Abstract
Evidence regarding the effects of subclinical hypothyroidism (SCH) on adverse pregnancy outcomes and the ability of levothyroxine (LT4) treatment to prevent them is unclear. Available recommendations for the management of SCH during pregnancy are inconsistent. We conducted a nationwide survey among physicians assessing their knowledge of and current practices in the care of SCH in pregnancy and compared these with the most recent American Thyroid Association (ATA) recommendations. In this cross-sectional study, an online survey was sent to active US members of the Endocrine Society. This survey included questions about current practices and clinical scenarios aimed at assessing diagnostic evaluation, initiation of therapy, and follow-up in pregnant women with SCH. In total, 162 physicians completed the survey. ATA guidelines were reviewed by 76%, of whom 53% indicated that these guidelines actually changed their practice. Universal screening was the preferred screening approach (54%), followed by targeted screening (30%). For SCH diagnosis, most respondents (52%) endorsed a TSH level >2.5 mIU/L as a cutoff, whereas 5% endorsed a population-based cutoff as recommended by the ATA. The decision to initiate treatment varied depending on the specific clinical scenario; however, when LT4 was initiated, respondents expected a small/very small reduction in maternofetal complications. In conclusion, despite recently updated guidelines, there is still wide variation in clinical practices regarding the care of women with SCH in pregnancy. Highly reliable randomized trials are required to evaluate the effectiveness of the most uncertain treatment practices on the care of pregnant women with SCH.
Keywords: hypothyroidism, subclinical, pregnancy, survey, guideline
Subclinical hypothyroidism (SCH) in pregnancy is a mild thyroid disorder defined by an elevated serum TSH level with a normal free thyroxine (FT4) level [1]. As a result of physiological changes in thyroid function during pregnancy leading to increased maternal thyroid hormone demand, SCH is a common condition among pregnant women [2–4]. During pregnancy, overt hypothyroidism, defined as an elevated TSH level with a low FT4 level, contributes to adverse maternofetal and offspring outcomes [5–9]. Accordingly, treatment with levothyroxine (LT4) is strongly recommended [1, 10]. For pregnant women with SCH, however, the evidence for both adverse outcomes and the ability of LT4 treatment to prevent them is unclear [11–15], and the clinical recommendations are inconsistent [1, 10, 16].
In 2012, the Endocrine Society published a clinical practice guideline for the management of thyroid diseases in pregnancy and recommended that all pregnant women with SCH be treated with LT4, independent of thyroid peroxidase antibody (TPO-Ab) status [10]. In the 2015 clinical management guidelines of the American College of Obstetricians and Gynecologists (ACOG), universal screening for thyroid disease in pregnancy was not recommended on the basis of evidence that identification and treatment of maternal SCH has not improved neurocognitive function in offspring [16]. In 2017, the American Thyroid Association (ATA) issued new guidelines that changed the TSH threshold used to define SCH and emphasized the use of TPO-Ab status to determine whether to treat SCH with LT4 [1]. Specifically, the TSH upper limit was raised from 2.5 to 4.0 mIU/L when no population-based cutoff is available, and evaluation of TPO-Ab status was recommended in all pregnant women with TSH concentrations >2.5 mIU/L, with the result contributing to the treatment decision.
The inconsistencies noted in the recommendations from different organizations may be due to different publication times, which allowed evaluation of more data in the more recent guidelines. The paucity of reliable evidence and variations in recommendations may contribute to unwarranted practice variations. A recent study using a US national administrative database showed that of 8040 pregnant women with SCH (TSH level of 2.5 to 10 mIU/L), only 15% were started on LT4 treatment. Furthermore, endocrinologists had a lower TSH threshold for starting LT4 treatment compared with internists, obstetricians, and other clinicians [17]. Moreover, previous studies assessing the management of thyroid disorders during pregnancy have shown wide variations in practice among physicians worldwide [18–22].
To better understand the effect of the most recent ATA guidelines on the care of pregnant women with SCH in the United States, we surveyed physicians nationwide to assess their knowledge and perceptions of the diagnosis, treatment, and effect of SCH in pregnancy and compared these findings with ATA recommendations for care.
1. Materials and Methods
A. Survey Design
Two authors (S.M. and F.J.K.T.) prepared an initial draft of the questionnaire according to the study objectives and previously issued surveys in the field [18–20]. The survey included demographic data (specialty, geographical location, years of clinical practice, community type) and multiple-choice questions based on two clinical scenarios describing variations in TSH levels, thyroid autoimmunity status, and thyroid physical examination results to widely assess the diagnostic evaluation, decision on initiation of therapy, and follow-up in pregnant women with SCH. The survey used in this project is publicly shared in an online repository [23]. The main topics covered by the survey were screening, TSH diagnostic cutoff, use of TPO to guide therapy, types of therapy, and follow-up. Because we intended to assess current clinical practices, an initial screening question was added to exclude clinicians who do not care for pregnant women with SCH. Most questions required a single best response to be selected from multiple choices and were constructed to omit phrasing that could lead respondents to the “right” answer. Some questions allowed multiple items to be simultaneously selected. We limited questions to achieve a survey response time of less than 15 minutes. Subsequent survey drafts were distributed among the coauthors, and after an iterative process of feedback and discussion, a final version was prepared. There was an additional review process by the Endocrine Society Clinical Affairs Committee, which provided feedback and ensured survey relevance to its members. The study was considered exempt by the institutional review board of the University of Arkansas for Medical Sciences.
B. Survey Distribution and Data Collection
An anonymous online survey was sent to 5914 US medical doctors who are members of the Endocrine Society between 5 September and 16 November 2018. They received an e-mail invitation to participate from society administrators, which described the survey and contained an electronic link to the survey website without offering incentives to participate. Three reminders were sent after the first e-mail, each 2 to 3 weeks apart. Survey responses were anonymously collected and stored electronically by an online survey service (Google Forms, Mountain View, CA), and data were password protected. Repeated submissions from the same IP address were automatically blocked by the survey service. Only members of the Endocrine Society were surveyed because according to previous survey-based studies in thyroidology that included members of the ATA and the American Association of Clinical Endocrinologists, the majority of survey respondents came from the Endocrine Society. In addition, there was substantial overlap between the respondents’ memberships, and a small percentage did not have Endocrine Society membership. [24–26]. We also attempted to collaborate with the ACOG regarding distribution of the same survey to its members; however, we were unsuccessful.
C. Statistical Analysis
Summary statistics are presented as frequencies (percentages) for categorical variables and as means and SD or median and interquartile range (IQR) for continuous variables according to the normality of the variables. The response rate was estimated for each question. Statistical analyses explored the relationships between respondents’ demographics and adherence to ATA guidelines or self-confidence level in the management of SCH in pregnant women. Differences in categorical variables were analyzed with the χ2 or Fisher’s exact test, and differences in continuous variables with the independent t test or Mann-Whitney test as appropriate. Simple linear regression was used to analyze correlations between guideline adherence and the guideline-reported strength of the recommendation or quality of evidence. ANOVA was used to assess differences between demographic characteristics of the respondents and self-confidence or adherence to ATA guidelines for the management of SCH during pregnancy. To assess the possible drivers of adherence to ATA guideline recommendations, a multivariate analysis adjusted for geographic location, specialty, years in clinical practice, number of pregnant women with SCH treated over the past 6 months, previous reading of ATA guidelines, and type of clinical practice was performed. All analyses were two-tailed, with α set at 0.05, and were conducted using IBM SPSS Statistics version 25.
2. Results
A. Demographics of Respondents
Of the 5914 survey invitations sent by e-mail, 5911 were successfully delivered and 1562 (26%) were opened. We received a total of 162 responses (10%), of which 147 (91%) came from physicians who have participated in the care of pregnant women with SCH (screening question). The demographic characteristics of the respondents are summarized in Table 1. Respondents had practiced for an average of 18 years (IQR, 9 to 28 years) and had evaluated ∼6 (IQR, 3 to 10) pregnant women with SCH over the past 6 months.
Table 1.
General Characteristics of Survey Respondents
| Characteristic | n (%) |
|---|---|
| Geographic location | |
| Northeast | 56 (38.1) |
| South | 36 (24.5) |
| West | 32 (21.8) |
| Midwest | 23 (15.6) |
| Community type | |
| Urban | 79 (53.7) |
| Suburban | 59 (40.2) |
| Rural | 9 (6.1) |
| Medical specialty | |
| Endocrinology, focused on thyroid disorders | 112 (76.2) |
| Endocrinology, not focused on thyroid disorders | 22 (15.0) |
| Reproductive endocrinology | 8 (5.4) |
| Internal medicine | 3 (2.0) |
| Obstetrics | 1 (0.7) |
| Other | 1 (0.7) |
| Family medicine | 0 (0) |
| Years in clinical practice | |
| <2 y | 4 (2.7) |
| 2–5 y | 23 (15.6) |
| 5–10 y | 17 (11.6) |
| >10 y | 103 (70.1) |
| Number of pregnant women with SCH treated over the past 6 mo | |
| <5 women | 73 (50.0) |
| 5–10 women | 39 (26.7) |
| 10–20 women | 16 (11.0) |
| >20 women | 18 (12.3) |
B. ATA Guideline Adherence
About 76%, 70%, and 18% of respondents had reviewed guidelines by the ATA, the Endocrine Society, and the ACOG, respectively. Only 53% of the respondents who had reviewed the ATA guidelines thought the recommendations had changed their practice.
The concordance between survey respondents’ current clinical practices and the ATA recommendations (ATA guideline adherence) is summarized in Table 2. Overall, we did not find a correlation between guideline adherence and the guideline-reported strength of the recommendations [P trend = 0.66] or quality of the evidence supporting the recommendations [P trend = 0.31]. However, when analyzing by recommendation topics (SCH diagnosis/treatment/follow-up), we found a significant correlation between guideline adherence and the guideline-reported strength of the recommendations related to treatment [P trend = 0.01], but not for those related to diagnostic evaluation or therapy follow-up.
Table 2.
Concordance of ATA 2017 Recommendations for SCH [1] With Survey Respondents’ Current Clinical Practices
| Recommendation No. | Brief Description of Recommendation | Recommendation Scope | Recommendation Grade | Survey Concordance (%)a |
|---|---|---|---|---|
| R26 | The pregnancy-specific TSH reference range should be defined as population- and trimester-specific reference ranges. When this goal is not feasible, pregnancy-specific TSH reference ranges obtained from similar patient populations or an upper reference limit of 4.0 mU/L may be used. | Diagnostic evaluation | Strong recommendation, high-quality evidence | 30.6 |
| R28 | Pregnant women with TSH concentrations >2.5 mU/L should be evaluated for TPO-Ab status. | Diagnostic evaluation | Strong recommendation, high-quality evidence | 20.4 |
| R29a | LT4 therapy is recommended for TPO-Ab‒positive women with a TSH concentration greater than the pregnancy-specific reference range or >4.0 mU/L if unavailable. | Treatment | Strong recommendation, moderate-quality evidence | 87.1 |
| R29b-1 | LT4 therapy may be considered for TPO-Ab‒positive women with TSH concentrations >2.5 mU/L and below the upper limit of the pregnancy-specific reference range. | Treatment | Weak recommendation, moderate-quality evidence | 57.8 |
| R29b-2 | LT4 therapy may be considered for TPO-Ab‒negative women and TPO-Ab‒negative women with TSH concentrations greater than the pregnancy-specific reference range and below 10.0 mU/L. | Treatment | Weak recommendation, low-quality evidence | 51.0 |
| R29c | LT4 therapy is not recommended for TPO-Ab‒negative women with a normal TSH (TSH within the pregnancy-specific reference range or <4.0 mU/L if unavailable). | Treatment | Strong recommendation, high-quality evidence | 81.6 |
| R31 | The recommended treatment of maternal hypothyroidism is administration of oral LT4. Other thyroid preparations such as T3 or desiccated thyroid should not be used in pregnancy. | Treatment | Strong recommendation, low-quality evidence | 95.2 |
| R32 | It is reasonable to target a TSH concentration in the lower half of the trimester-specific reference range. When this is not available, it is reasonable to target maternal TSH concentrations below 2.5 mU/L. | Follow-up | Weak recommendation, moderate-quality evidence | 85.7 |
| R33 | Women with overt and subclinical hypothyroidism should be monitored with a serum TSH measurement approximately every 4 wk until midgestation and at least once near 30 wk gestation. | Follow-up | Strong recommendation, high-quality evidence | 57.1 |
| R38 | Some women in whom LT4 is initiated during pregnancy may not require LT4 postpartum. Such women are candidates for discontinuing LT4, especially when the LT4 dose is <50 µg/d. | Follow-up | Weak recommendation, moderate-quality evidence | 17.7 |
Percentage of participants who follow the recommendation in the ATA guidelines.
In a multivariate analysis, the number of years in clinical practice was the only significant predictor of guideline adherence [nβ = −0.23; P = 0.008] after adjustments for geographic location, specialty, the number of pregnant women with SCH treated over the past 6 months, previous reading of ATA guidelines, and type of clinical practice.
C. Screening and Diagnostic Evaluation
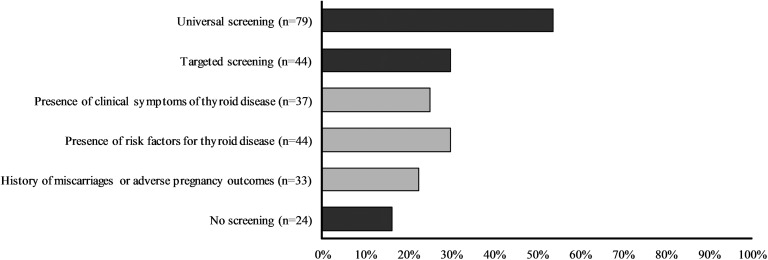
Most respondents recommended screening for thyroid dysfunction for every woman at the beginning of her pregnancy (54%), whereas 30% recommended targeted screening and 16% recommended no screening for SCH in pregnancy (Fig. 1). Survey findings regarding diagnostic evaluation of pregnant women with SCH are summarized in Table 3.
Figure 1.
Screening approaches for thyroid dysfunction during pregnancy according to survey respondents.
Table 3.
Detailed Survey Responses About Diagnostic Evaluation, Treatment, and Follow-Up of SCH During Pregnancy
| Survey Responses | n (%) |
|---|---|
| TSH cutoff | |
| Fixed cutoff of TSH >2.5 mIU/L | 77 (52.4) |
| Fixed cutoff of TSH >4.0 mIU/L | 37 (25.2) |
| Population-based cutoff | 8 (5.4) |
| According to TPO-Ab status | 7 (4.8) |
| According to clinical features | 7 (4.8) |
| Nonpregnant adult cutoff | 6 (4.1) |
| Other or unknown | 5 (3.3) |
| FT4 measurement | |
| When TSH is higher than pregnancy-specific cutoff | 72 (49.0) |
| Always | 53 (36.1) |
| Never | 9 (6.1) |
| When TSH >10.0 mIU/L | 7 (4.8) |
| Other or unknown | 6 (4.0) |
| TPO-Ab measurement | |
| When TSH is higher than pregnancy-specific cutoff | 64 (43.5) |
| Always | 57 (38.8) |
| Never | 12 (8.2) |
| Other or unknown | 12 (8.2) |
| When TSH >10.0 mIU/L | 2 (1.4) |
| Medication choicea | |
| LT4 | 143 (97.3) |
| LT4 + liothyronine (T3) | 4 (2.7) |
| Thyroid extracts | 3 (2.0) |
| Thyroid hormone initial dose | |
| Fixed small dose (25–50 µg/d) | 104 (70.8) |
| Dose based on patient’s TSH level | 17 (11.6) |
| Fixed full dose (75–100 µg/d) | 12 (8.2) |
| Dose based on patient’s weight | 11 (7.4) |
| Other dose | 3 (2.0) |
| TSH follow-up until midgestation | |
| On everyone, every 4–6 wk | 95 (64.6) |
| Only if TSH is not appropriate at first check after LT4 initiation | 26 (17.7) |
| On everyone, every 6–8 wk | 8 (5.4) |
| On everyone, every trimester | 7 (4.8) |
| On everyone, every 2–4 wk | 6 (4.0) |
| Never | 3 (2.0) |
| Other or unknown | 2 (1.4) |
| TSH treatment goal | |
| TSH <2.5 mIU/L | 108 (73.5) |
| TSH in the lower half of the trimester-specific reference range | 18 (12.2) |
| TSH <4.0 mIU/L | 13 (8.8) |
| Other or unknown | 7 (4.8) |
| TSH between normal limits for a nonpregnant adult | 1 (0.7) |
| Indication to stop LT4 treatment postpartum | |
| TSH level postpartum within normal limits for a nonpregnant adult | 51 (34.7) |
| Women who used LT4 <50 μg daily | 26 (17.7) |
| All the postpartum women | 25 (17.0) |
| Women with TPO-Ab negative | 19 (12.9) |
| No indication to stop LT4 treatment | 10 (6.8) |
| Other indications | 9 (6.1) |
| Women with normal thyroid function prior to pregnancy | 4 (2.7) |
| Decision according to patient preferences | 3 (2.0) |
Multiselect and multiple choice question.
For the diagnosis of SCH, most respondents endorsed a TSH level >2.5 mIU/L as the cutoff (52%), whereas only 5% endorsed a population-based cutoff as recommended by the ATA. Others required different thresholds depending on the presence of clinical features (5%) or TPO-Ab status (5%). The most frequent indication for measurements of FT4 and TPO-Ab during the initial diagnostic workup was a TSH level higher than the pregnancy-specific cutoff used in the responders’ clinical practice (49% and 44%, respectively).
D. Clinical Scenarios
Table 4 summarizes the treatment decisions among respondents for each clinical scenario and the expected risk reductions in adverse pregnancy outcomes and adverse health/cognitive outcomes of the offspring if treatment was provided.
Table 4.
Treatment Decision and Expected Maternofetal Risk Reduction According to Different Clinical Scenarios
| Patient 1 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A healthy 29-y-old woman presents for a prenatal visit at the 8th wk of her first pregnancy. She has no known history of thyroid disorder, infertility, or previous miscarriage. She currently takes no medication, and the pregnancy is going well. She had laboratories done the day of the prenatal visit. Please choose the most appropriate next step for each of the following scenarios: | Expected reduction in the risk of adverse pregnancy outcomes that pregnant women will gain from the treatmenta | Expected reduction in the risk of adverse health/cognitive outcomes to the offspring from the treatmenta | |||||||||||||||
| TSH (mIU/L) | FT4 | TPO-Abs | Neck Physical Exam | Start Thyroid Hormone Therapy Now | Repeat TSH Within 1 Mo | No Further Evaluation | None | Very Small (<10%) | Small (10%–20%) | Large (20%–40%) | Very Large (>40%) | None | Very Small (<10%) | Small (10%–20%) | Large (20%–40%) | Very Large (>40%) | |
| 1 | 4.4 | Normal limits | (+) | Normal | 128 (87.1) | 18 (12.2) | 1 (0.7) | 6 (4.7) | 56 (43.8) | 47 (36.7) | 17 (10.5) | 2 (1.2) | 14 (10.9) | 57 (44.5) | 37 (28.9) | 15 (11.7) | 5 (3.9) |
| 2 | 4.4 | Normal limits | (−) | Normal | 74 (50.3) | 65 (44.2) | 8 (5.4) | 7 (9.5) | 45 (60.8) | 11 (14.9) | 9 (12.2) | 2 (2.7) | 12 (16.2) | 39 (52.7) | 15 (20.3) | 5 (6.8) | 3 (4.1) |
| 3 | 4.4 | Normal limits | (−) | Small diffuse goiter | 91 (61.9) | 51 (34.7) | 5 (3.4) | 6 (6.6) | 50 (54.9) | 21 (23.1) | 11 (12.1) | 3 (3.3) | 14 (15.4) | 46 (50.5) | 21 (23.1) | 7 (7.7) | 3 (3.3) |
| 4 | 3.2 | Normal limits | (+) | Normal | 84 (57.1) | 58 (39.5) | 5 (3.4) | 7 (8.3) | 39 (46.4) | 29 (34.5) | 7 (8.3) | 2 (2.4) | 13 (15.5) | 40 (47.6) | 24 (28.6) | 5 (6.0) | 2 (2.4) |
| 5 | 3.2 | Normal limits | (−) | Normal | 26 (17.7) | 78 (48.1) | 43 (29.3) | 4 (14.8) | 12 (44.4) | 7 (25.9) | 2 (7.4) | 1 (3.7) | 4 (14.8) | 11 (40.7) | 7 (25.9) | 3 (11.1) | 1 (3.7) |
| Patient 2 | |||||||||||||||||
| A healthy 32-y-old woman presents for a prenatal visit at the 17th wk of her first pregnancy. She has no known history of thyroid disorder, infertility, or previous miscarriage. She currently takes no medication, and the pregnancy is going well. She had laboratories done the day of the prenatal visit. Please choose the most appropriate next step for each of the following scenarios: | Expected reduction in the risk of adverse pregnancy outcomes that pregnant women will gain from the treatmenta | Expected reduction in the risk of adverse health/cognitive outcomes to the offspring from the treatmenta | |||||||||||||||
| TSH (mIU/L) | FT4 | TPO-Abs | Neck Physical Exam | Start Thyroid Hormone Therapy Now | Repeat TSH Within 1 Mo | No Further Evaluation | None | Very Small (<10%) | Small (10%–20%) | Large (20%–40%) | Very Large (>40%) | None | Very Small (<10%) | Small (10%–20%) | Large (20%–40%) | Very Large (>40%) | |
| 1 | 4.4 | Normal limits | (+) | Normal | 116 (78.9) | 28 (19.0) | 3 (2.0) | 14 (12.1) | 56 (48.3) | 37 (31.9) | 6 (5.2) | 3 (2.6) | 20 (17.2) | 56 (48.3) | 29 (25.0) | 9 (7.8) | 2 (1.7) |
| 2 | 4.4 | Normal limits | (−) | Normal | 68 (46.3) | 64 (43.5) | 15 (10.2) | 7 (10.3) | 43 (63.2) | 13 (19.1) | 3 (4.4) | 2 (2.9) | 12 (17.6) | 36 (52.9) | 16 (23.5) | 3 (4.4) | 1 (1.5) |
| 3 | 4.4 | Normal limits | (−) | Small diffuse goiter | 79 (53.7) | 59 (40.1) | 9 (6.1) | 7 (8.9) | 52 (65.8) | 14 (17.7) | 4 (5.1) | 2 (2.5) | 16 (20.3) | 40 (50.6) | 17 (21.5) | 5 (6.3) | 1 (1.3) |
| 4 | 3.2 | Normal limits | (+) | Normal | 69 (46.9) | 65 (44.2) | 13 (8.8) | 6 (8.7) | 40 (58.0) | 18 (26.1) | 3 (4.3) | 2 (2.9) | 14 (20.3) | 39 (56.5) | 15 (21.7) | 0 (0.0) | 1 (0.6) |
| 5 | 3.2 | Normal limits | (−) | Normal | 19 (12.9) | 75 (51.0) | 53 (36.1) | 3 (15.8) | 10 (52.6) | 3 (15.8) | 2 (10.5) | 1 (5.3) | 3 (15.8) | 8 (42.1) | 7 (36.8) | 0 (0.0) | 1 (5.3) |
Data are presented as n (%).
Of the respondents who would treat the patient according to the clinical scenario.
When one-variable changes in a patient’s clinical characteristics were assessed in clinical scenarios, changes in TPO-Ab status [87% for positive vs 50% for negative; P < 0.001] and thyroid physical examination results [62% for goiter vs 50% for normal; P < 0.001] significantly increased LT4 prescription rates for a first-trimester pregnant woman with TSH level = 4.4 mIU/L. In the case of first-trimester pregnant women with TSH level = 3.2 mIU/L, a change in TPO-Ab status increased LT4 prescription rates as well [57% for positive vs 18% for negative; P < 0.001]. The clinical scenarios regarding a second-trimester pregnant woman showed similar results.
In a multivariate analysis, TSH level (2.5 to 4.0 mIU/L vs >4.0 mIU/L), TPO-Ab status (positive vs negative), physical examination findings (normal vs presence of small goiter), and pregnancy trimester (first vs second trimester) were all significant predictors for starting LT4 therapy throughout the clinical scenarios. The strongest predictor was TPO-Ab status [nβ = 0.35; P < 0.001], followed by TSH level [nβ = 0.31; P < 0.001], physical examination findings [nβ = 0.1; P = 0.004], and trimester of diagnosis [nβ = 0.07; P = 0.003].
More than 70% of the clinicians who would start LT4 thought that the treatment would have a small effect (10% to 20% reduction) or very small effect (<10% reduction) on maternofetal complications regardless of the clinical scenario.
E. Treatment and Follow-Up
Survey findings regarding treatment decision and follow-up of pregnant women with SCH are summarized in Table 3. The preferred therapy for the management of SCH during pregnancy was LT4 (97%), using an initial fixed dose of 25 to 50 µg/d (71%) or 75 to 100 µg/d (8%). A few respondents selected a dose based on TSH level (12%) or the patient’s weight (7%). The factors considered when deciding whether to start therapy in a pregnant woman with SCH are shown in Fig. 2.
Figure 2.
Factors considered by survey respondents for treatment initiation in women with SCH during pregnancy.
According to most respondents (65%), TSH levels should be rechecked every 4 to 6 weeks until midgestation; 18% would recheck TSH levels only if the TSH level was not appropriate at first check after LT4 initiation, and 2% would never reassess TSH levels during pregnancy. Most respondents followed ATA guidelines and endorsed a TSH goal for thyroid hormone therapy of <2.5 mIU/L (74%) during pregnancy or in the lower half of the trimester-specific reference range (12%).
F. Stopping Therapy
Respondents would stop LT4 therapy after delivery if postpartum TSH levels fell within normal limits for a nonpregnant adult (35%) of if the patient required an LT4 dose <50 μg daily (18%). Some would stop LT4 in every woman (17%), whereas others would stop it in all TPO-Ab‒negative women (13%) (Table 3).
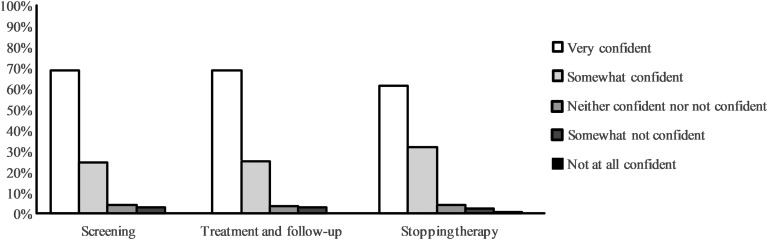
G. Self-Confidence
With clinical experience (in years or in numbers of women treated), respondents expressed greater self-confidence in the care of pregnant women with SCH (Fig. 3). Endocrinologists showed a higher self-confidence level than other clinicians, and internal medicine specialists had the lowest self-confidence level for the management of SCH in pregnancy.
Figure 3.
Self-confidence regarding screening, treatment and follow-up, and stopping therapy for SCH during pregnancy as reported by survey respondents.
3. Discussion
This study assessed clinicians’ knowledge, perceptions, and clinical practices regarding SCH diagnosis, treatment, and effect on pregnancy in relation to the most recently published ATA guidelines [1]. Across a demographically diverse sample of clinicians and members of the largest endocrinology society in the United States, we found an awareness of the ATA guidelines and evidence of their effects on practice, with low adherence to the recommended TSH cutoff for the diagnosis of SCH during pregnancy and the indications for TPO-Ab status assessment as part of the diagnostic evaluation. When LT4 treatment was chosen by respondents, there was a small or very small expected reduction in maternofetal complications. We also found that only 50% of the clinicians who responded take patient preferences into consideration when determining treatment, and 19% take therapy adverse effects into consideration.
Interventional studies [13, 27, 28] have been unable to document the same benefits of LT4 treatment of SCH in pregnancy as seen in observational studies [29–32]. In this uncertain context, the ATA issued an updated version of its guidelines encompassing major changes in clinical practices for the management of SCH during pregnancy [1, 33]. The new recommendations have been partially accepted [33–35]; as demonstrated here, practice is only partially concordant with ATA recommendations. This could be due to low-grade recommendations according to the guideline-grading hierarchy, which could be perceived by health care providers as lacking certainty in the evidence. However, we did not find a correlation between guideline adherence and the guideline-reported strength of recommendations or the quality of the evidence supporting the recommendations, except for those recommendations regarding treatment. Moreover, the extent to which adherence to these guidelines improves maternofetal outcomes remains uncertain.
One of the most controversial issues in the field of thyroid dysfunction and pregnancy is the appropriate screening approach in early pregnancy. The ATA guideline recommends neither for nor against universal screening for abnormal TSH concentrations in early pregnancy on the basis of uncertain benefits [36]. Contrary to this recommendation, according to our survey universal screening is the preferred method for pregnant women, a result that is consonant with findings from surveys of other medical societies [18, 19, 21, 37]. The preference for universal screening may be driven by the inability of clinicians to identify at-risk women [38] and the well-known benefit of LT4 treatment for overt thyroid dysfunction during pregnancy. Furthermore, universal screening for thyroid dysfunction in pregnancy has been supported by some authors [35, 39], who argue that universal screening [40] for overt thyroid disease is justifiable and lack of clarity on the effect of optimum management of SCH is not an adequate rationale for inaction after detection.
Of note, most respondents are still using a TSH level >2.5 mIU/L for the diagnosis of SCH in the first trimester of pregnancy, as recommended in the older guidelines [10, 41] and consistent with previous studies [18]. Although a TSH cutoff of >4.0 mIU/L was offered for cases in which a population-based cutoff is unavailable, only 5.4% of respondents selected this option. This may be because using a TSH cutoff of >4.0 mIU/L for diagnosis has been criticized, given that only 26% of the studies cited under this recommendation found an upper limit of normal for TSH ≥4.0 mU/L [1, 35, 42–45]. However, the benefit of detecting and treating women with a TSH level of 2.5 to 4.0 mIU/L remains uncertain while exposing patients to anxiety and treatment burden, factors that physicians rarely consider in treatment decisions as reported in the current study. In addition, although a TSH level >2.5 mIU/L was the most used cutoff for SCH diagnosis among respondents, only 18% of the respondents would treat a pregnant woman with a TSH level of 3.2 mIU/L and without thyroid autoimmunity. It is possible that for the respondents a TSH level >2.5 mIU/L is used mainly to define SCH and create awareness, leading to closer follow-up during pregnancy, but does not lead on its own to LT4 treatment initiation.
Although the decision to treat or not varied depending on the specific clinical scenario, when LT4 treatment was chosen, respondents expected a small or very small reduction in maternofetal complications across all clinical scenarios regardless of patients’ characteristics. This trend may be explained by the absence or small size of effects on LT4-treated patients shown in interventional studies [13, 27, 28, 46, 47].
Delivery of maternal T4 to the fetus through placental transference is crucial for optimal fetal brain development [48–50]. The use of LT4 + T3 or desiccated thyroid extracts produces a low T4/T3 ratio; as a result, the placental transfer of LT4 to the fetal brain may be insufficient [49, 51, 52].Therefore, LT4 is the preferred drug during pregnancy. Despite the strong recommendation to not use other thyroid preparations, a small portion of the respondents still choose LT4 + T3 or desiccated thyroid extracts as pharmacological therapy during pregnancy.
Our findings are limited in their applicability by the relatively low response rate of society members. However, previous studies have shown similar response rates by clinically active members of the Endocrine Society [24–26, 53]. This low response rate may have an effect on the generalizability of the present results, driven mainly by the potential selection bias of the respondents. Moreover, underrepresentation of non–endocrine clinicians (Obstetrics & Gynecology, internal medicine, and family medicine specialists) may have affected the results. In addition, we did not include history of miscarriages as a variable in the case scenarios, and this could have changed/influenced the respondents’ answers. A history of miscarriage, as demonstrated by our results, is a strong factor in the decision to initiate LT4 therapy during pregnancy. Finally, it is important to note that it takes ∼2 to 3 years to fully implement a new guideline in clinical practice. We performed this survey 18 months after the ATA guidelines were released, which may have contributed to the low adherence and may be a source of bias in the current study. Further studies in the field might intend to evaluate the practices of the other participating specialties in the care of pregnant women with SCH (obstetrics & gynecology, internal medicine, family medicine).
In summary, this national study assessed clinicians’ knowledge and reported practices regarding the diagnosis, treatment, and effect of SCH on pregnancy and their concordance with the latest ATA guidelines. Despite recently updated guidelines, there is still wide variation in clinical practice regarding the care of pregnant women with SCH. Improvement may require multicentric collaboration to produce highly reliable, practical randomized trials of the comparative effectiveness and the impact on maternofetal and offspring outcomes of the most uncertain and commonly used treatment practices in the care of SCH during pregnancy.
Acknowledgments
The Endocrine Society Clinical Affairs Committee provided valuable feedback during the preparation of this manuscript.
Financial Support: S.M. receives support from the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000. This material is the result of work supported with resources and the use of facilities at the Central Arkansas Veterans Healthcare System, Little Rock, Arkansas. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Additional Information
Disclosure Summary: The authors report no conflicts of interest in this work.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- ACOG
American College of Obstetricians and Gynecologists
- ATA
American Thyroid Association
- FT4
free thyroxine
- IQR
interquartile range
- LT4
levothyroxine
- SCH
subclinical hypothyroidism
- TPO-Ab
thyroid peroxidase antibody
References and Notes
- 1. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315–389. [DOI] [PubMed] [Google Scholar]
- 2. Dong AC, Stagnaro-Green A. Differences in diagnostic criteria mask the true prevalence of thyroid disease in pregnancy: a systematic review and meta-analysis. Thyroid. 2019;29(2):278–289. [DOI] [PubMed] [Google Scholar]
- 3. Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18(3):404–433. [DOI] [PubMed] [Google Scholar]
- 4. Springer D, Jiskra J, Limanova Z, Zima T, Potlukova E. Thyroid in pregnancy: from physiology to screening. Crit Rev Clin Lab Sci. 2017;54(2):102–116. [DOI] [PubMed] [Google Scholar]
- 5. Leung AS, Millar LK, Koonings PP, Montoro M, Mestman JH. Perinatal outcome in hypothyroid pregnancies. Obstet Gynecol. 1993;81(3):349–353. [PubMed] [Google Scholar]
- 6. Allan WC, Haddow JE, Palomaki GE, Williams JR, Mitchell ML, Hermos RJ, Faix JD, Klein RZ. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen. 2000;7(3):127–130. [DOI] [PubMed] [Google Scholar]
- 7. Davis LE, Leveno KJ, Cunningham FG. Hypothyroidism complicating pregnancy. Obstet Gynecol. 1988;72(1):108–112. [PubMed] [Google Scholar]
- 8. Wolfberg AJ, Lee-Parritz A, Peller AJ, Lieberman ES. Obstetric and neonatal outcomes associated with maternal hypothyroid disease. J Matern Fetal Neonatal Med. 2005;17(1):35–39. [DOI] [PubMed] [Google Scholar]
- 9. Wasserstrum N, Anania CA. Perinatal consequences of maternal hypothyroidism in early pregnancy and inadequate replacement. Clin Endocrinol (Oxf). 1995;42(4):353–358. [DOI] [PubMed] [Google Scholar]
- 10. De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(8):2543–2565. [DOI] [PubMed] [Google Scholar]
- 11. Maraka S, Ospina NM, O’Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, Erwin PJ, Coddington CC III, Stan MN, Murad MH, Montori VM. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26(4):580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson DB, Casey BM, McIntire DD, Cunningham FG. Subsequent pregnancy outcomes in women previously diagnosed with subclinical hypothyroidism. Am J Perinatol. 2014;31(1):77–84. [DOI] [PubMed] [Google Scholar]
- 13. Casey BM, Thom EA, Peaceman AM, Varner MW, Sorokin Y, Hirtz DG, Reddy UM, Wapner RJ, Thorp JM Jr, Saade G, Tita AT, Rouse DJ, Sibai B, Iams JD, Mercer BM, Tolosa J, Caritis SN, VanDorsten JP; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376(9):815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, Cunningham FG. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105(2):239–245. [DOI] [PubMed] [Google Scholar]
- 15. Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, Canick J, Porter TF, Luthy D, Gross S, Bianchi DW, D’Alton ME. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol. 2008;112(1):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American College of Obstetricians and Gynecologists. Practice Bulletin No. 148: Thyroid disease in pregnancy. Obstet Gynecol. 2015;125(4):996–1005. [DOI] [PubMed] [Google Scholar]
- 17. Maraka S, Mwangi R, Yao X, Sangaralingham LR, Singh-Ospina NM, O’Keeffe DT, Rodriguez-Gutierrez R, Stan M, Brito JP, Grubina McCoy R, Montori VM. Thyroid Hormone Prescription Patterns for the Treatment of Pregnant Women with Subclinical Hypothyroidism. Endocrine Society;2018 Available at: https://www.endocrine.org/meetings/endo-annual-meetings/abstract-details?ID=5348. [Google Scholar]
- 18. Koren R, Wiener Y, Or K, Benbassat CA, Koren S. Thyroid disease in pregnancy: a clinical survey among endocrinologists, gynecologists, and obstetricians in Israel. Isr Med Assoc J. 2018;20(3):167–171. [PubMed] [Google Scholar]
- 19. Vaidya B, Hubalewska-Dydejczyk A, Laurberg P, Negro R, Vermiglio F, Poppe K. Treatment and screening of hypothyroidism in pregnancy: results of a European survey. Eur J Endocrinol. 2012;166(1):49–54. [DOI] [PubMed] [Google Scholar]
- 20. Azizi F, Amouzegar A, Mehran L, Alamdari S, Subekti I, Vaidya B, Poppe K, San Luis T Jr, Akamizu T. Screening and management of hypothyroidism in pregnancy: results of an Asian survey. Endocr J. 2014;61(7):697–704. [DOI] [PubMed] [Google Scholar]
- 21. Medeiros MF, Cerqueira TL, Silva Junior JC, Amaral MT, Vaidya B, Poppe KG, Carvalho GA, Gutierrez S, Alcaraz G, Abalovich M, Ramos HE; Latin American Thyroid Society. An international survey of screening and management of hypothyroidism during pregnancy in Latin America. Arq Bras Endocrinol Metabol. 2014;58(9):906–911. [DOI] [PubMed] [Google Scholar]
- 22. Azizi F, Mehran L, Amouzegar A, Alamdari S, Subetki I, Saadat N, Moini S, Sarvghadi F. Prevalent practices of thyroid diseases during pregnancy among endocrinologists, internists and general practitioners. Int J Endocrinol Metab. 2015;14(1):e29601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toloza FJK, Singh Ospina NM, Rodriguez-Gutierrez R, O’Keeffe DT, Brito JP, Montori VM, Maraka S. Data from: Practice variation in the care of subclinical hypothyroidism during pregnancy: a national survey of physicians in the United States. figshare 2019. Deposited 9 May 2019. 10.6084/m9.figshare.8101670. [DOI] [PMC free article] [PubMed]
- 24. Burch HB, Burman KD, Cooper DSA. A 2011 survey of clinical practice patterns in the management of Graves’ disease. J Clin Endocrinol Metab. 2012;97(12):4549–4558. [DOI] [PubMed] [Google Scholar]
- 25. Burch HB, Burman KD, Cooper DS, Hennessey JVA. A 2013 survey of clinical practice patterns in the management of primary hypothyroidism. J Clin Endocrinol Metab. 2014;99(6):2077–2085. [DOI] [PubMed] [Google Scholar]
- 26. Burch HB, Burman KD, Cooper DS, Hennessey JV, Vietor NOA. A 2015 survey of clinical practice patterns in the management of thyroid nodules. J Clin Endocrinol Metab. 2016;101(7):2853–2862. [DOI] [PubMed] [Google Scholar]
- 27. Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, Chiusano E, John R, Guaraldo V, George LM, Perona M, Dall’Amico D, Parkes AB, Joomun M, Wald NJ. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(6):493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hales C, Taylor PN, Channon S, Paradice R, McEwan K, Zhang L, Gyedu M, Bakhsh A, Okosieme O, Muller I, Draman MS, Gregory JW, Dayan C, Lazarus JH, Rees DA, Ludgate M. Controlled antenatal thyroid screening II: effect of treating maternal suboptimal thyroid function on child cognition. J Clin Endocrinol Metab. 2018;103(4):1583–1591. [DOI] [PubMed] [Google Scholar]
- 29. Liu H, Shan Z, Li C, Mao J, Xie X, Wang W, Fan C, Wang H, Zhang H, Han C, Wang X, Liu X, Fan Y, Bao S, Teng W. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid. 2014;24(11):1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, Bisschop PH. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2011;17(5):605–619. [DOI] [PubMed] [Google Scholar]
- 31. Stagnaro-Green A, Chen X, Bogden JD, Davies TF, Scholl TO. The thyroid and pregnancy: a novel risk factor for very preterm delivery. Thyroid. 2005;15(4):351–357. [DOI] [PubMed] [Google Scholar]
- 32. Medici M, Korevaar TI, Schalekamp-Timmermans S, Gaillard R, de Rijke YB, Visser WE, Visser W, de Muinck Keizer-Schrama SM, Hofman A, Hooijkaas H, Bongers-Schokking JJ, Tiemeier H, Jaddoe VW, Visser TJ, Peeters RP, Steegers EA. Maternal early-pregnancy thyroid function is associated with subsequent hypertensive disorders of pregnancy: the generation R study. J Clin Endocrinol Metab. 2014;99(12):E2591–E2598. [DOI] [PubMed] [Google Scholar]
- 33. Rotondi M, Chiovato L, Pacini F, Bartalena L, Vitti P. Management of subclinical hypothyroidism in pregnancy: a comment from the Italian Society of Endocrinology and the Italian Thyroid Association to the 2017 American Thyroid Association guidelines‒“The Italian Way.” Thyroid. 2018;28(5):551–555. [DOI] [PubMed] [Google Scholar]
- 34. Goldberg AS, Sujana Kumar S, Greenblatt E, Lega IC, Shapiro H. Delaying thyroxine until positive beta-human chorionic gonadotropin is safe for patients receiving fertility therapy: applying new ATA guidelines to subclinical hypothyroidism. J Obstet Gynaecol Can. 2018;40(3):299–303. [DOI] [PubMed] [Google Scholar]
- 35. Stagnaro-Green A. Clinical guidelines: thyroid and pregnancy: time for universal screening? Nat Rev Endocrinol. 2017;13(4):192–194. [DOI] [PubMed] [Google Scholar]
- 36. Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab. 2010;95(4):1699–1707. [DOI] [PubMed] [Google Scholar]
- 37. Srimatkandada P, Stagnaro-Green A, Pearce EN. Attitudes of ATA survey respondents toward screening and treatment of hypothyroidism in pregnancy. Thyroid. 2015;25(3):368–369. [DOI] [PubMed] [Google Scholar]
- 38. Pop VJ, Broeren MA, Wiersinga WM, Stagnaro-Green A. Thyroid disease symptoms during early pregnancy do not identify women with thyroid hypofunction that should be treated. Clin Endocrinol (Oxf). 2017;87(6):838–843. [DOI] [PubMed] [Google Scholar]
- 39. Velasco I, Taylor P. Identifying and treating subclinical thyroid dysfunction in pregnancy: emerging controversies. Eur J Endocrinol. 2018;178(1):D1–D12. [DOI] [PubMed] [Google Scholar]
- 40. Wilson JM, Jungner YG. Principles and practice of mass screening for disease [in Spanish]. Bol Oficina Sanit Panam. 1968;65(4):281–393. [PubMed] [Google Scholar]
- 41. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li C, Shan Z, Mao J, Wang W, Xie X, Zhou W, Li C, Xu B, Bi L, Meng T, Du J, Zhang S, Gao Z, Zhang X, Yang L, Fan C, Teng W. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J Clin Endocrinol Metab. 2014;99(1):73–79. [DOI] [PubMed] [Google Scholar]
- 43. Medici M, de Rijke YB, Peeters RP, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VV, Hofman A, Hooijkaas H, Steegers EA, Tiemeier H, Bongers-Schokking JJ, Visser TJ. Maternal early pregnancy and newborn thyroid hormone parameters: the Generation R Study. J Clin Endocrinol Metab. 2012;97(2):646–652. [DOI] [PubMed] [Google Scholar]
- 44. Quinn FA, Gridasov GN, Vdovenko SA, Krasnova NA, Vodopianova NV, Epiphanova MA, Schulten M. Prevalence of abnormal thyroid stimulating hormone and thyroid peroxidase antibody-positive results in a population of pregnant women in the Samara region of the Russian Federation. Clin Chem Lab Med. 2005;43(11):1223–1226. [DOI] [PubMed] [Google Scholar]
- 45. Männistö T, Surcel HM, Ruokonen A, Vääräsmäki M, Pouta A, Bloigu A, Järvelin MR, Hartikainen AL, Suvanto E. Early pregnancy reference intervals of thyroid hormone concentrations in a thyroid antibody-negative pregnant population. Thyroid. 2011;21(3):291–298. [DOI] [PubMed] [Google Scholar]
- 46. Nazarpour S, Ramezani Tehrani F, Simbar M, Tohidi M, Alavi Majd H, Azizi F. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur J Endocrinol. 2017;176(2):253–265. [DOI] [PubMed] [Google Scholar]
- 47. Nazarpour S, Ramezani Tehrani F, Simbar M, Tohidi M, Minooee S, Rahmati M, Azizi F. Effects of levothyroxine on pregnant women with subclinical hypothyroidism, negative for thyroid peroxidase antibodies. J Clin Endocrinol Metab. 2018;103(3):926–935. [DOI] [PubMed] [Google Scholar]
- 48. James SR, Franklyn JA, Kilby MD. Placental transport of thyroid hormone. Best Pract Res Clin Endocrinol Metab. 2007;21(2):253–264. [DOI] [PubMed] [Google Scholar]
- 49. de Escobar GM, Obregón MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18(2):225–248. [DOI] [PubMed] [Google Scholar]
- 50. Henrichs J, Ghassabian A, Peeters RP, Tiemeier H. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol (Oxf). 2013;79(2):152–162. [DOI] [PubMed] [Google Scholar]
- 51. Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004;151(Suppl 3):U25–U37. [DOI] [PubMed] [Google Scholar]
- 52. Lev-Ran A. Part-of-the-day hypertriiodothyroninemia caused by desiccated thyroid. JAMA. 1983;250(20):2790–2791. [PubMed] [Google Scholar]
- 53. Davidge-Pitts C, Nippoldt TB, Danoff A, Radziejewski L, Natt N. Transgender health in endocrinology: current status of endocrinology fellowship programs and practicing clinicians. J Clin Endocrinol Metab. 2017;102(4):1286–1290. [DOI] [PubMed] [Google Scholar]