Abstract
Electronic nicotine delivery system (e-cigarette) use is prevalent among pregnant women as a seemingly safe alternative to traditional tobacco use, known to result in fetal developmental abnormalities and impaired fertility of male offspring. However, little is known about the effects of e-cigarette use on fertility or pregnancy outcomes. A successful pregnancy is initiated by a multitude of dynamic molecular alterations in the uterus resulting in embryo implantation at day 4.5 in the mouse. We examined whether e-cigarette exposure impairs implantation and offspring health. Pregnant C57BL/6J mice were exposed five times a week to e-cigarette vapor or sham. After 4 months, e-cigarette exposed dams exhibited a significant delay in the onset of the first litter. Furthermore, exposure of new dams in early pregnancy significantly impaired embryo implantation, as evidenced by nearly complete absence of implantation sites in e-cigarette–exposed animals at day 5.5, despite exhibiting high levels of progesterone, an indicator of pregnancy. RNA microarray from day 4.5 pseudopregnant mice revealed significant changes in the integrin, chemokine, and JAK signaling pathways. Moreover, female offspring exposed to e-cigarettes in utero exhibited a significant weight reduction at 8.5 months, whereas males exhibited a slight but nonsignificant deficiency in fertility. Thus, e-cigarette exposure in mice impairs pregnancy initiation and fetal health, suggesting that e-cigarette use by reproductive-aged women or during pregnancy should be considered with caution.
Keywords: pregnancy, e-cigarette, implantation, exposure, tobacco
The use of tobacco products during pregnancy correlates with an increased risk of poor perinatal and obstetrical outcomes [1]. Additionally, babies exposed to cigarette smoke in utero exhibit a higher risk of developmental abnormalities [1]. Increasing in popularity among adolescents and pregnant women [2, 3], electronic nicotine delivery systems (e-cigarettes) are tobacco delivery devices that aerosolize a mixture of propylene glycol and vegetable glycerin with customizable additions of nicotine and flavors into an inhaled vapor [3]. Currently, the England Public Health system has ruled e-cigarettes to be 95% safer than conventional cigarettes [4], despite the fact that long-term and second-generation clinical studies of e-cigarette exposure do not exist [5]. As a result, the public is encouraged to use e-cigarettes as an effective smoking cessation tool [6]. Contrastingly, the European Respiratory Society reported that the safety of extended use of e-cigarettes compared with conventional tobacco is unknown [7]. Thus, studies of the safety of e-cigarettes are needed, especially among pregnant women and future offspring.
Proper functioning of the receptive uterus and successful embryo implantation is necessary for normal pregnancy. Multiple factors facilitate the preparation of the uterine luminal epithelium for reception of the implanting embryo at day 4.5 in the mouse, a critical period referred to as the window of receptivity [8, 9]. Implantation of the blastocyst elicits a dynamic morphological and molecular change of the epithelial and stromal compartments of the uterus [8, 9]. Although difficult to quantify, a predicted 60% of pregnancy losses result from improper uterine receptivity and implantation [10]. Thus, identifying factors that influence uterine and blastocyst biology is of great clinical interest.
Recent literature in the mouse reports that e-cigarette exposure in utero causes changes in metabolic, inflammatory, neurologic, and pulmonary factors in exposed offspring [11, 12]. However, the reproductive fitness and fetal outcomes of dams exposed to e-cigarettes before and during pregnancy have yet to be determined. Here, we directly examine the effects of e-cigarettes on pregnancy initiation and second-generation fetal reproductive health and find that e-cigarettes delay implantation and impair the health of future offspring.
1. Materials and Methods
A. E-Cigarette Exposure
C57BL/6J mice were cared for according to the guidelines of the Institution of Animal Care and Use Committee at the University of North Carolina. Mice were exposed to e-cigarette aerosol in a SCIREQ inExpose whole-body inhalation system (Montréal, QC, Canada) for 3 h/d with two puffs per minute. Puff duration was ∼2 seconds. Vapor was generated with a Joyetech eVic VTC Mini with a 0.15-Ω adjustable-temperature atomizer set to 245°C. The propylene glycol and vegetable glycerin mixture was made fresh in a 55:45 ratio with 24 mg/mL nicotine. No flavorings or other adulterants were added. Sham mice were exposed to room air. For fertility trial, females were mated and exposed on the first day of mating to e-cigarette or sham for 5 days a week, for 4 months. For implantation studies, females were primed with e-cigarettes 5 days a week, for 4 weeks, before mating to intact or vasectomized males. The presence of a copulatory plug was recorded as day 0.5, and mice were exposed for each day of pregnancy until euthanasia.
B. Progesterone Measurements
Progesterone levels were measured from mouse serum via ELISA performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
C. RNA Microarray
RNA quality was assessed on the Agilent 4200 Tapestation and hybridized to the Affymetrix Clariom S Mouse Array at the Functional Genomics Core, University of North Carolina. Raw data were analyzed via one-way ANOVA and normalized, and hierarchal clustering was performed on the Gene Expression platform in the Partek Genomics Suite. Top pathways were identified with Ingenuity Pathway Analysis. Raw data are publicly available on the National Center for Biotechnology Information GEO database, accession number GSE131077.
D. Histology
Tissue was fixed overnight in 4% paraformaldehyde, processed, and embedded in paraffin. Testes were stained overnight in Bouin solution and washed in ethanol for 3 days before embedding. Periodic acid–Schiff staining was performed at Lineberger Cancer Center Histopathology. For implantation studies, the uterus was sectioned, with every fifth slide stained for hematoxylin and eosin (H&E). Embryo presence was scored microscopically.
E. Sperm Analysis
Sperm was harvested, counted on a hemocytometer, and measured for motility on the Hamilton-Thorne CASA system with CASAnova [13].
2. Results
A. E-Cigarette Exposure Delays the Onset of First Litter
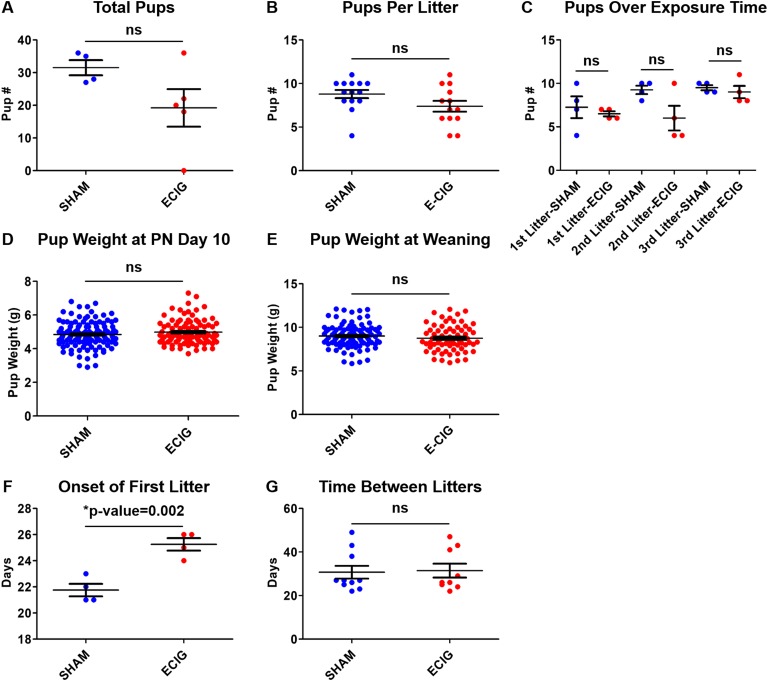
To assess how e-cigarettes affect pregnancy, a fertility trial was performed. To limit the number of confounding variables, female mice were mated to males and exposed to e-cigarettes at the same time, day 0 of the fertility trial. Female mice were left with male mice in mating continuously for 4 consecutive months. After exposure to e-cigarettes or sham while mating, litter incidence and pup number and weight were recorded (Fig. 1, n = 5). E-cigarette dams exhibited a trend for reduced number of total pups and pups per litter, although neither reached statistical significance (Fig. 1A and 1B). Indeed, one e-cigarette dam failed to deliver any pups. Additionally, pup number over time of exposure increased gradually in the sham group as expected in a fertility trial initiated with virgin mice (Fig. 1C). However, animals exposed to e-cigarettes exhibited a slight, insignificant reduction in mean pup number by the second litter, with the highest pup numbers occurring by the third litter. Although the e-cigarette pup numbers per litter across exposure time are slightly lower than those of the sham-exposed group, this decrease was not statistically significant. Thus, e-cigarette exposure mildly decreases offspring number per litter, yet 4-month exposure did not dramatically impair pup number compared with sham. Pup weights exhibited no change (Fig. 1D and 1E). However, e-cigarette dams exhibited a delay in the onset of their first litter by 3 to 4 days (P = 0.002), with no differences in time between subsequent litters (Fig. 1F and 1G). Therefore, e-cigarette exposure significantly delays the onset of the first litter and may contribute to slightly reduced pup numbers.
Figure 1.
E-cigarette (ECIG) exposed mice exhibit slightly reduced offspring number and weight and delayed onset of first litter. Graphs depicting (A) total pups per female; (B) pups born per litter; (C) pups born per litter over exposure time; and pup weights (D) at postnatal (PN) day 10 and (E) at weaning; (F) onset of first litter; and (G) time between litters for e-cigarette–exposed vs sham-exposed mice during a 4-mo fertility trial. Data were analyzed via Student t test. ns, not significant.
B. Embryo Attachment Is Delayed in E-Cigarette Dams
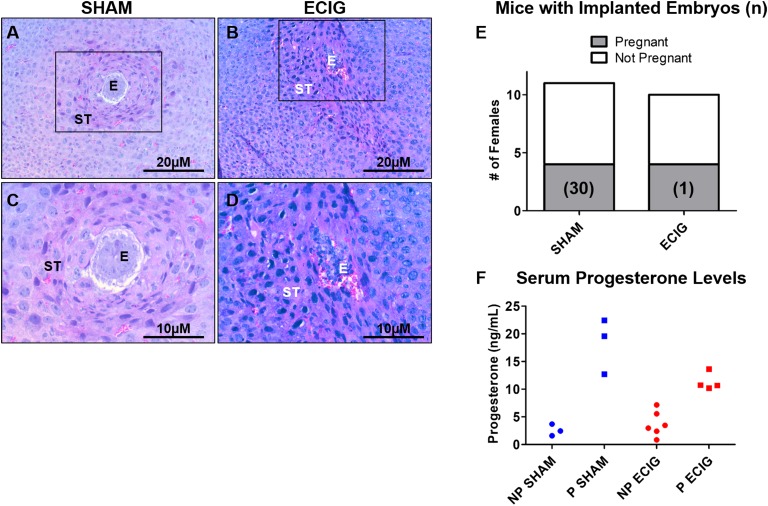
To identify the cause of pregnancy delay observed in the fertility trial, the timing of embryo attachment was examined. To accurately represent e-cigarette exposure in women, virgin mice were exposed to e-cigarette or sham treatment for 4 weeks before mating. Upon successful mating, mice were exposed to e-cigarettes or sham each day of pregnancy and euthanized 5 days after the presence of the copulatory plug (day 5.5). This exposure protocol was designed to mirror the actions of women who initiate use of e-cigarettes while not pregnant and then subsequently become pregnant and continue use of e-cigarettes throughout their pregnancy. After 5 days of exposure, implantation sites were observed in sham animals, yet e-cigarette–exposed uteri did not reveal implantation sites. The sham-exposed implantation sites displayed normal morphology, with surrounding decidualized stromal cells (Fig. 2A and 2C), whereas e-cigarette uteri exhibited only one disorganized implantation site, with hemorrhagic blood cells (Fig. 2B and 2D). No floating embryos were observed. Sham mice displayed a total of 30 embryos representing four dams, and e-cigarette mice exhibited one embryo for 10 dams (Fig. 2E). Because pregnancy was difficult to ascertain in the e-cigarette uteri because of the lack of embryos, we used serum progesterone levels as an indicator of the pregnant state of the dams. Consequently, 4 out of 10 e-cigarette dams were pregnant, with progesterone levels >10 ng/mL, similar to the levels observed in the sham-exposed dams (Fig. 2F). Thus, e-cigarette–exposed mice exhibit a delay in embryo attachment, as few embryos implant by day 5.5 of pregnancy.
Figure 2.
E-cigarettes impair embryo attachment at day 5.5. (A–D) H&E staining from (A, C) sham and (B, D) e-cigarette (ECIG) mice at day 5.5, with higher magnification in boxed regions. (E) Total number of pregnant mice with implanted embryos (n). (F) Serum progesterone results for nonpregnant (NP) and pregnant (P) sham- and e-cigarette–exposed animals. E, embryo; ST, stroma.
C. E-Cigarettes Modulate Receptive Genes at Implantation
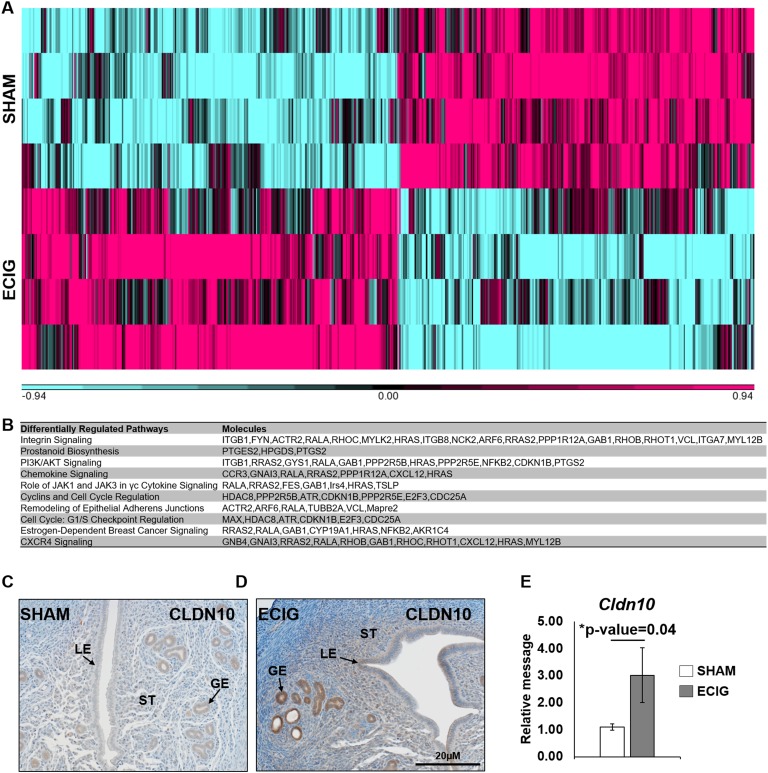
To assess how e-cigarette exposure delays implantation, an RNA microarray was performed on sham and e-cigarette–exposed uteri at pseudopregnant day 4.5 (n = 4). In total, 767 genes were differentially regulated, 395 upregulated and 372 downregulated, hierarchically clustered in Fig. 3A. The top pathways changed included major pathways important for uterine receptivity: integrin, prostanoid biosynthesis, proliferation, JAK, and chemokine signaling (Fig. 3B) [8]. Furthermore, the tight junction protein claudin 10 (CLDN10) was upregulated in the epithelium and stroma of e-cigarette uteri (Fig. 3C and 3D), with increased RNA levels (Fig. 3E). Although the function of CLDN10 in the uterus remains uncharacterized [14], it is known to play important functions in the kidney related to the epithelial transport of ions [15]. Thus, e-cigarette maternal inhalation regulates pathways important for uterine receptivity, including genes involved in ion transport.
Figure 3.
E-cigarette (ECIG) exposure misregulates receptive signaling pathways. RNA microarray on day 4.5 pseudopregnant uteri was analyzed via one-way ANOVA, and significant genes were determined with a P ≤ 0.05 and a fold change >1.14 and <0.714. (A) Hierarchal clustering of 767 significant genes, 395 upregulated and 372 downregulated. (B) Top 10 gene pathways involved in receptivity from the top 50 canonical pathways identified via Ingenuity Pathway Analysis. (C, D) CLDN10 protein expression and (E) RNA level. RNA levels were analyzed via Student t test. GE, glandular epithelium; LE, luminal epithelium; ST, stroma.
D. In Utero E-Cigarette Exposure of Males Results in Minor Reduction in Fertility
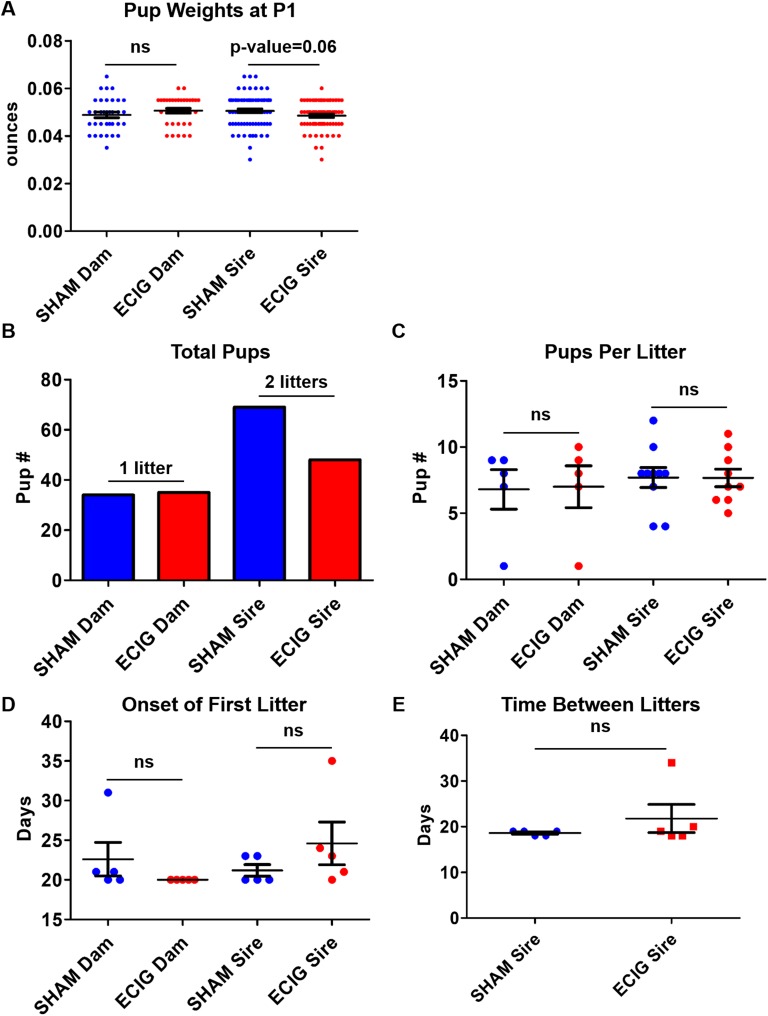
Offspring of women who smoke during their pregnancy demonstrate a higher risk of growth restriction, metabolic disorders, and, specifically in males, impaired reproduction [1]. To assess whether e-cigarettes affect fetal health, litters from exposed dams were aged 8 to 12 weeks and mated. In utero e-cigarette exposure of dams had no effect on reproductive fitness and F2 generation health (Fig. 4A–4D, n = 5). However, exposed sires exhibited a slight reduction in F2 offspring weight (P = 0.06) and pups produced (Fig. 4A and 4B, n = 5). Yet none were significant due to individual variation. Nonetheless, one e-cigarette pair took 35 days to produce their first litter, resulting in a slight variation in litter timing (Fig. 4D and 4E). Thus, in utero e-cigarette exposure does not affect female reproductive fitness, yet males exhibit a slight reduction in fertility and offspring weight and number.
Figure 4.
In utero e-cigarette–exposed males demonstrate slight reduction in fertility rate and offspring number. Graphs depict (A) pup weights at postnatal day 1 (P1), (B) total pup summary, (C) pups generated per litter, (D) onset of first litter, and (E) time between litters for male and female mice exposed to sham and e-cigarettes (ECIG) in utero. One-way ANOVA and Student t test were used to determine significance. ns, not significant.
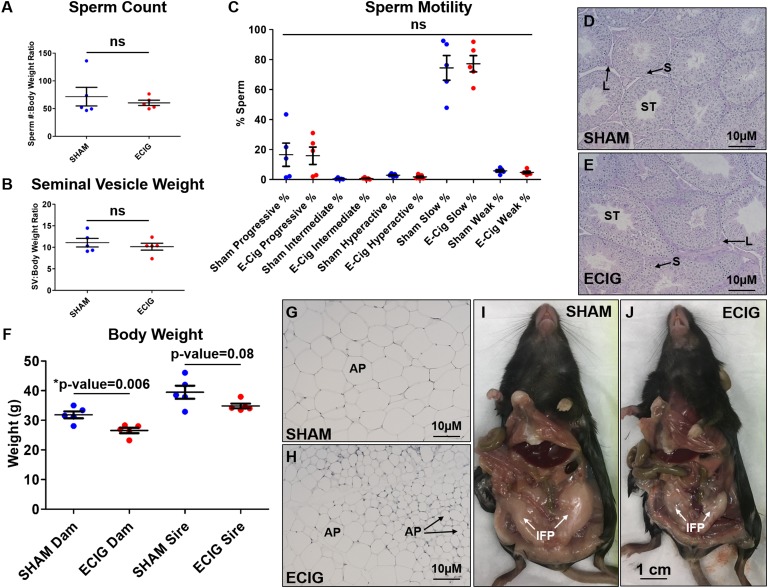
To assess this impairment, in utero sham and e-cigarette–exposed adult males were euthanized, and sperm function and testes morphology were evaluated. There was no significant difference in sperm count, motility, and seminal vesicle weight, an established measure of testosterone [16] (Fig. 5A–5C, n = 5). Additionally, testes morphology appeared normal (Fig. 5D and 5E, n = 5). Therefore, although in utero e-cigarette–exposed males exhibited a slight reduction in fertility and pup number and weight, no phenotype was identified in sperm function or testes morphology.
Figure 5.
E-cigarette exposure in utero has no effect on male reproductive function but reduces weight gain in adulthood. (A) Sperm count to body weight ratios, (B) seminal vesicle weights, (C) sperm motility results from CASAnova analysis, and (D, E) periodic acid–Schiff staining of testes from male offspring exposed to sham or e-cigarettes (ECIG) in utero. (F) Total body weights for sham vs e-cigarette animals at 8.5 mo of age. (G, H) Representative images from sham- vs e-cigarette–exposed females of H&E-stained inguinal adipocytes (AP) and (I, J) gross morphology of inguinal fat pads (IFP). One-way ANOVA and Student t test were used to determine significance. L, Leydig cells; ns, not significant; S, Sertoli cells; ST, seminiferous tubule.
E. In Utero E-Cigarette Exposure Results in Adult Weight Loss in Females
Although no reproductive impairment was identified for the female mice exposed in utero to e-cigarette, upon euthanasia at 8.5 months of age, these mice were significantly smaller in body weight compared with sham-exposed mice (P = 0.006) (Fig. 5F, n = 5). Male mice demonstrated only a minor reduction in body weight (P = 0.08) (Fig. 5F, n = 5). E-cigarette animals had visibly smaller inguinal fat pads and adipocytes compared with sham (Fig. 5G–5J). Thus, e-cigarette exposure in utero results in decreased weight gain in females.
3. Discussion
Because e-cigarettes are increasingly used by reproductive-aged and pregnant women [2, 3], it is important to evaluate whether and how e-cigarettes contribute to pregnancy success and fetal health. By exposing mated mice to e-cigarettes for 4 months, we demonstrated that e-cigarettes delayed the onset of the first litter, with no delay in future pregnancies. Furthermore, total pup and litter number were slightly reduced upon e-cigarette exposure but not significantly different. Additionally, one female exposed to e-cigarettes failed to deliver a litter throughout the fertility trial. Thus, e-cigarette exposure is sufficient to delay a first-time pregnancy and may affect fetal survival. Although minimal, these effects may be exacerbated in people because of individual genetic variability and predispositions, confounding health conditions, or additional environmental effectors, resulting in an unfavorable state of fertility. Thus, further clinical investigations are needed to examine how e-cigarettes may affect pregnancy initiation in women.
To determine how e-cigarette exposure delayed pregnancy, we studied sham- and e-cigarette–exposed mice the day after implantation, day 5.5. E-cigarette–exposed mice did not exhibit implantation sites, despite their ability to maintain successful pregnancies. The absence of implantation sites at this time in mice is rare and typically associated with infertility or lack of pregnancy. Yet we confirmed that e-cigarette–exposed animals were pregnant by serum progesterone levels. Thus, because both treatment groups exhibited similar pregnancy rates and litter sizes, we conclude that e-cigarette exposure permits fertilization yet delays implantation, because only one embryo was identified in four pregnant uteri the day after implantation.
RNA microarray analysis revealed differential expression of established pathways involved in embryo reception. Integrin and JAK/STAT signaling pathways are important for governing the attachment and adherence of the embryo to the epithelial surface [8]. Furthermore, the prostanoid biosynthesis, proliferation, and chemokine signaling pathways are critical for trophoblast invasion and the decidualization process [8]. Additionally, CLDN10 may regulate critical ion transport functions important for implantation. Thus, e-cigarette maternal inhalation regulates the expression of ion transport and receptive signaling pathways, which upon misregulation can perturb the uterine environment and prevent timely initiation of embryo attachment [8, 17].
Because cigarette smoking during pregnancy is associated with fetal abnormalities [1], the long-term health of in utero e-cigarette–exposed offspring was examined. Male offspring exposed to e-cigarettes in utero exhibited a slight impairment of fertility, with one mating pair failing to produce a litter until 35 days after mating. However, no histological testicular phenotype was identified. These data suggest that e-cigarette usage during pregnancy may be safer for male offspring reproductive outcomes than traditional tobacco cigarette smoking. However, e-cigarette usage in combination with other environmental or genetic factors could reduce male reproductive fitness, and these factors were not evaluated in the current study.
Upon in utero exposure to e-cigarettes, female offspring exhibited no difference in weight at birth. However, the same mice were significantly smaller than sham controls at 8.5 months of age. Indeed, e-cigarette mice exhibited smaller inguinal fat pads and adipocytes, suggesting that e-cigarette exposure in utero may regulate metabolic function in adulthood. Other studies have shown that e-cigarette exposure modulates weight in young mice [11, 18, 19]. Thus, our data are consistent with these findings and further imply that genetic and epigenetic programs that affect metabolic dysregulation may be initiated via in utero exposure. However, metabolic effects of e-cigarettes in adulthood are less understood, and future investigation is needed to elucidate the second-generation implications of e-cigarette exposure on metabolism.
Our results indicate that e-cigarettes negatively influence implantation success and the future health of the in utero exposed fetus, resulting in abnormal pregnancy outcomes. These data imply that e-cigarette usage by reproductive-aged women may directly and negatively affect their ability to become pregnant, with potentially deleterious effects on the fetus. Thus, advocacy of e-cigarette usage as a safe alternative during pregnancy or for young reproductive-aged women should be interpreted with caution.
Acknowledgments
The authors acknowledge support from the Caron and Doerschuk laboratories and Debbie O’Brien.
Financial Support: Supported by grants RO1HD060860 (K.M.C.), 18POST33960353 (M.W.), R01HL145396 (C.M.D.), and P50HL120100 (R.T.) from the National Institutes of Health (NIH), American Heart Association (AHA), and US Food and Drug Administration (FDA) Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, AHA, or FDA.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- e-cigarette
electronic nicotine delivery system
- H&E
hematoxylin and eosin
References and Notes
- 1. Holbrook BD. The effects of nicotine on human fetal development. Birth Defects Res C Embryo Today. 2016;108(2):181–192. [DOI] [PubMed] [Google Scholar]
- 2. Wagner NJ, Camerota M, Propper C. Prevalence and perceptions of electronic cigarette use during pregnancy. Matern Child Health J. 2017;21(8):1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suter MA, Mastrobattista J, Sachs M, Aagaard K. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth Defects Res A Clin Mol Teratol. 2015;103(3):186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. East K, Brose LS, McNeill A, Cheeseman H, Arnott D, Hitchman SC. Harm perceptions of electronic cigarettes and nicotine: a nationally representative cross-sectional survey of young people in Great Britain. Drug Alcohol Depend. 2018;192:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jankowski M, Brożek G, Lawson J, Skoczyński S, Zejda JE. E-smoking: emerging public health problem? Int J Occup Med Environ Health. 2017;30(3):329–344. [DOI] [PubMed] [Google Scholar]
- 6. McNeill A, Brose LS, Calder R, Bauld L, Robson D. Evidence Review of E-Cigarettes and Heated Tobacco Products 2018: A Report Commissioned by Publich Health England. London, UK: Public Health England; 2018:6. [Google Scholar]
- 7. Bals R, Boyd J, Esposito S, Foronjy R, Hiemstra PS, Jiménez-Ruiz CA, Katsaounou P, Lindberg A, Metz C, Schober W, Spira A, Blasi F. Electronic cigarettes: a task force report from the European Respiratory Society. Eur Respir J. 2019;53(2):1801151. [DOI] [PubMed] [Google Scholar]
- 8. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol. 2012;357(1–2):108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013;11(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen H, Li G, Chan YL, Chapman DG, Sukjamnong S, Nguyen T, Annissa T, McGrath KC, Sharma P, Oliver BG. Maternal e-cigarette exposure in mice alters DNA methylation and lung cytokine expression in offspring. Am J Respir Cell Mol Biol. 2018;58(3):366–377. [DOI] [PubMed] [Google Scholar]
- 12. Whittington JR, Simmons PM, Phillips AM, Gammill SK, Cen R, Magann EF, Cardenas VM. The use of electronic cigarettes in pregnancy: a review of the literature. Obstet Gynecol Surv. 2018;73(9):544–549. [DOI] [PubMed] [Google Scholar]
- 13. Goodson SG, Zhang Z, Tsuruta JK, Wang W, O’Brien DA. Classification of mouse sperm motility patterns using an automated multiclass support vector machines model. Biol Reprod. 2011;84(6):1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schumann S, Buck VU, Classen-Linke I, Wennemuth G, Grümmer R. Claudin-3, claudin-7, and claudin-10 show different distribution patterns during decidualization and trophoblast invasion in mouse and human. Histochem Cell Biol. 2015;144(6):571–585. [DOI] [PubMed] [Google Scholar]
- 15. Milatz S, Breiderhoff T. One gene, two paracellular ion channels-claudin-10 in the kidney. Pflugers Arch. 2017;469(1):115–121. [DOI] [PubMed] [Google Scholar]
- 16. Deanesly R, Parkes AS. Size changes in the seminal vesicles of the mouse during development and after castration. J Physiol. 1933;78(4):442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruan YC, Chen H, Chan HC. Ion channels in the endometrium: regulation of endometrial receptivity and embryo implantation. Hum Reprod Update. 2014;20(4):517–529. [DOI] [PubMed] [Google Scholar]
- 18. Chen H, Li G, Chan YL, Nguyen T, van Reyk D, Saad S, Oliver BG. Modulation of neural regulators of energy homeostasis, and of inflammation, in the pups of mice exposed to e-cigarettes. Neurosci Lett. 2018;684:61–66. [DOI] [PubMed] [Google Scholar]
- 19. McGrath-Morrow SA, Hayashi M, Aherrera A, Lopez A, Malinina A, Collaco JM, Neptune E, Klein JD, Winickoff JP, Breysse P, Lazarus P, Chen G. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS One. 2015;10(2):e0118344. [DOI] [PMC free article] [PubMed] [Google Scholar]