This cohort study examines whether there is a dose-response association between lifetime indoor tanning and risk of cutaneous squamous cell carcinoma.
Key Points
Question
Is there a dose-response association between lifetime indoor tanning and risk of cutaneous squamous cell carcinoma?
Findings
In this prospective cohort study of 159 419 women in Norway, a significant dose-response association was found between indoor tanning and risk of squamous cell carcinoma. The association between cumulative exposure to indoor tanning and risk of squamous cell carcinoma was the same regardless of duration of use and age at initiation.
Meaning
The findings provide supporting evidence that indoor tanning is associated with increased risk of squamous cell carcinoma, with a greater risk among women with higher cumulative number of indoor tanning sessions.
Abstract
Importance
No study, to our knowledge, has prospectively investigated a dose-response association between lifetime indoor tanning and risk of cutaneous squamous cell carcinoma (SCC).
Objective
To investigate the dose-response association between lifetime indoor tanning and SCC risk, the association between duration of use and age at initiation with SCC risk, and the association between age at initiation and age at diagnosis.
Design, Setting, and Participants
This cohort study included data from women born from 1927 to 1963 from the Norwegian Women and Cancer study, established in 1991 with follow-up through December 31, 2015. Baseline questionnaires were issued to participants from 1991 to 2007, with follow-up questionnaires given every 5 to 7 years. Data analysis was performed from January 2, 2018, to March 2, 2019.
Exposures
Participants reported pigmentation factors. Sunburns, sunbathing vacations, and indoor tanning were reported for childhood, adolescence, and adulthood.
Main Outcomes and Measures
Information on all cancer diagnoses and dates of emigration or death were obtained through linkage to the Cancer Registry of Norway, using the unique personal identification number of Norwegian citizens.
Results
A total of 159 419 women (mean [SD] age at inclusion, 49.9 [8.3] years) were included in the study. During follow-up (mean [SD], 16.5 [6.4] years), 597 women were diagnosed with SCC. Risk of SCC increased with increasing cumulative number of indoor tanning sessions. The adjusted hazard ratio (HR) for highest use vs never use was 1.83 (95% CI, 1.38-2.42; P < .001 for trend). A significantly higher risk of SCC was found among women with 10 years or less of use (HR, 1.41; 95% CI, 1.08-1.85) and more than 10 years of use (HR, 1.43; 95% CI, 1.16-1.76) and among women with age at initiation of 30 years or older (HR, 1.36; 95% CI, 1.11-1.67) and younger than 30 years (HR, 1.51; 95% CI, 1.18-1.92) vs never users. No significant association was found between age at initiation and age at diagnosis (estimated regression coefficient, −0.09 [95% CI, −1.11 to 0.94] for age at initiation of ≥30 years and −0.02 [95% CI, −1.27 to 1.22] for <30 years vs never use).
Conclusion and Relevance
The findings provide supporting evidence that there is a dose-response association between indoor tanning and SCC risk among women. The association between cumulative exposure to indoor tanning and SCC risk was the same regardless of duration of use and age at initiation. These results support development of policies that regulate indoor tanning.
Introduction
Cutaneous squamous cell carcinoma (SCC) is one of the most common types of cancer worldwide.1 Norway, one of the few countries with national high-quality SCC incidence data, has had a 9-fold increase in age-standardized incidence among women and a 6-fold increase among men since 1963.2
Although development of SCC has been associated with cumulative solar UV radiation (UVR) exposure,1,3,4 few studies have investigated its association with cumulative exposure to UVR from indoor tanning. Four meta-analyses5,6,7,8 (2006-2012), based on 3 to 6 studies, reported a significantly increased risk of SCC in ever vs never users of indoor tanning devices (summary relative risk estimates, 1.67-2.25). Two of the 6 studies9,10 investigated age at first indoor tanning exposure, and only 1 study10 examined a dose-response association. In a report11 from the Nurses’ Health Study, a significant dose-response association was found for indoor tanning in adolescence and adulthood, but information about indoor tanning was obtained several years after entry into the cohort, increasing the risk of recall bias. In the Norwegian-Swedish Women's Lifestyle and Health cohort study,12 cumulative use of indoor tanning devices from 10 to 49 years of age was significantly associated with increased SCC risk. That cohort study included the first one-third of women enrolled in the Norwegian Women and Cancer (NOWAC) study, the cohort assessed in this article.
A previous study13 examined the association between indoor tanning and cutaneous melanoma in the NOWAC study. This cohort similarly provides a unique opportunity to examine prospectively the association between indoor tanning and long-term risk of SCC. We aimed to investigate the dose-response association between cumulative number of indoor tanning sessions and SCC, the association of duration of use and age at indoor tanning initiation with SCC risk, and the association between age at initiation and age at diagnosis.
Methods
The NOWAC Cohort
This cohort study included data from the NOWAC study, which was established in 1991, with follow-up through December 31, 2015, and has been described in detail elsewhere.13,14 For the NOWAC study, women were selected randomly from the Norwegian Population Register. Baseline questionnaires were issued in 1991 to 2007, and 171 725 of approximately 320 000 women (53.7%) answered. First and second follow-up questionnaires were sent after 5 to 7 years (response rates, 80% for the first questionnaire and 79% for the second questionnaire). Data analysis was performed from January 2, 2018, to March 2, 2019. All women filled in an informed consent for participation in the study. The research group received only anonymous data, and the data were handled according to the permission given by the national Data Inspection Board. The national Data Inspection Board and the Medical Ethical Committees of North Norway approved the NOWAC study.
Study Sample
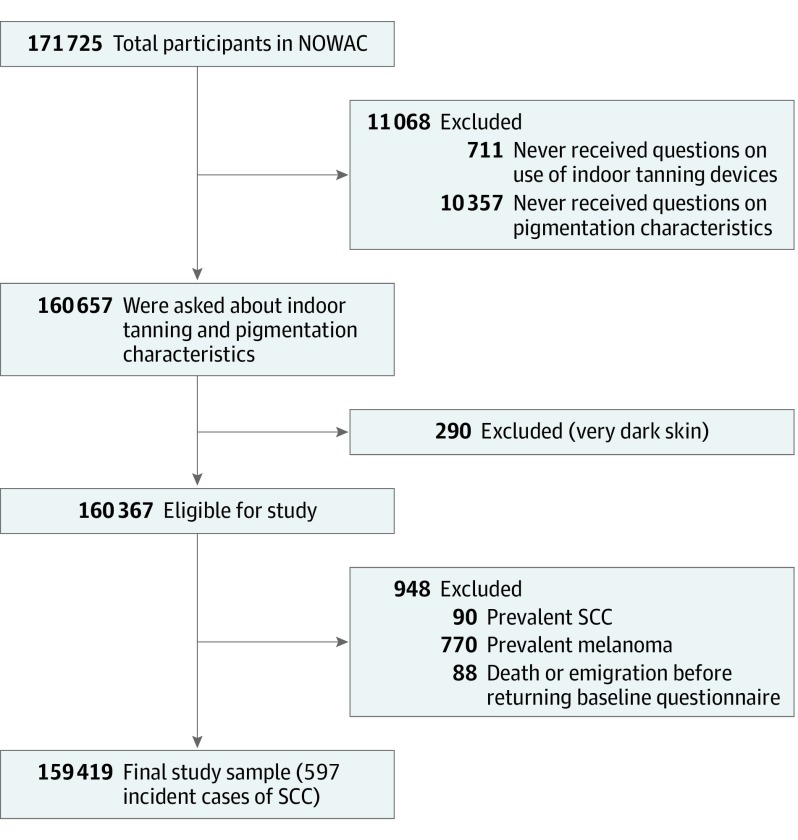
Of the 171 725 women who completed and returned questionnaires, 160 657 were asked about indoor tanning use and pigmentation characteristics. We excluded women with very dark skin (n = 290), prevalent SCC (n = 90), or cutaneous melanoma (n = 770) (Figure). The Cancer Registry of Norway (CRN) does not routinely record information on basal cell carcinoma. We further excluded 88 women who emigrated or died before the date of questionnaire return, resulting in 159 419 women born from 1927 to 1963.
Figure. Flowchart of the Study Sample From the Norwegian Women and Cancer Study (NOWAC).
SCC indicates squamous cell carcinoma.
Follow-up and End Points
The cohort was linked to the CRN using the unique personal identification number of Norwegian citizens.15 Mandatory reporting of malignant diseases from independent sources (hospitals, laboratories, general practitioners, and the Cause of Death Registry) to the CRN ensures virtual completeness and high-quality data, with 99.7% of the nonmelanoma skin cancers (excluding basal cell carcinoma) being morphologically verified.16 Cutaneous SCC cases were identified by the International Classification of Diseases, Seventh Revision (ICD-7), code 191 and the morphology codes 80703, 80713, 80763, 80953, 80513, 80723, and 80743. We excluded cases with the ICD-7 code 1914 (perineum, perianal) because they are unlikely to be related to UVR exposure. Primary anatomical location of the tumor was categorized as head and/or face (code 1910: outer ear; code 1911: eyelids [including eyelets]; code 1912: face and the rest of the head [including scalp, orbital region, chin, and cheek]), neck and/or trunk (code 1913), upper limbs (code 1915), lower limbs (code 1916), multiple localizations (code 1918, based on clinical notification of >1 tumor, within 4 months), and unspecified site (code 1919).
Indoor Tanning
Data on use of an indoor tanning device (never; rarely; 1, 2, or 3-4 times per month; >1 time per week) were obtained at baseline for childhood (<10 years of age), adolescence (10-19 years of age), and adulthood and were updated in the follow-up questionnaires. We created 5 variables for indoor tanning exposure: cumulative number of sessions, ever or never use, current use (no or yes), duration of use (never, 1-10, or >10 years), and age at initiation (never, ≥30 years, or <30 years).13 Cumulative number of sessions was calculated by converting reported frequencies for all age periods from 10 years of age to a yearly amount (never, 0 sessions per year; rarely, 1 session per year; 1 time per month, 12 sessions per year; 2 times per month, 24 sessions per year; 3-4 times per month, 42 sessions per year; >1 time per week, 60 sessions per year) and multiplying this with the number of years for the given period.13 The sum was categorized to capture the heavy tail of the distribution (never use, 0 sessions; lowest use, 1-38 sessions; medium use, 39-240 sessions; and highest use, >240 sessions; 38 was the highest tertile and 240 the highest sextile).
Covariates
Ambient UVR exposure was categorized based on the mean ambient UVR hours of the region of residence (latitudes, 70°-58°) as low (northern Norway), medium-low (central Norway), medium (southwestern Norway), and highest (southeastern Norway).13,17 Participants reported educational level (≤10, 11-13, or ≥14 years), smoking (never, former, or current smoker), hair color (black or dark brown, brown, blond or yellow, or red), freckling when sunbathing (no or yes), and untanned skin color (color scale from 1 [very fair] to 10 [very dark]; categorized as light [grades 1-3], medium [grades 4-5], dark [grades 6-8], and very dark skin [grades 9-10]). Skin reactions to acute and chronic sun exposure were recorded for a subsample of the cohort.
The annual number of sunburns that resulted in pain or blisters and subsequent peeling (never, 1, 2-3, 4-5, or ≥6) and the mean annual number of weeks spent on sunbathing vacations (never, 1, 2-3, 4-6, or ≥7 weeks per year) in low latitudes or within Norway or northern countries were reported for the same age periods as for indoor tanning and updated by follow-up questionnaires. The cumulative number of sunburns was calculated similarly to indoor tanning and categorized as none, lowest tertile (1-30 sunburns), middle tertile (31-54 sunburns), or highest tertile (>54 sunburns). The cumulative weeks spent on sunbathing vacations was calculated similarly and categorized as never, lowest tertile (1-74 weeks), middle tertile (75-149 weeks), or highest tertile (>149 weeks).13 We calculated the number of indoor and outdoor tanning sessions by dividing the cumulative number of indoor tanning sessions and sunbathing vacations into quartiles, which were then summed (score, 0-8) and categorized into 5 groups (1, lowest; 5, highest).
Reproducibility was good for freckling when sunbathing (κ = 0.77), skin color (intraclass correlation, 0.59), indoor tanning (weighted κ [κw] = 0.70), sunbathing vacations in low latitudes (κw = 0.71), fair for sunburns (κw = 0.49), and sunbathing vacations in Norway or northern countries (κw = 0.47) in the first follow-up questionnaire.18 Age, educational level, and skin color did not affect reproducibility. The NOWAC study is representative of Norwegian women aged 45 to 74 years with regard to total cancer incidence,14 with no major selection bias19 and with almost no selection of participants from the baseline questionnaire to the first follow-up questionnaire.14
Statistical Analysis
The association between use of indoor tanning devices and SCC was estimated by hazard ratios (HRs) and 95% CIs using Cox proportional hazards regression with age as the time scale. We stratified by birth cohort (1927-1944, 1945-1952, and 1953-1963) because irradiance levels from indoor tanning devices have changed over time.12 Person-years were calculated from date of entry to date of first primary SCC diagnosis, melanoma diagnosis (ie, censoring at melanoma diagnosis), emigration, death, or end of follow-up (December 31, 2015), whichever occurred first. We conducted sensitivity analysis excluding all prevalent cancers and censoring for all other cancers to ensure that a history of cancer did not affect the estimates.
We modeled indoor tanning variables (except age at initiation) and cumulative number of sunburns and sunbathing vacations as time-varying variables. All exposure and covariate information was collected before disease diagnosis. The proportionality assumption was checked using Schoenfeld residuals. A likelihood ratio test was used to test for interaction between ever use of indoor tanning devices or cumulative number of indoor tanning sessions (collapsing medium and highest use) and birth cohort, residential ambient UVR exposure, hair color, untanned skin color, and sunbathing vacations. We tested for trend by modeling the variables as continuous.
On the basis of a directed acyclic graph20 (eFigure in the Supplement), we adjusted for hair color, residential ambient UVR exposure, sunburns, and sunbathing vacations in the multivariable models. We conducted sensitivity analysis based on a directed acyclic graph in which the arrow between sunbathing and indoor tanning was reversed (eFigure in the Supplement) and adjusted for hair color and residential ambient UVR exposure only. Additional adjustment for educational level, smoking, freckling, untanned skin color, and skin reactions to acute and chronic sun exposure did not change the results.
We investigated age at indoor tanning initiation and age at diagnosis using linear regression and present regression coefficient estimates (β̂ ) and 95% CIs. The multivariable model included birth year, hair color, residential ambient UVR exposure, cumulative number of sunburns, and sunbathing vacations.
We had missing information in 13% of participants for the cumulative number of indoor tanning sessions and up to 20% missing in the covariates of the multivariable model. We used multiple imputation with chained equations21 to impute 40 data sets.
All tests were 2-sided and deemed to be significant at P < .05. Statistical analyses were conducted using R software, version 3.5.2 (R Foundation for Statistical Computing).
Results
A total of 159 419 women (mean [SD] age at inclusion, 49.9 [8.3] years) were included in the study. The mean (SD) follow-up was 16.5 (6.4) years (range, <1-25 years), during which 597 women were diagnosed with incident SCC. The first primary SCC was the first cancer diagnosis for 481 women, second for 98 women, third for 12 women, fourth for 3 women, and fifth for 3 women. The mean (SD) age at SCC diagnosis was 66.4 (9.1) years. Mean age at SCC diagnosis was similar for women with SCC as their first (66 years), second (67 years), or third to fifth (67 years) cancer diagnosis. Head (n = 248) was the most common site (outer ear [n = 13], eyelids [n = 9], face and/or rest of the head [n = 226]), followed by neck and/or trunk (n = 141), lower limbs (n = 82), upper limbs (n = 66), multiple localizations (n = 50), and unspecified site (n = 10).
In total, 95 552 women (69.0%) reported ever use of indoor tanning. Indoor tanning was more common in the younger birth cohorts, women living in northern and central Norway, current smokers, women with lighter hair color, and women with darker skin color (Table 1). Host characteristics were similar among women who answered the baseline, first follow-up, and second follow-up questionnaires (eTable 1 in the Supplement) except for birth cohort, because of the sampling procedure (women recruited earlier had more time to receive follow-up questionnaires).
Table 1. Host Characteristics Stratified by Cumulative Number of Indoor Tanning Sessions, Norwegian Women and Cancer Study, 1991-2015a.
| Characteristic | Indoor Tanning (n = 138 474) | |||
|---|---|---|---|---|
| Never Use | Lowest Use | Medium Use | Highest Use | |
| Participants, No. (%) | 42 922 (31.0) | 63 979 (46.2) | 15 666 (11.3) | 15 907 (11.5) |
| Person-years of follow-up | ||||
| Total | 694 707 | 1 036 626 | 285 634 | 262 108 |
| Mean | 16.2 | 16.2 | 18.2 | 16.5 |
| Mean (SD) age, y | ||||
| At inclusion | 51.3 (8.7) | 49.1 (7.8) | 47.8 (7.9) | 49.0 (7.6) |
| At diagnosis | 68.6 (9.7) | 64.0 (8.6) | 63.3 (7.1) | 64.9 (8.1) |
| Incident SCC cases | 152 (30.8) | 207 (42.0) | 65 (13.2) | 69 (14.0) |
| Birth cohort | ||||
| 1927-1944 | 43.7 | 36.3 | 10.6 | 9.4 |
| 1945-1952 | 29.7 | 46.5 | 11.8 | 12.0 |
| 1953-1963 | 25.0 | 51.9 | 11.1 | 12.0 |
| Residential ambient UVR exposure | ||||
| Low (northern Norway) | 25.8 | 47.6 | 13.4 | 13.1 |
| Medium-low (central Norway) | 25.5 | 49.5 | 13.2 | 11.8 |
| Medium (southwestern Norway) | 36.2 | 47.0 | 8.5 | 8.3 |
| Highest (southeastern Norway) | 32.5 | 44.5 | 11.0 | 11.9 |
| Educational level, y (n = 131 909) | ||||
| ≤10 | 33.7 | 41.8 | 11.2 | 13.2 |
| 11-13 | 26.1 | 48.7 | 12.3 | 12.8 |
| ≥14 | 31.9 | 48.5 | 10.7 | 8.9 |
| Smoking status at baseline (n = 138 093) | ||||
| Never | 39.6 | 42.5 | 9.5 | 8.4 |
| Former | 27.2 | 48.8 | 12.0 | 12.0 |
| Current | 25.4 | 47.4 | 12.6 | 14.5 |
| Hair color (n = 137 521) | ||||
| Black or dark brown | 35.2 | 44.8 | 9.7 | 10.2 |
| Brown | 29.5 | 47.3 | 11.6 | 11.6 |
| Blond or yellow, red | 30.4 | 45.9 | 11.7 | 12.0 |
| Freckling when sunbathing (n = 121 018)b | ||||
| No | 30.5 | 46.3 | 11.4 | 11.7 |
| Yes | 27.2 | 48.0 | 12.5 | 12.2 |
| Untanned skin color (n = 118 311)b | ||||
| Light | 31.8 | 46.2 | 10.6 | 11.4 |
| Medium | 28.1 | 47.7 | 12.2 | 11.9 |
| Dark | 26.7 | 47.7 | 12.8 | 12.7 |
| Skin reaction to acute sun exposure (n = 74 874)b | ||||
| Brown | 27.9 | 42.1 | 15.4 | 14.6 |
| Red | 31.0 | 43.3 | 14.7 | 11.0 |
| Red with pain | 35.7 | 43.7 | 12.5 | 8.2 |
| Red with pain and blisters | 37.5 | 41.3 | 12.0 | 9.1 |
| Skin reaction to chronic sun exposure (n = 74 930)b | ||||
| Deep brown | 25.4 | 45.2 | 15.4 | 13.9 |
| Brown | 29.4 | 43.5 | 15.0 | 12.1 |
| Light brown | 36.6 | 41.3 | 13.0 | 9.1 |
| Never brown | 60.9 | 28.8 | 6.3 | 4.1 |
| Cumulative No. of sunburns (n = 118 642) | ||||
| None | 39.6 | 41.1 | 9.2 | 10.1 |
| Lowest tertile | 28.6 | 48.5 | 10.9 | 12.0 |
| Middle tertile | 28.1 | 48.5 | 12.2 | 11.2 |
| Highest tertile | 29.6 | 45.9 | 11.9 | 12.6 |
| Cumulative No. of weeks on sunbathing vacations (n = 129 810) | ||||
| None | 67.1 | 24.9 | 4.4 | 3.6 |
| Lowest tertile | 32.2 | 46.5 | 12.2 | 9.1 |
| Middle tertile | 25.3 | 50.4 | 11.8 | 12.6 |
| Highest tertile | 24.8 | 48.5 | 11.6 | 15.2 |
Abbreviations: SCC, squamous cell carcinoma; UVR, UV radiation.
Data are presented as row percentages or participants unless otherwise indicated. Because of rounding, percentages may not sum to 100%.
Recorded for a subsample of the cohort.
We present the results from the multiple imputation analyses based on the multivariable model except P values for interaction, which were based on the complete-case analysis. The risk of SCC was significantly higher among ever users of indoor tanning devices than among never users (HR, 1.43; 95% CI, 1.17-1.74) (Table 2). Significant increased risk was also found among current users (HR, 1.27; 95% CI, 1.06-1.53). We found a significant dose-response association between cumulative number of indoor tanning sessions and SCC risk (HR, 1.83; 95% CI, 1.38-2.42; highest vs never use, P < .001 for trend). A significantly higher risk of SCC was found among women with 10 years or less of use (HR, 1.41; 95% CI, 1.08-1.85) and more than 10 years of use (HR, 1.43; 95% CI, 1.16-1.76) and among women with age at initiation of 30 years or older (HR, 1.36; 95% CI, 1.11-1.67) and younger than 30 years (HR, 1.51; 95% CI, 1.18-1.92) vs never users. The dose-response association between indoor tanning and SCC risk was evident in women with 10 years or less and more than 10 years of indoor tanning use, with no significant interaction (P = .19 for interaction) (Table 2). We found no significant interaction between cumulative indoor tanning and age at initiation (P = .82 for interaction). None of the tests for interactions between ever use of indoor tanning devices or cumulative number of sessions and birth cohort, residential ambient UVR exposure, hair color, untanned skin color, and sunbathing vacations were significant (P = .23 to P = .84 for interaction). There was a significant association between increase in SCC risk and increasing number of indoor and outdoor tanning sessions combined (HR, 2.43; 95% CI, 1.74-3.39 for highest vs lowest category; P < .001 for trend) (Table 2). In the linear regression analysis, we found no significant association between age at initiation and age at diagnosis (β̂ = −0.09; 95% CI, −1.11 to 0.94 for age ≥30 years and β̂ = −0.02; 95% CI, −1.27 to 1.22 for age <30 years vs never use) (Table 3).
Table 2. HRs (95% CIs) for Indoor Tanning and Risk of Cutaneous Squamous Cell Carcinoma, Norwegian Women and Cancer Study, 1991-2015.
| Variable | No. of Participants (N = 113 290) | No. of Cases (n = 366) | HR (95% CI) | ||
|---|---|---|---|---|---|
| Complete-Case Analyses | Multiple-Imputation Analysisc | ||||
| Age Adjusteda | Multivariable Modelb | ||||
| Ever use of indoor tanning device | |||||
| Never | 33 721 (29.8) | 101 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Ever | 79 569 (70.2) | 265 | 1.50 (1.19-1.90) | 1.45 (1.14-1.85) | 1.43 (1.17-1.74) |
| Current use of indoor tanning device | |||||
| No | 44 971 (39.7) | 146 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 68 319 (60.3) | 220 | 1.27 (1.02-1.57) | 1.22 (0.98-1.52) | 1.27 (1.06-1.53) |
| Cumulative No. of sessions | |||||
| Never use | 33 721 (29.8) | 101 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Lowest use | 53 458 (47.2) | 167 | 1.41 (1.10-1.82) | 1.38 (1.07-1.78) | 1.29 (1.04-1.60) |
| Medium use | 12 809 (11.3) | 47 | 1.57 (1.11-2.23) | 1.54 (1.08-2.19) | 1.60 (1.20-2.15) |
| Highest use | 13 302 (11.7) | 51 | 1.78 (1.27-2.51) | 1.68 (1.19-2.38) | 1.83 (1.38-2.42) |
| P value for trend | NA | NA | <.001 | .002 | <.001 |
| Duration of use | |||||
| Never | 33 721 (29.8) | 101 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| ≤10 y | 15 682 (13.8) | 49 | 1.56 (1.10-2.22) | 1.54 (1.08-2.20) | 1.41 (1.08-1.85) |
| >10 y | 63 887 (56.4) | 216 | 1.49 (1.17-1.89) | 1.43 (1.12-1.84) | 1.43 (1.16-1.76) |
| P value for trend | NA | NA | .002 | .007 | .001 |
| Age at initiation, y | |||||
| Never | 33 652 (29.7) | 101 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| ≥30 | 52 910 (46.7) | 188 | 1.50 (1.17-1.91) | 1.45 (1.13-1.86) | 1.36 (1.11-1.67) |
| <30 | 26 728 (23.6) | 77 | 1.41 (1.04-1.91) | 1.36 (1.00-1.86) | 1.51 (1.18-1.92) |
| P value for trend | NA | NA | .011 | .028 | <.001 |
| Duration of Use and Cumulative No. of Tanning Sessions | |||||
| Duration ≤10 y of use | |||||
| Never use | 33 721 (29.8) | 101 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Lowest use | 13 362 (11.8) | 37 | 1.39 (0.95-2.05) | 1.38 (0.93-2.03) | 1.27 (0.94-1.71) |
| Medium/highest use | 2320 (2.0) | 12 | 2.49 (1.36-4.56) | 2.45 (1.33-4.49) | 1.99 (1.28-3.08) |
| Duration >10 y of use | |||||
| Never use | 33 721 (29.8) | 101 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Lowest use | 40 096 (35.4) | 130 | 1.42 (1.09-1.85) | 1.38 (1.05-1.80) | 1.30 (1.03-1.63) |
| Medium/highest use | 23 791 (21.0) | 86 | 1.60 (1.20-2.15) | 1.53 (1.14-2.07) | 1.66 (1.29-2.13) |
| P value for interactiond | NA | NA | .204 | .194 | NA |
| Cumulative No. of indoor and outdoor tanning sessionse,f | |||||
| 1 (Lowest) | 14 897 (13.1) | 44 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 | 30 122 (26.6) | 95 | 1.47 (1.03-2.11) | 1.39 (0.97-1.99) | 1.42 (1.02-1.98) |
| 3 | 37 127 (32.8) | 106 | 1.43 (1.00-2.04) | 1.34 (0.94-1.92) | 1.48 (1.08-2.02) |
| 4 | 14 970 (13.2) | 57 | 2.02 (1.36-3.00) | 1.89 (1.27-2.82) | 1.79 (1.24-2.59) |
| 5 (Highest) | 16 174 (14.3) | 64 | 2.21 (1.50-3.27) | 2.03 (1.38-3.01) | 2.43 (1.74-3.39) |
| P value for trend | NA | NA | <.001 | <.001 | <.001 |
Abbreviations: HR, hazard ratio; NA, not applicable; UVR, UV radiation.
Cox proportional hazards regression with age as the time scale and stratified by birth cohort.
Additional adjustments for residential ambient UVR exposure, hair color, and cumulative number of sunburns and sunbathing vacations.
Analysis with multiple imputation of missing data conducted using chained equations and a total of 40 imputed data sets, with the same adjustments as the multivariable model (n = 159 419; 597 cases).
Testing interaction between number of sessions and duration of use.
On the basis of the sum of quartiles of both variables (resulting in a score from 0 to 8), categorized as 0 to 1, 2 to 3, 4 to 5, 6, or 7 to 8.
For this variable, the multivariable model included residential ambient UVR exposure, hair color, and cumulative number of sunburns.
Table 3. Linear Regression Analysis of the Association Between Age at Initiation of Indoor Tanning and Age at Diagnosis, Norwegian Women and Cancer Study, 1991-2015.
| Age at Initiation y | No. of Cases (n = 366) | β̂ (95% CI) | ||
|---|---|---|---|---|
| Complete-Case Analyses | Multiple-Imputation Analysisb | |||
| Crude | Adjusteda | |||
| Never | 101 | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| ≥30 | 188 | −2.78 (−4.83 to −0.73) | −0.33 (−1.47 to 0.82) | −0.09 (−1.11 to 0.94) |
| <30 | 77 | −3.00 (−5.52 to −0.49) | 0.18 (−1.24 to 1.61) | −0.02 (−1.27 to 1.22) |
Adjusted for birth year, residential ambient UV radiation exposure, hair color, cumulative number of sunburns, and sunbathing vacations.
Analysis with multiple imputation of missing data conducted using chained equations and a total of 40 imputed data sets, with the same adjustments as the adjusted complete-case analysis (n = 597 cases).
Results from the sensitivity analysis based on the directed acyclic graph in which the arrow between indoor tanning and sunbathing was reversed (eFigure in the Supplement) were similar but with slightly higher HRs (eTable 2 in the Supplement). In the sensitivity analysis restricted to women with no history of cancer (n = 148 444; 481 incident SCC cases; mean follow-up, 15.9 years), the associations between indoor tanning and SCC were similar but with slightly weaker estimates (eTable 3 in the Supplement). Moreover, the analysis of age at initiation and age at diagnosis also gave almost identical estimates (eTable 4 in the Supplement).
Discussion
In this large, prospective cohort study, we found a significant dose-response association between cumulative number of indoor tanning sessions and SCC risk. The results suggest that the association between cumulative exposure to indoor tanning and SCC risk was the same regardless of duration of use and age at initiation.
Randomized controlled clinical trials are the gold standard for investigating causal associations.22,23 In our context, randomized controlled clinical trials would be unethical, and cohort studies therefore provide the highest level of evidence. NOWAC is a well-characterized cohort of women randomly selected from the general population, with information about indoor tanning, sunburns, and sunbathing vacations from all decades of life and complete follow-up through the CRN (>99% of SCCs morphologically verified15,16) linked by the unique personal identification number. Previous cohort studies10,11,12 had data on indoor tanning exposure only for limited periods. We had updated information on indoor tanning during follow-up and used cutoffs that took the heavy tail of the distribution into account, which make our results unique.
Our study provides supportive evidence of a significant association between ever use of indoor tanning devices and SCC risk, with an HR in agreement with the latest meta-analysis.8 Moreover, we found a dose-response association after adjusting for sunburns and sunbathing vacations.
To our knowledge, no previous study has investigated the association between duration of indoor tanning and SCC risk. We found significant and similar HRs for 10 years or less and more than 10 years of use and no significant interaction between cumulative number of sessions and duration of use. Three studies9,10,24 investigated age at initiation of indoor tanning and SCC risk. One found a significant association with initiation at 35 years or younger but not for initiation later in life,9 whereas no significant association was found between age at initiation and SCC risk in the other 2 studies.10,24 In our study, initiation at 30 years or older and younger than 30 years was significantly associated with SCC risk compared with never use. However, we found no significant interaction between cumulative number of sessions and age at initiation.
The Norwegian-Swedish Women's Lifestyle and Health cohort study12 evaluated the association between combined indoor and outdoor UVR exposure and SCC risk and found a significant association between increased SCC risk and increased exposure. There is some overlap of women with the present study. We used the whole NOWAC cohort with updated exposure during follow-up, which provided stronger evidence that cumulative indoor and outdoor UVR exposure are important factors associated with SCC.1 We found no evidence of an association between age at initiation and age at SCC diagnosis, in contrast to a previous finding for melanoma.13 To our knowledge, no other study has investigated the association between age at initiation and age at SCC diagnosis.
We conducted sensitivity analysis among women with no history of cancer, resulting in a more direct, less confounded association of indoor tanning with SCC risk. The results were in line with the results in the whole cohort but with slightly lower HRs and wider CIs because of the reduced sample size and person-years of follow-up. A previous cohort study10,11 included only women with no history of cancer, but the study did not censor by date of diagnosis of any incident non-SCC cancer, as was done in the present study.
Limitations
This study has limitations. Type (UV-A, UV-B) and intensity of UVR vary largely among tanning devices,25,26,27 and we did not have information on the types of indoor tanning devices used and lengths of sessions. The NOWAC cohort includes only women, and although indoor tanning is more common among women than men,28,29 another study9 found similar estimates for the association between indoor tanning and SCC for men and women. Nonetheless, generalizability to men may be limited. The information on exposure was collected retrospectively; thus, some misclassification is likely to have occurred. However, the chosen cutoffs should limit this by placing low users in the same category and differentiating higher users, thereby focusing on cumulative indoor tanning exposure. Of importance, all information was collected before SCC diagnosis. In contrast, case-control studies may be limited by the potential for differential bias in recall of exposure between cases and controls.30,31 In our age-adjusted model, assuming nondifferential, nonsystematic errors, misclassification would attenuate the HR of the higher indoor tanning use category,32 that is, that the true association of this category (compared to the lower) is likely to be even higher than reported in Table 2. Moreover, in this model, under the same assumptions, the test for trend would be valid.32
We could not distinguish lip SCC, which may be associated with smoking,33 but there was no change in estimates when smoking was included as a covariate; thus, it is unlikely to have affected the results. This cohort is still young with respect to SCC incidence. Excluding basal cell carcinoma and melanoma, the median age at diagnosis for skin cancers (mostly SCC) in Norwegian women was 80 years in 2012 to 2016 (obtained from the CRN), compared with 66 years in our study.
Conclusions
The results from this large prospective study provide supporting evidence of a dose-response association of indoor tanning with SCC risk. The association between cumulative exposure to indoor tanning and SCC risk was the same regardless of duration of use and age at initiation. Avoidance of indoor tanning may help prevent not only melanoma13 but also SCC, and our results support the development of policies that regulate indoor tanning.
eFigure. Directed Acyclic Graphs (DAGs) on the Association of Indoor Tanning and Risk of Cutaneous Squamous Cell Carcinoma (SCC)
eTable 1. Distribution of Host Characteristics Among Women Who Answered the Baseline Questionnaire, First Follow-up Questionnaire, and Second Follow-up Questionnaire, Norwegian Women and Cancer Study, 1991-2015
eTable 2. Hazard Ratios and 95% Confidence Intervals (CIs) for Indoor Tanning and Risk of Squamous Cell Carcinoma, Norwegian Women and Cancer Study, 1991-2015
eTable 3. Women Without a History of Cancer: Hazard Ratios (HRs) and 95% Confidence Intervals (CIs) for Indoor Tanning and Risk of Cutaneous Squamous Cell Carcinoma, Norwegian Women and Cancer Study, 1991-2015
eTable 4. Women Without a History of Cancer: Linear Regression Analysis of the Association Between Age at Initiation and Age at Diagnosis, Norwegian Women and Cancer Study, 1991-2015
References
- 1.Green AC, Olsen CM. Cutaneous squamous cell carcinoma: an epidemiological review. Br J Dermatol. 2017;177(2):373-381. doi: 10.1111/bjd.15324 [DOI] [PubMed] [Google Scholar]
- 2.Robsahm TE, Helsing P, Veierød MB. Cutaneous squamous cell carcinoma in Norway 1963-2011: increasing incidence and stable mortality. Cancer Med. 2015;4(3):472-480. doi: 10.1002/cam4.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63(1-3):8-18. doi: 10.1016/S1011-1344(01)00198-1 [DOI] [PubMed] [Google Scholar]
- 4.Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379(4):363-374. doi: 10.1056/NEJMra1708701 [DOI] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer Exposure to Artificial UV Radiation and Skin Cancer. Geneva, Switzerland : World Health Organization; 2006. [Google Scholar]
- 6.Gordon L, Hirst N. The Health Effects of Using Solaria and Potential Cost-Effectiveness of Enforcing Solaria Regulations in Australia. Queensland, Australia: Queensland Institute of Medical Research; 2007. [Google Scholar]
- 7.Boniol M, Autier P, Boyle P, Gandini S. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757. doi: 10.1136/bmj.e4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wehner MR, Shive ML, Chren MM, Han J, Qureshi AA, Linos E. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909. doi: 10.1136/bmj.e5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karagas MR, Stannard VA, Mott LA, Slattery MJ, Spencer SK, Weinstock MA. Use of tanning devices and risk of basal cell and squamous cell skin cancers. J Natl Cancer Inst. 2002;94(3):224-226. doi: 10.1093/jnci/94.3.224 [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Qureshi AA, Geller AC, Frazier L, Hunter DJ, Han J. Use of tanning beds and incidence of skin cancer. J Clin Oncol. 2012;30(14):1588-1593. doi: 10.1200/JCO.2011.39.3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S, Han J, Laden F, Qureshi AA. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: a cohort study. Cancer Epidemiol Biomarkers Prev. 2014;23(6):1080-1089. doi: 10.1158/1055-9965.EPI-13-0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veierød MB, Couto E, Lund E, Adami HO, Weiderpass E. Host characteristics, sun exposure, indoor tanning and risk of squamous cell carcinoma of the skin. Int J Cancer. 2014;135(2):413-422. doi: 10.1002/ijc.28657 [DOI] [PubMed] [Google Scholar]
- 13.Ghiasvand R, Rueegg CS, Weiderpass E, Green AC, Lund E, Veierød MB. Indoor tanning and melanoma risk: long-term evidence from a prospective population-based cohort study. Am J Epidemiol. 2017;185(3):147-156. doi: 10.1093/aje/kww148 [DOI] [PubMed] [Google Scholar]
- 14.Lund E, Dumeaux V, Braaten T, et al. . Cohort profile: the Norwegian Women and Cancer Study–NOWAC—Kvinner og kreft. Int J Epidemiol. 2008;37(1):36-41. doi: 10.1093/ije/dym137 [DOI] [PubMed] [Google Scholar]
- 15.Larsen IK, Småstuen M, Johannesen TB, et al. . Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218-1231. doi: 10.1016/j.ejca.2008.10.037 [DOI] [PubMed] [Google Scholar]
- 16.Cancer Registry of Norway. Cancer in Norway 2017: Cancer Incidence, Mortality, Survival and Prevalence in Norway. Oslo, Norway: Institution of Population-Based Cancer Research; 2018.
- 17.Edvardsen K, Veierød MB, Brustad M, Braaten T, Engelsen O, Lund E. Vitamin D-effective solar UV radiation, dietary vitamin D and breast cancer risk. Int J Cancer. 2011;128(6):1425-1433. doi: 10.1002/ijc.25463 [DOI] [PubMed] [Google Scholar]
- 18.Veierød MB, Parr CL, Lund E, Hjartåker A. Reproducibility of self-reported melanoma risk factors in a large cohort study of Norwegian women. Melanoma Res. 2008;18(1):1-9. doi: 10.1097/CMR.0b013e3282f120d2 [DOI] [PubMed] [Google Scholar]
- 19.Lund E, Kumle M, Braaten T, et al. . External validity in a population-based national prospective study: the Norwegian Women and Cancer Study (NOWAC). Cancer Causes Control. 2003;14(10):1001-1008. doi: 10.1023/B:CACO.0000007982.18311.2e [DOI] [PubMed] [Google Scholar]
- 20.Veierod MB, Lydersen S, Laake P. Causal inference. Med Stat Clin Epidemiol Res. 2012;1:493-527. [Google Scholar]
- 21.Buuren Sv, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2010;45(3):1-68. [Google Scholar]
- 22.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887-1892. doi: 10.1056/NEJM200006223422507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murad MH, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evid Based Med. 2016;21(4):125-127. doi: 10.1136/ebmed-2016-110401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iannacone MR, Wang W, Stockwell HG, et al. . Patterns and timing of sunlight exposure and risk of basal cell and squamous cell carcinomas of the skin: a case-control study. BMC Cancer. 2012;12(1):417. doi: 10.1186/1471-2407-12-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsen LT, Hannevik M, Aalerud TN, Johnsen B, Friberg EG, Veierød MB. Trends in UV irradiance of tanning devices in Norway: 1983-2005. Photochem Photobiol. 2008;84(5):1100-1108. doi: 10.1111/j.1751-1097.2008.00330.x [DOI] [PubMed] [Google Scholar]
- 26.Nilsen LT, Aalerud TN, Hannevik M, Veierød MB. UVB and UVA irradiances from indoor tanning devices. Photochem Photobiol Sci. 2011;10(7):1129-1136. doi: 10.1039/c1pp05029j [DOI] [PubMed] [Google Scholar]
- 27.Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174(4):730-740. doi: 10.1111/bjd.14388 [DOI] [PubMed] [Google Scholar]
- 28.Grange F, Mortier L, Crine A, et al. . Prevalence of sunbed use, and characteristics and knowledge of sunbed users: results from the French population-based Edifice Melanoma survey. J Eur Acad Dermatol Venereol. 2015;29(s2)(suppl 2):23-30. doi: 10.1111/jdv.12899 [DOI] [PubMed] [Google Scholar]
- 29.Norwegian Cancer Society and Norwegian Radiation Protection Authority Survey of Sun Exposure Habits. [in Norwegian] Oslo, Norway: TNS Gallup; 2014. [Google Scholar]
- 30.de Vries E, Boniol M, Severi G, et al. . Public awareness about risk factors could pose problems for case-control studies: the example of sunbed use and cutaneous melanoma. Eur J Cancer. 2005;41(14):2150-2154. doi: 10.1016/j.ejca.2005.04.042 [DOI] [PubMed] [Google Scholar]
- 31.Rothman KJ. Epidemiology: An Introduction. 2nd ed Oxford, UK: Oxford University Press; 2012. [Google Scholar]
- 32.Buonaccorsi JP. Measurement Error: Models, Methods, and Applications. Boca Raton, FL: Chapman and Hall/CRC; 2010. doi: 10.1201/9781420066586 [DOI] [Google Scholar]
- 33.Perea-Milla López E, Miñarro-Del Moral RM, Martínez-García C, et al. . Lifestyles, environmental and phenotypic factors associated with lip cancer: a case-control study in southern Spain. Br J Cancer. 2003;88(11):1702-1707. doi: 10.1038/sj.bjc.6600975 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Directed Acyclic Graphs (DAGs) on the Association of Indoor Tanning and Risk of Cutaneous Squamous Cell Carcinoma (SCC)
eTable 1. Distribution of Host Characteristics Among Women Who Answered the Baseline Questionnaire, First Follow-up Questionnaire, and Second Follow-up Questionnaire, Norwegian Women and Cancer Study, 1991-2015
eTable 2. Hazard Ratios and 95% Confidence Intervals (CIs) for Indoor Tanning and Risk of Squamous Cell Carcinoma, Norwegian Women and Cancer Study, 1991-2015
eTable 3. Women Without a History of Cancer: Hazard Ratios (HRs) and 95% Confidence Intervals (CIs) for Indoor Tanning and Risk of Cutaneous Squamous Cell Carcinoma, Norwegian Women and Cancer Study, 1991-2015
eTable 4. Women Without a History of Cancer: Linear Regression Analysis of the Association Between Age at Initiation and Age at Diagnosis, Norwegian Women and Cancer Study, 1991-2015