the control group. # P<0.05, ## P<0.01 versus the IL-6 group. After the stimulation of adipocytes by IL-11, the relative protein expression of p-STAT3, p-AKT, and p-mTOR were detected with Western blot (C). The expression of cell cycle-related protein, cyclin D1, and CDK4, were detected by Western blot (D). When the STAT3/AKT pathway was blocked, the relative expression of pathway proteins and cell cycle-related proteins was determined by Western blot (C, D). Data are presented as the mean ± standard deviation (SD). *** P<0.001 versus the control group. # P<0.05, ## P<0.01 versus the IL-11 group.
Discussion
Polycystic ovary syndrome (PCOS) is associated with several physiological abnormalities that include hyperglycemia and increased secretion of testosterone. The findings from the present study showed that the rat model of PCOS, created by subcutaneous injection of dehydroepiandrosterone (DHEA), resulted in hyperglycemia and increased serum levels of testosterone. In addition to its physiological properties for storing and releasing energy, adipose tissue is involved in metabolic regulation, immune function, and has neuroendocrine properties. The presence of excessive adipose tissue directly contributes to the pathogenesis of obesity-related disorders [11]. Women with PCOS tend to have abdominal obesity, which is an independent factor for inducing endocrine abnormalities, which may result in insulin resistance [12,13]. Because women with PCOS are exposed to excess androgens, adipocytes are also prone to hypertrophy, resulting in the release of free fatty acids, apoptosis, fibrosis, and inflammation [14]. Functional abnormalities in adipose tissues are also closely associated with PCOS [11]. In the present study, adipocytes were isolated from adipose tissues in the rat model of PCOS.
In inflammation associated with PCOS, IL-6 acts as an important inflammatory cytokine and is also involved in the regulation of bone marrow function, and immune regulation. IL-6 does not directly promote testosterone secretion of ovarian follicular cells, but can upregulate the expression of testosterone receptors in ovarian tissue, indirectly increases androgen activity, and is involved in the pathogenesis of PCOS [15], which supports the findings of the present study. IL-11 is an immunomodulator that may downregulate the expression of some pro-inflammatory cytokines and has been reported to be highly expressed in the follicular fluid of atretic ovarian follicles, indicating its involvement in PCOS [16]. In this study, in a rat model of PCOS, IL-11 was also overexpressed in rat adipose tissue.
IL-6, IL-11, IL-27, oncostatin M, and leukemia inhibitory factor (LIF), are members of the IL-6 cytokine family, which is closely linked with several biological responses, including the immune response, inflammation, and oncogenesis by modulation of cell growth and differentiation [17]. The IL-6 family also includes the use of the gp130 receptor β-subunit, which binds to IL-6 or IL-11 and induces the activation of the latent transcription factor, STAT3 [18]. STAT3 has an important role in many cellular processes that include cell growth and apoptosis [18]. Previous studies have shown that IL-6 secreted from adipose stromal cells enhanced the migration of ovarian cancer cells and contributed to ovarian cancer progression by activating the STAT3 pathway [19]. Also, the expression of IL-6 is regulated in an AKT-dependent way in airway epithelial cells [20]. IL-6, regulated by AKT activity, has previously been shown to activate PI3K/AKT and the anti-apoptotic signaling cascade [21,22]. IL-11, as a member of IL-6 family, is present in solid tumors and has a role in cancer progression by activating the JAK/STAT3 pathway [23,24]. In the present study, the cell proliferation of adipocytes from rat adipose tissues was significantly increased when stimulated by IL-6 and IL-11, and the STAT3 and AKT pathways were activated. Inhibition of these pathways reduced the expression of the cell cycle proteins, cyclin D1, and CDK4. Therefore, the findings from this study showed that IL-6 and IL-11 could stimulate cell proliferation by modulating the STAT3/AKT pathway.
This study had several limitations. The study included a rat model of PCOS and focused on the role of IL-6 and IL-11. Adipocytes stimulated by IL-6 or IL-11 showed increased adipocyte proliferation and activation of STAT3/AKT signaling, but adipocytes were not stimulated by combined treatment with IL-6 and IL-11. Therefore, it remains unclear whether or not combined stimulation with IL-6 and IL-11 exhibit any additive or synergistic effect on adipocyte proliferation and STAT3/AKT signaling. Also, changes in ovarian morphology and testosterone levels associated with PCOS affect the normal function of the ovaries [25]. Therefore, the identification of small molecules and compounds that have promising effects in the treatment of PCOS have been developed, and include eicosapentaenoic acid (EPA) [26] and melatonin [25]. In the present study, in a rat model of PCOS, IL-6, and IL-11 were associated with the changes in the rat ovary in the model group. However, whether approaches that suppress the expression of IL-6 and IL-11 may have potential as therapeutic approaches for women with PCOS require further studies.
Conclusions
In a rat model of polycystic ovary syndrome (PCOS), increased expression of IL-6 and IL-11 was associated with the AKT/STAT3 pathway. Increased levels of IL-6 and IL-11 stimulated adipocytes from adipose tissue of the rat model, which promoted cell proliferation by activating AKT/STAT3 signaling.
Footnotes
Source of support: This study was supported by the fund project from Xuzhou Science and Technology Bureau (No. KC14SH031)
Conflicts of interest
None.
References
- 1.Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- 2.Amer SAK. Polycystic ovarian syndrome: Diagnosis and management of related infertility. Obstet Gynaecol Reprod Med. 2009;19(10):263–70. [Google Scholar]
- 3.Al-Musawy SH, Al-Samairy IE, Flaifil MS. Levels of cytokines profile in polycystic ovary syndrome. Med J Babylon. 2018;15(2):124–28. [Google Scholar]
- 4.Orio F, Jr, Palomba S, Cascella T, et al. Adiponectin levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(6):2619–23. doi: 10.1210/jc.2002-022033. [DOI] [PubMed] [Google Scholar]
- 5.Vural P, Degirmencioglu S, Saral NY, Akgul C. Tumor necrosis factor alpha (-308), interleukin-6 (-174) and interleukin-10 (-1082) gene polymorphisms in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2010;150(1):61–65. doi: 10.1016/j.ejogrb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Lee JY, Tae JC, Kim CH, et al. Expression of the genes for peroxisome proliferator-activated receptor-gamma, cyclooxygenase-2, and proinflammatory cytokines in granulosa cells from women with polycystic ovary syndrome. Clin Exp Reprod Med. 2017;44(3):146–51. doi: 10.5653/cerm.2017.44.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez F, Nair KS, Daniels JK, et al. Hyperandrogenism sensitizes leukocytes to hyperglycemia to promote oxidative stress in lean reproductive-age women. J Clin Endocrinol Metab. 2012;97(8):2836–43. doi: 10.1210/jc.2012-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu DH, Zhu Z, Wakefield MR, et al. The role of IL-11 in immunity and cancer. Cancer Lett. 2016;373(2):156–63. doi: 10.1016/j.canlet.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Campbell CL, Guardiani R, Ollari C, et al. Interleukin-11 receptor expression in primary ovarian carcinomas. Gynecol Oncol. 2001;80(2):121–27. doi: 10.1006/gyno.2000.6064. [DOI] [PubMed] [Google Scholar]
- 10.Putoczki TL, Thiem S, Loving A, et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell. 2013;24(2):257–71. doi: 10.1016/j.ccr.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Delitala AP, Capobianco G, Delitala G, et al. Polycystic ovary syndrome, adipose tissue and metabolic syndrome. Arch Gynecol Obstet. 2017;296(3):405–19. doi: 10.1007/s00404-017-4429-2. [DOI] [PubMed] [Google Scholar]
- 12.Agacayak E, Tunc SY, Sak S, et al. Levels of neopterin and other inflammatory markers in obese and non-obese patients with polycystic ovary syndrome. Med Sci Monit. 2015;21:2446–55. doi: 10.12659/MSM.894368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmina E, Bucchieri S, Esposito A, et al. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab. 2007;92(7):2500–5. doi: 10.1210/jc.2006-2725. [DOI] [PubMed] [Google Scholar]
- 14.Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction. 2015;149(5):R219–27. doi: 10.1530/REP-14-0435. [DOI] [PubMed] [Google Scholar]
- 15.Xiong YL, Liang XY, Yang X, et al. Low-grade chronic inflammation in the peripheral blood and ovaries of women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;159(1):148–50. doi: 10.1016/j.ejogrb.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Ledee-Bataille N, Lapree-Delage G, Taupin JL, et al. Follicular fluid concentration of leukaemia inhibitory factor is decreased among women with polycystic ovarian syndrome during assisted reproduction cycles. Hum Reprod. 2001;16(10):2073–78. doi: 10.1093/humrep/16.10.2073. [DOI] [PubMed] [Google Scholar]
- 17.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121(9):3375–83. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putoczki TL, Thiem S, Loving A, et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell. 2013;24(2):257–71. doi: 10.1016/j.ccr.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Kim B, Kim HS, Kim S, et al. Adipose stromal cells from visceral and subcutaneous fat facilitate migration of ovarian cancer cells via IL-6/JAK2/STAT3 pathway. Cancer Res Treat. 2017;49(2):338–49. doi: 10.4143/crt.2016.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang L, Chen Q, Meng Z, et al. Suppression of Sirtuin-1 increases IL-6 expression by activation of the Akt pathway during allergic asthma. Cell Physiol Biochem. 2017;43(5):1950–60. doi: 10.1159/000484119. [DOI] [PubMed] [Google Scholar]
- 21.Bharadwaj U, Marin-Muller C, Li M, et al. Mesothelin confers pancreatic cancer cell resistance to TNF-alpha-induced apoptosis through Akt/PI3K/NF-kappaB activation and IL-6/Mcl-1 overexpression. Mol Cancer. 2011;10:106. doi: 10.1186/1476-4598-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen HY, Lin LT, Wang ML, et al. Musashi-1 regulates AKT-derived IL-6 autocrinal/paracrinal malignancy and chemoresistance in glioblastoma. Oncotarget. 2016;7(27):42485–501. doi: 10.18632/oncotarget.9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Putoczki TL, Ernst M. IL-11 signaling as a therapeutic target for cancer. Immunotherapy. 2015;7(4):441–53. doi: 10.2217/imt.15.17. [DOI] [PubMed] [Google Scholar]
- 24.Zheng H, Yang Y, Han J, et al. TMED3 promotes hepatocellular carcinoma progression via IL-11/STAT3 signaling. Sci Rep. 2016;6:37070. doi: 10.1038/srep37070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pai SA, Majumdar AS. Protective effects of melatonin against metabolic and reproductive disturbances in polycystic ovary syndrome in rats. J Pharm Pharmacol. 2014;66(12):1710–21. doi: 10.1111/jphp.12297. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, He J, Yang J. Eicosapentaenoic acid improves polycystic ovary syndrome in rats via sterol regulatory element-binding protein 1 (SREBP-1)/toll-like receptor 4 (TLR4) pathway. Med Sci Monit. 2018;24:2091–97. doi: 10.12659/MSM.909098. [DOI] [PMC free article] [PubMed] [Google Scholar]